Abstract
A rapid, efficient, and environmentally friendly method using quick, easy, cheap, effective, rugged, and safe (QuEChERS) combined with ionic liquid-based dispersive liquid-liquid microextraction (QuEChERS-IL-DLLME) prior to high-performance liquid chromatography coupled with photodiode array detection (HPLC-PDA) has been developed for the determination of six triazole fungicides (triazolone, triadimenol, epoxiconazole, flusilazole, tebuconazole, and diniconazole) in various fruits (pear, apple, and grapefruit). Several parameters affecting the extraction efficiency in IL-DLLME, such as type and volume of ionic liquid and acetonitrile volumes and extraction time, were investigated by single factor experiments. Then, the extractant volume, dispersant volume, and extraction time were optimized using response surface methodology (RSM). The optimal values were determined to be within an extractant volume of 63.7 μL, a dispersant volume of 0.43 mL, and an extraction time of 1.7 min, respectively. Under the optimum conditions, an excellent linearity with determination coefficient higher than 0.997 was obtained. The average recoveries in three concentration levels (0.2, 0.5, and 1 mg kg−1) ranged from 63.8 to 119.1 %, respectively, and the relative standard deviations (RSDs) from 1.1 to 12.6 %. The limits of detection (LODs) (S/N = 3) and limits of quantification (LOQs) (S/N = 10) for the six triazole fungicides ranged from 3.4 to 26.8 μg kg−1 and 9.8 to 50.3 μg kg−1, respectively. The proposed method was successfully applied for the determination of trace amounts of triazole fungicides in various fruits including pear, apple, and grapefruit.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pesticides have gained extensive applications to control and improve the quality of agricultural products in modern agriculture, which include insecticides, fungicides, herbicides, and other (LeDoux 2011). Among the major fungicides, triazole fungicides have been widely used in fruits, vegetables, and grain crops during cultivation and storage, thanks to their excellent protective, curative, and eradicant power against a wild spectrum of crop diseases (Kahle et al. 2008; Zhang et al. 2012). However, they are powerful endocrine disruptors and have been demonstrated to change the liver function, decrease kidney weight, and alter urinary bladder structure (Guducu et al. 2011). Therefore, it is necessary to develop sensitive and selective methods for the analysis of triazole residues usually present in trace amounts. Potential analytical techniques include high-performance liquid chromatography (HPLC) with UV, diode array detection (DAD), VWD, and photodiode array (PDA), respectively (Bordagaray et al. 2013; Bordagaray et al. 2014; Gao et al. 2012; Luo et al. 2013; Ye et al. 2012; Zhang et al. 2015), HPLC with tandem mass spectrometry (HPLC-MS/MS) (Li et al. 2013; Zhang and Xu 2014) and capillary electrophoresis (CE) with DAD (Rodriguez et al. 2001), gas chromatography (GC) with FID (Farajzadeh et al. 2010; Farajzadeh et al. 2011; Farajzadeh et al. 2012; Farajzadeh et al. 2013; Freitas et al. 2014; Sarafraz-Yazdi et al. 2012), gas chromatography-mass spectrometry (GC-MS) (Farajzadeh et al. 2012; Freitas et al. 2014; Sarafraz-Yazdi et al. 2012), and gas chromatography-tandem mass spectrometry (GC-MS/MS) (Li et al. 2011).
Quick and effective sample preparation coupled with a reliable analytical technique is imperative. Liquid-liquid extraction (LLE) (Rezaee et al. 2006) and solid-phase extraction (SPE) (Sharif et al. 2006) are the most common sample preparation methods widely used for residue analysis. Recently, a growing number of studies have focused on two kinds of microextractions termed as liquid-phase microextraction (LPME) (Psillakis and Kalogerakis 2003) and solid-phase microextraction (SPME) (Kataoka et al. 2000), based on miniaturization of conventional LLE and SPE, respectively. As a novel LPME, DLLME has been recognized as a very popular preparation technique due to the simplicity of operation, time-saving, low cost, and high enrichment factor (Rezaee et al. 2006). However, hazardous solvents such as halohydrocarbon were frequently used as extraction solvents in the conventional DLLME. To overcome this problem, some low toxic and green solvents such as low-density alcohols and ionic liquids (ILs) have been successfully used as extraction solvents (Leong et al. 2014). Unfortunately, the lack of purification for samples with more complex matrices, such as fruits and vegetables, has caused DLLME to be limited to those with simpler matrices, specifically water and a few fruit juices (Zhang et al. 2014).
At present, “quick, easy, cheap, effective, rugged, and safe” (QuEChERS) sample preparation is the most common technique for multi-residue pesticide analysis in food, especially fruits and vegetables (Anastassiades et al. 2003). Although this technique has rapid cleanup ability, its poor enrichment capacity can lead to higher detection limits, i.e., lower sensitivity, compared with other techniques. Researchers proposed a new method comprised of DLLME preconcentration after QuEChERS extraction (Cunha and Fernandes 2011; Zhang et al. 2014; Zhao et al. 2007). Coupling these techniques takes advantages of the benefits of both methods while reducing some of their drawback. As a novel coupling sample preparation technique, QuEChERS-DLLME has been used for extracting and enriching contaminant residues in not only water and fruit juices but also more complex matrices such as fruits and vegetables compared with DLLME.
To the best of our knowledge, there is no report on the extraction and enrichment of triazole residues in fruit using QuEChERS-DLLME or QuEChERS-IL-DLLME method. In this paper, a simple, rapid, and environmentally friendly method using QuEChERS-IL-DLLME followed by HPLC was applied for the determination of six triazole fungicides in fruits (pear, apple, and grapefruit). Figure 1 shows their structures. Several experimental parameters have been optimized by response surface methodology, and the optimized method was successfully applied to real samples.
Materials and Methods
Chemicals and Reagents
Triadimenol (98.0 % purity), epoxiconazole (98.5 % purity), flusilazole (98.0 % purity), tebuconazole (99.0 % purity), and diniconazole (99.0 % purity) were from Dr. Ehrenstorfer GmbH (Germany). Triazolone (99.7 % purity) was purchased from Sigma-Aldrich (USA).
HPLC-grade methanol, acetonitrile, 1-butyl-3-methylimidazolium hexafluorophosphate ([C4MIM][PF6], >98 % purity), 1-hexyl-3-methylimidazolium hexafluorophosphate ([C6MIM][PF6], ≥98 % purity), and 1-octyl-3-methylimidazolium hexafluorophosphate ([C8MIM][PF6], >98 % purity) were from CNW (Germany). Sodium chloride and anhydrous magnesium sulfate were analytical reagent obtained from Sinopharm Chemical Reagent (Shanghai, China).
The standard stock solution of six triazole fungicides was prepared at the concentration of 100 mg L−1 in methanol and stored in a glass volumetric flask at −50 °C. Standard working solutions at a series of concentrations were prepared by the dilution of aliquots of the stock solution with methanol and stored at 4 °C in a freezer. Deionized water (18 MΏ cm resistivity) from a Milli-Q Advantage A10 SP Reagent Water System (Millipore, Bedford, MA, USA) was used throughout.
Apparatus
A Waters Alliance e2695 Separations Module high-performance liquid chromatography (Waters Co., Milford, MA, USA) equipped with a PDA detector. Data were obtained and processed using Empower chromatography workstation. The separations were carried out in an XBridgeTM-C18 column (Waters Co., Milford, MA, USA) (4.6 × 150 mm, 5 μm). A CL31R refrigerated centrifuge (Thermo Scientific, USA) and GENIUS 3 vortex agitator (IKA, Germany) were used for the sample preparation. A Waters Quatrro-Premier XE UPLC-MS/MS (USA) equipped with an Acquity BEH C18 column (2.1 × 50 mm, 1.7 μm) was used to perform the confirmation.
HPLC Analysis
The chromatographic separation was carried in gradient elution with a mobile phase of (A) water and (B) methanol as follows: 0 min, 60:40; 0.2 min, 60:40; 15 min, 40:60; 25 min, 60:40. The column temperature was 30 °C; the injection volume was 10 μL, and the flow rate was 1 mL min−1. Selected as monitor wavelength for six analytes was 220 nm.
Validation Study
A test mixture with standard triazoles at a series of concentrations of 0.01, 0.1, 0.2, 0.5, 2, and 10 mg kg−1 (triazolone and diniconazole) and 0.03, 0.1, 0.2, 0.5, 2, and 10 mg kg−1 (the other four triazoles) was prepared in pure methanol not blank extract and analyzed under optimized conditions to determine linearity. Instrument precision and repeatability (intra- and inter-day variation) were determined using three replicates of the standard working solution (0.5 mg L−1). The precision was expressed as relative standard deviation (RSD, %).
A detailed study of matrix effects was performed by comparison of standards prepared in solvent and in matrix, a common way to test matrix effects. The differences were calculated using following equation (Botero-Coy et al. 2015). A positive difference value indicated matrix-induced signal enhancement, whereas a negative difference indicated signal suppression. We assumed that no relevant matrix effect occurred when differences were within ±20 %.
Recovery was performed by spiked blank samples (pear, apple, and grapefruit) at three different concentration levels (0.2, 0.5, and 1 mg kg−1) with six replicates. Precision was expressed as RSD.
UPLC-MS/MS Confirmation
UPLC-MS/MS confirmation was performed according to the previously reported procedure (Zhang et al. 2015).
Samples
Pear, apple, and grapefruit were purchased from local supermarkets. Samples were homogenized before extraction to remove the sediments. Pear and apple were prepared in the form of whole fruit, while grapefruit pulp (2 kg each) was separate carefully into peel. A representative portion of these samples (200 g each) was chopped and homogenized in a food chopper (HR 2095, Philips Electronics Co., Hong Kong, China).
QuEChERS-DLLME Procedure
The QuEChERS procedure described below was followed for extraction and cleanup (Zhang et al. 2014): (1) Weigh 5.00 ± 0.01 g of sample into a 50-mL fluorinated ethylene propylene (FEP) centrifuge tube. (2) Add 5.00 mL acetonitrile into each tube to all samples and shake vigorously by hand for 1 min. (3) Keep the tubes in a refrigerator at least for 15 min at −20 °C. (4) Add 2.0 g anhydrous MgSO4 and 0.5 g NaCl and shake vigorously by hand for 1 min. (5) Centrifuge at 10,000 rpm for 5 min. (6) Decant 1.00 mL extracts (upper layer) into the centrifuge tube containing 50 mg PSA. (7) Cap the tubes well and vortex them for 1 min. (8) Centrifuge at 4000 rpm for 5 min. (9) Transfer 0.43 mL extracts (upper layer) into a centrifuge tube, add 63.7 μL of [C6MIM][PF6] (as extraction solvent), and vortex for 1 min.
The DLLME procedure described below was followed for enrichment: (1) Weigh 5.00 g ± 0.01 g of deionized water into a sharp-bottom 15-mL FEP centrifuge tube. (2) Inject the above mixture quickly into water with a syringe to form cloudy solution. (3) Whirl vigorously for 1.7 min. (4) Centrifuge at 4000 rpm for 5 min. (5) Remove the sedimented phase and mix with methanol (1:1, v/v) in a trace intubation.
Results and Discussion
Optimum Separation Conditions
Six triazoles can be separated within 26 min. Although peaks of triadimenol 1 and 2 partially overlapped, they were together integrated without difficulty because the quantitative method depended on peak area sum of triadimenol enantiomers.
Table 1 summarizes the linearity, limits of detection (LODs), and reproducibility of peak area and retention time. The linearity of the method was tested using five different concentrations within the range of 10–15,000 μg L−1 (triazolone and diniconazole) and 30–15,000 μg L−1 (the other four triazoles), executing at least three replication injections. The results reveal a satisfactory linearity for all the analytes with the correlation coefficients (R 2) higher than 0.997 in linear regression equation. Data on the regression equations are listed in Table 1. The LODs (S/N = 3) of the six triazoles were between 5 and 10 μg L−1. The precision (RSDs) of the proposed method in terms of peak area for six replicate injections was 0.8–2.4 %. The RSDs in terms of retention time were between 0.02 and 0.07 %.
Optimization of QuEChERS-DLLME
Since the extraction solution of acetonitrile obtained after QuEChERS was used for dispersive solvent, the types and volumes of extraction solvents were the only parameters to be optimized (Zhang et al. 2014). Several parameters affecting the extraction efficiency in IL-DLLME, such as type and volume of ionic liquid and acetonitrile volumes and extraction time, were investigated by single factor experiments.
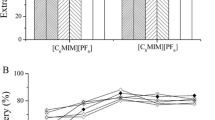
The appropriate extraction solvent in IL-DLLME should meet several requirements including low water solubility, low volatility, and high extraction capability of analytes. In our study, three common ILs including [C4MIM][PF6], [C6MIM][PF6], and [C8MIM][PF6] were used. Unfortunately, when [C4MIM][PF6] was used, no sediment phase was found at the bottom of the tube after centrifugation, which was due to the higher solubility of [C4MIM][PF6] than the other two ILs. Figure 2a showed that the better extraction recoveries were obtained using [C6MIM][PF6] compared with [C8MIM][PF6]. Therefore, [C6MIM][PF6] was used in subsequent experiments.
A series volumes of [C6MIM][PF6] were evaluated for enrichment as follows: 5 g of pear was spiked with the standard solution at 0.5 mg kg−1, 0.5 mL of acetonitrile was used for dispersant, and the extractant volume was changed from 40 to 80 μL in the interval of 20 μL. Observably, the extraction recoveries of the analytes were improved with the increase of the volume of [C6MIM][PF6] (Fig. 2b). When [C6MIM][PF6] was increased from 40 to 80 μL, the recoveries of the analytes were gradually enhanced. The maximal recoveries were mainly obtained at 80 μL. Thereby, 80 μL of [C6MIM][PF6] was selected as the optimal extraction solvent volume.
In order to investigate the effect of dispersant volume, acetonitrile was varied from 0.3 to 0.7 mL in the interval of 0.2 mL while the extractant solvent ([C6MIM][PF6]) was kept at 80 μL. As shown in Fig. 3a, with the increase of acetonitrile from 0.3 to 0.5 mL, the extraction efficiency of most analytes increased gradually while the extraction efficiency of most analytes dropped down slightly above 0.5 mL. Therefore, 0.5 mL of acetonitrile was selected as the optimum dispersant volume to obtain an acceptable recovery.
In DLLME process, an appropriate extraction time is an important stage at which the extraction solvent is well dispersed into the sample solution, meanwhile enlarging the contacting area between the analytes and solution. The effect of extraction time was studied in the range from 0.5 to 4 min (Fig. 3b). The results revealed that the extraction efficiency increased from 0.5 to 2 min and then decreased slowly from 2 min. So, an extraction time of 2 min was chosen.
Optimization of Extraction Conditions by Response Surface Methodology
Response surface methodology (RSM) was carried out in order to establish regression equations between the dependent variables (the average recovery of six analytes) and three effectively independent variables, i.e., extractant volume (X 1), dispersant volume (X 2), and extraction time (X 3). Table 2 shows the variables and levels in the Box-Beknhen design matrix. The lower and upper levels for each variable were selected after running preliminary experiments for the variable.
The analysis of variance for RSM was carried out using the Design-Expert program and is shown in Table 3. In this experiment, the coefficients of X 2, X 3, X 2 X 3, X 2 2 and X 3 2 were statistically significant (p < 0.05), while the other coefficients were not statistically significant. The results were fitted with a second-order polynomial equation. The values of the regression coefficients were calculated, and the response variable and the test variables are related by the following second-order polynomial equation:
Compared with the single factor experiments, the response surface methodology is more accurate. The RSM, which is based on the single factor experiments, further optimizes the conditions. Moreover, the optimum conditions of single factor experiments which came from the designed conditions could not indicate the interactions between two parameters. The 3D response surface plot shown in Fig. 4 indicates the interactions of each two factors on the variation tendency of the average recovery of six analytes. We can get the significance of each parameter from the contour lines of the 3D response surfaces in Fig. 4.
The optimum conditions predicated by RSM were the extractant volume of 63.7 μL, the dispersant volume of 0.43 mL, and the extraction time of 1.7 min. To validate the predicted model, experiments were performed under the modified conditions.
Method Validation
Table 4 showed the average recovery value of the studied triazole fungicides ranging from 63.8 to 119.1 % (RSDs, 1.1–12.6 %). The LODs (S/N = 3) of the six triazoles were between 3.4 and 26.8 μg kg−1. The LOQs (S/N = 10) of the six triazoles were between 9.8 and 50.3 μg kg−1. The enrichment factors were in a range of 10.4 to 14.2 (pear, apple, and grapefruit). Figure 5 shows the chromatogram from the spiked pear at 0.5 mg kg−1 of each triazole fungicide obtained after QuEChERS-IL-DLLME. Obviously, QuEChERS-IL-DLLME is a very simple and effective method for preconcentrating triazole fungicides in fruit. Additionally, there was no interference peak in the typical chromatogram of blank pear after QuEChERS-IL-DLLME.
Table 5 summarizes the details of the proposed method and some other microextraction methods which were applied for triazole fungicide determination in water and fruit juices. Compared with most of the existing reports, more triazole fungicides were analyzed in this study. Most importantly, it was the first report on the extraction and enrichment of triazole residues in fruits using QuEChERS-IL-DLLME method.
Conclusions
In this study, a rapid, simple, and environmentally friendly QuEChERS-IL-DLLME method followed by HPLC-PDA was established to detect six triazole fungicides in pear, apple, and grapefruit. IL was selected as extraction solvent, and less-toxic organic solvent was used compared with the conventional DLLME process. Response surface methodology was used for the optimization of the extraction parameters affecting the extraction efficiency. The results of the method evaluation confirmed the method calibration, precision, and accuracy. The proposed method was successfully applied in the analysis of triazole fungicides in pear, apple, and grapefruit with satisfactory recoveries.
Reference
Anastassiades M, Lehotay SJ, Stajnbaher D, Schenck FJ (2003) Fast and easy Multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J AOAC Int 86:421
Bordagaray A, Garcia-Arrona R, Millán E (2013) Development and application of a screening method for triazole fungicide determination in liquid and fruit samples using solid-phase microextraction and HPLC-DAD. Anal Methods 5:2565
Bordagaray A, Garcia-Arrona R, Millán E (2014) Determination of triazole fungicides in liquid samples using ultrasound-assisted emulsification microextraction with solidification of floating organic droplet followed by high-performance liquid chromatography. Food Anal Methods 7:1195
Botero-Coy AM, Marín JM, Serrano R, Sancho JV, Hernández F (2015) Exploring matrix effects in liquid chromatography-tandem mass spectrometry determination of pesticide residues intropical fruits. Anal Bioanal Chem 407:3667
Cunha SC, Fernandes JO (2011) Multipesticide residue analysis in maize combining acetonitrile-based extraction with dispersive liquid-liquid microextraction followed by gas chromatography-mass spectrometry. J Chromatogr A 1218:7748
Farajzadeh MA, Bahram M, Jafary F, Bamorowat M (2011) Combination of extraction by silylated vessel-dispersive liquid-liquid microextraction as a high-enrichment factor technique: optimization and application in preconcentration of some triazole pesticides from aqueous samples followed by GC-FID determination. Chromatographia 73:393
Farajzadeh MA, Djozan D, Khorram P (2012) Development of a new dispersive liquid-liquid microextraction method in a narrow-bore tube for preconcentration of triazole pesticides from aqueous samples. Anal Chim Acta 713:70
Farajzadeh MA, Djozan D, Nouri N, Bamorowat M, Shalamzari MS (2010) Coupling stir bar sorptive extraction-dispersive liquid-liquid microextraction for preconcentration of triazole pesticides from aqueous samples followed by GC-FID and GC-MS determinations. J Sep Sci 33:1816
Farajzadeh MA, Mogaddam MRA, Aghdam AA (2013) Comparison of air-agitated liquid-liquid microextraction technique and conventional dispersive liquid-liquid micro-extraction for determination of triazole pesticides in aqueous samples by gas chromatography with flame ionization detection. J Chromatogr A 1300:70
Freitas LAD, Vieira AC, Mendonca JAFR, Figueiredo EC (2014) Molecularly imprinted fibers with renewable surface for solid-phase microextraction of triazoles from grape juice samples followed by gas chromatography mass spectrometry analysis. Analyst 139:626
Gao YY, Zhou QX, Xie GH, Yao ZW (2012) Temperature-controlled ionic liquid dispersive liquid-phase microextraction combined with HPLC with ultraviolet detector for the determination of fungicides. J Sep Sci 35:3569
Guducu HE, Inam R, Aboul-Enein HY (2011) Determination of organophosphorus and triazole pesticides by gas chromatography and application to vegetable and commercial samples. J Liq Chromatogr Relat Technol 34:2473
Kahle M, Buerge IJ, Hauser A, Muller MD, Poiger T (2008) Azole fungicides: occurrence and fate in wastewater and surface waters. Environ Sci Technol 42:7193
Kataoka H, Lord HL, Pawliszyn J (2000) Applications of solid-phase microextraction in food analysis. J Chromatogr A 880:35
LeDoux M (2011) Analytical methods applied to the determination of pesticide residues in foods of animal origin: a review of the past two decades. J Chromatogr A 1218:1021
Leong MI, Fuh MR, Huang SD (2014) Beyond dispersive liquid-liquid microextraction. J Chromatogr A 1335:2
Li J, Dong FS, Xu J, Liu XG, Li YB, Shan WL, Zheng YQ (2011) Enantioselective determination of triazole fungicide simeconazole in vegetables, fruits, and cereals using modified QuEChERS (quick, easy, cheap, effective, rugged and safe) coupled to gas chromatography/tandem mass spectrometry. Anal Chim Acta 702:127
Li YB, Dong FS, Liu XG, Xu J, Chen X, Han YT, Liang XY, Zheng YQ (2013) Studies of enantiomeric degradation of the triazole fungicide hexaconazole in tomato, cucumber, and field soil by chiralliquid chromatography-tandem mass spectrometry. Chirality 25:160
Luo M, Liu DH, Zhou ZQ, Wang P (2013) A new chiral residue analysis method for triazole fungicides in water using dispersive liquid-liquid microextraction (DLLME). Chirality 25:567
Psillakis E, Kalogerakis N (2003) Developments in liquid-phase microextraction. Trends Anal Chem 22:565
Rezaee M, Assadi Y, Hosseini MRM, Aghaee E, Ahmadi F, Berijani S (2006) Determination of organic compounds in water using dispersive liquid-liquid microextraction. J Chromatogr A 1116:1
Rodriguez R, Pico Y, Font G, Manes J (2001) Analysis of post-harvest fungicides by micellar electrokinetic chromatography. J Chromatogr A 924:387
Sarafraz-Yazdi A, Assadi H, Ibrahim WAW (2012) Determination of triazole fungicides using hollow fiber liquid phase microextraction prior to gas chromatography-mass spectrometry. Anal Ind Eng Chem Res 51:3101
Sharif Z, Man YBC, Hamid NSA, Keat CC (2006) Determination of organochlorine and pyrethroid pesticides in fruit and vegetables using solid phase extraction clean-up cartridges. J Chromatogr A 1127:254
Ye CL, Liu QL, Wang ZK, Fan J (2012) Dispersive liquid-liquid microextraction combined with high-performance liquid chromatography-ultraviolet detection for the determination of three triazole derivatives in environmental water samples. Int J Environ Anal Chem 92:1196
Zhang H, Qian MG, Wang XQ, Wang XY, Xu H, Wang Q, Wang MH (2012) HPLC-MS/MS enantioseparation of triazole fungicides using polysaccharide-based stationary phases. J Sep Sci 35:773
Zhang Y, Xu H (2014) Determination of triazoles in tea samples using dispersive solid phase extraction combined with dispersive liquid-liquid microextraction followed by liquid chromatography-tandem mass spectrometry. Food Anal Methods 7:189
Zhang YH, Zhang XL, Jiao BN (2014) Determination of ten pyrethroids in various fruit juices: comparison of dispersive liquid-liquid microextraction sample preparation and QuEChERS method combined with dispersive liquid-liquid microextraction. Food Chem 159:367
Zhang YH, Zhang Y, Zhao QY, Chen WJ, Jiao BN (2015) Vortex-assisted ionic liquid dispersive liquid-liquid microextraction coupled with high-performance liquid chromatography for the determination of triazole fungicides in fruit juices. Food Anal Methods (in press)
Zhao E, Zhao W, Han L, Jiang S, Zhou Z (2007) Application of dispersive liquid-liquid microextraction for the analysis of organophosphorus pesticides in watermelon and cucumber. J Chromatogr A 1175:137
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This study was funded by National Risk Assessment Program for Agricultural Products Quality and Safety (no. GJFP2015002, no. GJFP2015004) and Scientific and Technological Innovation Project of Chinese Academy of Agricultural Sciences, China Agriculture Research System (CARS-27), and Natural Science Foundation of Chongqing (no. cstc2013jcyjA80004).
Conflict of Interest
Yaohai Zhang declares that he has no conflict of interest. Yan Zhang declares that she has no conflict of interest. Jiyun Nie declares that he has no conflict of interest. Bining Jiao declares that he has no conflict of interest. Qiyang Zhao declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Additional information
Yaohai Zhang and Yan Zhang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhang, Y., Zhang, Y., Nie, J. et al. Determination of Triazole Fungicide Residues in Fruits by QuEChERS Combined with Ionic Liquid-Based Dispersive Liquid-Liquid Microextraction: Optimization Using Response Surface Methodology. Food Anal. Methods 9, 3509–3519 (2016). https://doi.org/10.1007/s12161-016-0548-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-016-0548-9