Abstract
An ultrasound-assisted emulsification microextraction with solidification of organic droplet method followed by high-performance liquid chromatography with diode array detection for six triazole fungicide determination (diniconazole, fluquinconazole, flusilazole, myclobutanil, tebuconazole, and tetraconazole) was developed. After some preliminary experiments, undecanol was chosen as extracting solvent using 50 μL for 10 mL of liquid sample. A central composite design was performed to obtain the best experimental conditions for the following variables NaCl concentration (250 g L−1), extraction time (18 min), and temperature (30 °C) in ultrasonic bath. After the ultrasound-assisted extraction, two steps considering centrifugation (4,200 rpm, 10 min) and solidification (5 min, 3 °C) were done. Following these conditions, the method showed linearity higher than 0.9930 with the concentration ranged from 20 to 890 μg L−1. The limits of detection obtained using calibration curves were from 10.9 to 17.2 μg L−1 and the intra- and inter-day repeatability at two levels showed RSD values between 1.9 and 10.6 %. The enrichment factors for the studied triazoles were between 226 (flusilazole) and 255 (tebuconazole). Recovery studies at two spiked levels in apple and grape juices gave values from 64 to 112 %.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Triazole fungicides are systemic pesticides widely used to control fungus diseases in field crops. These triazole compounds inhibit ergosterol biosynthesis needed for membrane structure and function (Tomlin 2000). The widespread use of triazoles has increased the concern about the detrimental effects in ecosystems and human health. Some toxicological studies in rats have shown tumorigenic (Wolf et al. 2006) and endocrine disrupting (Goetz et al. 2007) effects. Since the fungicide residues have been found on food for human consumption, the accurate determination of the residue levels is necessary for food safety monitoring and regulatory purposes. In order to protect the health of consumers, maximum residue levels (MRLs) in raw products have been established. European Union has fixed MRLs ranging from 0.01 to 2 mg kg−1 in apples and grapes for diniconazole, fluquinconazole, flusilazole, myclobutanil, tebuconazole, and tetraconazole (EC Commission Regulation 149/2008 of 29 January 2008).
The sample preparation is a critical step in the analytical process for determination of pesticides residues. In the previous years, considerable efforts have been made in trying to develop new sample preparation techniques that save time and require less amount of hazardous organic solvent, which allow improving the quality and sensitivity of the analytical procedures (Tankiewicz et al. 2011). Sorptive extraction techniques mainly include solid-phase extraction (SPE), solid-phase microextraction (SPME), and stir bar sorptive extraction (SBSE). SPE is a widely used sample preparation technique; and modification and development of new adsorbent are a major goal to improve the extraction efficiency. The main drawbacks of SPE are the cost of cartridges and the relatively high consumption of sample and solvent that can be reduced using miniaturized SPE. SPME and SBSE are both solvent-free extraction techniques and show some other advantages such as the high sensitivity and wide range of compound application. The main drawbacks are due to the long time required for a single extraction, the difficulties in variables optimization and reliable calibration, and the cost of the fibers or the magnetic stirrers (Hyötyläinen 2009).
Liquid-phase microextraction (LPME) avoids at least the great problem of the solvent consumption associated with conventional liquid-liquid extraction. LPME can be done in several modes, where the extraction is performed by using small amount (drop) of water immiscible solvent suspended in a sample or the extraction is done via a membrane which can be a selective barrier between two phases (Stocka et al. 2011). The dispersive liquid-liquid microextraction (DLLME), with dispersion of very fine droplets of organic solvents into the aqueous phase, has emerged as a valuable alternative for the traditional liquid-liquid extraction. DLLME employs a ternary component solvent system composed of an aqueous solution containing the analytes, a water immiscible solvent, and a water–miscible disperser solvent. The extraction solvent is generally collected at the bottom of the tube after centrifugation. DLLME is fast, cheap, simple, requires minute amounts of organic solvent, and provides high enrichment factors (Bosch Ojeda and Sánchez Rojas 2011). However, the extraction solvents used in DLLME were generally highly toxic. A valuable LPME method based on solidification of a floating organic solvent droplet (SFO) was presented by Khalili Zanjani et al. (2007). In this method, a small volume of an organic solvent with a melting point near room temperature is floated on the surface of the agitated aqueous sample. Later, the sample vial is placed in an ice bath and the solidified organic droplet is collected and immediately melted for analyte determination. The technique is cheap, quick, and sensitive; but the rate of extraction is slightly slow. A posterior modification considering DLLME methodology (DLLME-SFO) achieved faster mass transfer and better extraction times (Leong and Huang 2008). As previously mentioned, one of the main disadvantages of DLLME is the use of relatively high volumes of disperser organic solvents (Bosch Ojeda and Sánchez Rojas 2011). With the goal of avoiding the environmentally unfriendly disperser solvents, the ultrasound-assisted emulsification microextraction (USAEME) has emerged to assist the dispersion of extraction solvent in the aqueous solution (Regueiro et al. 2008).
Some of the abovementioned extraction techniques have been used for the determination of triazoles residues in different types of water and liquid fruit juices. After the treatment step, the procedures mostly use gas chromatography or liquid chromatography (LC) with diode array detectors (DAD) or mass spectrometry (MS). A meliorated SPE procedure was used for simultaneous multiclass pollutants, including triazoles, in water (Baugros et al. 2008). A graphene-based magnetic nanoparticles was showed as adsorbent for triazole fungicides in environmental water (Wang et al. 2012). Other sorptive techniques were also used: SPME in liquid samples (Bordagaray et al. 2013) and SBSE combined with DLLME in aqueous samples (Farajzadeh et al. 2010). Working with DLLME, other alternatives have been showed; addition of ionic liquids (Ravelo-Pérez et al. 2009), use of a narrow-bore tube (Farajzadeh et al. 2012), and dispersion with methanol followed by SFO (Wang et al. 2011a). Until now, there is no application of ultrasound-assisted emulsification microextraction with solidification of organic droplet (USAEME-SFO) without disperser solvent for the triazole fungicides determination in water and fruit juice samples.
There are several variables affecting the extraction procedure, among them are the type and volume of extraction solvent, salt addition, stirring rate, sample solution temperature, and extraction time (Ghambarian et al. 2013). Experimental designs that take into account simultaneously several variables and its interaction effects seem to be an appropriate way to find the convenient experimental conditions with a reduced number of experiments. Response surface designs, including central composite design (CCD), are used during method optimization to determine optimal conditions for the factors that have the most influence on the interest response (Tranter 2000; Stalikas et al. 2009). This approach was presented in developing an IL-DLLME-HPLC-DAD for pesticide determination (Ravelo-Pérez et al. 2009), and used with DLLME for simultaneous determination of carbamates and organophosphorus pesticides in water (Sousa et al. 2013).
The aim of the work was to develop an appropriate green method for the determination of triazoles fungicides in water and fruit juices samples using USAEME-SFO followed by high-performance liquid chromatography with diode array detection (HPLC-DAD). The method only used a minute amount of alcohol organic solvent and avoided the use of the hazardous disperser solvent. After selection of some important extraction variables (among them type and volume of extraction solvent and sample liquid volume), the optimization of time and temperature extraction conditions and sodium chloride amount was performed using a CCD approach. The method was validated and used in the analysis of different spiked apple and grape juice samples.
Experimental
Chemicals and Samples
Diniconazole (99.8 %, Pestanal) and tebuconazole (99.6 %, Pestanal) were acquired in Sigma-Aldrich (Madrid, Spain), flusilazole (99.3 %) and myclobutanil (99.4 %) were supplied by LGC Standards (Barcelona, Spain), and tetraconazole (97.5 %) and fluquinconazole (98.5 %) from Dr. Ehrenstorfer GmbH (Augsburg, Germany). Individual stock solutions of triazoles were prepared in methanol (SpS) supplied by Teknokroma (Barcelona, Spain) at a concentration of 6,000 mg L−1. Working standard solutions were prepared in a range from 20 to 890 μg L−1 by appropriate dilutions in salted water. HPLC-grade methanol was from Teknokroma and sodium acetate (PA grade), and acetic acid used for buffer were supplied by Panreac (Barcelona, Spain). The buffer made adjusting with 0.01 mol L−1 acetic acid/sodium acetate solution to pH 4 was stored at 4 °C. 1-undecanol (purity 99 %), hexadecane (purity 99 %), and 1-bromohexadecane (purity 97 %) were acquired from Sigma-Aldrich (Madrid, Spain), while 1-dodecanol (purity 98 %) and sodium chloride (PA) were acquired from Panreac (Barcelona, Spain).
Homemade and commercial apple and grape juices were used. Commercial products were acquired in a store, while the homemade juice was made by squeezing the apples and filtering the liquid.
Equipment
HPLC analyses were performed on a LC-20AD system equipped with a SPD-M20A DAD (Shimadzu Corporation, Duisburg, Germany). Data were collected and processed using LC solution 2.1 version software. Separations were carried out using XDB-C18 column (250 mm × 4.6 mm, 5 μm) from Agilent (Wilmington, DW, USA). The analysis was done at ambient temperature, and the injection volume was 20 μL. A binary mobile-phase gradient with methanol and sodium acetate/acetic acid buffer solution was used at a flow rate of 0.5 mL min−1. The initial mobile phase was held for 1 min with 78 % methanol, followed by a decrease to 71.5 % methanol from 1 to 13 min, then raised to 85 % methanol from 13 to 14 min, and kept for 10 min. The system was re-equilibrated at the initial conditions (78 % methanol) from 24 to 30 min. In order to homogenize faster the circuit, the flow rate was increased to 1.5 mL min−1 in the last 2.5 min. With these conditions, the triazole sequence and elution time was myclobutanil (9.9 min), fluquinconazole (10.6 min), tetraconazole (TT) (11.2 min), flusilazole (FS) (12.2 min), tebuconazole (15.4 min), and diniconazole (D) (23.0 min). The UV–Vis spectra were recorded from 190 to 500 nm, using 249 nm as a working wavelength for diniconazole and 221 nm for the other 5 analytes.
The extraction was carried out in a Bandelin Sonorex Digitec DT100H ultrasound bath (ALLPAX GmbH & Co. KG, Papenburg, Germany) with 35 kHz ultrasound frequency. The cooling bath used was a Julabo F26 from GmbH (Augsburg, Germany). Experimental design was performed, and results were evaluated using Statistica software (StatSoft, Tulsa, USA).
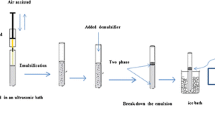
USAEME-SFO Procedure
Ten milliliter of the 250 g L−1 NaCl solution was placed in a 40 mL screw cap glass vial and a mixed solution of triazole standards was spiked. Then, 50 μL of 1-undecanol as a extraction solvent was added to the solution, shacked by hand, placed into ultrasound bath previously heated to 30 ± 1 °C, and maintained at 18 min. After, the vial was first placed into a centrifuge for 10 min at 4,200 rpm and later into the cooling thermostatic bath at 3 °C for 5 min. The formed solidified organic drop was carefully collected with a spatula and transferred to an Eppendorf vial where it melted rapidly at room temperature. Twenty-five microliter of the melted solution was collected and mixed with 20 μL of methanol before the injection into the HPLC.
For the fruit juices, 0.5 mL of the filtrate sample was added to 9.5 mL salted water before spiking the solution of analytes and the extracting solvent. All the rest of the procedure followed the above indicated conditions. Several previous tries with 10 mL of salted sample and lower dilutions (i.e., 5 mL sample/5 mL water, 2.5 mL sample/7.5 mL water) were done, but the drop did not properly form and was hardly managed.
Enrichment Factor
Enrichment factor (EF) was calculated as the relation between the concentration of each analyte in the initial aqueous sample (C0) and the final concentration in the extracting phase (Cfinal).
The initial concentration (C0) is the concentration spiked into the aqueous solution before the extraction. The extracting phase concentration was calculated after the extraction process (Cfinal), taking into account an external calibration made by injecting directly standards into the HPLC-DAD.
Results and Discussion
Selection of Extracting Solvent
For extraction process, it is important to choose an extracting solvent that fits well with the analytes and experimental working conditions. The solvent should be immiscible in water and present a melting point (MP) near to the room temperature (Ganjali et al. 2010). Taking into account these characteristics, 1-bromohexadecane (BRHEX, MP 17 °C), n-hexadecane (HEX, MP 18 °C), 1-dodecanol (DOD, MP 22 °C), and 1-undecanol (UND, MP 14 °C) were chosen as extracting solvents.
Firstly, the compatibility of the extracting solvent with the HPLC mobile phase was checked. After several assays, with different proportions of methanol with water, UND and DOD showed better solubility than BRHEX and HEX. Hence, only the alcohols were considered in the following experiments. UND showed better miscibility than DOD with less proportion of organic solvent, and it was considered miscible from the proportion above 60 %. In the comparison of extraction efficiency for triazole analytes, UND showed better results than DOD as it can be seen in Fig. 1. Therefore, further experiments were carried out considering UND as the extracting solvent.
Effect of 1-undecanol (UND) and 1-dodecanol (DOD) extracting solvent on the extraction efficiency following LPME-SFO method. Extraction conditions: triazole concentration, 80 μg L−1; sample volume, 10 mL; volume of extracting solvent, 15 μL; extraction time, 30 min; extraction temperature, 60 °C; no salt addition; agitation, 500 rpm. Solidification conditions: time, 10 min; cooling temperature, 3 °C. Triazole abbreviations: D diniconazole, FQ fluquinconazole, FS flusilazole, M myclobutanil, TB tebuconazole, TT tetraconazole
Comparison with LPME-SFO
USAEME was compared with LPME both followed by SFO. In all the runs, the following extractions conditions were considered: concentration of triazoles, 80 μg L−1; sample volume, 10 mL; volume of extraction solvent, 20 μL; extraction temperature, 25 °C; and salt addition, NaCl 180 g L−1. The solidification conditions (3 °C for cooling temperature during 5 min) were also similar. For LPME, 500 rpm agitation during extraction was used. And, for USAEME, extraction followed by centrifugation (4,200 rpm, 10 min) was done. Different extraction times, up to 60 min for LPME and up to 15 min for USAEME, were considered to compare the extraction efficiency. As it can be seen in Fig. 2, the extraction efficiency for the six triazole analytes is better using ultrasound assistance. Even in the comparison of 1-h extraction time with LPME with 5 min in USAEME, the results are higher using the ultrasound-assisted microextraction. The application of ultrasonic energy facilitates the emulsification phenomenon and accelerates the mass-transfer process that leads to an increment in the extraction efficiency in a very short time (Ghambarian et al. 2013).
Effect of incorporation of ultrasound assistance on extraction efficiency in LPME-SFO method. Extraction conditions: concentration of triazoles, 80 μg L−1; sample volume, 10 mL; volume of extraction solvent, 20 μL, extraction temperature, 25 °C; salt addition, NaCl 180 g L−1. Solidification conditions: time, 5 min, and cooling temperature, 3 °C. For LPME, agitation during extraction 500 rpm. For USAEME, extraction followed by centrifugation (4,200 rpm, 10 min). Triazole abbreviations: D diniconazole, FQ fluquinconazole, FS flusilazole, M myclobutanil, TB tebuconazole, TT tetraconazole
Central Composite Design: Application in Selection of Experimental Conditions for Influent Extraction Factors
The experimental conditions for some of the variables that affect the extraction process were selected according to working characteristics. For example, drop volume was big enough to collect sufficient volume before injecting to HPLC, but as small as possible to avoid the dilution of analytes in the drop. Centrifugation speed and time values were chosen as minimum to recollect the cloudy solution into a drop, and changes with longer time or different speed did not affect in extraction efficiency. The cooling step conditions were fixed in order to freeze the drop in a good way to collect with the spatula. Thus, the drop was cooled at 3 °C for 5 min with agitation, and the changes of conditions in cooling step did not show differences.
Other variables are not so easy to fix in a reduced amount of experiments. Response surface methodologies, including CCD, are used to optimize simultaneously some variables following established series of experiments that considerably reduce the number of the experiments comparing with the approach if each variable is taken in an univariate way. Taking into account the variables reported in previous works (Leong and Huang 2008; Wang et al. 2011b; Ghambarian et al. 2013), in this study, three variables were selected for CCD design. Those were NaCl concentration, extraction time, and temperature in ultrasonic bath. The variables and its low, medium, and high levels are shown in Table 1.
The CCD done was based in a factorial design (23) increased by a [(2 × 3) + 1] star design considering the rotatability conditions (α = ±1.682). Each experimental point was performed twice, except the central point that was run out five times. In total, the matrix of CCD design consisted of 33 experimental runs that are presented in Table 1. Due the large amount of experiments, the design was randomly carried out in 2 days according to blocks. The obtained values (in units of peak areas) for each studied triazole are also shown in Table 1.
The most common way to present the results of a CCD is with a response surface. This can be done in 3D plots representing results and selecting two independent variables (i.e., temperature and time). Instead of the independent response surfaces for each studied triazoles, the global desirability surface was chosen, since it can provide an overall view of the considered analytes. The desirability function for each dependent variable was fixed by assigning desirability values of 0.0 (for undesirable, lowest area), 0.5 (for medium), and 1.0 (for very desirable, highest area). Therefore, the conditions are better when the desirability surface shows values closer to 1. Otherwise, the conditions are less favorable when the graph shows the desirability values close to 0. The response surface for global desirability considering NaCl concentration and extraction time is showed in Fig. 3. The extraction temperature variable for this response surface was fixed at 30 °C. As it can be seen, in the studied experimental domain, the best responses were obtained working with high NaCl concentrations and extraction times. The extraction temperature showed a similar behavior with the best responses at high levels. Hence, 250 g L−1 NaCl, 18 min extraction time, and 30 °C extraction temperatures were selected as the experimental conditions.
Method Performance
Important analytical parameters such as linearity, limit of detection (LOD), precision, EF, and recovery were determined to evaluate the performance of the USAEME-SFO method following the previously detailed experimental conditions. The obtained data for the analytical characteristics are summarized in Table 2. All analytes showed a good linearity between 20 and 890 μg L−1 with R2 correlation coefficients ranging from 0.9931 (flusilazole) to 0.9971 (myclobutanil, tetraconazole).
There are several methods to calculate LODs (Konieczka and Namiesnik 2009). In chromatographic analysis, the simplest and widely applied way is based considering the lowest concentration of an analyte that yields a signal to noise ratio of 3. This method is applicable when analyte concentration is measurable for blank sample or for a sample with a very low analyte concentration. The method used in this work follows the next equation and take into consideration the calibration equation and its properties. LOD = 3 s y/x /b; where b is the slope of calibration curve and s y/x is the residual standard deviation of the calibration curve. The LODs obtained were between 10.9 μg L−1 (TT) and 17.2 μg L−1 (FS).
The precision of the method was evaluated considering the repeatability of the measurements and expressed as relative standard deviation (RSD) in percentages. The repeatability was run out at two different analyte concentrations (50 and 400 μg L−1). For intraday repeatability, six experiments were carried out in the same day under same conditions. For interday repeatability, 15 runs were performed in 3 days in two different weeks. The RSD obtained ranged from 1.9 to 6.0 % for intraday repeatability and was from 3.1 to 10.6 % for interday repeatability.
Taking into account the selected experimental conditions, the EF of USAEME-SFO for the studied triazole fungicides were from 226 (flusilazole) to 255 (tebuconazole). The EFs were calculated as an average of six independent samples with 80 μg L−1 of each analyte, and the data are shown in Table 2.
The recovery studies were carried out in commercial and homemade apple juice and commercial grape juice. The juice samples were spiked with the standards of six triazoles at two concentrations (80 μg L−1 and with 400 μg L−1), and each analysis was done with four independent samples. The average recoveries of the studied triazoles are shown in Table 3. The recovery values were from 82 to 112 % in apple juices and ranged from 64 to 112 % in grape juice. Figure 4 shows the HPLC-DAD chromatograms that belong to the non-spiked commercial apple sample and the spiked sample with 80 and 400 μg L−1 of triazole standards.
HPLC-DAD chromatograms (λ 221 nm) obtained from a commercial apple juice spiked with 80 μg L−1 (dotted line) and 400 μg L−1 (discontinuous line) of the six triazole compounds following the USAEME-SFO optimized procedure. Continuous line belongs to the blank of apple juice analysis. Peak assignment (1) myclobutanil, (2) fluquinconazole, (3) tetraconazole, (4) flusilazole, (5) tebuconazole, and (6) diniconazole
The performance of the proposed USAEME-SFO method was compared with other reported extraction methods for triazoles in liquid matrices that are listed in Table 4. The best LODs were obtained with SPE followed by HPLC using UV or MS detectors (Zhou et al., Baugros et al. 2008). Working with different extraction techniques (IL-DLLME, DLLME-SFO, SPME) and HPLC-DAD, the LODs ranged from around 0.1 to 10 μg L−1. The obtained values with the proposed method are slightly higher. However, it has to be taken into consideration that LODs were evaluated considering the calibration curve instead of the signal to noise relation of three. The LODs estimated based on calibration curve are more realistic but gave higher results.
The RSDs obtained with the proposed procedure were less than 11 % and were in the order of the other listed procedures. The enrichment factors calculated in this work agreed with most of the studies where the EFs were showed. The results of recovery studies taken from consulted references were made both in water and in liquid juices. The comparison of obtained recovery data with results of similar juices was satisfactory.
Conclusions
A reliable method based on USAEME-SFO was developed. The extracting solution was later injected in a HPLC-DAD in order to separate and determine six triazole fungicides. The method provided good linearity, repeatability, and enrichment factors. The LODs are in the order of a few micrograms per liter. Also, satisfactory recoveries were achieved in fruit juice samples.
Compared to other extraction processes, this method provides the advantages of simplicity and low cost (regarding chemicals and equipment). Also, it is relatively fast since the extraction is made in 18 min. Furthermore, it can be considered an environmentally friendly method due to the use of only 1-undecanol as organic solvent in the extraction process.
References
Baugros JB, Giroud B, Dessalces G, Grenier-Loustalot MF, Cren-Olivé C (2008) Anal Chim Acta 607:191
Bordagaray A, Garcia-Arrona R, Millán E (2011) Food Anal Methods 4:293
Bordagaray A, Garcia-Arrona R, Millán E (2013) Anal Methods 5:2565
Bosch Ojeda C, Sánchez Rojas F (2011) Chromatographia 74:651
EC Commission Regulation 149/2008 of 29 January 2008 amending regulation (EC) No 396/2005 of the European Parliament and of the Council by establishing Annexes II, III and IV setting maximum residue levels for products covered by Annex I thereto. OJ L 58, 1.3.2008, p.1. MRLs available at: http://ec.europa.eu/sanco_pesticides
Farajzadeh MA, Djozan D, Nouri N, Bamorowat M, Shalamzhari MS (2010) J Sep Sci 33:1816
Farajzadeh MA, Bahram M, Jafary F, Bamorowat M (2011) Chromatographia 73:393
Farajzadeh MA, Djozan D, Khorram P (2012) Anal Chim Acta 713:70
Ganjali MR, Sobhi HR, Farahani H, Norouzi P, Dinarvand R, Kashtiaray A (2010) J Chromatogr A 1217:2337
Ghambarian M, Yamini Y, Esrafili A (2013) Michrochim Acta 180:519
Goetz AK, Ren H, Schmid JE, Blystone CR, Thillainadarajah L, Best DS, Nichols HP, Strader LF, Wolf DC, Narotsky MG, Rockett JC, Dix DJ (2007) Toxicol Sci 95:227
Hyötyläinen T (2009) Anal Bioanal Chem 394:743
Khalili Zanjani MR, Yamini Y, Shariati S, Jönsson JA (2007) Anal Chim Acta 585:286
Konieczka P, Namiesnik J (2009) Quality assurance and quality control in the analytical chemical laboratory. CRC Press, Boca Raton, USA
Leong MI, Huang SD (2008) J Chromatogr A 1211:8
Ravelo-Pérez LM, Hernández-Borges J, Asensio-Ramos M, Rodríguez-Delgado MA (2009) J Chromatogr A 1216:7336
Regueiro J, Llompart M, Garcia-Jares C, Garcia-Monteagudo JC, Cela R (2008) J Chromatogr A 1190:27
Sarafraz-Yazdi A, Assadi H, Ibrahim WAW (2012) Ind Eng Chem Res 51:3101
Sousa R, Homem V, Moreira JL, Madeira LM, Alves A (2013) Anal Methods 5:2736
Stalikas C, Fiamegos Y, Sakkas V, Albanis T (2009) J Chromatogr A 1216:175
Stocka J, Tankiewicz M, Biziuk M, Namiesnik J (2011) Int J Mol Sci 12:7785
Tang T, Quian K, Shi T, Wang F, Li J, Cao Y (2010) Anal Chim Acta 680:26
Tankiewicz M, Fenik J, Biziuk M (2011) Talanta 86:8
Tomlin CDS (2000) The pesticide manual, 12th edn. British Crop Protection Council, Farnham, UK
Tranter RL (ed) (2000) Design and analysis in chemical research. Sheffield Academic Press Ltd, Sheffield, England
Wang C, Wu Q, Wu C, Wang Z (2011a) J Hazard Mater 185:71
Wang S, Ren L, Xu Y, Liu F (2011b) Microchim Acta 173:453
Wang W, Ma X, Wu Q, Wang C, Zang X, Wang Z (2012) J Sep Sci 35:2266
Wolf DC, Allen JW, George MH, Hester SD, Sun GB, Moore T, Thai SF, Delker D, Winkfield E, Leavitt S, Nelson G, Roop BC, Jones C, Thibodeaux J, Nesnow S (2006) Toxico Pathol 34:895
Zhou Q, Xiao J, Ding Y (2007) Anal Chim Acta 602:223
Acknowledgments
Authors are grateful to the University of Basque Country (UPV/EHU) for financial support (pre-doctoral fellowship for A. B. and EHU 13/52 project).
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Conflict of Interest
Ane Bordagaray has no conflict of interest. Rosa Garcia-Arrona has no conflict of interest. Esmeralda Millán has no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bordagaray, A., Garcia-Arrona, R. & Millán, E. Determination of Triazole Fungicides in Liquid Samples Using Ultrasound-Assisted Emulsification Microextraction with Solidification of Floating Organic Droplet Followed by High-Performance Liquid Chromatography. Food Anal. Methods 7, 1195–1203 (2014). https://doi.org/10.1007/s12161-013-9733-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-013-9733-2