Abstract
As a thermochemical conversion process, biomass pyrolysis has received a lot of interest for energy recovery by generating clean fuels, valuable compounds, and advanced materials. Innovative and novel pyrolysis procedures have arisen over time, and these processes may be optimized to produce high-quality end products. Substantial progress has been achieved in the development of analytical pyrolysis systems during the last few decades. However, due to a lack of knowledge of the reaction process, the current mechanism of biomass pyrolysis, as well as its economic feasibility, is far from a complete and thorough explanation. This review systematically covers biomass pyrolysis for energy recovery, the most recent advances in biomass pyrolysis, and the numerous factors responsible for the end products. Furthermore, the various feedstock compositions, as well as the techno-economic analyses, have also been reported. This review emphasizes discernment into future paths, intending to overcome existing deficiencies. This review may also be employed to get new insights into this field and be useful for future studies on biomass pyrolysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the rise in human population, urbanization, and, subsequently, energy need, availability of fossil fuels has been decreasing. Therefore, meeting the need for energy in a reliable alternative manner is becoming a major socioeconomic problem for the entire world [1]. According to reports, the social and economic developments of the twenty-first century are direct outcomes of the industrial revolution of 1837, which caused a fast growth in the use of energy since it was the main input for manufacturing activities [2]. The global economy’s energy requirements have been shown to increase by 2.5% every year since 1850. Due to the exhaustion of fossil fuels, rising energy consumption, industrialization, and pollution of the soil, air, and water, the globe is currently confronted with several issues regarding the need for energy [1, 3]. According to United Nations (UN) world population aspects 2022, around 7875 million people are living in the globe, with 4378 million of them living in urban areas, or 55.6% of the total [4]. Asian and European countries have a maximum growth rate in the globe for energy consumption [2]. The majority of the world’s energy comes from fossil fuels. Combining manufacturing, importation, sales, and reserves, 81% of the world’s major energy supply is comprised of coal, crude oil, and natural gas [5, 6]. In 2021, just 13.8% of the major energy source came from renewable energy sources like solar, wind, hydro, biomass, and other types of energy [2]. Especially in poor or developing countries, the demand for coal roughly doubled between 2000 and 2022, making up about 50% of the overall growth in primary energy consumption [2, 7]. A sizable portion of the world’s power comes from coal. 39% of the power generated worldwide in 2021 came from coal-based sources. With a proportion of 62%, hydropower leads all other renewable sources of electricity generation, with the wind coming in second at 19% [7]. The third-largest renewable form of power generation is bioenergy [6]. The poor or developing countries’ economic development has benefited from coal, but it has also increased greenhouse gas (GHG) emissions and air pollution. Since 2000, the oil demand has more than doubled, mostly as a result of rising automobile ownership and the usage of roads for transportation [2]. The transportation industry utilizes about 27% of all energy used worldwide. 92% of the energy requirements for the transportation industry are covered by crude oil and oil products [2, 8]. Currently, the most environmentally friendly and effective choices for the sector are liquid biofuels and biogas. Biofuels have a market share of more than 3% and have grown by 13%, which is about six times greater than the total energy required in the transportation sector [8]. Because the government has subsidized and promoted liquefied petroleum gas (LPG) usage in culinary applications, it has also helped to drive up oil use.
The Asian and European country’s dependency on imported crude oil has steadily grown due to a lack of domestic resources, reaching almost 75% in 2022. In 2000, African and Asian countries’ major energy source was coal, followed by traditional biomass, which made up about one-fourth of the country’s main energy mix [2]. Presently, according to reports (our world in data), the world’s entire energy source depends on coal 32%, petroleum oil 30%, gas 24.2%, and renewable energy sources, making up just 13.8% (Fig. 1b) [9]. Per capita energy consumed across the globe from fossil fuels, renewable and nuclear, and by different sources is shown in Fig. 1. Traditional biomass is primarily fuel wood, but it also includes animal waste and charcoal. In 2021, wood chips, wood pellets, and other conventional biomass sources accounted for 85% of home consumption. In contrast, municipality and corporate businesses accounting for 4%, following biogas for 4%, and liquid biofuels for 8% [9]. Global production of wood fuel reached 1.93 billion m3 in 2020 [10]. The production of wood fuel was mostly concentrated in Africa and the Americas, contributing 36% and 37%, respectively. One of the bioenergy industries with the fastest global growth is wood pellets. Thirty-nine million tonnes of pellets were produced worldwide in 2021 [11]. Another important bioenergy industry that produces huge amounts of wood charcoal is this one. In 2021, Africa produced 53.1 million tonnes of wood charcoal, approximately 65% of the world’s total production. Agriculture might benefit significantly from the usage of bioenergy in the future. There are several opportunities to increase significant agricultural yields from various regions worldwide [10, 11]. As a result, more food and fuel could be supplied, and the agricultural industry has a major impact on the worldwide use of bioenergy. After woods and farming, the third feedstock area for energy production uses urban and industry wastes.
Even though opinions and estimates of the total amount of available fossil fuel resources vary greatly, it is fair to conclude that they will be exhausted over the next 50 to 100 years, with a production peak occurring far in advance of that time. The “climatic hazard” of more carbon being released into the environment is a significant one, though. According to International Energy Projection (IEA) projections, America dominates biofuel production worldwide. Collectively, North and South America generate 76% of the world’s biofuels, with only 15% coming from Europe [12]. Millions of employment are also created by renewable energy technology across the whole production chain [2]. Approximately 12.7 million people were engaged in the renewable energy industry in 2021, with bioenergy being the second-largest jobs provider, with approximately 3.59 million employees [13]. A thousand gigatons of carbon might be emitted into the environment if the current proportions of fossil fuels are maintained up to 2040 without carbon sequestration. This is particularly troubling because the current total cumulative emissions of carbon, which amount to around 500 gigatonnes, have already created serious problems for the global weather trade [14]. A major global worry closely tied to energy issues is the development of various wastes, which is parallel to the concern about the need for energy. The production of bio-waste is the most concerning type of waste. Most of the bio-waste is thrown away after usage. Only some percentage of bio-waste has ever been converted into other sources, placing our capacity to handle these wastes at a severe disadvantage. The majority of bio-waste slowly degrades into smaller particles and is mixed into the soil giving no use. Therefore, the effective use of these bio-wastes will lead to creating major benefits for the ecosystems, the environment, the economy, and human health [2, 8]. So, bio-waste generation needs to be managed with added technology. There are different ways of waste management, which include prevention, minimization, reuse, energy recovery, landfills, and controlled deposit [15, 16]. Out of these, energy recovery could be a good solution for reducing waste and developing the energy sector. The pyrolysis of bio-waste is a good option for energy recovery as bio-waste is a source of energy. Global energy consumption has more than doubled, but traditional biomass’s share of the energy mix in 2022 is just 12% [12].
The primary objective of this review is to explore pyrolysis as a process for energy recovery and the potential of numerous waste biomasses as a feedstock to recover energy through extensive surveys of the literature available. Different parameters, as well as different reactor environment, affect the final product. So, the different pyrolysers, their working, and their roles in the yield of output are discussed. The techno-economic aspect is one most important parameters to look after so that the products of the pyrolysis process can be usable in reality. In this regard, the techno-economic aspects that have been reported by a few researchers are also reviewed. Furthermore, the use or application of oil from the pyrolysis process in different engine performance scenarios is also reported. It will help the potential users to further studies in choosing alternate feedstock for energy recovery, input parameters that have a significant effect on the output, and methodologies that have been adopted by different researchers during the pyrolysis process.
Pyrolysis for Energy Recovery
The term “pyrolysis” originated from the Greek words “pyr” (fire) and “lysis” (breakdown), which emphasizes the disintegration of substances by heating. When used more explicitly, pyrolysis denotes the thermal breakdown of a material’s molecules in the absence of air [17,18,19].
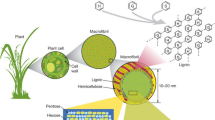
The pyrolysis of biomass is a popular technique for producing solid (charcoal), condensable vapours which transformed into bio-oil at ambient temperature, and non-condensable (permanent) gaseous products for the purpose of generating energy because biomass is a renewable source that is broadly available in the world and these outputs are of interest as they are potential substitute sources of energy [18, 19]. These products have various applications in generating electricity and heat, making transporting fuels, chemicals, and blends (Fig. 2). The majority of non-condensable gases are made up of stable gases, including CO, CO2, and CH4 [20]. Chemical makeup of non-condensable gases is significantly influenced by chemical compositions of the feedstock being pyrolysed. Because hemicellulose has high carboxyl content, pyrolysis of it produces a sizable amount of CO2. On the other hand, cellulose’s carbonyl groups cause it to produce more carbon monoxide. Due to its aromatic compositions and methoxyl functional groups, lignin generates more H2 and CH4 when it undergoes pyrolysis [21].
The fundamental processes that occur during pyrolysis are as follows: (i) temperature inside the fuel rises due to heat transfer from a heat source; (ii) as a result of the temperature increase, pyrolysis processes begin, releasing volatiles and producing char; (iii) volatiles are released, causing heat to pass from the heated volatiles to the colder un-pyrolysed fuel; (iv) tar is created when certain volatiles condense in the colder areas of the fuel; and (v) these interactions result in autocatalytic secondary pyrolysis processes [22]. The pores of the particle allow gases and volatiles to pass through and take part in the heat transfer process. The pace at which the pyrolysis processes take place is based on ambient temperature. Since biomass turns into gases during the pyrolysis process, solid’s pores increase rather than becoming more porous [23]. The pyrolyzing solid’s larger pores provide several places for the volatile and gaseous pyrolysis products to react, which facilitates their contact with the heated solid. The following methods convey heat inside the pyrolyzing particle: (i) internal particle conductivity, (ii) the particle pores’ internal convection, and (iii) radiation and convection from the pellet’s surface [24].
The key feature of pyrolysis is the disintegration of solid fuel results in the dissolution of carbon-carbon bonds and development of carbon-oxygen bonds. Pyrolysis needs temperatures between 400 and 550 °C, while it may be carried out at temperatures considerably higher [25,26,27]. The yield as a percentage during pyrolysis of biomass is shown in Fig. 3. In recent years, pyrolysis has drawn increased attention as a practical and efficient way to turn biomass into biofuel [6, 28, 29]. Both the combustion and gasification processes start with pyrolysis, which is also a part of both. CO, CO2, and light hydrocarbons make up the gas. Bio-oil and char are two names given to this black liquid. The operating circumstances have an impact on product yields and quality [24]. Depending on the circumstances, multiple designations are given to pyrolysis. Low temperatures and lengthy residence durations are used in slow pyrolysis or carbonization, which favours the creation of charcoal. Long operation durations and high temperatures encourage production of gases [30]. In contrast, low gas residence times and moderate temperatures encourage the formation of bio-oil. Figure 4 depicts the pyrolysis process’ chemical reaction.
Pyrolysis is classified into three kinds based on the working conditions: slow, medium, and fast; as illustrated in Table 1, process temperature, solid residence time, biomass particle size, and heating rate change across different categories [17].
Advantages of Pyrolysis
When compared to other viable technologies for bio-waste resource recovery, a pyrolysis-based process has significant advantages [30]. The study of pyrolysis is becoming more and more important since it is a precursor to gasification and combustion as well as a separate process. It is more responsive to changes in feedstock composition than competing processes and is suitable for all types of solid commodities. It may be executed as a batch, low-pressure process with a negligible amount of pre-treatment of feedstock [31].
To some extent, sustainable use of biomass energy can replace nuclear and fossil fuel consumption. Biomass energy, which is used in village locations of developing nations, is used by almost half of the globe’s population. Biomass contributes to worldwide efforts to minimize greenhouse gas emissions [29]. Because of recent technology advances and increased availability, biomass may now be used as a renewable energy resource with little environmental impact [32]. Many different power and chemical production processes have made use of high calorific value pyrolysis by-products, such as bio-oil, acid extract, gases, and coal fines. The principal by-products of the pyrolysis process can be utilized directly or chemically transformed into high-quality fuel or other chemical compounds [29, 32]. Because most biomass feedstocks are physicochemical complex, their constituent elements react differently and create different end products. A portion of the feedstock is turned into carbon, while the remaining amount is oxidized and hydrolysed to produce sugars, phenols, aldehydes, ketones, alcohols, and carboxylic acids. These compounds combine to make more complex compounds like esters and polymer products [33]. The technique of allowing air to obtain pyrolysis is acquired by supplying air in an amount below stoichiometric; the heat created during combustion is then utilized to maintain reactor’s temperature while processing the pyrolysis-related processes [34].
Technology of Biomass Pyrolysis to Value-Added Products
Due to the existence of a significant number of carbon-oxygen bonds, side reactions like polycondensation are more likely to occur in the upgrading and deoxygenation process of biomass pyrolysis to create liquid fuels and high-value-added chemicals. As a result, the product’s quality and yield are impacted [1]. The dark brown liquid bio-oil can be created by condensing a part of the pyrolysis gases. The majority of the components of the biomass that were able to leave the reactor environment are found in this liquid product, together with moisture and oxygenated hydrocarbon [35]. The wet source material and subsequent interactions between bio-constituent oil’s parts during storage both contribute to its moisture content. This moisture content causes the crude bio-oil to split up into two phases: high-density aqueous and low-density organic. To provide fuel with a high calorific value, the organic phase can be increased or combined with fuel derived from petroleum. Acetic acid, hydroxyl acetone, and phenol are just a few examples of water-soluble substances that make up the majority of the aqueous phase. Although this phase could not be utilized as fuel, it may be catalytically converted to make hydrogen [36].
Two pyrolysis techniques are primarily used in modern research and innovation. The first is to produce high-grade bio-oil by directly catalysing the pyrolysis of biomass, improving the specificity of the bio-oil constituents by employing suitable catalysts in the pyrolysis process [37]. Second, fast pyrolysis is used to create bio-oil, which is then refined and modified in two steps to create liquid fuel and high-value chemicals [35]. The conversion of these platform compounds into liquid fuels and high-value-added chemicals like gasoline and aviation kerosene through pre-aldol condensation and catalytic hydrogenation has been proposed by some researchers in recent years. This new synthetic method involves first catalysing the pyrolysis of biomass to produce ketones, furans, and other platform compounds [35].
Direct Pyrolysis of Biomass
A one-step technique for manufacturing high-quality liquid fuels and chemicals from biomass is direct catalytic pyrolysis with a catalyst to create high-grade fuel oil or high-value-added compounds. Deoxygenation, pyrolysis, aromatization, ketylation, alcohol aldehyde condensation, and catalytic cracking are the main chemical reactions that take place during the catalytic pyrolysis of biomass [38]. These reactions occur selectively depending on the type of catalyst used and the creation of necessary reaction conditions. By changing the kind of catalyst and pyrolysis settings, the lignocellulose structure is catalysed to directly participate in the process and enhance the selectivity of desired products in bio-oil [39]. Catalytic pyrolysis of biomass refers to improving the quality of pyrolysis vapour while pyrolyzing biomass by adding various catalysts with deep deoxygenation activity into the fast pyrolysis process, controlling various pyrolysis paths from the source, arbitrarily adjusting the proportion of components, and raising the added value of products [40]. The biomass catalytic pyrolysis catalyst can either be utilized with the raw biomass materials or just the pyrolysis vapour. Based on the different ways the catalyst and biomass source materials interact, the process is divided into in situ catalytic pyrolysis and ex situ catalytic pyrolysis [6].
In Situ Catalytic Pyrolysis
The instantaneous mixing of the catalyst with the raw materials before heating and pyrolyzing in the reactor is the fundamental aspect of in situ catalytic pyrolysis. This method is simple. It is an intensive pyrolysis technique that has undergone substantial investigation [37]. By ensuring that the pyrolysis fragments come into touch with the catalyst first during in situ catalytic pyrolysis, the degree of pyrolysis is increased, and the possibility of pyrolysis products reacting later is reduced. Eucalyptus was subjected to in situ catalytic pyrolysis using a catalyst made of nickel [39]. By encouraging the decarbonylation process and lowering the oxygen level, Ni increases the stability of bio-oil. Catalytic pyrolysis investigations are carried out in a fixed-bed reactor by mixing HZSM-5 and chlorella residue in a certain ratio. When compared to oil generated by direct pyrolysis, the oxygen content of catalytic bio-oil decreased from 30.2 to 19.6%, and as a result, the calorific value increased from 24.7 to 32.8 MJ/kg [39]. While the oil generated by direct pyrolysis is largely made up of long carbon chain compounds with a variety of end groups, the oil produced by catalytic bio-oil is primarily made up of aromatic hydrocarbons. The bulk of the bio-oil is composed of a benzene series when mixing HZSM-5 and lignin at a weight ratio of 2, and catalytic pyrolysis performed at 400–550 °C with air pressure which includes benzene, naphthalene, alkylbenzene, and phenol, as well as other hydrocarbons or oxygenated hydrocarbon components. The bio-oil was then hydrogenated using a palladium carbon catalyst at 90 to 180 °C [39]. It was feasible to get biological aviation fuel’s naphthenic blending component, which has an average of around 11.2 carbon atoms. Gopakumar et al. [41] studied the catalytic pyrolysis products of freshwater green algae and Chlorella. The carbon output of aromatic hydrocarbons rose during catalytic pyrolysis from 0.97 to 25.9 wt% as the amount of catalyst used grew from 0 to 9 times the biomass mass. In situ catalytic pyrolysis has the drawback of making it more difficult to create and deposit charcoal and separate catalysts, which raises the coke concentration. According to Yildiz et al. [42], in situ catalytic processes perform better at boosting the yield of aromatic hydrocarbon products and decreasing the generation of coke. However, compared to ex situ catalytic pyrolysis, in situ catalytic pyrolysis necessitates a higher catalyst loading. Wang et al. [43] used a HZSM-5 molecular sieve in a series microreactor to research the in situ and ex situ catalytic pyrolysis of poplar. The in situ catalytic pyrolysis mode exhibited better benefits in boosting the production of aromatic hydrocarbons, while the ex situ catalytic mode greatly outperformed it in terms of olefin output. Additionally, compared to employing the ex situ method, the yield of the pyrolytic catalytic mode (31.4%) was much greater (18.7%).
Ex Situ Catalytic Pyrolysis
Ex situ catalytic pyrolysis is direct combustion of biomass to produce main pyrolysis gas, which is subsequently brought into connection with a catalyst to produce components by secondary pyrolysis [35]. The catalyst does not come into touch with the basic ingredients directly. Ex situ catalytic pyrolysis divides the reaction procedure into separate sections, allowing each segment to be finished on its own and improving process control. The process is more adaptable, and it is simpler to get the ideal reaction conditions and a superior pyrolysis impact [40]. Additionally, because it is more readily separated, the biochar produced during biomass pyrolysis has less impact on the succeeding pyrolysis’s volatile products’ catalytic reforming procedure. Hu et al. [44] catalytically pyrolysed ex-situ pine sawdust biomass on an HZSM-5 catalyst. The findings demonstrated that light compounds might be efficiently converted into aromatics and olefins by the shape specificity in the HZSM-5 channel. At the acid centre on the outside of HZSM-5, heavy compounds may also be aggregated and broken simultaneously, but the catalytic action is not very high. Gungor et al. [45] compared the in situ and ex situ catalytic pyrolysis of pine bark using ReUS-Y as a catalyst. Ex situ catalytic pyrolysis is superior to in situ catalytic pyrolysis in terms of increasing the generation of bio-oil and stimulating the deoxygenation of volatile pyrolysis products at high reaction temperatures. Nguyen et al. [46] studied the in situ and ex situ catalytic pyrolysis of wood using octahedral zeolite as a catalyst. Deoxygenation of pyrolysis products may be done more effectively using the ex-situ catalytic pyrolysis method. This shift can be attributed to the catalyst going through an ex situ preheating procedure in a catalytic reactor. A significant temperature difference exists between catalyst and biomass source components during the in situ catalytic process. Gamliel et al. [47] investigated the catalytic pyrolysis of Miscanthus in situ and ex situ using a Py-GC/MS microreactor (HZSM-5 molecular sieve as a catalyst). While the in situ catalytic reaction mode enhanced the yield of bio-oil more than ex situ reaction settings, the amounts of aromatic compounds in the bio-oil and pyrolysis gas were much greater. The yield of biochar under the two distinct catalytic regimes showed minimal change in terms of composition [47].
Catalytic Upgrading of Bio-oil
The preparation of high-quality liquid fuels and chemicals from biomass involves two steps. Fast pyrolysis is used to create bio-oil from biomass raw materials, which is then catalytically upgraded to create the desired products [48]. More than 400 different types of organic chemicals, mostly phenols, ketones, carboxylic acids, esters, aldehydes, alcohols, furans, anhydrous-sugars, and compounds containing nitrogen, are included in the complex chemical makeup of the liquid compounds generated from quick pyrolysis of biomass. The bulk of the components is oxygen-containing, which results in a poor bio-oil value [45]. Due to the fact that it only possesses 41 to 44% of the calorific content of fossil fuels like diesel, it cannot completely substitute high-quality fuels. However, there are also a number of possible pathways for biomass conversion provided by the presence of these oxygenates. For instance, oxygenates derived from biomass can be transformed into liquid hydrocarbon fuels by catalytic hydrogenation, into high-value compounds through oxidation, or into high-quality fuels by lengthening the carbon chain through condensation [49]. It is challenging to use biomass bio-oil directly as fuel due to several drawbacks. The crude bio-oil must be upgraded and refined to create high-value liquid fuel. Catalytic hydrogenation, esterification, cracking, and other processes are the most common [50].
Analytical pyrolysis systems are created with the following features in order to ensure quick degradation of the biomass macromolecules and precise product analysis. To quickly heat the sample to the desired temperature, micro-pyrolysers can generally heat it at a maximum rate of over 100 °C/s. Typically, the pyrolysis vapours have a retention duration of fewer than 1/10 s [37]. To avoid subsequent reactions, pyrolysis vapours are swiftly sucked into connected analytical equipment. Another need for an analytical pyrolysis system is good repeatability. According to reports, an analytical pyrolysis system may provide programmes with a relative standard deviation of 2% or less [50].
Instrumentation
Numerous kinds of micro-pyrolysers are being used in research on biomass thermochemical conversion. Depending on the type of heating source they use, micro-pyrolysers can be differentiated into a variety of groups, with the most common being micro-furnace, filament, and Curie-point pyrolysers.
Micro-furnace Pyrolyser
The initial study on a vertical-type micro-furnace pyrolyser was carried out in 1977. Although other designs for micro-furnace pyrolysers have been documented, the vertical-type model is the most often used [19]. Samples are dispensed into the reaction area of the vertical-type micro-furnace pyrolyser using a sample cup or a liquid/gas syringe. Materials such as metals, glass, or quartz are used to produce sample cups. The reaction zone is commonly heated electrically, and temperature sensors and mechanisms for feedback can be used to control the reaction zone’s temperature precisely. With a temperature variation range of 1 °C, the temperature of a vertical-type micro-furnace pyrolyser may be accurately controlled throughout a wide temperature range. This kind of pyrolyser has a reaction temperature maximum of 1050 °C. The interface temperature can be changed as needed between 40 and 450 °C to restrict the vapours of pyrolysis from condensing as they exit the furnace [51].
In earlier experiments, quick pyrolysis of biomass was typically accomplished using micro-furnaces. The sample dropper is affixed to the furnace’s top for one run. To avoid sample volatilization or thermal degradation, sample cup can be held in carrier gas at room temperature [19]. This enables the exact pyrolysis of heat-sensitive materials without denaturing them. By sliding the sample cup up and down, the double-shot pyrolyser, a kind of micro-furnace pyrolyser, enables samples to go through two subsequent pyrolysis processes under different reaction circumstances. As a result, heat-sensitive materials may be precisely pyrolyzed without becoming denaturized [19, 51].
Resistively Heated Filament Pyrolyser
The first resistively heated filament pyrolyser was used in 1961 to pyrolyze polymers. Nowadays, the platinum-coil pyrolyser emerges to be the most popular commercial resistively heated filament pyrolyser variant [19].
The reaction zone and sample put in reaction zone are typically heated simultaneously by these pyrolysers from the preheated temperature (200–300 °C) to required temperature. In order to prevent the condensation of low volatile pyrolysates, which might result in denaturing the sample prior to pyrolysis, the chamber of pyrolysis is often warmed [51]. At a very rapid rate of heating, filament can achieve specified pyrolysis temperatures. As per the study, ribbon filament can go from ambient temperature to 1000 °C in just 7 ms [52]. For resistively heated filament pyrolysers, a variety of materials, including platinum, nickel, and nichrome, are employed as filament material. Due to its elevated electrical resistance and wide working range, platinum is one of these materials that is frequently employed in resistively heated filament pyrolysers [53]. Using platinum-coil pyrolysers, elevated pyrolysis temperatures of up to 1400 °C may be achieved. The physical condition of the sample dictates the two most popular filament forms, which are coil and ribbon [54]. Most samples are pyrolysed using a ribbon filament, while some samples are pyrolysed using a coil filament. In the pyrolyser, the sample is immediately spread out on the ribbon filament’s surface [55, 56].
Resistively heated filament pyrolysers have certain inherent drawbacks. Since the filament resistance may change over the course of its lifetime, the reaction zone’s temperature differential between the specified temperature and the real temperature will fluctuate appropriately [19]. To achieve the proper correlation, these two temperatures need to be calibrated often. Additionally, the filament’s length may not be heated evenly. The sample should be positioned the same way in every experiment to ensure precision and repeatability.
Curie-Point Pyrolyser (Inductively Heated Filament Pyrolyser)
Ferromagnetic metals are used in Curie-point pyrolysers to heat samples quickly. Curie-point pyrolysers heat materials in between 0.2 to 0.4 s, which is substantially quicker than micro-furnace pyrolysers [56]. There is a 1:1 relationship between reaction temperature and Curie-point temperature. The pyrolysis temperature reproducibility in each experiment is good when the foil alloy is consistent [56].
The sample that is put onto a ferromagnetic foil is heated inductively inside an RF coil. It is not necessary to connect the ferromagnetic foil to a power source. The Curie point, or temperature at which ferromagnetic foil turns from ferromagnetic to paramagnetic, is reached when ferromagnetic foil is heated by induction, at which point it immediately loses its magnetic characteristics [57]. The RF coil must be switched off before the temperature changes. For different Curie-points, different foil alloys may be produced by combining several ferromagnetic metals. Each foil alloy has a single, extremely constant Curie point temperature. The initial study on Curie-point pyrolyser was conducted in 1964 by taking temperatures ranging from 160 to 1040 °C. The advancement of Curie-point pyrolysers is constrained by the lack of control over reaction temperature. Curie-point pyrolysers do not have the option of preset heating [57, 58].
Hybrid Pyrolyser
The hybrid pyrolyser combines two different pyrolyser types to give two distinct modes of pyrolysis. The initial research on a hybrid pyrolyser was conducted in Japan in the year 2015 [19]. The Curie-point and microfurnace pyrolysers in this hybrid pyrolyser enable two pyrolysis modes. Depending on the goal and sample morphologies, one of two pyrolysis modes, Curie-point pyrolysis or microfurnace pyrolysis, can be utilized. The hybrid pyrolysis mode, which requires continuing Curie-point pyrolysis after sample thermal extraction, is another option [19].
Standard and Norms
The characteristics of bio-oils may differ significantly due to changes in the chemical composition of biomass. Processing conditions and the properties of the biomass both have an impact on the quality of the bio-oil [59]. Because of this, a set of laws and requirements must be put in place for bio-oil and bio-oil blends to be introduced and effectively marketed on the market. Since bio-properties oils are so different from the typical liquid fuels that drive the world economy, standardization is even more important for it. Even though efforts to standardize the characteristics of bio-oils have been continuing since 1985, the fundamental issue with these efforts is that traditional testing methods for mineral oils cannot be instantly applied to bio-oils without the necessary validation [37]. The first fast pyrolysis oil standards were established by ASTM. Two grades have been established thus far. Grades D and G can only be distinguished by their maximum permissible ash and solids content. Table 2 contains a list of the grades for fast pyrolysis bio-oil [59].
Pyrolysis Process Parameters
The initial research on pyrolysis was used to create tar, which was applied to wooden vessels to protect them from moisture and rot [60]. The pyrolysis of coal, wood, and petroleum can result in the production of tar. Tar made from wood was a major economic driver in Northern Europe and America. The Royal Navy mainly employed tar to protect wooden ships. The emergence of ships made of steel and iron decreased the demand for tar [61]. Since then, solutions have been created to get around the bioresources’ time-based application restrictions. Researchers have been working hard to create stable, high-quality renewable fuels that may be used effectively in a variety of applications without endangering food security and producing less waste. The parameters for the product yield in plastic pyrolysis, the product yield in biomass pyrolysis, and their fuel qualities have been evaluated because pyrolysis is one of the acceptable energy conversion technologies [49]. Additionally, different feedstock has various physio-chemical properties; a few of them are included in Table 3 and were documented by various studies using ultimate and proximate analyses.
The thermo-chemical breakdown of biomass in an inert environment is influenced by the feedstock type, operational circumstances, and physicochemical properties of the biomass [49]. The distribution and quality of the final product, as well as the biomass conversion time or pyrolysis rate, are influenced by these elements as well. A few of these crucial process variables are briefly discussed below:
Feedstock
Extractives, which are often smaller organic molecules or polymers, and minerals, which are inorganic substances, are present in diverse amounts in various biomass types, and these proportions have an impact on the product distributions during pyrolysis [85]. The following are some ways that the main biomass components influence product yields at pyrolysis temperatures: While lignin mostly produces sacharred residue, cellulose and hemicellulose components produce volatile pyrolysis products [86]. However, holocellulose (cellulose and hemicellulose) mostly changes to liquids (tars), whereas lignin breakdown is responsible for the production of char and gas [87]. Minerals, especially alkali metals, typically remain in char and have a catalytic effect on pyrolysis reactions, increasing char yields based on other conditions, in addition to effect of ash on char yield [88]. By simple volatilization or breakdown, extractives contribute to liquid and gas products. Due to numerous catalytic processes, this elemental contribution in ash also degrades the pyrolysis oil’s quality, and its removal has an impact on the procedure by increasing the pyrolysis liquid and decreasing the gas products [37].
The constituents of lignocellulosic biomass include cellulose (25–50 wt%), hemicellulose (15–40 wt%), lignin (10–40 wt%), extractives (0–15 wt%), and often a minor amount of inorganic mineral materials [38, 89]. The kind of biomass has a variety of effects on the pyrolysis procedure and end products. First, the relative mass ratios of the organic and inorganic components change depending on the type of biomass, the environment in which it grows, and the time of harvest. Each constituent’s pyrolysis has distinct reaction routes and thermochemical properties, resulting in diverse products [90,91,92,93]. The output of bio-oil is increased by cellulose and hemicelluloses, whereas lignin produces a higher percentage of solid char [94]. The average molecular weight and viscosity of the bio-oils may rise with higher lignin content, while their water concentration may drop [48]. The non-structural components of lignocellulosic biomass known as extractives, including fatty acids, simple sugars, waxes, and sterols, may be extracted using solvents such as water, ethanol, acetone, benzene, and toluene [95, 96]. It was reported in an experiment utilising corn stalk and wheat straw as the feedstocks for pyrolysis that the extractives might increase the bio-oil yield and decrease the formation of char and gas. As compared to the bio-oils from the original samples, those from the extracted samples with decreased extractives likewise had higher oxygen and lower alkane concentrations [38]. Another research found that while pyrolyzing Mongolian pine and Manchurian ash, extractives reduced the activation energy and yields of CO2, CO, and aldehydes while increasing acid production. The structural arrangement of the components varies from biomass to biomass in general, which causes the interactions between components to fluctuate depending on the kind of biomass and consequently impacts the performance of pyrolysis [92, 93]. Due to its catalytic action during bio-mass pyrolysis, the composition and amount of mineral matter in the biomass types can also be variables that affect product distribution and attributes.
Heating Rate and Temperature
Primary vapours must be heated and cooled quickly to minimize the number of secondary reactions that lower liquid yield and degrade its quality. Higher char yields are another benefit of slow heating [89]. On char yields and characteristics, temperature also has a considerable effect. In all pyrolysis processes, higher temperatures lead to lower char yields. The main cause of this is that at higher temperatures, significant volatile material is stripped from the char, which lowers yields. For instance, as temperature rises from 638 to 879 K, the char output drops from 31 to 17% [97]. Lower temperatures might also be detrimental since they could lead to incomplete biomass breakdown, which would increase the amount of unpyrolysed solid in the char content. The composition of the char is also influenced by temperature, with higher temperatures producing chars with higher carbon levels [38]. For temperatures higher than 773 K, the solid product char comprises more than 85% carbon by weight [91]. Up to a maximum temperature, typically between 673 and 823 K, liquid yields rise, although this is greatly sensitive on other operating circumstances. The greatest production of pyrolysis oil has been shown to be attained for a range of feedstock types at temperatures of about 673 to 823 K, with a continuous drop in char yield and matching rise in gas yield [89]. The condensed liquid yields were decreased beyond this temperature range due to secondary processes that cause vapour breakdown becoming more prevalent [97]. Below the peak temperature (773 K) for liquid yield, gas yields are typically modest; above this temperature, gas yields dramatically rise with increasing temperatures since gases are the primary products of vapour decomposition [85]. Below the peak temperature (773 K) for liquid yield, gas yields are typically modest; above this temperature, gas yields dramatically rise with increasing temperatures since gases are the primary products of vapour decomposition [89]. However, in pyrolysis, higher heating values of non-condensable gases are achieved at reaction temperatures higher than 723 K because CO and CH4 are produced at a higher rate than CO2 does [6].
Volatile Residence Time and Pressure
The gas flow rate through the reactor determines the residence time of volatiles, which in turn influences the contact time between primary vapours and hot char, which in turn impacts the intensity of secondary reactions as well as the volatile product qualities [98, 99]. The cracking and polymerization reactions of vapours to gases and solids, respectively, cause lower oil yields for longer vapour residence times. However, it was shown that for vapour residence times between 1 and 5 s at temperatures of about 500 K, the loss of tar yield is only around 10%. By inducing incomplete depolymerization of lignin due to random bond breakage and macromolecule interaction, short volatile residence durations < 1 s have an impact on the biomass breakdown process, ultimately leading to a less uniform liquid product [100]. The operating pressure, which, when raised, results in a lower specific volume of volatiles and a longer intra-particle residence time that favours their breakdown while scaping the biomass particle, also has a substantial impact on the pyrolysis process [101]. Additionally, this causes volatiles to be more concentrated (under partial pressure), which accelerates the breakdown reaction rate via subsequent reactions. Greater pressures have been observed to result in higher char fractions [101]. It was also claimed that the chars produced under conditions of increased pressure led to higher yields of fixed carbon. This result is helpful in optimising biochars’ capacity for carbon sequestration [102].
Particle size, Shape, and Orientation
The pyrolysis process’s heat and mass transmission characteristics are significantly influenced by particle size. The heat gradients are simply bigger for larger particles, and the fluid residence periods are long enough to promote secondary reactions. For external temperatures more than 800 K, the increasing particle size also decreases the liquid yields due to the activity of secondary processes, which increases gas yields [103]. Biomass size leads to a smaller char fraction. However, experimental studies reported that there is no significant influence of the increase in particle size (0.7–17 mm) on the product yields. However, it decreased the heating rate from 1000 to1.5 K/s; there was a marginal decrease in the liquid yields and around a 5% decrease in the gas yields with a corresponding increase in the char yields when the particle size was increased from 17 to 20 mm. Increasing the particle size also led to increase in the water content (40 to 55 wt%) of pyrolysis liquid and a decrease in the carbon content (78.5 to 75 wt%) of the solid char product [89]. Particle shape also influences the pyrolysis process. Spherical particles have lesser char yield and conversion time when compared to slab-shaped and cylindrical particles. However, another study showed that spherical particles have the smallest surface-to-volume ratio, leading to a slower rate of heat and mass transfer and higher conversion time in comparison to other spherical particles. At small particle diameters (typically less than 0.2 mm), the rate of reaction becomes dominant, and the different particle shapes show nearly equal conversion times [103].
Grain orientation is an important parameter in biomass pyrolysis due to the anisotropic behaviour of biomass. The permeability of flow along the grains was 104 times that across the grain, and thermal conductivity along the grains was twice that across the grains [104]. The perpendicular grain heating decreased the tar yield with a corresponding increase in the char, water (dehydration reaction), and gas yields. They attributed this to lower thermal conductivity (almost one-third as compared to parallel or tangential grain direction) but not due to an increase in residence time owing to a reduction in porosity [87]. Branca and Blasi [105] proposed that the secondary reactions occur to a larger extent for perpendicular grain heating as compared to parallel grain heating.
Reactor Configuration
Many reactor layouts have been investigated for the thermal breakdown of biomass in the absence of air. The medium used to transport heat from the reactor to the biomass particles during the decomposition process is the most significant component affecting reactor choice. Heat transmission in the ablative kind occurs when biomass particles come into touch with the heated surface [100]. The pace of heat delivery to the reactor and the size of the biomass particles used in this process are also factors. Although the heat transfer gas or carrier gas is not necessary, it is difficult to heat biomass due to substantial heat losses. Getting extended residence durations for the biomass particles to enable high conversion, high char attrition, and high carbon carry-over into pyrolysis liquid products is the main problem of this arrangement [98]. Heat is transferred by direct contact with a heated surface during vacuum pyrolysis. Larger biomass particles and volatiles with short residence times are used in this procedure. However, this approach needs more sophisticated equipment and has low heat and mass transmission rates. The circulating fluidized bed (CFB) employs a heat source, a fluidizing or carrier gas or solid, to heat the biomass particles by convection as well as conduction. Because of its restrictions on heat transmission, this method cannot produce acceptable liquid yields with particles larger than roughly 3 mm [100]. The CFB configuration has some drawbacks related to uneven biomass particle residence times, the need for solid recycling of partially reacted feed, the need for post-pyrolysis liquid treatment to reduce char content due to carryover, high char attrition, ash build-up in circulating solids leading to the cracking of organic molecules in volatile products, and decreased pyrolysis oil yield [37]. The same method of heat transmission to biomass particles is used in a bubbling fluidized bed (BFB); however, convective and conductive heat transfer contributions vary. With a high concentration of desired compounds and a low carryover of char (micro-carbon) into the liquid product, this technique improves the quality of the pyrolysis oil. Additionally, there is a quick elution of char from the actor, lowering the likelihood of volatile cracking [98].
Biomass Pre-treatment
Before pyrolysis, the biomass feedstock often has to undergo some sort of pre-treatment. To improve the effectiveness of pyrolysis, the pre-treatment aims to alter or even destroy the lignocellulosic structure. Five main categories can be used to categorize biomass pre-treatment technologies: (1) physical (such as milling, grinding, and extrusion), (2) thermal (such as torrefaction, steam explosion/liquid hot water pre-treatment, and ultrasound/microwave irradiation), (3) chemical (such as treatment with acids, bases, and ionic liquids), (4) biological (such as fungal, microbial consortium, and enzymatic), and (5) above combined pre-treatments [106].
Physical Pre-treatment
A common practice to simplify biomass feeding into reactors and enhance pyrolysis performance is milling or grinding the biomass into smaller particles. Given that most biomass is a poor conductor of heat, the biomass pyrolysis mechanism will be influenced by the temperature gradient across the particle [107]. In general, smaller particles aid in the establishment of homogeneous temperature inside particles during pyrolysis, which increases the production of bio-oil by reducing the formation of char and subsequent cracking of vapours. The entire cost of the biomass pyrolysis process might be greatly raised by particle size reduction, which can be expensive [108]. The volumetric energy density of biomass is increased, while the moisture content is decreased by extruding biomass under higher pressure to create biomass pellets, which typically have the shape of tiny cylinders. Xue et al. [109] found that the yields of char, gas, and char density rose with bigger particle diameters, while the yield of tar decreased. Additionally, mixed biomass resources could be utilized during pyrolysis procedures. Mixing pellets pyrolysis of pine (25%), fir (25%), wood and cotton (50%), or corn (50%) at 400–750 °C resulted in gas products with a generation of high CO and H2 and low CO2 contents and the gas heating value around 14–15 MJ/m3.
Chemical Pre-treatment
The process of biomass pyrolysis is thought to be affected by the presence of inorganic minerals, particularly alkali (K, Na) and alkaline-earth (Mg, Ca) metal salts [110]. For instance, during the first pyrolysis of cellulose, K in the biomass mineral matter catalytically promotes the synthesis of lower molecular weight molecules and inhibits the creation of levoglucosan. As catalysts, the cations cause the biomass monomers to fragment rather than depolymerize, favouring char production and reducing bio-oil yields. The build-up of salts on the inside walls of the reactor and pipeline also leads to corrosion and engineering challenges [111]. Additionally, the presence of ash in bio-oils impairs their subsequent uses and hastens the ageing process. The aforementioned disadvantages can be remedied by lowering the ash level with water or acid washing. During the harvesting, transport, and storage of biomass, water washing is used to remove the dirt and minerals from the surface of the biomass particles. The biomass matrix will still include the structural minerals, though. Acid washing using HNO3 and HF can further lower the ash concentration [50]. Water washing, however, reduces char formation and boosts bio-oil output. The cellulose feedstock was pre-treated with phosphoric acid to increase the generation of levoglucosan and levoglucosenone in the bio-oils. Concentrated acids, such as H2SO4, have occasionally been used to hydrolyse and solubilize the carbohydrates in biomass in order to remove the lignin, and alkaline solutions (e.g., NaOH) were employed to remove lignin, hemicellulose, and/or cellulose [106]. Ionic liquids are a class of recently discovered chemicals that can assume the form of/turn into liquids at temperatures below 100 °C and are mostly composed of organic cations and inorganic/organic anions (especially at room temperature) [112]. They are considered green solvents having distinct physical and chemical properties such as low vapour pressure, high chemical stability, and non-flammability [113]. Ionic liquids have found uses not only in catalysis, chemical synthesis, and engineering fluids but also in the deconstruction and dissolving of cellulose, hemicellulose, and lignin [114]. It has been used to pre-treat lignocellulosic biomass for the production of sugars from enhanced enzymatic hydrolysis of oil palm fronds [115]; renewable chemicals of vanillin, syringyl, and ally lguaiacol from eucalyptus, switchgrass, and pine respectively [116]; levulinic acid from cellulose [117]; and biogas from improved anaerobic digestion of water hyacinth, rice straw, mango leaves, and spruce [118]. The thermal behaviour of biomass materials can also be altered once they have been pre-treated with ionic solutions. Zhang et al. [119] reported that after pre-treatment with 1-butyl-3-methylimidazolium acetate, the Avicel and switchgrass samples had greater heat resistance due to cellulose crystal modification and mineral removal, respectively.
Thermal Pre-treatment
Prior to pyrolysis, biomass is dried to boost energy efficiency and enhance the quality of bio-oil products. There are a variety of industrial dryers available for drying biomass [120, 121] that are made to take the moisture out of the biomass while reusing the fugitive heat created during the heated pyrolysis process. The water content of the biomass is completely eliminated, and the oxygen content is slightly decreased when the thermal pre-treatment, also known as torrefaction, is carried out at temperatures between 200 and 300 °C [122]. Torrefied biomass is superior to untreated biomass in a number of ways. It has a greater energy density, better grind ability, reduced hygroscopicity when kept outdoors, reduces the danger of self-ignition and biological deterioration, and enhances feeding in the reactors [123]. Some breakdown processes start to occur during torrefaction, resulting in the formation of levoglucosan, CO, acetic acid, and CO2. According to Boateng et al. [124], torrefied hardwood and switchgrass pellets generate bio-oils with lower acidity and better energy density but lower liquid yield and carbon-to-oil conversion than oils from untreated biomass. It has been found that torrefaction increases the H2 and CH4 concentrations of generated syngas while decreasing their CO2 content. In order to “explode” the biomass structure, steam explosion (SE) involves exposing biomass to saturated steam for a brief period of time at a temperature between 150 to 260 °C and a pressure of 1.5 to 5 MPa in a sealed vessel [125]. The physical characteristics of lignocellulose and the breakdown of carbohydrate bonds caused by SE change the behaviour of biomass pyrolysis and affect the qualities of the final product. In a study of the pyrolysis of willow chips following SE pre-treatment at 205 °C using thermogravimetric analysis at 10 °C/min, it was found that the treated material had enhanced cellulose crystallinity. Hemicellulose breakdown became more active and migrated to a lower temperature area throughout this process, although cellulose and lignin saw an improvement in thermal stability. In a further experiment, loblolly pine chips were pre-treated by SE (1.3 MPa and 173–193 °C), and the pre-treated and untreated materials were then individually pyrolysed in a specialized auger reactor [124] [125]. The results revealed that the chips following SE pre-treatment had higher cellulose and lignin levels while having a lower hemicellulose content when compared to the untreated feedstock. A bio-oil product with viscosity (at 40 °C) from 6.5 to 3.9 cSt, and water content from 20.8 to 29.3%, was also produced by the SE pre-treatment. Similar to this, hot liquid water may be used to partially dissolve the hemicellulose in biomass feedstock, which helps to lower the amount of acetic acid present and stabilize bio-oils [124]. For pre-treating biomass, unconventional thermal methods, including ultrasound and microwave irradiation, are used. Ultrasonography’s main goal is to boost the production of biogas from the anaerobic digestion of sludge, namely methane [126]. The lignocellulosic biomass has also been the subject of several investigations. Due to the cavitation effects, which might facilitate the movement of enzyme molecules and the opening up of the substrate’s surface, ultrasound aid can effectively speed up the enzymatic hydrolysis of cellulose in maize stover and sugar cane bagasse to produce sugars. In an investigation, it was shown that hemicellulose generated using ultrasonic aid had more linearity and less acidity than hemicellulose produced by traditional KOH extraction [127]. Further research is required to determine how ultrasonic pre-treatment affects biomass pyrolysis. Nowadays, microwave irradiation is a popular substitute for conventional heating of lignocellulosic biomass because it may produce “hot spots” in the biomass. Although pyrolysis of bio-mass with a microwave has been extensively researched [128]. The use of microwave irradiation to pre-treat biomass before pyrolysis is not well understood. Due to the inhibition of secondary reactions during pyrolysis following biomass drying in a microwave oven, it was suggested that microwave drying at 600 W and for 6 min would increase the bio-oil and char yields. Performance demonstrated superior yields than standard electrical oven drying [129].
Biological Pre-treatment
Physical and chemical pre-treatments are faster, while biological approaches have a better environmental impact and use less energy [130]. It has been demonstrated that pre-treating lignocellulose with fungi before pyrolysis increases the performance of the reaction. To pre-treat the natural lignocellulose, white-rot fungus was chosen because it has the potential to selectively breakdown the refractory lignin component during pyrolysis. Three distinct white-rot fungus species (Pleurotus ostreatus BP2, Echinodontium taxodii 2538, and Irpex lacteus CD2) were used in an experiment to biopretreat maize stover. After that, the thermal properties of the pyrolyzed corn stover were examined using a TGA instrument. The results demonstrated that this bio-pre-treatment was capable of lowering the pyrolysis temperature by 1–35 °C and reducing the emission of hazardous SOx by reducing the sulphur content of the feedstock by 30–45%. Using ZSM-5 zeolite, Yu et al. [130] examined the rapid pyrolysis of maize stover that had been pre-treated with the white-rot fungus I. lacteus CD2. They found that the yields of useful aromatic products improved by 10%, and the deposition of unwanted coke on catalysts was reduced by 20%. Pre-treating lignocellulosic biomass using a microbial consortium has often been employed to increase the generation of biogas. It uses certain bacteria that have been chosen from the environment’s natural resources to primarily destroy the cellulose and hemicellulose components [106]. The procedure can increase the methane output by 25% to about 100% and lasts for several hours to many days. Prior to its pyrolysis, lignin’s hydrolysis using enzymes has been suggested to increase the synthesis of aromatic phenols and hydrocarbons. The chars that were created also looked to have vesicles and to be quite porous [131].
Techno-economic Assessment for Pyrolysis of Biomass
Few studies have looked at the techno-economic performance of pyrolyzing biomass or petroleum waste, and even fewer studies have estimated the amount of energy that can be produced from the by-products of rapid pyrolysis or examined the conversion of bio-oil into transportation fuels. Patel et al. [132] used aspen hardwood to model a 2000-MT/day plant in order to study the techno-economics of producing renewable gasoline and diesel. The second stage involved converting the bio-oil produced in the first stage into gasoline and diesel fuel using an alumina-supported sulfidic nickel-molybdenum catalyst. It was determined that the expenses of manufacturing gasoline and diesel were $1.04 and $1.09 per litre, respectively. A 1-MT rice straw batch power plant was the subject of a comparative techno-economic and environmental assessment. Similar to this, Shabazz et al. [133] modelled the techno-economic performance of the pyrolysis of individual biomass components (holocellulose and lignin) and reported that the pyrolysis of lignin was more cost-effective and environmentally friendly, producing more biochar than the pyrolysis of hemicellulose and cellulose components. The Iowa State University (ISU) reported technical and economic feasibility of the fast pyrolysis and hydro-processing of biomass which concluded that the pathway could produce cellulosic biofuels for a minimum fuel selling price (MFSP) of $2.11/gal [134]. The expanded work of this research was done by Brown et al. [134] by performing an updated techno-economic analysis of the fast pyrolysis and hydro-processing pathway. They have calculated the MFSP for a 2000 metric tonnes per day (MTPD) facility employing fast pyrolysis and hydro-processing to convert corn stover to gasoline and diesel fuel to quantify the economic feasibility of the pathway. They reported the MFSP of gasoline and diesel fuel produced via fast pyrolysis and hydro processing to be $2.57/gal which indicates the competitiveness of the pathway with petroleum. Shabangu et al. [135] assessed the feasibility of the co-production of methanol and biochar from slow pyrolysis at 300 °C and 450 °C and gasification at 800 °C of pine to produce biochar and volatiles and the processing of the volatiles to produce methanol using process data for large-scale conversions based on natural gas. They reported that from gasification, methanol could be generated at or below current prices of methanol produced from fossil fuel ($422/tonne) from a plant size of 100 tonne/h upwards. Interestingly, they did not find the pyrolysis as competitive without valuing the biochar as a product [135]. They found that their profitability is sensitive to the biochar selling price, with a break-even at a biochar price of about $220/tonne for the pyrolysis at 300 °C and about $280/tonne for pyrolysis at 450 °C. The comparison of capital and operating cost for six near-term biomass-to-liquid fuel technology scenarios representing three conversion platforms: pyrolysis, gasification, and biochemical were reported in which the feedstock is assumed to be corn stover and plant capacity was 2000 tonne/day for each plant [134]. It was found large differences in the total capital investments required among the three platforms, and the stand-alone biomass-to-liquid fuel plants were expected to produce fuels with a product value in the range of $2.00–5.50 per gallon ($0.53–1.45 per litre) gasoline equivalent, with pyrolysis the lowest and bio-chemical the highest in which the relatively high production values were driven primarily by an assumed feedstock cost of $75 per dry tonne and the cost of capital for the plants [132]. They have reported that by taking into account increased capital costs and decreased plant performance associated with first-of-a-kind plants, increases estimated product values to $2.00–12.00 per gallon ($0.53–3.17 per litre) gasoline-equivalent. Similarly, six types of commercial-scale pyrolysis and co-pyrolysis plants for rice straw (RS) and waste tire (WT), with a capacity of 20 tonne/h, have been modelled by Khan et al. [136] based on experimental data in which the capital investment of plants ranged between $17.0 and $19.9 million with Plant A (RS only) having the lowest and Plant E (20% RS and 80% WT) having the highest value. They have found that the operating cost was the lowest for Plant A and highest for Plant F (100% WT) due to the procurement cost of WT, and Plant E was the most economical alternative with the highest gross margin, highest net present value, and lowest payback time of 7.06%, $ 5.63 million, and 6.23 years respectively. Jaroenkhasemmeesuk et al. [137] analysed the production and energy consumption of bio-oil production based on a small biomass pyrolysis plant (capacity of 20–30 dm3 of bio-oil per day) by carrying out the calculation on mass and energy balance to assess the performance and improve process design. They have found that the operation cost of crude bio-oil production was about 30–35 Thai baht (THB) per dm3 which implies the excessively high operating cost of the fast pyrolysis units. The bio-oil production cost was estimated to be about 30–35 THB per dm3. The estimated crude bio-oil price from other plants was 15–20 THB per dm3 for the 100 kg/h plant [137]. The capacity of the plant affected production costs directly. Their results showed that when the sale of the project reaches 86.5% of the predicted value, the payback period of the plant is about 7.5 years, which is much shorter than the operational life. Unfortunately, the sale of the bio-oil has not yet reached the expected point. Figure 5 shows the break-even analysis for the pyrolysis plant. A breakeven point is based on the projection proceeds of sale and expected proceeds of sale after upgrading. Even though the operation cost of bio-oil with upgrading will increase, higher proceeds of the sale will reduce the production capacity to 66.5% [137].
Break-even analysis. “Reprinted from Technical and Economic Analysis of A Biomass Pyrolysis Plant, 79, Chawannat Jaroenkhasemmeesuk and Nakorn Tippayawong, Energy Procedia, 950-955, 2015, with permission from Elsevier” [137]
Oudenhoven et al. [138] evaluated the technical and economic feasibility of pyrolysis of pinewood, bagasse, and straw by considering a plant of biomass capacity of 5 to 50 tonne/h in which the target products were heating oil and/or additional pyrolytic sugars. They have reported a very sensitive economics approach to the plant scale, capital cost, and biomass price. They also found that the production of heating oil and sugars from bagasse at a biomass scale of 50 tonne/h is the most economical option. The techno-economic performance analysis of biofuel production and electric power generation from fast biomass pyrolysis and bio-oil hydro processing was done by Shemfe et al. [139] through process simulation by considering a process model of 72 MT/day pine wood fast pyrolysis and bio-oil hydro-processing plant. They have found from the simulation results that 1 kg/s pine wood generated 0.64 kg/s bio-oil, 0.22 kg/s gas, and 0.14 kg/s char. They also reported that the energy required for drying and fast pyrolysis operations could be provided from the combustion of pyrolysis by-products, mainly char and non-condensable gas, with sufficient residual energy for miniature electric power generation. They found that about 0.24 kg/s of gasoline and diesel range products and 96 W of electric power can be produced from 1 kg/s of pine wood. The effect of initial bio-mass moisture content on the amount of electric power generated and the effect of biomass feed composition on product yields was also reported in this study [139]. They estimated from discounted cash flow analysis assuming the plant operates for 20 years at a 10% annual discount rate, that the plant would require £16.6 million of capital investment and product value estimated to be at £6.25/GGE (gasoline gallon equivalent).
Since the majority of pyrolysis plants are currently not operating on a commercial scale, it is crucial to evaluate the techno-economic viability of pyrolysis biofuel production in comparison to conventional petroleum fuels [138]. Few studies have used a process simulation platform to undertake a techno-economic analysis of the fast pyrolysis process and bio-oil hydroprocessing for the manufacture of transportation fuels. In order to assess the production of hydrocarbon biofuel from a 2000-MT/day plant of hybrid poplar wood chips, Jones et al. [140] undertook a design case study in 2009. A capital expenditure of US$303 million with a minimum fuel selling price of US$2.04 was projected in their assessment. Wright et al. [141] conducted another techno-economic analysis on a 2000-MT/day of corn Stover fast pyrolysis plant and subsequent bio-oil upgrading via hydrotreating and hydrocracking processes to determine fuel product value and capital costs. In order to evaluate the techno-economic performance of the process, a 72-MT/day fast pyrolysis plant for pine wood and subsequent bio-oil hydro processing are modelled using rate-based chemical processes [139]. Fast pyrolysis (FP) and hydro processing were found to be the most economically feasible of the three cellulosic biofuel pathways. Despite the significantly higher capital and operating costs involved, techno-economic analyses suggest that using bio-oil as a feedstock for the production of renewable hydrocarbon fuels and chemicals is the most economically viable use of the substance. However, this is dependent on input costs and output values.
Discussions and Future Outlooks
Biomass thermochemical conversion has made considerable use of analytical pyrolysis. The three most popular kinds of micro-pyrolysers are Curie-point, filament, and micro-furnace models. Micro-pyrolysers may be used to analyse solid, liquid, and gas samples and have several analysis modes, such as pyrolysis mode, EGA mode, and offline mode [29]. Analytical pyrolysis has been used to study biomass pyrolysis, including non-catalytic pyrolysis, in situ catalytic pyrolysis, ex situ catalytic pyrolysis, and hydropyrolysis. Analytical pyrolysis, which defines pyrolysis, is a potent tool for enhancing reaction conditions. It is critical to assess the techno-economic viability of pyrolysis biofuel production in comparison to traditional petroleum fuels because the bulk of pyrolysis facilities is not currently functioning on a commercial scale. The impact of the equipment’s lifetime and analytical precision restricts its applicability in studies of oxidative pyrolysis, gasification, and solvent liquefaction. Given the potential for turning agricultural waste, wood waste, and municipal solid waste into clean energy, the pyrolysis of biomass deserves a lot of studies [132]. Based on the anticipated product output, this study determined the pyrolysis technological path, including the choice of pyrolysis operating modes, reactor types, etc. (bio-oil, bio-char or syngas). However, the potential of the pyrolysis products may be realized with a solid grasp of the underlying process. The application scope of analytical pyrolysis systems may be expanded by the variable usage of pyrolysis modes. The temperature-programmed pyrolysis mode and the stepwise pyrolysis mode can be used to explore the impact of heat treatment conditions on the physical characteristics of precious materials because only a small number of samples are needed. Currently, the discipline of pyrolysis is where analytical pyrolysis has been used the most. By adjusting the configuration of micro-pyrolysers, such as the sampling technique, the combined configuration of the catalytic system and the carrier gas system, and the method of product collection and analysis, analytical pyrolysis can be applied to more fields. An analytical pyrolysis device may be used to examine biomass solvent liquefaction by adding the solvent and sample to a specifically made sample cup. Purging in the analytical pyrolysis system with an inert carrier gas at a high split ratio can mitigate the impact of residual oxygen and water in the combustion products on the column in biomass combustion experiments. Some micro-pyrolysers’ designs allow for the construction of several pyrolysis zones with various carrier gases, which may be used to explore how various atmospheric conditions affect the pyrolysis process [75]. The breakdown of biomass by UV and visible light is a significant issue that needs in-depth research. Studies examining the characteristics of material degradation by UV are useful for combining analytical pyrolysis with UV pre-treatment. Using an analytical pyrolysis system, it is feasible to examine biomass solvent liquefaction by adding the solvent and sample to a sample cup that has been particularly made for the purpose. For investigations on biomass combustion, purging the analytical pyrolysis system with an inert carrier gas at a high split ratio can minimize the impact of any remaining oxygen and water in the combustion products on the column. One way to explore the impact of various atmospheres on the pyrolysis mechanism is to configure distinct pyrolysis zones with various carrier gases using the design of some micro-pyrolysers. An important issue that needs in-depth research is the destruction of biomass by UV and visible light. Studies investigating the UV material degrading qualities can benefit from combining analytical pyrolysis with UV pre-treatment [19]. Torrefaction and hydrothermal pretreatment of biomass might be a way to improve its physicochemical properties, which could increase its conversion efficiency, reduce the creation of coke, and boost the generation of aromatic compounds during the catalytic pyrolysis of biomass. For instance, cellulose content and physicochemical features of biomass, such as fewer oxygenated chemicals and high heating value, can be improved by torrefaction to provide bio-oil with low oxygenated compounds, low acidity, high energy content, and high monoaromatic hydrocarbons [75]. Crystalline cellulose may be created by hydrothermal treatment, which also removes alkali and alkaline metals, particularly K and Na metals, which creates an ideal environment for the synthesis of aromatic compounds.
However, significant attempts have been undertaken to create a viable bio-oil usage strategy. Bio-oil still has a hard time finding useful commercial use. The product stream is more intricate when compared to many other methods. Because pyrolytic oil cannot be used directly in IC engines, further expensive upgrading is required [142]. It is feasible to examine biomass solvent liquefaction using an analytical pyrolysis system by introducing the solvent and sample to a sample cup that has been particularly made for the purpose. Purging with an inert carrier gas at a high split ratio in the analytical pyrolysis system may be used to conduct biomass combustion experiments without having to worry about the column being impacted by residual oxygen and water from the combustion products. The architecture of certain micro-pyrolysers allows for the formation of several pyrolysis zones with various carrier gases, which can be useful for researching the effects of various atmospheric conditions on the pyrolysis process. It is necessary to research in depth the phenomena of biomass breakdown by UV and visible light. Studies examining the characteristics of materials degrading under UV light can benefit from combining analytical pyrolysis with UV pre-treatment.
Conclusion
This work investigated the process of biomass pyrolysis for energy recovery in the form of oil, gas, and char by thoroughly analysing its different process parameters and techno-economic aspects. It also discusses the advantages as well limitations of the current biomass pyrolysis process. Different feedstock has a different potential to give useful end products based on their physical and chemical constituents, which have been shown in Table 3. Very few analyses on the economy of biomass pyrolysis have been done to date, research on which can give a boost in this field in economic conversion processes. Based on the discussion that has just taken place, we can conclude that energy can be easily recovered through pyrolysis in the form of oil, gas, or any other valuable solid products, so long as the potential feedstock and input variables that are essential for the desired output are identified. The working methods are also essential for achieving this aim. We must consider each of these factors to get the desired outcome during pyrolysis.
References
Kumar Mishra R, Mohanty K (2020) Co-pyrolysis of waste biomass and waste plastics (polystyrene and waste nitrile gloves) into renewable fuel and value-added chemicals. Carbon Resour Convers 3:145–155. https://doi.org/10.1016/j.crcon.2020.11.001
Alarik Sandrup, Moxham B, Rakos C (2022) Global bioenergy statistics. In: World Bioenergy Assoc. https://www.worldbioenergy.org/global-bioenergy-statistics/. Accessed 17 Dec 2022
Fang J, Gozgor G, Mahalik MK et al (2021) The impact of economic complexity on energy demand in OECD countries. Environ Sci Pollut Res 28:33771–33780. https://doi.org/10.1007/s11356-020-12089-w
United Nations Department of Economic and Social Affairs (2022) World population prospects. In: United Nations Publ. https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/wpp2022_summary_of_results. Accessed 20 Dec 2022
Sehrawat M (2021) Modelling the nexus between human capital, income inequality, and energy demand in India: new evidences from asymmetric and non-linear analysis. Environ Sci Pollut Res 28:3632–3643. https://doi.org/10.1007/s11356-020-10733-z
Qiu B, Tao X, Wang J et al (2022) Research progress in the preparation of high-quality liquid fuels and chemicals by catalytic pyrolysis of biomass: a review. Energy Convers Manag 261:115647. https://doi.org/10.1016/j.enconman.2022.115647
Kumar A, Agrawal A (2020) Recent trends in solid waste management status, challenges, and potential for the future Indian cities — a review. Curr Res Environ Sustain 2:100011. https://doi.org/10.1016/j.crsust.2020.100011
Pinto Da Costa J, Rocha Santos T, Duarte A (2020) The environmental impacts of plastics and micro-plastics use, waste and pollution: EU and national measures. Eur Union 3:10–62
Ritchie H, Rosado M, Pablo R (2022) Renewable energy. In: Our world data. https://ourworldindata.org/renewable-energy. Accessed 21 Nov 2022
Jaganmohan M (2022) Production of wood fuel worldwide from 2000 to 2020. In: Statista. https://www.statista.com/statistics/481654/wood-fuel-production-globally/. Accessed 2 Nov 2022
Jaganmohan M (2022) Export volume of wood pellets worldwide in 2021, by major country. In: Statista. https://www.statista.com/statistics/477086/exports-of-wood-pellets-volume-by-key-country/. Accessed 2 Nov 2022
Bouckaert S, Spencer T (2022) World Energy Outlook. In: International energy agency. https://www.iea.org/reports/world-energy-outlook-2022. Accessed 5 Nov 2022
ILO I (2022) Renewable energy and jobs: Annual Review 2022. In: Int. Renew. energy agency. https://www.irena.org/News/pressreleases/2022/Sep/Renewable-Energy-Jobs-Hit-12-7-Million-Globally. Accessed 11 Nov 2022
Granholm JM (2022) Energy efficiency and renewable energy. In: Dep. Energy. https://www.energy.gov/eere/office-energy-efficiency-renewable-energy. Accessed 11 Dec 2022
Achim Steiner, Newman D (2022) Global waste management outlook. In: UN Environ. Program. https://www.unep.org/ietc/resources/publication/global-waste-management-outlook#:~:text=The Global Waste Management Outlook%2C a collective effort,a call for action to the international community. Accessed 17 Dec 2022
Qiu B, Yang C, Shao Q et al (2022) Recent advances on industrial solid waste catalysts for improving the quality of bio-oil from biomass catalytic cracking: a review. Fuel 315:123218. https://doi.org/10.1016/j.fuel.2022.123218
Dhyani V, Bhaskar T (2019) Pyrolysis of biomass. In: Pandey A, Larroche C, Dussap C-G et al (eds) Biofuels: alternative feedstocks and conversion processes for the production of liquid and gaseous biofuels, 2nd edn. Elsevier Inc., Amsterdam, pp 217–244
Pandey U, Stormyr JA, Hassani A et al (2020) Pyrolysis of plastic waste to environmentally friendly products. In: Tallinn E (ed) WIT transactions on ecology and the environment, 3rd edn. WIT Press, Billerica, pp 61–74
Li T, Su J, Wang C et al (2022) Advances in the development and application of analytical pyrolysis in biomass research: a review. Energy Convers Manag 271:116. https://doi.org/10.1016/j.enconman.2022.116302
Ma M, Xu D, Zhi Y et al (2022) Co-pyrolysis re-use of sludge and biomass waste: development, kinetics, synergistic mechanism and industrialization. J Anal Appl Pyrolysis 168:105–119. https://doi.org/10.1016/j.jaap.2022.105746
Chen D, Cen K, Zhuang X et al (2022) Insight into biomass pyrolysis mechanism based on cellulose, hemicellulose, and lignin: evolution of volatiles and kinetics, elucidation of reaction pathways, and characterization of gas, biochar and bio-oil. Combust Flame 242:112–124. https://doi.org/10.1016/j.combustflame.2022.112142
Ghodake GS, Shinde SK, Kadam AA et al (2021) Review on biomass feedstocks, pyrolysis mechanism and physicochemical properties of biochar: state-of-the-art framework to speed up vision of circular bioeconomy. J Clean Prod 297:126645. https://doi.org/10.1016/j.jclepro.2021.126645
Deng C, Liaw SB, Gao X, Wu H (2020) Differences in soot produced from rapid pyrolysis of xylan, cellulose and lignin under pulverized-fuel conditions. Fuel 265:116991. https://doi.org/10.1016/j.fuel.2019.116991
Fakayode OA, Wahia H, Zhang L et al (2022) State-of-the-art co-pyrolysis of lignocellulosic and macroalgae biomass feedstocks for improved bio-oil production—a review. Fuel 332:126071. https://doi.org/10.1016/j.fuel.2022.126071
Escalante J, Chen WH, Tabatabaei M et al (2022) Pyrolysis of lignocellulosic, algal, plastic, and other biomass wastes for biofuel production and circular bioeconomy: a review of thermogravimetric analysis (TGA) approach. Renew Sustain Energy Rev 169:112914. https://doi.org/10.1016/j.rser.2022.112914
Choi D, Jung S, Lee DJ et al (2021) A new upgrading platform for livestock lignocellulosic waste into syngas using CO2-assisted thermo-chemical process. Energy Convers Manag 236:114084. https://doi.org/10.1016/j.enconman.2021.114084
Naveiro M, Romero Gómez M, Arias-Fernández I, Baaliña Insua Á (2022) Thermodynamic and environmental analyses of a novel closed loop regasification system integrating ORC and CO2 capture in floating storage regasification units. Energy Convers Manag 257:115410. https://doi.org/10.1016/j.enconman.2022.115410
Silveira Junior EG, Silveira T, Perez VH et al (2022) Fast pyrolysis of elephant grass: intensification of levoglucosan yield and other value-added pyrolytic by-products. J Energy Inst 101:254–264. https://doi.org/10.1016/j.joei.2022.02.003
Uddin MN, Techato K, Taweekun J et al (2018) An overview of recent developments in biomass pyrolysis technologies. Energies 11:3115. https://doi.org/10.3390/en11113115
Perkins G, Bhaskar T, Konarova M (2018) Process development status of fast pyrolysis technologies for the manufacture of renewable transport fuels from biomass. Renew Sustain Energy Rev 90:292–315. https://doi.org/10.1016/j.rser.2018.03.048
Kantorek M, Jesionek K, Polesek-Karczewska S et al (2021) Thermal utilization of meat-and-bone meal using the rotary kiln pyrolyzer and the fluidized bed boiler — the performance of pilot-scale installation. Renew Energy 164:1447–1456. https://doi.org/10.1016/j.renene.2020.10.124
Cen K, Zhuang X, Gan Z et al (2022) Biomass pyrolysis polygeneration with bio-oil recycling: co-pyrolysis of heavy bio-oil and pine wood leached with light bio-oil for product upgradation. Fuel 335:127057. https://doi.org/10.1016/j.fuel.2022.127057
Hasnzadeh A, Mehrara M, Irani M et al (2022) An innovative biomass-fueled gas turbine-orc system equipped with electrochemically mediated amine regeneration for CO2 capture. SSRN Electron J 68:102365. https://doi.org/10.2139/ssrn.4156651
Mesa-Perez JM, Cortez LAB, Rocha JD et al (2005) Unidimensional heat transfer analysis of elephant grass and sugar cane bagasse slow pyrolysis in a fixed bed reactor. Fuel Process Technol 86:565–575. https://doi.org/10.1016/j.fuproc.2004.05.014
Liaw SS, Perez VH, Westerhof RJM et al (2020) Biomethane production from pyrolytic aqueous phase: biomass acid washing and condensation temperature effect on the bio-oil and aqueous phase composition. Bioenergy Res 13:878–886. https://doi.org/10.1007/s12155-020-10100-3
Bidir MG, Millerjothi NK, Adaramola MS, Hagos FY (2021) The role of nanoparticles on biofuel production and as an additive in ternary blend fuelled diesel engine: a review. Energy Reports 7:3614–3627. https://doi.org/10.1016/j.egyr.2021.05.084
Zhong S, Zhang B, Liu C et al (2022) A minireview on catalytic fast co-pyrolysis of lignocellulosic biomass for bio-oil upgrading via enhancing monocyclic aromatics. J Anal Appl Pyrolysis 164:105544. https://doi.org/10.1016/j.jaap.2022.105544
Zhang Z, Li Y, Luo L et al (2022) Insight into kinetic and thermodynamic analysis methods for lignocellulosic biomass pyrolysis. Renew Energy 202:154–171. https://doi.org/10.1016/j.renene.2022.11.072
Bharath G, Rambabu K, Hai A et al (2020) Catalytic hydrodeoxygenation of biomass-derived pyrolysis oil over alloyed bimetallic Ni3Fe nanocatalyst for high-grade biofuel production. Energy Convers Manag 213:112859. https://doi.org/10.1016/j.enconman.2020.112859
Fan L, Chen P, Zhou N et al (2018) In-situ and ex-situ catalytic upgrading of vapors from microwave-assisted pyrolysis of lignin. Bioresour Technol 247:851–858. https://doi.org/10.1016/j.biortech.2017.09.200
Thangalazhy-Gopakumar S, Adhikari S, Chattanathan SA, Gupta RB (2012) Catalytic pyrolysis of green algae for hydrocarbon production using H+ZSM-5 catalyst. Bioresour Technol 118:150–157. https://doi.org/10.1016/j.biortech.2012.05.080
Yildiz G, Ronsse F, Vercruysse J et al (2016) In situ performance of various metal doped catalysts in micro-pyrolysis and continuous fast pyrolysis. Fuel Process Technol 144:312–322. https://doi.org/10.1016/j.fuproc.2016.01.012
Shirazi Y, Viamajala S, Varanasi S (2020) In situ and ex situ catalytic pyrolysis of microalgae and integration with pyrolytic fractionation. Front Chem 8:1–12. https://doi.org/10.3389/fchem.2020.00786
Hu C, Zhang H, Wu S, Xiao R (2020) Molecular shape selectivity of HZSM-5 in catalytic conversion of biomass pyrolysis vapors: the effective pore size. Energy Convers Manag 210:112678. https://doi.org/10.1016/j.enconman.2020.112678
Güngör A, Önenç S, Uçar S, Yanik J (2012) Comparison between the “one-step” and “two-step” catalytic pyrolysis of pine bark. J Anal Appl Pyrolysis 97:39–48. https://doi.org/10.1016/j.jaap.2012.06.011
Nguyen TS, Zabeti M, Lefferts L et al (2013) Conversion of lignocellulosic biomass to green fuel oil over sodium based catalysts. Bioresour Technol 142:353–360. https://doi.org/10.1016/j.biortech.2013.05.023
Gamliel DP, Du S, Bollas GM, Valla JA (2015) Investigation of in situ and ex situ catalytic pyrolysis of miscanthus × giganteus using a PyGC-MS microsystem and comparison with a bench-scale spouted-bed reactor. Bioresour Technol 191:187–196. https://doi.org/10.1016/j.biortech.2015.04.129
Cao J, Yu Y, Wu H (2022) Primary release and transformation of inorganic and organic sodium during fast pyrolysis of sodium-loaded lignin. Proc Combust Inst. https://doi.org/10.1016/j.proci.2022.07.196
Fahmy TYA, Fahmy Y, Mobarak F et al (2020) Biomass pyrolysis: past, present, and future. Environ Dev Sustain 22:17–32. https://doi.org/10.1007/s10668-018-0200-5
Jiang J, Tie Y, Deng L, Che D (2022) Influence of water-washing pretreatment on ash fusibility of biomass. Renew Energy 200:125–135. https://doi.org/10.1016/j.renene.2022.09.121
Pang X, Qiu J, Zhang Z et al (2022) Wide-scope multi-residue analysis of pesticides in beef by gas chromatography coupled with quadrupole Orbitrap mass spectrometry. Food Chem 407:135171. https://doi.org/10.1016/j.foodchem.2022.135171
Suriapparao DV, Tejasvi R (2022) A review on role of process parameters on pyrolysis of biomass and plastics: present scope and future opportunities in conventional and microwave-assisted pyrolysis technologies. Process Saf Environ Prot 162:435–462. https://doi.org/10.1016/j.psep.2022.04.024
Tamburini D (2021) Analytical pyrolysis applied to the characterisation and identification of Asian lacquers in cultural heritage samples — a review. J Anal Appl Pyrolysis 157:105202. https://doi.org/10.1016/j.jaap.2021.105202
Weir A, del Barco J, Carrión A, Queffélec C et al (2022) Renewable binders from waste biomass for road construction: a review on thermochemical conversion technologies and current developments. Constr Build Mater 330:127076. https://doi.org/10.1016/j.conbuildmat.2022.127076
Sabatini F, Corsi I, Ceccarini A et al (2022) Pyrolysis gas chromatography mass spectrometry: a promising tool for disclosing metal-free tanning agents used in leather industry. J Anal Appl Pyrolysis 169:105803. https://doi.org/10.1016/j.jaap.2022.105803
Wei J, Wang M, Tang G et al (2022) Advances on in-situ analysis of char structure evolution during thermochemical conversion of coal/biomass: a review. Fuel Process Technol 230:107221. https://doi.org/10.1016/j.fuproc.2022.107221
Picó Y, Barceló D (2020) Pyrolysis gas chromatography-mass spectrometry in environmental analysis: focus on organic matter and microplastics. TrAC - Trends Anal Chem 130:115964. https://doi.org/10.1016/j.trac.2020.115964
Chen W, Yu H-Q (2021) Advances in the characterization and monitoring of natural organic matter using spectroscopic approaches. Water Res 190:116759. https://doi.org/10.1016/j.watres.2020.116759
Oasmaa A, Van De Beld B, Saari P et al (2015) Norms, standards, and legislation for fast pyrolysis bio-oils from lignocellulosic biomass. Energy and Fuels 29:2471–2484. https://doi.org/10.1021/acs.energyfuels.5b00026
Vuppaladadiyam AK, Vuppaladadiyam SSV, Awasthi A et al (2022) Biomass pyrolysis: a review on recent advancements and green hydrogen production. Bioresour Technol 364:128087. https://doi.org/10.1016/j.biortech.2022.128087
Liu Z, Hughes M, Tong Y et al (2021) Enhanced energy and resource recovery via synergistic catalytic pyrolysis of byproducts from thermal processing of wastewater solids. Renew Energy 177:475–481. https://doi.org/10.1016/j.renene.2021.05.125
Bhatnagar A, Singhal A, Tolvanen H et al (2022) Effect of pretreatment and biomass blending on bio-oil and biochar quality from two-step slow pyrolysis of rice straw. Waste Manag 138:298–307. https://doi.org/10.1016/j.wasman.2021.12.013
Ilo OP, Nkomo SL, Mkhize NM et al (2022) Optimisation of process parameters using response surface methodology to improve the liquid fraction yield from pyrolysis of water hyacinth. Environ Sci Pollut Res 29:55–89. https://doi.org/10.1007/s11356-022-22639-z
Guan Y, Ma Y, Zhang K et al (2015) Co-pyrolysis behaviors of energy grass and lignite. Energy Convers Manag 93:132–140. https://doi.org/10.1016/j.enconman.2015.01.006
Liu W, Niu S, Tang H (2022) Structural characteristics of pores and fractures during lignite pyrolysis obtained from X-ray computed tomography. J Pet Sci Eng 220:111150. https://doi.org/10.1016/j.petrol.2022.111150
Rousset P, Aguiar C, Labbé N, Commandré JM (2011) Enhancing the combustible properties of bamboo by torrefaction. Bioresour Technol 102:8225–8231. https://doi.org/10.1016/j.biortech.2011.05.093
Wannapeera J, Fungtammasan B, Worasuwannarak N (2011) Effects of temperature and holding time during torrefaction on the pyrolysis behaviors of woody biomass. J Anal Appl Pyrolysis 92:99–105. https://doi.org/10.1016/j.jaap.2011.04.010
Fekhar B, Zsinka V, Miskolczi N (2020) Thermo-catalytic co-pyrolysis of waste plastic and paper in batch and tubular reactors for in-situ product improvement. J Environ Manage 269:110741. https://doi.org/10.1016/j.jenvman.2020.110741
Madhu P, Livingston TS, Kanagasabapathy H (2018) Flash pyrolysis of lemon grass (Cymbopogon flexuosus) for bio-oil production in an electrically heated fluidized bed reactor. Waste and Biomass Valorization 9:1037–1046. https://doi.org/10.1007/s12649-017-9872-6
Mishra RK, Mohanty K (2019) Thermal and catalytic pyrolysis of pine sawdust (Pinus ponderosa) and Gulmohar seed (Delonix regia) towards production of fuel and chemicals. Mater Sci Energy Technol 2:139–149. https://doi.org/10.1016/j.mset.2018.12.004
Santos NAV, Magriotis ZM, Saczk AA et al (2013) Kinetic study of pyrolysis of castor beans ( Ricinuscommunis L.) Presscake : an alternative use for solid waste arising from the biodiesel production. Energy & Fuels 20:555–595
Raj T, Kapoor M, Gaur R et al (2015) Physical and chemical characterization of various indian agriculture residues for biofuels production. Energy and Fuels 29:3111–3118. https://doi.org/10.1021/ef5027373
Arias B, Pevida C, Fermoso J et al (2008) Influence of torrefaction on the grindability and reactivity of woody biomass. Fuel Process Technol 89:169–175. https://doi.org/10.1016/j.fuproc.2007.09.002
Pradhan D, No SL (2013) Bio-oil from biomass: thermal pyrolysis of Mahua seed. Int Conf Energy Effic Technol Sustain 2:487–490
Zulkafli AH, Hassan H, Ahmad MA, Din ATM (2022) Co-pyrolysis of palm kernel shell and polypropylene for the production of high-quality bio-oil: product distribution and synergistic effect. Biomass Convers Biorefinery 12:111–135. https://doi.org/10.1007/s13399-022-03476-z
Gayathri G, Uppuluri KB (2022) The comprehensive characterization of Prosopis juliflora pods as a potential bioenergy feedstock. Sci Rep 12:1–13. https://doi.org/10.1038/s41598-022-22482-9
Dubey S, Kumar R, KumarMondal M (2022) Pyrolysis kinetics and thermodynamics of pomegranate peel usingTG/DTG analysis. Biomass Convers Biorefinery 12:125–141. https://doi.org/10.1007/s13399-022-03288-1
Kaushik VS, Dhanalakshmi CS, Madhu P, Tamilselvam P (2022) Co-pyrolysis of neem wood bark and low-density polyethylene: influence of plastic on pyrolysis product distribution and bio-oil characterization. Environ Sci Pollut Res 29:125–139. https://doi.org/10.1007/s11356-022-21746-1
Bridgeman TG, Jones JM, Shield I, Williams PT (2008) Torrefaction of reed canary grass, wheat straw and willow to enhance solid fuel qualities and combustion properties. Fuel 87:844–856. https://doi.org/10.1016/j.fuel.2007.05.041
Kim SW, Koo BS, Ryu JW et al (2013) Bio-oil from the pyrolysis of palm and Jatropha wastes in a fluidized bed. Fuel Process Technol 108:118–124. https://doi.org/10.1016/j.fuproc.2012.05.002
Park YK, Yoo ML, Lee HW et al (2012) Effects of operation conditions on pyrolysis characteristics of agricultural residues. Renew Energy 42:125–130. https://doi.org/10.1016/j.renene.2011.08.050
Bharath G, Hai A, Rambabu K et al (2020) Systematic production and characterization of pyrolysis-oil from date tree wastes for bio-fuel applications. Biomass and Bioenergy 135:105523. https://doi.org/10.1016/j.biombioe.2020.105523
Greenhalf CE, Nowakowski DJ, Harms AB et al (2013) A comparative study of straw, perennial grasses and hardwoods in terms of fast pyrolysis products. Fuel 108:216–230. https://doi.org/10.1016/j.fuel.2013.01.075
Liu W-J, Tian K, Jiang H et al (2013) Preparation of liquid chemical feedstocks by co-pyrolysis of electronic waste and biomass without formation of polybrominated dibenzo-p-dioxins. Bioresour Technol 128:1–7. https://doi.org/10.1016/j.biortech.2012.10.160
Ruwoldt J, Toven K (2022) Alternative wood treatment with blends of linseed oil, alcohols and pyrolysis oil. J Bioresour Bioprod 7:278–287. https://doi.org/10.1016/j.jobab.2022.07.002
Kamau-Devers K, Miller SA (2022) Using a micromechanical viscoelastic creep model to capture multi-phase deterioration in bio-based wood polymer composites exposed to moisture. Constr Build Mater 314:125252. https://doi.org/10.1016/j.conbuildmat.2021.125252
Wang Z, Gao Y, Zhou Y et al (2022) Pyrolysis and combustion behaviors of densified wood. Proc Combust Inst 38:532–546. https://doi.org/10.1016/j.proci.2022.08.057
Zhang M, He T, Jin B (2022) Effect of mineral additives on pyrolytic characteristics and heavy metal behavior during co-pyrolysis of industrial sludge and hyperaccumulator plant. J Anal Appl Pyrolysis 169:105827. https://doi.org/10.1016/j.jaap.2022.105827
Wang J, Ku X, Lin J (2022) Numerical investigation of biomass pyrolysis performance in a fluidized-bed reactor by a TFM-DEM hybrid model. Chem Eng Sci 260:117922. https://doi.org/10.1016/j.ces.2022.117922
Yang X, Zhang J, Zheng J et al (2022) In-situ and ex-situ catalytic pyrolysis of cellulose to produce furans over red mud-supported transition metal catalysts. J Anal Appl Pyrolysis 169:105830. https://doi.org/10.1016/j.jaap.2022.105830
Zhong D, Zeng K, Li J et al (2022) Characteristics and evolution of heavy components in bio-oil from the pyrolysis of cellulose, hemicellulose and lignin. Renew Sustain Energy Rev 157:111989. https://doi.org/10.1016/j.rser.2021.111989
Li L, Cai D, Zhang L et al (2022) Synergistic effects during pyrolysis of binary mixtures of biomass components using microwave-assisted heating coupled with iron base tip-metal. Renew Energy 203:312–322. https://doi.org/10.1016/j.renene.2022.12.076
Amen R, Yaseen M, Mukhtar A et al (2020) Lead and cadmium removal from wastewater using eco-friendly biochar adsorbent derived from rice husk, wheat straw, and corncob. Clean Eng Technol 1:100006. https://doi.org/10.1016/j.clet.2020.100006
Charusiri W, Phowan N, Vitidsant T (2022) Pyrolysis of lignocellulosic biomass with high-density polyethylene to produce chemicals and bio-oil with high liquid yields. Sustain Chem Pharm 25:100567. https://doi.org/10.1016/j.scp.2021.100567
Da Cruz JER, Saldanha HC, E Freitas GRO, Morais ER (2022) A review of medicinal plants used in the Brazilian Cerrado for the treatment of fungal and bacterial infections. J Herb Med 31:100523. https://doi.org/10.1016/j.hermed.2021.100523
McLaughlin LP, Belmont EL (2022) Primary aerosol emissions from lignocellulosic biomass and major constituents under well-defined pyrolysis conditions. J Aerosol Sci 166:106067. https://doi.org/10.1016/j.jaerosci.2022.106067
Wang W, Lemaire R, Bensakhria A, Luart D (2022) Thermogravimetric analysis and kinetic modeling of the co-pyrolysis of a bituminous coal and poplar wood. Chinese J Chem Eng 53:667–689. https://doi.org/10.1016/j.cjche.2022.10.015
Bajpai P (2021) Biomass conversion processes. In: Bajpai P (ed) Future Energy, 3rd edn. Elsevier Inc., Amsterdam, pp 41–151
Chen L, Yang K, Huang J et al (2022) Experimental and kinetic study on flash pyrolysis of biomass via on-line photoionization mass spectrometry. Appl Energy Combust Sci 9:100057. https://doi.org/10.1016/j.jaecs.2022.100057
Guilhaume N, Schuurman Y, Geantet C (2021) The role of catalysis in the valorization of woody biomass fast pyrolysis liquids: overview and contribution of IRCELYON. Catal Today 373:5–23. https://doi.org/10.1016/j.cattod.2021.03.030
Maziarka P, Anca-Couce A, Prins W, Ronsse F (2022) A meta-analysis of thermo-physical and chemical aspects in CFD modelling of pyrolysis of a single wood particle in the thermally thick regime. Chem Eng J 446:137088. https://doi.org/10.1016/j.cej.2022.137088
Liu H, Wang C, Zhang A (2020) Numerical simulation of the wood pyrolysis with homogenous/heterogeneous moisture using FireFOAM. Energy 201:117624. https://doi.org/10.1016/j.energy.2020.117624
Cai L, Wang Q, Xiong Q et al (2022) Numerical simulation and multi-process coupling analysis for biomass pyrolysis fluidized bed reactor based on synergistic effects between biomass and nitrogen inlet modes. J Anal Appl Pyrolysis 169:105801. https://doi.org/10.1016/j.jaap.2022.105801
Pietraccini M, Badu P, Tait T et al (2022) Study of flash pyrolysis and combustion of biomass powders using the Godbert-Greenwald furnace: an essential step to better understand organic dust explosions. Process Saf Environ Prot 169:458–471. https://doi.org/10.1016/j.psep.2022.11.041
Branca C, Colomba DB (2022) Effects of heat/mass transfer limitations and process exothermicity on the kinetic parameters of the devolatilization and oxidation reactions of wood chars. Thermochim Acta 716:179321. https://doi.org/10.1016/j.tca.2022.179321
Zheng Y, Zhao J, Xu F, Li Y (2014) Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog Energy Combust Sci 42:35–53. https://doi.org/10.1016/j.pecs.2014.01.001
Zha Z, Wu K, Ge Z et al (2022) Effect of oxygen on thermal behaviors and kinetic characteristics of biomass during slow and flash pyrolysis processes. Combust Flame 247:112481. https://doi.org/10.1016/j.combustflame.2022.112481
He J, Strezov V, Zhou X et al (2020) Pyrolysis of heavy metal contaminated biomass pre-treated with ferric salts: product characterisation and heavy metal deportment. Bioresour Technol 313:123641. https://doi.org/10.1016/j.biortech.2020.123641
Xue A, Pan J, Tian M (2013) Experimental study of impact of biomass pellet size on the pyrolysis products. Adv Mater Res 641–642:756–759. https://doi.org/10.4028/www.scientific.net/AMR.641-642.756
Karkania V, Fanara E, Zabaniotou A (2012) Review of sustainable biomass pellets production — a study for agricultural residues pellets’ market in Greece. Renew Sustain Energy Rev 16:1426–1436. https://doi.org/10.1016/j.rser.2011.11.028
Kumar P, Subbarao PMV, Kala L, Vijay VK (2022) Influence of physical, mechanical, and thermal properties of biomass pellets from agriculture residue: Pearl millet cob and mix. Bioresour Technol Reports 20:101278. https://doi.org/10.1016/j.biteb.2022.101278
Chu G, Wang W, Zhao J, Zhou D (2022) Transformation of phosphorus species during phosphoric acid-assisted pyrolysis of lignocellulose. Sci Total Environ 865:161010. https://doi.org/10.1016/j.scitotenv.2022.161010
Kalel RA, Gaikwad DS (2022) Cooperative catalysis: condensation-aromatization for synthesis of 2-(4-nitrophenyl)-1H-benzimidazole by silica immobilized Brønsted-Lewis acidic ionic liquid (Si-BLAIL). J Indian Chem Soc 99:100550. https://doi.org/10.1016/j.jics.2022.100550
Khachatrian AA, Solomonov BN (2022) The comparative analysis of solvation thermochemistry of organic non-electrolytes in ionic liquids and molecular solvents. J Mol Liq 368:120765. https://doi.org/10.1016/j.molliq.2022.120765
Singh S, Kumar A, Sivakumar N, Verma JP (2022) Deconstruction of lignocellulosic biomass for bioethanol production: recent advances and future prospects. Fuel 327:125109. https://doi.org/10.1016/j.fuel.2022.125109
Fernández-Rodríguez J, de Diego-Díaz B, Tapia-Martín ME (2021) Biomethanization of agricultural lignocellulosic wastes: pretreatments. In: Tyagi V, Aboudi K (eds) Clean Energy and Resources Recovery, 2nd edn. Elsevier Inc., Amsterdam, pp 155–202
Muranaka Y, Suzuki T, Sawanishi H et al (2014) Effective production of levulinic acid from biomass through pretreatment using phosphoric acid, hydrochloric acid, or ionic liquid. Ind Eng Chem Res 53:11611–11621. https://doi.org/10.1021/ie501811x
Gao J, Chen L, Yuan K et al (2013) Ionic liquid pretreatment to enhance the anaerobic digestion of lignocellulosic biomass. Bioresour Technol 150:352–358. https://doi.org/10.1016/j.biortech.2013.10.026
Zhang J, Feng L, Wang D et al (2014) Thermogravimetric analysis of lignocellulosic biomass with ionic liquid pretreatment. Bioresour Technol 153:379–382. https://doi.org/10.1016/j.biortech.2013.12.004
Wincy WB, Edwin M, Sekhar SJ (2022) Optimization of process parameters to implement biomass gasifier for drying high moisture paddy in reversible flatbed dryer. Energy 249:123771. https://doi.org/10.1016/j.energy.2022.123771
Niu M, Xie J, Liang S et al (2021) Simulation of a new biomass integrated gasification combined cycle (BIGCC) power generation system using Aspen Plus: Performance analysis and energetic assessment. Int J Hydrogen Energy 46:22356–22367. https://doi.org/10.1016/j.ijhydene.2021.04.076
Mauro C, Rentizelas AA, Chinese D (2018) International vs. domestic bioenergy supply chains for co-firing plants: the role of pre-treatment technologies. Renew Energy 119:712–730. https://doi.org/10.1016/j.renene.2017.12.034
Cheng F, Bayat H, Jena U, Brewer CE (2020) Impact of feedstock composition on pyrolysis of low-cost, protein- and lignin-rich biomass: a review. J Anal Appl Pyrolysis 147:104780. https://doi.org/10.1016/j.jaap.2020.104780
Boateng AA, Mullen CA (2013) Fast pyrolysis of biomass thermally pretreated by torrefaction. J Anal Appl Pyrolysis 100:95–102. https://doi.org/10.1016/j.jaap.2012.12.002
Carvalheiro F, Duarte LC, Gírio F, Moniz P (2016) Hydrothermal/liquid hot water pretreatment (autohydrolysis): a multipurpose process for biomass upgrading. In: Mussatto SI (ed) Biomass fractionation technologies for a lignocellulosic feedstock based biorefinery, 1st edn. Elsevier Inc., Amsterdam, pp 315–347
Bundhoo ZMA, Mudhoo A, Mohee R (2013) Promising unconventional pretreatments for lignocellulosic biomass. Crit Rev Environ Sci Technol 43:2140–2211. https://doi.org/10.1080/10643389.2012.672070
Canales-Flores RA, Prieto-García F (2020) Taguchi optimization for production of activated carbon from phosphoric acid impregnated agricultural waste by microwave heating for the removal of methylene blue. Diam Relat Mater 109:108027. https://doi.org/10.1016/j.diamond.2020.108027
Su G, Ong HC, Cheah MY et al (2022) Microwave-assisted pyrolysis technology for bioenergy recovery: mechanism, performance, and prospect. Fuel 326:124983. https://doi.org/10.1016/j.fuel.2022.124983
Potnuri R, Suriapparao DV, Sankar Rao C et al (2022) Effect of dry torrefaction pretreatment of the microwave-assisted catalytic pyrolysis of biomass using the machine learning approach. Renew Energy 197:798–809. https://doi.org/10.1016/j.renene.2022.08.006
Yu Y, Zeng Y, Zuo J et al (2013) Improving the conversion of biomass in catalytic fast pyrolysis via white-rot fungal pretreatment. Bioresour Technol 134:198–203. https://doi.org/10.1016/j.biortech.2013.01.167
Lou R, Wu S (2011) Products properties from fast pyrolysis of enzymatic/mild acidolysis lignin. Appl Energy 88:316–322. https://doi.org/10.1016/j.apenergy.2010.06.028
Patel M, Oyedun AO, Kumar A, Gupta R (2019) A techno-economic assessment of renewable diesel and gasoline production from aspen hardwood. Waste and Biomass Valorization 10:2745–2760. https://doi.org/10.1007/s12649-018-0359-x
Shahbaz M, AlNouss A, Parthasarathy P et al (2022) Investigation of biomass components on the slow pyrolysis products yield using Aspen Plus for techno-economic analysis. Biomass Convers Biorefinery 12:669–681. https://doi.org/10.1007/s13399-020-01040-1
Brown TR, Thilakaratne R, Brown RC, Hu G (2013) Techno-economic analysis of biomass to transportation fuels and electricity via fast pyrolysis and hydroprocessing. Fuel 106:463–469. https://doi.org/10.1016/j.fuel.2012.11.029
Shabangu S, Woolf D, Fisher EM et al (2014) Techno-economic assessment of biomass slow pyrolysis into different biochar and methanol concepts. Fuel 117:742–748. https://doi.org/10.1016/j.fuel.2013.08.053
Khan SR, Ciolkosz D, Vasco-Correa J, Zeeshan M (2022) A techno-economic study to evaluate the impacts of feedstock ratio on commercial scale co-pyrolysis plants of biomass and waste tire. J Anal Appl Pyrolysis 167:105699. https://doi.org/10.1016/j.jaap.2022.105699
Jaroenkhasemmeesuk C, Tippayawong N (2015) Technical and economic analysis of a biomass pyrolysis plant. Energy Procedia 79:950–955. https://doi.org/10.1016/j.egypro.2015.11.592
Oudenhoven SRG, van der Ham AGJ, van den Berg H et al (2016) Using pyrolytic acid leaching as a pretreatment step in a biomass fast pyrolysis plant: process design and economic evaluation. Biomass and Bioenergy 95:388–404. https://doi.org/10.1016/j.biombioe.2016.07.003
Shemfe MB, Gu S, Ranganathan P (2015) Techno-economic performance analysis of biofuel production and miniature electric power generation from biomass fast pyrolysis and bio-oil upgrading. Fuel 143:361–372. https://doi.org/10.1016/j.fuel.2014.11.078
Suiuay C, Sudajan S, Katekaew S et al (2019) Production of gasoline-like-fuel and diesel-like-fuel from hard-resin of Yang (Dipterocarpus alatus) using a fast pyrolysis process. Energy 187:115967. https://doi.org/10.1016/j.energy.2019.115967
Wang W-C, Jan J-J (2018) From laboratory to pilot: design concept and techno-economic analyses of the fluidized bed fast pyrolysis of biomass. Energy 155:139–151. https://doi.org/10.1016/j.energy.2018.05.012
Hadiya V, Popat K, Vyas S et al (2022) Biochar production with amelioration of microwave-assisted pyrolysis: current scenario, drawbacks and perspectives. Bioresour Technol 355:127303. https://doi.org/10.1016/j.biortech.2022.127303
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gogoi, D., Kumar, M. & Lakshmi, Y.G. A Comprehensive Review on “Pyrolysis” for Energy Recovery. Bioenerg. Res. 16, 1417–1437 (2023). https://doi.org/10.1007/s12155-023-10568-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-023-10568-9