Abstract
A novel dispersive micro-solid-phase extraction (DMSPE) method is established for the determination of seven acaricides (clofentezine, benzomate, fenpyroximate, diafenthiuron, pyridaben, doramectin, and ivermectin) in fruit juice and functional food by ultra-high-performance liquid chromatography. In this method, these analytes were extracted from apple juice and curcuma wenyujin using cucurbituril used as the adsorbent, water as the extraction solvent, and methanol as the elution solvent. The primary parameters affecting the extraction efficiency were optimized by experiments for concentration of adsorbent, oscillation time, desorption solvent, and pH value and by response surface methodology. Under the optimal conditions, all target analytes had a good linear relationship, with correlation coefficients ranging from 0.9900 to 0.9940, the limits of detection were 0.42–5.16 ng/mL, the limits of quantification were 1.39–17.18 ng/mL, and the spiked recoveries of seven target compounds in real samples ranged from 70.04 to 94.95%. The intra-day precision RSD variations ranged from 0.13 to 0.70%, and the inter-day precision RSDs were between 0.38 and 1.4%, which had good reproducibility and repeatability. In general, DMSPE is a convenient, efficient, and environmentally friendly method for sample preparation, which has the potential for microextraction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Clofentezine, fenpyroximate, diafenthiuron, pyridoxal, and ivermectin are broad-spectrum acaricides that have been developed to control insects and mites (Kasiotis et al. 2018). These acaricides are used in the cultivation of fruit trees and herb to minimize the possible impact of diseases, weeds, and pests, but they are worth noting that the widespread use of these pesticides has led to direct food and environmental pollution (Zhang et al. 2013). In addition, acaricide residues in fruits and natural products have aroused public health concerns because many people drink commercial fruit juice in daily life (Zeng et al. 2017). The Association of the Industry of Juices and Nectars and the European Union Directive on Fruit Juice Quality stipulate that the maximum allowable concentration is 0.010 mg/kg for total pesticides. Therefore, it is necessary to monitor the quality of fruit juice and herb. It is required to establish a sensitive, efficient, and suitable analytical method for the determination of acaricides in samples. In recent years, the methods for detecting acaricides residues in actual fruit juice and herb samples need to be prepared before instrument analysis. In previous studies, some extraction methods, such as dispersive liquid–liquid extraction (Zhang et al. 2021), magnetic solid-phase extraction (MSPE) (Wang et al. 2018), single drop microextraction (Pakade and Tewary 2010), and solid-phase extraction (SPE) (Liang et al. 2018), have been established for various sample pretreatment to enrich and separate pesticide residue–containing samples. However, the most obvious disadvantage of the above-mentioned method is that the amount of solvent used is large and the operation is complicated. Thus, it makes sense to develop efficient and miniaturized pre-treatment methods.
In recent years, many extraction methods have been reported, such as MSPE technology (Mohamed et al. 2019) and SPE technology (El-Wekil et al. 2018a, b). In addition, a small extraction model of dispersive micro-solid-phase extraction (DMSPE) has also been proposed. This model disperses the adsorbent into the sample so that the adsorbent is in close contact with the target, which improves the adsorption kinetics and improves the extraction efficiency (Sahebi et al. 2020). For example, the developed method was relied on dispersive solid-phase extraction using synergistic effect of reduced graphene oxide and cobalt hydroxide nanoparticles in addition to cloud point extraction using polyethylene glycol 6000 as non-ionic surfactant (El-Wekil et al. 2018a, b). Compared with traditional SPE, it utilizes the dispersion of adsorbent particles and shortens the extraction time. In addition, DMSPE has been widely used in the fields of pharmacy (Ali et al. 2021), food (Bozorgzadeh et al. 2020), and environment (Nascimento et al. 2019) due to its significant advantages such as low organic solvent consumption, short extraction time, simple operation, and low cost. In the DMSPE method, an important factor to be considered in terms of efficiency and extraction capacity is the type of adsorbent material used. Adsorbents have received extensive attention because of their strong adsorption capacity for adsorbents and generally do not react with adsorbents. According to previous studies, the common adsorbent materials used in the proposed method include phenyl methanethiol nanomagnetic composite (Shirkhanloo et al. 2021), nanometer-sized titanium dioxide (Hu et al. 2020), anionic surfactant sodium dodecyl sulfate (Alyami et al. 2021), and cationic gemini surfactant (Hu et al. 2020).
Cucurbituril is one of the representative macrocyclic supramolecules after crown ethers, cyclodextrins, and calixarene, which is obtained by the reaction of glycoluril and paraformaldehyde under strong acid conditions (Zhang et al. 2018). The excellent host–guest recognition ability of cucurbituril is determined by the larger hydrophobic cavity and multi-electron carbonyl port in its structure (Cui et al. 2021). During the process of identifying and binding small molecules, the hydrophobic interaction between the cucurbituril cavity and the molecule is an important driving force for recognition and binding, and the hydrophobic part of the small molecule is wrapped in the cavity. The electrostatic interaction and hydrogen bond between the carbonyl port and the positively charged part of the guest molecule play a role in stabilizing the composite structure and finally form a diverse and stable composite system. Based on the novel chemical structure and unique properties of cucurbituril, it is widely used in the fields of molecular recognition (Li et al. 2020), catalytic reactions (Mustafa et al. 2021), drug carriers (Breve et al. 2020), molecular packaging (Hasanzade and Raissi 2020), separation materials (Papadourakis et al. 2018), and sewage treatment (Ruz et al. 2021). Some previous studies have shown that cucurbituril as a new type of adsorption material can efficiently adsorb dye molecules and metal ions (Sun et al. 2016), but there is no research report on the adsorption of pesticide residues.
This study mainly aims to develop a rapid, sensitive, and accurate microextraction method for the detection and quantification of acaricides in fruit juice and functional food. In order to achieve this goal, the cucurbituril as DMSPE adsorbent was studied to evaluate the applicability of this method. The proposed method had been developed and verified from the aspects of single factor optimization (concentration of adsorbent, oscillation time, desorption solvent, and pH value), response surface methodology, and methodology verification and so on. Under the optimized conditions, the proposed method was effectively applied to the extraction and enrichment of seven acaricides from apple juice and curcuma wenyujin.
Experimental
Chemicals and Reagents
The analytical reagents were supplied by Sigma-Aldrich Shanghai Trading Co., Ltd. (Shanghai, China), including analytical grade C18, anhydrous magnesium sulfate (MgSO4), sodium chloride (NaCl), hydrochloric acid (HCl), sodium hydroxide (NaOH), and ethyl acetate (EtOAc). HPLC-grade methanol (MeOH), ethanol (EtOH), and acetonitrile (ACN) were obtained from Merck Millipore (Darmstadt, USA). Cucurbituril (99.3%) was acquired from Hubei Jusheng Technology Co., Ltd (Hubei, China). Standards containing clofentezine (98.6%), fenpyroximate (99.5%), pyridaben (99%), doramectin (98%), and ivermectin (95%) were purchased from Aladdin Bio-Chem Technology Co., Ltd. (Shanghai, China), while benzomate (99.1%) and diafenthiuron (98.1%) were purchased from Tan Mo Quality Inspection Technology Co., Ltd (Beijing, China). The pure water for the whole experiment was provided by Hangzhou Wahaha Group Co., Ltd. (Hangzhou, Zhejiang, China). The apple and curcuma wenyujin were purchased from a local market (Hangzhou, Zhejiang, China).
Instrumentation and Chromatographic Conditions
The entire experiment was performed on an Agilent 1290 ultra-high-performance liquid chromatography (UHPLC) system (Agilent Technologies, Santa Clara, CA, USA) with a binary pump, a diode array detector (DAD), and an automatic sampler. The chromatographic separation was performed on an Agilent Eclipse Plus C18 (2.1 mm × 100 mm i.d., 3.5 μm). The water and ACN were taken as mobile phase A and B, respectively. Therefore, the elution gradient is as follows: 0–2 min, 15–50% B, 2–4 min, 50–80% B, 4–10 min, 80–100% B. The mobile phase flow rate at 0.4 mL/min and injection volume was 2 μL. The detection wavelength of all analytes was at 240 nm, and the temperature of the column was kept at 30 °C.
Preparation of Standard and Sample Solutions
Individual stock standard solutions of benzomate, fenpyroximate, diafenthiuron, pyridaben, doramectin, and ivermectin were prepared at a concentration of 1000 μg/mL, while clofentezine was prepared at a concentration of 250 μg/mL and was stored at 4 °C in the dark before the working standard solutions were prepared by diluting the stock solutions in methanol.
First, crush the apples into apple juice and crush the functional food of curcuma wenyujin into powder. A portion of 1 mL apple juice and 1 g functional food powder sample were weighed into a 30-mL wide-mouth bottle, and 15 mL ACN was then added. The 2.0 g of NaCl and 4.0 g MgSO4 were added and the mixture was ultrasound with an KQ-300DE (Kun Shan Ultrasonic Instruments Co., Ltd) for 30 min. Finally, the supernatant is stored in a centrifuge tube (4 °C). One hundred milligrams of C18 sorbent prewashed with ACN was mixed with apple juice and curcuma wenyujin powder extract (1.0 mL) in a 3.0 mL the SPE column. The analytes were eluted from the SPE cartridge with 5 × 1 mL of ACN, and the eluate was collected in a centrifuge tube. After centrifugation, the supernatant was filtrated by a 0.22-μm nylon filter for UHPLC analysis.
Analytical Procedure of DMSPE
The seven acaricides in samples were extracted by DMSPE using cucurbituril as adsorbent material. The accurately weighed cucurbituril (1.0 mg) was added into 10 mL distilled water and put into an ultrasonic bath (100 W, 40 Hz) for 30 min to obtain a cucurbituril solution with a concentration of 100 μg/mL. The samples containing seven acaricides at a certain final concentration 0.4–2 μg/mL and 60 μL of the cucurbituril dispersion at a final concentration of 0.6 μg/mL were quantified to 10 mL of water into 30-mL wide-mouth bottle and using the oscillating shaker at a maximum speed (500 rpm) for 60 s. In this step, the analytes interact with cucurbituril and were separated and concentrated on the adsorbent material. Transfer the liquid in wide-mouth bottle to a 10.0-mL syringe with a precision pipette, filter it with a 0.45-μm disposable nylon filter, and retain the nylon filter. Then, a 1.0-mL syringe was selected and eluted the analyte on the disposable nylon filter with 100 μL of methanol. Finally, moderate samples (2 μL) were injected into the UHPLC for identification and quantification of the acaricides. In addition, after the cucurbituril solution is prepared, it is relatively stable within 2 h of standing at room temperature. And the diagram for the DMSPE procedure is in Fig. 1.
Optimization of the DMSPE Procedure
In order to acquire the best extraction condition and great sensitivity, several parameters that may influence the proposed DMSPE method are considered in-depth prior to conducting experiments, such as concentration of adsorbent, oscillation time, desorption solvent, or pH value. During the extraction optimization process, the concentration of cucurbituril is optimized from 0.2 to 1.0 μg/mL, oscillation time is optimized from 30 to 150 s, pH is optimized from 2.0 to 12.0, and the optimization of the elution solvent includes methanol, ethanol, acetonitrile, chloroform, and ethyl acetate. Then, the response surface methodology (RSM) analysis method is carried out. After preliminarily determining the range of each variable through previous experiments, three experimental factors including concentration of cucurbituril (X1, 0.2–1.0 μg/mL), extraction time (X2, 30–150 s), and pH (X3, 2–12) were chosen as the independent variables, and the content of analytes was set as response variables (Y).
Calculations
Enrichment Factors and Extraction Recovery
To evaluate the performance of the proposed methods, the enrichment factors (EFs) and extraction recovery (ER%) were calculated using Eqs. (1) and (2) (Peng et al. 2013).
where Cfinal and Cinitial are the concentrations related to the eluting phase and the initial sample solution, respectively; Vs is the volume of the sample solution; and Ve is the volume of the eluting phase.
Adsorption Capacity
Adsorption capacity (Qe, μg/mg), each analyte adsorbed per unit mass of adsorbent could be calculated by Eq. (3) (Xiao et al. 2020):
C0 and Ce are the initial concentration and equilibrium concentration (μg/mL) of the analyte; V is the volume of the mixed solution (mL); and W is the weight of cucurbituril (mg).
Results and Discussion
Optimization of DMSPE Procedure Conditions
Effect of Cucurbituril Concentration
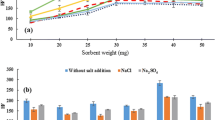
The amount of cucurbituril was a key factor affecting the enrichment factor because the amount of adsorbent influenced the extraction process and the ability of the adsorbent (Khezeli and Daneshfar. 2017). In this study, a series of experiments were carried out in the range of 0.2–1.0 μg/mL by changing the concentration of the adsorbent (cucurbituril) in the solution. The other conditions were that methanol was the desorption solvent, the oscillation time was 1 min, elution solvent was methanol (100 μL), and the pH was 7.0. The obtained results in Fig. 2A show that the content increases with enhancing the concentration of the sorbent from 0.2 to 0.6 μg/mL. This result may be high concentration of cucurbituril enhance the surface area and the interaction between the analytical compound and the adsorbent, and then increasing the extraction efficiency. When the concentration of the adsorbent increased from 0.6 to 0.8 μg/mL, the content of seven acaricides decreased. This phenomenon indicates that the higher the concentration of cucurbituril, the stronger the adsorption capacity of the target compound, and the target compound is difficult to be eluted by the eluent, the lower the extraction efficiency (Karcher et al. 2001). And then 0.8 to 1.0 μg/mL the content nearly remained unchanged. According to the above test results, 0.6 μg/mL cucurbituril solution was selected as the best concentration of adsorbent for further study. To guarantee the extraction of target compounds, the optimal extraction time was 60 s.
Effect of Extraction Time
Extraction time is one of the primary factors in the DMSPE which was investigated by using oscillation method. In order to identify the optimal time obtained for the extraction, extraction time (30, 60, 90, 120, and 150 s) was assessed under the same conditions (cucurbituril concentration 0.6 μg/mL, oscillation speed 500 rpm, elution solvent methanol 100 μL, pH 7). The effect of extraction time on the content of acaricides is shown in Fig. 2B. The results showed that the content of all analytes increased with the increase of oscillation time from 30 to 60 s. Generally speaking, sufficient oscillation time in a certain range could the adsorbent fully contact with the compounds, which will increase the extraction efficiency. However, from 60 to 150 s, the content of the target analytes decreased with the increase of oscillation time. This may be due to the equilibrium of dynamic distribution at 60 s, after which a longer oscillation time may lead to the redissolution of the target analytes into the solution, which may adversely affect the extraction efficiency. To guarantee the extraction of target compounds, the optimal extraction time was 60 s.
Effect of Elution Solvent
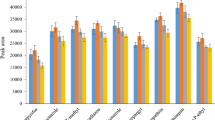
The selection of elution solvent is a crucial parameter that directly affects the extraction efficiency, because the elution solvent could be strong enough to break the interaction between the adsorbent and the analytes. In order to find the optimal elution solvent, there are five kinds of organic solvents: methanol, ethanol, acetonitrile, chloroform, and ethyl acetate were used as solvents to evaluate the influence the extraction performance of target compounds, and other conditions remain the same (cucurbituril concentration 0.6 μg/mL, oscillation speed 500 rpm, oscillation time 60 s, elution solvent volume 100 μL, pH 7). As seen in Fig. 2C, the dates indicated that the methanol showed the highest content, followed by ethyl acetate, ethanol, acetonitrile, and chloroform. This phenomenon could be explained by methanol having the characteristics of high polarity; thus, the elution ability is strong. It can be seen from the figure that chloroform has strong elution ability for clofentezine, but chloroform has poor elution ability for other acaricides, so chloroform is not selected as the elution solvent. Therefore, based on the above experimental results, methanol was selected as the optimum extraction solvent because of its high extraction efficiency. In the previous pre-experiment, the elution volume was greater than 100 μL; it had a dilution effect, which could obviously affect the extraction efficiency. The elution volume was less than 100 μL, the elution could be incomplete and also affect the experimental effect, so choose the elution volume for 100 μL.
Effect of pH Value
The pH value of sample solution is one of the key factors that influenced the existing form of analytes in sample solution (Sun et al. 2015). In this work, the extraction efficiency of the target analytes was studied in the pH range of 2.0–12.0 and the pH value of the sample solution was adjusted with 1.0 mol/L HCl or 1.0 mol/L NaOH. As can be seen from Fig. 2D, maximum content were obtained when pH was 3.0. Figure 2D shows that the extraction efficiency of the seven target compounds increases significantly with the increase of pH value from 2.0 to 3.0 and decreases when pH value exceeds 3.0. The influence of pH on the adsorption efficiency is due to its effect on the surface properties of the adsorbent in aqueous solution. When pH was 3.0, the cucurbituril has a positive surface charge; the adsorbent protonated with hydrogen ion in solution. The interaction between the analytes and the adsorbent is strong, the desorption of acaricide by the eluent was facilitated, resulting in greater extraction efficiency.
The pKa values of the clofentezine, benzomate, fenpyroximate, diafenthiuron, pyridaben, doramectin, and ivermectin were 1.68, 1.78, 1.58, 12.19, 2.69, 12.42, and 12.80, respectively. The pKa reflects the ability of an acid to transfer protons to water to form H3O+, which reflects the strength of the acid (Babić et al. 2007). The smaller the pKa value, the stronger the acidity. It can be seen from Fig. 2D that the acidic conditions (pH 3) favored the interactions of the analytes with cucurbituril by intermolecular interactions, as hydrogen bond, which is the major role by the high recovery of analytes. In particular, the extraction efficiency of clofentezine and benzomate were inhibited under alkaline conditions, and the other compounds did not change significantly. Therefore, acidic environment is conducive to adsorption, and the pH value was adjusted 3.0 for the following experiment.
Optimization of Extraction Conditions Based on RSM
In order to optimize the extraction conditions, the RSM was established by describing the relationship between each variable and the response in the experiment (Song et al. 2008). Box-Behnken design (BBD) is a commonly used experimental design method for response surface optimization. After preliminarily determining the range of each variable through previous experiments, three experimental factors including concentration of cucurbituril (X1, 0.2–1.0 μg/mL), extraction time (X2, 30–150 s), and pH (X3, 2–12) were chosen as the independent variables, and the content of analytes was set as response variables (Y). Table S1 showed the experimental data and content of 17 random ordered runs. According to the quadratic polynomial model, different variable combinations were used to optimize the extraction conditions of DMSPE. The Desk-Expert software (Version 8.0.6) was used to evaluate the coefficients in the multiple linear regression model, and the final models of the seven target analytes were as follows:
-
Y(Clofentezine) = 4.10 + 0.47X1 + 0.23X2–0.1X3 + 0.40X1X2–0.031X1X3 + 0.29X2X3–0.64X12–0.36X22–0.41X32.
-
Y(Benzomate) = 8.06 + 0.78X1–0.052X2–0.36X3 + 0.90X1X2 + 0.18X1X3 + 0.83X2X3–1.35X12–0.50X22 + 0.077X32.
-
Y(Fenpyroximate) = 9.67 + 0.61X1 + 0.54X2–0.16X3 + 0.96X1X2 + 0.18X1X3 + 0.68X2X3–1.21X12–0.79X22–0.14X32.
-
Y(Diafenthiuron) = 9.66 + 0.63X1 + 0.64X2–0.26X3 + 1.00X1X2 + 0.55X1X3 + 0.72X2X3–1.29X12–0.91X22–1.76E-003X32.
-
Y(Pyridaben) = 9.67 + 0.51X1 + 0.69X2–0.19X3 + 1.06X1X2 + 0.26X1X3 + 0.68X2X3–1.27X12–0.88X22–0.066X32.
-
Y(Doramectine) = 9.25 + 0.79X1 + 0.37X2–0.15X3 + 0.78X1X2 + 0.13X1X3 + 0.74X2X3–1.19X12–0.79X22–0.20X32.
-
Y(Ivermectine) = 9.19 + 0.93X1 + 0.53X2–0.091X3 + 1.12X1X2 + 0.053X1X3 + 0.74X2X3–1.24X12–0.37X22–0.16X32.
Analysis of variance (ANOVA) test was used to analyze the suitability and evaluation of the regression model. Analysis of variance, goodness of fit, and adequacy of seven acaricides (clofentezine, benzomate, fenpyroximate, diafenthiuron, pyridaben, doramectin, and ivermectin) are concluded in Table S2. The p values were applied to test the significance of the differences between the statistical variables, in order to understand the interaction strength between the variables (Pourmortazavia et al. 2019). According to ANOVA, Table S2 showed that the p values of seven acaricide compounds were less than 0.0500, which indicated that the quadratic polynomial regression model was significant. In addition, the p values for lack of fit (0.8469–0.9909) were non-significant, which demonstrated that the regression model greatly expressed the experimental results. The determination coefficients (R2) of clofentezine, benzoate, fenpyroximate, diafenthiuron, pyridaben, doramectin, and ivermectin for the response were 0.9706, 0.9410, 0.9786, 0.9669, 0.9799, 0.9801, and 0.9370, which implied 93.70–98.01% of total variation could be interpreted by this model. The adjusted coefficient of multiple determination (Radj2) of acaricide compounds ranged from 0.9370 to 0.9801, which demonstrated that the model could predict the change of extraction efficiency, and the coefficient of variation (C.V%) varied from 2.95 to 6.02, which verified the availability of the experimental results of the regression model.
The three-dimensional (3D) response surface diagram could intuitively reflect the influence of various factors on the response value in order to find the optimal extraction parameters and the interaction between the parameters. The response surface of the model described that by adjusting these two factors at the same time, the third variable was kept at the central level (level 0), which could intuitively reflect the influence of the independent variable on the dependent variable. Thus, extraction efficiency of seven compounds were affected by the concentration of adsorbent (X1), extraction time (X2), and pH (X3) as shown in Fig. S1. On the basis of the statistical analysis and the 3D response plots, the variables X1 and X2 had a significant effect on the extraction rate in this model, while the variable X3 had non-significant effect on the extraction efficiency. According to RSM, the optimal extraction conditions were predicted as follows: concentration of cucurbituril was 0.63 μg/mL, extraction time was 70 s, and pH was 2.8 for the subsequent sample experiments.
Sorption Capacity of Cucurbituril
The adsorption capacity of the adsorbent is an important factor, which determines the amount of adsorbent needed in the sample solution to quantitatively adsorb the target analyte (Rojas et al. 2013). In order to determine the adsorption capacity of cucurbituril for the acaricide analyte, adsorption tests were performed at room temperature. In this experiment, about 0.3-mg cucurbituril particles were added to a 10-mL sample solution, where the concentration of clofentezine was 2.5 to 20 μg/mL, and the concentration of other analytes was 10–80 μg/mL. All the experiments were carried out in triplicate. The relationship between adsorption capacity and target analyte concentration is shown in Figure S2. It can be seen from the curve that as the initial concentration of the target analyte in the sample solution increases, the amount of the target analyte adsorbed per unit mass of cucurbituril increases significantly, and then reaches the plateau value, which determines the adsorption capacity of the relevant adsorbent. In this study, the sorption capacities of clofentezine, benzomate, fenpyroximate, diafenthiuron, pyridaben, doramectin, and ivermectin were determined to be 601, 1665, 1995, 1933, 2322, 2659, and 2326 μg/mg, respectively.
Method Validation
Based on the previous optimal experimental conditions, linear range (LR), correlation coefficient (R2), the limit of detection (LOD) and quantification (LOQ), relative standard deviation (RSD), and other quantitative characteristics of the proposed method were evaluated to verify the effectiveness of the DMSPE method. The results of the calibration curves are shown in Table 1, and the selected acaricides had a good linear relationship with the method (R2 ranged from 0.9900 to 0.9940). The extraction efficiency ranged from 78.89 to 98.36%. In order to evaluate the precision of the method, the 2 μg/mL acaricides was used as the standard solution, and the experiment was repeated six times on 1 day and 3 consecutive days. The intra-day precision RSD variations of retention time ranged from 0.007 to 0.033% and peak areas ranged from 0.126 to 0.695%, while the inter-day precision RSD variations of retention times were between 0.018% and 0.307% and peak areas were between 0.377 and 1.357%. According to the signal–noise ratio, the LOD and LOQ (S/N) of each compound were 3 and 10, respectively. The LOD values of seven analytes ranged from 0.418 to 5.155 ng/mL, and LOQ values ranged 1.394 to 17.182 ng/mL, demonstrating that the established method had ultrahigh sensitivity. The EFs as for clofentezine, benzomate, fenpyroximate, diafenthiuron, pyridaben, doramectin, and ivermectin were 308, 208, 1975, 2156, 2529, 1362 and 1479, respectively. Obviously, compared with traditional methods, the developed method has significant advantages.
Application to Real Samples
The applicability and accuracy of the DMSPE method in actual samples were studied by extracting of acaricides from apple juice and curcuma wenyujin samples under the optimal conditions. Figure 3 showed UHPLC chromatograms of DMSPE method for 7 acaricides. And UHPLC typical chromatograms of apple juice samples with 0.1 μg/mL spiked and after DMSPE method are shown in Fig. 4. The results indicated that clofentezine, benzomate, fenpyroximate, diafenthiuron, pyridaben, doramectin, and ivermectin residues in all samples were lower than the detection level. Thus, the accuracy of the proposed method was evaluated by the recovery rate of standard addition. In the recovery experiment, seven acaricide pesticide standards with concentrations of 0.1 and 1 μg/mL were added to the samples. The quantitative results and recovery of target compounds are summarized in Table S3. The spiked recoveries of seven target compounds in real samples ranged from 70.04 to 94.95%. And the RSD variations of retention times ranged from 0.54 to 1.66%, and peak areas ranged from 3.44 to 9.82%. In conclusion, this method had good effectiveness and high sensitivity for the detection of acaricides.
Comparison of the Proposed Method with Other Methods
The DMSPE acaricide determination method proposed in this article is compared with other published methods and is summarized in Table 2. It could be concluded from Table 2 that there are seven compounds analyzed in this experiment, the analysis time is the least compared with other methods. The extraction solvent was water, and few organic solvents were used in the entire extraction process, which is safer and more environmentally friendly. At the same time, the experimental process was simple and convenient, significantly shortening the extraction time, and the LOD was significantly lower than other methods, greatly improving the efficiency of the experiment. In conclusion, compared with the previously reported methods, this method has the advantages of safety, reliability, high economic efficiency, and less harm to the environment.
Conclusion
In this work, cucurbituril was used as a novel adsorbent in the DMSPE program for the first time and used in combination with UHPLC to determine acaricides in fruit juice and functional food. The experimental results showed that this method had several advantages, such as convenient operation, good repeatability, low detection limit, use of small amounts of organic solvents, and less sample consumption. And owning to the use of adsorbents that do not require any synthesis, adjustment, and pretreatment, the preparation time is very short. Finally, based on the above advantages and reliable experimental results, it is shown that the DMSPE method is a viable option for the determination of acaricide residues in real samples for future research.
References
Ali HRH, Hassan AI, Hassan YF, El-Wekil MM (2021) Mannitol capped magnetic dispersive micro solid-phase extraction of polar drugs sparfloxacin and orbifloxacin from milk and water samples followed by selective fluorescence sensing using boron-doped carbon quantum dots. J Environ Chem Eng 9:105078. https://doi.org/10.1016/j.jece.2021.105078
Alyami BA, Mahmoud AM, Alkahtani SA, El-Wekil MM (2021) NiFe2O4 nanospheres functionalized with 2-(2, 4-dihydroxyphenyl)-3, 5, 7-trihydroxychromen-4-one for selective solid-phase microextraction of aluminium. Talanta 226:122167. https://doi.org/10.1016/j.talanta.2021.122167
Babić S, Horvat AJM, Mutavdžić P, Dragana K-M, Marija, (2007) Determination of pKa values of active pharmaceutical ingredients. Trends Anal Chem. https://doi.org/10.1016/j.trac.2007.09.004
Bozorgzadeh E, Pasdaran A, Ebrahimi-Najafabadi H (2020) Determination of toxic heavy metals in fish samples using dispersive micro solid phase extraction combined with inductively coupled plasma optical emission spectroscopy. Food Chem 346(1):128916. https://doi.org/10.1016/j.foodchem.2020.128916
Breve TG, Filius M, Araman C, Helm MPVD, Hagedoorn P, Joo C, Kasteren SV, Eelkema R (2020) Conditional copper-catalyzed azide alkyne cycloaddition by catalyst encapsulation. Angew Chem Int Ed 59:9340–9344. https://doi.org/10.1002/anie.202001369
Cui JQ, Yin J, Meng JS, Liu Y, Liao MY, Wu T, Dresselhaus M, Xie YM, Wu JH, Lu CZ, Zhang XC (2021) Supermolecule cucurbituril subnanoporous carbon supercapacitor. Nano Lett 21:2156–2164. https://doi.org/10.1021/acs.nanolett.0c04938
El-Wekil MM, Ali HRH, Marzoukb AA, Ali R (2018a) Enhanced dispersive solid phase extraction assisted by cloud point strategy prior to fluorometric determination of anti-hepatitis C drug velpatasvir in pharmaceutical tablets and body fluids. RSC Adv 8:13292–13300. https://doi.org/10.1039/C7RA13719B
El-Wekil MM, Ali HRH, Marzoukb AA, Ali R (2018b) Synthesis of Fe3O4 nanobead-functionalized 8-hydroxyquinoline sulfonic acid supported by an ion-imprinted biopolymer as a recognition site for Al3+ ions: estimation in human serum and water samples. New J Chem 42:9828. https://doi.org/10.1039/C8NJ01141A
Hasanzade Z, Raissi H (2020) Molecular mechanism for the encapsulation of the doxorubicin in the cucurbit[n]urils cavity and the effects of diameter, protonation on loading and releasing of the anticancer drug: Mixed quantum mechanical/molecular dynamics simulations. Comput Meth Prog Bio 196:105563. https://doi.org/10.1016/j.cmpb.2020.105563
Hu K, Li P, Yang SC, Wen XD (2020) Slurry sampling thermospray flame furnace atomic absorption spectrometric determination of bismuth in water and geological samples combined with ultrasound-assisted dispersive micro solid phase extraction. J Anal at Spectrom 35(3):526–533. https://doi.org/10.1039/C9JA00436J
Hu YQ, Li XL, Zhang L, Zhou M, Wang GM, Zhang YH, Xi CX, Cao SR (2014) Mesoporous alumina as a solid phase extraction adsorbent for the determination of abamectin and ivermectin in vegetables by liquid chromatography-tandem mass spectrometry. Anal Methods 4734-4741. https://doi.org/10.1039/C4AY00107A
Karcher S, Kornmüller A, Jekel M, (2001) Cucurbituril for water treatment. Part I: Solubility of cucurbituril and sorption of reactive dyes. Water Research 35(14):3309–3316. https://doi.org/10.1016/S0043-1354(01)00038-0.
Kasiotis KM, Tzouganaki ZD, Machera K (2018) Chromatographic determination of monoterpenes and other acaricides in honeybees: prevalence and possible synergies. Sci Total Environ 625 (JUN.1): 96–105. https://doi.org/10.1016/j.scitotenv.2017.12.244.
Khezeli T, Daneshfar A, (2017) Development of dispersive micro-solid phase extraction based on micro and nano sorbents. Trac Trends in Analytical Chemistry 89(Complete):99–118. https://doi.org/10.1016/j.trac.2017.01.004.
Li YW, Li QF, Miao XR, Qin CY, Chu D, Cao LP (2020) Adaptive chirality of an achiral cucurbit[8]uril-based supramolecular organic framework for chirality induction in water. Angew Chem Int Ed 60:6744–6751. https://doi.org/10.1002/anie.202012681
Liang DP, Liu WJ, Raza R, Bai Y, Liu HW (2018) Applications of solid-phase micro-extraction with mass spectrometry in pesticide analysis. J Sep Sci 42:330–341. https://doi.org/10.1002/jssc.201800804
Mi YD, Cui XY, Jia CD, Liu XY, Zhang SB, Zhou WF, Gao HX, Lu RH (2020) Humic acid functionalized hyperbranched polytriazine based dispersive solid-phase extraction for acaricides determination in tea matrix. J Sep Sci 43(2):373–564. https://doi.org/10.1002/jssc.201900558
Mohamed FA, Khashaba PY, El-Wekil MM (2019) Spectrodensitometric determination of rivastigmine after vortex assisted magnetic solid phase extraction. Microchem J 147:764–774. https://doi.org/10.1016/j.microc.2019.03.085
Mustafa SFZ, Arsad SR, Mohamad H, Abdallah HH, Maarof H (2021) Host-guest molecular encapsulation of cucurbit [7] uril with dillapiole congeners using docking simulation and density functional theory approaches. Struct Chem 1:11. https://doi.org/10.1007/s11224-020-01708-4
Nascimento MM, Rocha G, Andrade J (2019) Simple and effective dispersive micro-solid phase extraction procedure for simultaneous determination of polycyclic aromatic compounds in fresh and marine waters. Talanta 204:776–791. https://doi.org/10.1016/j.talanta.2019.06.061
Pakade YB, Tewary DK (2010) Development and applications of single-dropmicroextraction for pesticide residueanalysis: a review. J Sep Sci 33:3683–3691. https://doi.org/10.1002/jssc.201000331
Papadourakis M, Bosisio S, Michel J (2018) Blinded predictions of standard binding free energies: lessons learned from the SAMPL6 challenge. J Comput-Aided Mol 32:1047–1058. https://doi.org/10.1007/s10822-018-0154-6
Peng B, Zhang JH, Lu RH, Zhang SB, Zhou WF, Gao HX (2013) Dispersive micro-solid phase extraction based on self-assembling, ionic liquid-coated magnetic particles for the determination of clofentezine and chlorfenapyr in environmental water samples. Analyst 138(22):6834. https://doi.org/10.1039/C3AN00814B
Pourmortazavia SM, Sahebib H, Zandavara H, Mirsadeghi S (2019) Fabrication of Fe3O4 nanoparticles coated by extracted shrimp peels chitosan as sustainable adsorbents for removal of chromium contaminates from wastewater: the design of experiment. Compos B: Eng 175:107130. https://doi.org/10.1016/j.compositesb.2019.107130
Rojas R, Morillo J, Usero J, Delgado-Moreno L, Gan J (2013) Enhancing soil sorption capacity of an agricultural soil by addition of three different organic wastes. Sci Total Environ 458–460:614–623. https://doi.org/10.1016/j.scitotenv.2013.04.032
Ruz P, Banerjee S, Khurana R, Barooah N, Sudarsan V, Bhasikuttan AC, Mohanty J (2021) Metal-free supramolecular catalytic hydrolysis of ammonia borane through cucurbituril nanocavitands. ACS Appl Mater Interfaces 13:16218–16226. https://doi.org/10.1021/acsami.0c22213
Sahebi H, Konoz E, Ezabadi A, Niazi A, Ahmadi SH (2020) Simultaneous determination of five penicillins in milk using a new ionic liquid-modified magnetic nanoparticle based dispersive micro-solid phase extraction followed by ultra-performance liquid chromatography-tandem mass spectrometry. MicrochemI J 154:104605. https://doi.org/10.1016/j.microc.2020.104605
Shirkhanloo H, Abbasabadi MK, Hosseini F, Zarandi AF (2021) Nanographene oxide modified phenyl methanethiol nanomagnetic composite for rapid separation of aluminum in wastewaters, foods, and vegetable samples by microwave dispersive magnetic micro solid-phase extraction - ScienceDirect. Food Chem 347:129042. https://doi.org/10.1016/j.foodchem.2021.129042
Song JZ, Qiao CF, Li SL, Han QB, Xu HX (2008) Purity determination of yunaconitine reference standard using HPLC with experimental design and response surface optimization. J Sep Sci 31(22):3809–3816. https://doi.org/10.1002/jssc.200800455
Sun T, Jin Y, Yang J, Li L, Shi X, Li X (2015) Dispersive solid-phase extraction of organophosphorus pesticides from apple, cucumber and water samples using reduced graphene oxide coated with ZnO nanocomposites as a sorbent. Anal Methods 7:6095–6102. https://doi.org/10.1039/C5AY00866B
Sun XZ, Li B, Wan DJ, Wang N (2016) Using a novel adsorbent macrocyclic compound cucurbit[8]uril for Pb2+ removal from aqueous solution. J Environ Sci 50(012):3–12. https://doi.org/10.1016/j.jes.2016.04.029
Wang P, Luo M, Liu DH, Zhan J, Liu XK, Wang F, Zhou ZQ (2018) Application of a magnetic graphene nanocomposite for organophosphorus pesticide extraction in environmental water samples. J Chromatogr A 1535:9–16. https://doi.org/10.1016/j.chroma.2018.01.003
Xiao ZG, Wang LS, Zhang YR et al (2020) Synthesis and characterization of a novel rice bran protein-cerium complex for the removal of organophosphorus pesticide residues from wastewater. Food Chem 320:126604. https://doi.org/10.1016/j.foodchem.2020.126604
Yang MY, Zeng HZ, Wu XL, Yang XL, Zhou WF, Zhang SB, Lu RH, Li J, Gao HX (2016) Magnetic zinc oxide nanoflower-assisted ionic liquid-based nanofluid dispersive liquid–liquid microextraction for the rapid determination of acaricides in tea infusions. RSC Adv 10:1039. https://doi.org/10.1039/C6RA22353B
Yang XL, Qiao KX, Liu F, Wu XL, Yang MY, Li J, Gao HX, Zhang SB, Zhou WF, Lu RH (2017) Magnetic mixed hemimicelles dispersive solid-phase extraction based on ionic liquid-coated attapulgite/ polyaniline-polypyrrole/ Fe3O4 nanocomposites for determination of acaricides in fruit juice prior to high-performance liquid chromatography-diode array d. Talanta 166(Complete): 93–100. https://doi.org/10.1016/j.talanta.2017.01.051.
Zeng HZ, Yang XL, Yang MY, Wu XL, Zhou WF, Zhang SB, Lu RH, Li J, Gao HX (2017) Ultrasound-assisted, hybrid ionic liquid, dispersive liquid–liquid microextraction for the determination of insecticides in fruit juices based on partition coefficients. J Sep Sci 40:3513–3521. https://doi.org/10.1002/jssc.201700464
Zhang JH, Li M, Li YB, Li ZY, Wang FF, Li Q, Zhou WF, Lu RH, Gao HX (2013) Application of ionic-liquid-supported magnetic dispersive solid-phase microextraction for the determination of acaricides in fruit juice samples. J Sep Sci 36(19):3249–3255. https://doi.org/10.1002/jssc.201300358
Zhang SJ, Xu T, Liu Q, Liu JM, Lu FL, Yue MB, Li YX, Sun ZW, You JM (2018) Cationic gemini surfactant-resorcinol-aldehyde resin and its application in the extraction of endocrine disrupting compounds from food contacting materials. Food Chem 277:407–413. https://doi.org/10.1016/j.foodchem.2018.10.132
Zhang LY, Yu RZ, Zhang ZX, DJ, (2021) Ionic liquid–based dispersive liquid–liquid micro-extraction of five organophosphorus pesticides in coarse cereals. Food Anal Methods 14(1):1–8. https://doi.org/10.1007/s12161-020-01851-y
Funding
This study was supported by Hangzhou Social Development of Scientific Research Project (20191203B13), the Public Welfare Research Project of Zhejiang Province (LGF18H280006), National Natural Science Foundation of China (81573552), and Hangzhou 131 Middle aged and Young Talent Training Plan (China, 2017).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Informed consent not applicable.
Conflict of Interest
Yu-xin Gu, Tian-Ci Yan, Zi-Xuan Yue, Min-Hui Li, and Shu-Ling Wang declare that they have no conflict of interest. Jun Cao and Hui Zheng declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gu, YX., Yan, TC., Yue, ZX. et al. Dispersive Micro-solid-Phase Extraction of Acaricides from Fruit Juice and Functional Food Using Cucurbituril as Sorbent. Food Anal. Methods 15, 1356–1367 (2022). https://doi.org/10.1007/s12161-021-02209-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-021-02209-8