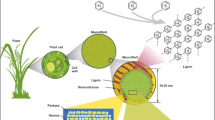
Abstract
Lignocellulosic biomass has a complex and rigid cell wall structure that makes biomass recalcitrant to biological and chemical degradation. Among the three major structural biopolymers (i.e., cellulose, hemicellulose, and lignin) in plant cell walls, lignin is considered the most recalcitrant component and generally plays a negative role in the biochemical conversion of biomass to biofuels. The conversion of biomass to biofuels through a biochemical platform usually requires a pretreatment stage to reduce the recalcitrance. Pretreatment renders compositional and structural changes of biomass with these changes ultimately governing the efficiency of the subsequent enzymatic hydrolysis. Dilute acid, hot water, steam explosion, and ammonia fiber expansion pretreatments are among the leading thermochemical pretreatments with a limited delignification that can reduce biomass recalcitrance. Practical applications of these pretreatment are rapidly developing as illustrated by recent commercial scale cellulosic ethanol plants. While these thermochemical pretreatments generally lead to only a limited delignification and no significant change of lignin content in the pretreated biomass, the lignin transformations that occur during these pretreatments and the roles they play in recalcitrance reduction are important research aspects. This review highlights recent advances in our understanding of lignin alterations during these limited delignification thermochemical pretreatments, with emphasis on lignin chemical structures, molecular weights, and redistributions in the pretreated biomass.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The growing demands on materials and energy consumption, coupled with diminishing fossil fuel resources and environmental concerns, have spurred global efforts into pursuing the utilization of renewable and sustainable energy resources for the production of fuels, chemicals, and materials. Lignocellulosic biomass represents one of the most abundant biopolymer sources on earth that can deliver a renewable and sustainable bioenergy resource. Over the past decade, the conversion of biomass to biofuels has garnered extensive research interest as a potentially viable option to petroleum-derived fuels [1, 2]. Currently, the biochemical route to produce biofuels usually involves three steps: pretreatment, enzymatic hydrolysis, and fermentation. Despite advances in the development of pretreatment technologies, it remains among the most costly processes and a key challenge for the efficient and cost-competitive production of cellulosic biofuels.
Lignocellulosic biomass is comprised primarily of plant cell wall materials with cellulose, hemicellulose, and lignin as the major structural biopolymers. The structural complexities of plant cell walls together with their unique chemical and physical properties render biomass resistant to biological degradation [3, 4]; therefore, a pretreatment step is generally required to reduce biomass recalcitrance [5, 6]. Pretreatment can cause compositional and structural changes of cell walls, thus enabling the polysaccharides embedded in the cell walls more accessible to hydrolytic enzymes. Based on the biomass sources and pretreatment methods, the initial characteristics of biomass and the resulted compositional/structural alterations due to pretreatments ultimately determine the efficacy of the subsequent enzymatic hydrolysis. Several key biomass properties such as biomass accessibility, cellulose crystallinity/ultrastructure and degree of polymerization, lignin content and structure, lignin-carbohydrate complex, and hemicellulose content are reported to impact the biomass recalcitrance [7–9]. Dependent upon the pretreatment type and severities, various biomass structural features are altered and their contributions to the reduction of biomass recalcitrance are different. A combination of structural features resulting from pretreatments is usually observed to contribute collectively to overcoming biomass recalcitrance. For example, mechanical pretreatment usually causes a reduction in biomass size, an increase in specific surface area and a decrease in cellulose crystallinity [10]. Organosolv pretreatment leads to a removal of lignin/hemicellulose and an increase in accessibility in the pretreated biomass [11–14].
Lignin is considered the most recalcitrant component in a plant cell wall and negatively impacts the efficiency of biochemical conversion of biomass to biofuels. Lignin is believed to be a major impediment to cellulose accessibility in biomass and its removal can make biomass amenable to enzymatic hydrolysis. Many efforts on genetic engineering and pretreatment technologies have been carried out directed at either reducing lignin content and/or inducing lignin structural changes to reduce biomass recalcitrance [15–20]. Some reports suggest that lignin content has significant impact on biomass digestibility and lignin removal benefits enzymatic hydrolysis and ideal pretreatments should maximize lignin removal and minimize polysaccharide modification or degradation [21, 22]. On the other hand, other studies have found no apparent correlation between lignin content and cellulose digestibility, suggesting that lignin removal is not a necessity to achieve significant reduction in biomass recalcitrance [23–27]. Various pretreatment technologies that feature no lignin removal or with only limited delignification have been developed to reduce biomass recalcitrance, including dilute acid pretreatment (DAP) [28–31], hot water pretreatment (HWP) [32–35], catalyzed and uncatalyzed steam explosion pretreatment (SEP) [36–38], pH-controlled hot water pretreatment [39], ammonia fiber expansion (AFEX) pretreatment [40–45], CO2 explosion pretreatment [46, 47], mechanical [48–50] and biological [51] pretreatment. All these pretreatments are reported to reduce the biomass recalcitrance and enhance sugar release in the subsequent enzymatic hydrolysis stage, and each has its own strength and disadvantages [52–54]. Many mechanical pretreatments are usually associated with high energy use, high operating and capital costs, and are not considered economically sustainable and practical in an industrial scale. Biological pretreatment is a safe and eco-friendly process for enhancement of biomass enzymatic digestibility and offers some advantages such as low chemical and energy use. Unfortunately, to date, there are some drawbacks for biological pretreatment such as long residence time (up to ~10–14 days), hardly controlled growth conditions, and high cost of enzymes, which make this process economically less attractive and limit its commercial applications. In addition, biological methods suffer from poor selectivity in that carbohydrates (i.e., cellulose and hemicellulose) are partially consumed by microorganisms. Mechanical pretreatments and biological pretreatments of a variety of lignocellulosic biomasses were thoroughly discussed and highlighted in recent reviews [10, 55–57].
Compared to mechanical and biological methods, thermochemical pretreatments are promising options for the future biorefinery with advantages such as comparatively short reaction time, high effectiveness, high sugar yields, and lower energy requirements. Dilute acid, hot water, steam explosion, and ammonia fiber expansion pretreatment are considered the leading thermochemical technologies that have attracted great interest due to their potential for viable and economical applications. Practical applications of these pretreatment are rapidly developing as illustrated by recent commercial scale cellulosic ethanol plants (http://www.eia.gov/todayinenergy/detail.cfm?id=17851). With only limited delignification in these pretreated biomass, the lignin structural changes that occur during these pretreatment are of great importance for both our understanding of its relevance to reduction in recalcitrance and the potential for further application/valorization of the lignin fraction. Extensive studies have been carried out to investigate the lignin structural features as a result of these pretreatment. A recent paper has provided an overview on structural transformations of biomass including cellulose, hemicellulose, and lignin during dilute acid and hydrothermal pretreatments [9]. Accordingly, this review will specifically focus on lignin-related structural alterations during these aqueous thermochemical pretreatments, with emphasis on lignin chemical structures, molecular weights, and redistributions. It is expected to serve as a reference for future advances in pretreatment technology development and lignin utilization from the emerging biorefinery process.
Thermochemical Pretreatment with Limited Delignification
Over the years, extensive efforts have been directed to develop low-cost and viable pretreatment approaches that can reduce biomass recalcitrance and realize high yields of fermentable sugars from both cellulose and hemicellulose fractions. Dilute acid, hot water, steam explosion, and ammonia fiber expansion pretreatment are among the leading aqueous thermochemical pretreatment technologies. One common feature from these pretreatment is that they typically result in only limited delignification in the pretreated biomass while achieving an improvement in enzymatic saccharification. It should be noted that while lignin removal is typical low during DAP, HWP, SEP, and AFEX in batch reactors, high lignin removal could still be achieved if severe conditions are applied under these pretreatments. For each of these technologies, a brief process description is given in this section and their representative pretreatment conditions and enzymatic hydrolysis results on various biomass feedstock are highlighted in Table 1.
Dilute Acid Pretreatment
DAP can significantly reduce lignocellulosic recalcitrance and it has been successfully applied to a wide range of feedstock, including softwoods, hardwoods, herbaceous crops, and agricultural residues [59, 69–72]. DAP process typically involves the treatment of biomass with a combination of an acidic pH, heat, and pressure. Although a variety of acids such as hydrochloric acid, nitric acid, phosphoric acid, and peracetic acid have been employed, sulfuric acid has been most widely used since it is inexpensive and effective. It is usually performed over a temperature range of 120 to 210 °C, with acid concentration typically less than 4 wt%, and residence time from a few seconds to an hour in different types of reactors.
Hot Water Pretreatment
HWP, also called hydrothermal pretreatment or autohydrolysis, refers to the use of water in the liquid or vapor phase to pretreat lignocellulosic materials without adding additional chemicals. Typically, biomass undergoes high temperature (~160-240 °C) cooking in water with high pressure over lengths of time ranging from a few minutes up to several hours [73]. While it is called near neutral condition, the HWP is usually carried out under a mild acidic condition which results from the release of organic acids from biomass components and a decrease in the pKw of water at the elevated temperature [74]. HWP provides several advantages such as no requirement for acid or chemical catalysts, low formation of inhibitory products, and high xylose recovery (i.e., up to 88–98 %), which make it economically and environmentally attractive. However, it also requires high energy due to high pressure and a large amount of water supplied to the system.
Steam Explosion Pretreatment
SEP is one of the most commonly used pretreatment methods to enhance biomass enzymatic saccharification and has been successfully applied to various types of lignocellulosic biomass including softwoods, hardwoods, and agricultural residues [66, 75–77]. In this process, biomass is treated with high-pressure saturated steam typically at temperatures of about 160–240 °C and pressures of 0.7–4.8 MPa for a short duration of time (ranging from several seconds to a few minutes). After pretreatment, the pressure is explosively released. The rapid reduce of pressure makes the biomass undergo an explosive decompression, disrupting the structure of the biomass fibrils and increasing the porosity of pretreated biomass. Addition of an acid catalyst (e.g., sulfuric acid, sulfur dioxide) has been reported to increase its hemicelluloses sugar yield and improve the enzymatic hydrolysis of pretreated biomass [64, 78–80]. The advantages of SEP include low environmental impact, low capital investment, high energy efficiency, less hazardous process conditions, and complete recovery of all biopolymers in biomass cell walls [81].
Ammonia Fiber Expansion Pretreatment
AFEX pretreatment, also known as ammonia fiber explosion pretreatment, is a physicochemical process, in which the biomass material is typically treated with liquid ammonia at moderate temperatures (60–100 °C) under high pressure for a variable period of time (5–60 min) and is then rapidly depressurized [40–44]. This swift pressure release leads to a rapid expansion of the ammonia gas that causes swelling and physical disruption of biomass fibers. Ammonia as a pretreatment reagent offers several advantages including being able to effectively swell lignocellulosic material, showing high reaction selectivity with lignin over carbohydrates, and its ease to recover and recycle due to high volatility. During AFEX pretreatment, about 95 % of the ammonia can be recovered in the gas phase and recycled, with a small amount of ammonia remaining in the lignocellulosics which can serve as a nitrogen source for microbes in the fermentation process [42].
Lignin Transformations During Thermochemical Pretreatments with Limited Delignification
Lignin is a three-dimensional amorphous polyphenolic polymer which is primarily biosynthesized from three typical types of phenylpropanoid precursors: coniferyl, sinapyl, and p-coumaryl alcohol depicted in Fig. 1. Once incorporated into the lignin polymer, these three monomers give rise to guaiacyl (G), syringyl (S), and p-hydroxyphenyl (H) lignin subunits in its structure, respectively. The lignin macromolecule is primarily linked via ether bonds and carbon-carbon bonds among phenylpropanoid units. Figure 2 shows the typical interunit linkages in lignin and a schematic representation of softwood lignin structure (https://public.ornl.gov/site/gallery/originals/Contemporary_View_of_L.jpg) [82]. The lignin content, monolignol composition, and the interunit linkages abundance vary with plant species and cell walls. Woody biomass generally has higher lignin content than agricultural residues and herbaceous plants. Among the interunit linkages, β-O-4 is the dominant linkage typically accounting for ~40–65 % of the total linkage structures dependent on the biomass species. Depending on the pretreatment types and severities, lignin undergoes chemical and physical structural changes as well as its relocation or redistribution in the pretreated biomass, contributing to the reduction of recalcitrance. The data showing typical types and trends of chemical structural alterations of lignin during dilute acid, hot water, steam explosion, and AFEX pretreatments are summarized in Table 2. The comparison of lignin molecular weights from a variety of biomass substrates before and after these pretreatments is highlighted in Table 3. As a complement of chemical structural changes, please see “Lignin Relocation and Redistribution” below.
Typical interunit linkages and substructures reported in lignin (left) [82], and a schematic representation of lignin structure (right, https://public.ornl.gov/site/gallery/originals/Contemporary_View_of_L.jpg). A: β-O-4 ether linkage; B: phenylcoumaran (β-5/α-O-4); C: resinol (β-β); D: dibenzodioxocin; E: spirodienone; G: 4-O-5; H: 5-5; K: cinnamaldehyde; M: cinnamyl alcohol; N: p-coumarate; P: ferulate
Lignin Chemical Structural Changes
Dilute Acid Pretreatment
Under acidic pretreatment conditions, the predominant reactions in lignin are fragmentation by acidolysis of aryl ether linkages and acid catalyzed condensation [9, 87, 92]. The β-O-4 aryl ether linkages (A in Fig. 2) in lignin are susceptible to acidic hydrolysis, leading to depolymerization and loss of β-O-4 bonds in lignin (Fig. 3). On the other hand, carbonium ion intermediates can be formed with a high affinity for nucleophiles within the lignin macromolecules. The reactions between carbonium ions and nucleophiles lead to lignin recondensation. After dilute acid pretreatment, a lower β-O-4 content in lignin is usually observed in the pretreated biomass, while linkages such as phenylcoumaran (B in Fig. 2) and resinol (C in Fig. 2) subunits are observed fairly stable [9, 84]. For example, Samuel et al. [83] reported that dilute acid pretreatment led to a 36 % decrease of β-O-4 linkages in pretreated switchgrass lignin. Evidence has also suggested that syringyl units were more readily degraded than guaiacyl units under dilute acid pretreatment conditions, resulting in a lower S/G ratio in lignin in pretreated biomass [9]. In addition, DAP results in the formation of new phenolic groups due in part to the cleavage of β-O-4 aryl ether bonds [85]. Other structural changes such as a decrease in methoxyl group content, hydrolysis of acetyl groups, and cinnamaldehyde units are also observed during dilute acid pretreatment [9].
Hot Water Pretreatment
Compared to dilute acid pretreatment, hot water pretreatment has milder acidic reaction conditions as the hydrolysis conditions are primarily catalyzed by the release of organic acids from biomass. Given the similarity to DAP conditions, HWP induces comparable lignin fragmentation and condensation reactions as reported for DAP. Studies have demonstrated that hot water pretreatment leads to a decrease in β-O-4 linkages, an increase in phenolic OH groups, a decrease in aliphatic hydroxyl group, and depletion of acetyl groups in lignin [89–91]. A comparison of the two-dimensional HSQC NMR data shows that autohydrolysis of aspen wood during hot water pretreatment at 177 °C for 210 min leads to the destruction of all beta-O-4 linkages in aspen lignin [87]. Unlike dilute acid pretreatment, Leschinsky et al. [91] documented that the S/G ratio in Eucalyptus globulus wood remained relatively constant during autohydrolysis at 170 °C, suggesting no preferential hydrolysis of S or G units in biomass under the HWP conditions employed. Likewise, Pu et al. [89] reported that hot water pretreatment of poplar at 180 °C results in relatively unchanged S/G ratio in lignin. On the other hand, Li et al. [96] investigated lignin deposition on cellulose during hydrothermal pretreatment that was carried out at 200 °C for 15 min. More syringyl units were observed in the deposited lignin than that in the untreated poplar wood lignin, suggesting syringyl units are more prone to acidic cleavage. Using isolated poplar lignin as raw material, Samuel et al. [90] reported a reduction in syringyl, guaiacyl, and p-hydroxybenzoyl units after hot water pretreatment at 200 °C with 30 min residence time. This study also demonstrated that the cleavage of lignin side-chain units is relatively fast in autohydrolysis of isolated lignin as compared to the autohydrolysis of biomass, revealing that the surrounding biomass impacts lignin reactivity during pretreatment. Trajano et al. [97] compared the fate of lignin during hydrothermal pretreatment using poplar wood versus isolated cellulolytic enzyme lignin (CEL). Flowthrough and batch reactors were employed and the pretreatments were conducted under the conditions summarized in Table 4. Hydrolysate was collected over every sampling intervals during the run for flowthrough pretreatments. The results showed that the poplar wood hydrolysates contained more syringyl type products, while the CEL hydrolysates contained more guaiacyl type products. The authors proposed that the cross-linking between lignin and hemicellulose modified the reactivity of guaiacyl and syringyl units, resulting in a different behavior from isolated poplar lignin and wood samples. These results suggest that the lignin reactivity and reaction behavior are significantly influenced by autohydrolysis pretreatment severity as well as the nature of the matrix that lignin is present in. Gao et al. reported that hydrothermal pretreatment also influenced the thermal stability and structure of lignin in corncob [98].
Steam Explosion Pretreatment
Steam explosion pretreatment has many of the same features that hot water pretreatment has, primarily autohydrolysis, which is catalyzed by organic acids released from biomass components in the presence of steam. From a chemistry point of review, the lignin in biomass undergoes a very similar chemical changes that occur in hot water pretreatment, i.e., lignin fragmentation and recondensation reactions caused by the formation of carbonium ions in lignin [92]. Chemical features of structural alterations that were introduced in steam explosion pretreatment include a decrease in β-O-4 structures, an increase in C–C condensed structures, a decrease in aliphatic OH groups, an increase in phenolic OH groups, and a loss of acetyl groups in lignin. For example, Li et al. [92] reported a successive decrease in the content of β-O-4 structures in aspen wood lignin with increasing severity of the steam explosion conditions (Table 2). In another study by Li et al. [94], steam explosion was applied to both softwood (Norway spruce and Scots pine) and hardwood (Birch and aspen) and the mechanism of lignin structural modification was investigated. The results showed that almost all the β-O-4 structures were depleted in steam explosion with SO2 impregnation, while less than half of such structures could survive the same steam explosion that had no SO2 impregnation. In addition, both the softwood and hardwood species showed an increase in the amount of carboxylic acids and phenolic hydroxyl groups in lignin after steam explosion. Heikkinen et al. [93] have suggested the steam explosion of wheat straw cause a substantial decrease of S/G ratio (~33 % decrease) and hydroxycinnamates (~54 % decrease), and an increase in the level of β-5 interunit linkages. By contrast, Yelle et al. [99] observed no significant changes in the p-hydroxyphenyl/guaiacyl/syringyl units distribution and hydroxycinnamates after the steam explosion treatment of wheat straw. They also have suggested that phenylcoumarans and resinols withstand the steam explosion while some deacylation of p-coumarates occurs. The pretreatment severity and conditions as well as biomass species appear to impact the lignin structural alterations that occur during steam explosion.
AFEX Pretreatment
During a typical AFEX pretreatment, the composition of biomass does not change significantly and the lignin in biomass was not significantly degraded with the core lignin polymer remaining intact [41, 42, 45, 100]. However, ammonolysis (amide-forming) and hydrolysis (acid-forming) reactions were reported to occur and these reactions can cleave the lignin-carbohydrate complexes, which are the main barriers for enzymes [42, 45, 62]. Studies by Chundawat et al. [45] demonstrated that p-coumaroyl and feruloyl amides were formed during AFEX pretreatment of corn stover due to ammonolytic cleavage of p-coumarate (N in Fig. 2) and ferulate (P in Fig. 2) esters in lignin. Most of the p-coumarate esters were cleaved and only ~10 to 15 % of these units remained in the extractable lignin after AFEX. Ferulate–polysaccharide esters in corn stover cell walls were also observed to cleave, resulting in significantly lower ferulate groups in the residual lignin after AFEX process. Liu et al. [101] examined structural features of lignin after aqueous ammonia pretreatment of miscanthus and reported an increase of S/G ratio, suggesting a selective removal of G type lignin. Using dehydrodiferulates as lignin model compounds, Azarpira et al. [102] investigated the chemical reactions involved in the cleavage of lignin-polysaccharides and documented that the diferulates were converted to amides due to ester cleavage under conditions similar to AFEX process. The chemical reactions and detailed lignin structural changes occurring during AFEX pretreatment are still not fully understood and need further investigation.
Lignin Molecular Weights
Lignin is a very polydisperse polymer in nature and most pretreatments often increase its molecular weights (MW). Although there is no one standard method for the analysis of lignin molecular weights, gel permeation chromatography (GPC) calibrated with external standards, or coupled with the use of vapor pressure osmometry or light scattering (static and dynamic) to determine MW are common approaches. These and other methodologies were recently reviewed in detail by Tolbert et al. [103] highlighting the strengths and limitations of current lignin MW determinations.
Under acidic pretreatment conditions (i.e., DAP, HWP, and SEP), the cleavage of β-O-4 linkages is often anticipated to result in a decrease in molecular weights of lignin; however, lignin repolymerization reactions can cause a more condensed and heterogeneous lignin structure resulting in little change and/or even an increase in lignin molecular weights. Cao et al. [84] reported that poplar lignin showed an initial slight decrease (by ~12 %) in weight-average molecular weight (M w) at the early stage of dilute acid pretreatment (170 °C, 0.3 min). This was likely due to the dominance of aryl ether linkage cleavage over recondensation. As the pretreatment time extended, condensation reactions became competitive to fragmentation and this number started to increase. Dilute acid pretreatment of poplar at longer time (5.4–26.8 min) resulted in no significant change in lignin’s overall molecular weights and molecular weights distribution when compared to the untreated lignin sample. With the milder acidic conditions, hot water and steam explosion pretreatments typically cause a decrease of molecular weights in biomass lignin possibly due to fragmentation reaction dominating over condensation under the conditions investigated. For example, when E. globulus was subjected to hot water pretreatment at 170 °C, size exclusion chromatograph (SEC) analysis showed a reduction of lignin molecular weights, suggesting degradation dominated over condensation reactions [91]. Similarly, hot water pretreatment of poplar at 180 °C led to a decrease in molecular weights in lignin [89]. Using size exclusion chromatograph, Li et al. [94] reported a substantial decrease of lignin molecular weights both in number-average molecular weight (M n, by ~ 46 %) and weight-average molecular weight (M w, by ~ 72 %) with a narrower molar mass distribution after steam explosion of Norway spruce. SO2 impregnation prior to steam explosion appeared to have little impact on the lignin size in Norway spruce. Likewise, comparison of the SEC analytical data for the reference lignin samples with those of aspen lignin after hot water autohydrolysis revealed a decrease of molecular weights of lignin with the peak in the molecular weight distribution curve shifting from about 104 to 103 Da, as a result of the destruction of beta-O-4 linkages in lignin [92]. Samuel et al. [90] investigated the fate of an isolated poplar lignin under varying autohydrolysis pretreating conditions (i.e., 150–200 °C, 0–30 min residence time) and found a drastic decrease in its molecular weights after pretreatment. The average molecular weights, M n and M w, of poplar lignin were decreased by ca. 46 and 65 % respectively, for pretreatment at 150 °C. As the hydrothermal pretreatment temperature increased from 150 to 180 °C and time extended from 0 to 30 min, the decrease in M n and M w in lignin was enhanced (M n decrease by ~82–85 %, and M w by ~86–91 %). The results suggested that the biomass matrix influenced lignin reactivity under autohydrolysis pretreatment.
On the other hand, HWP and SEP pretreatment can result in no significant decrease and/or even an increase in the molecular weights in lignin if under higher severity conditions in which condensation dominates over fragmentation [92]. Kaparaju et al. [95] found that no significant difference in M w for wheat straw and corn stover lignin after hydrothermal pretreatment. Li et al. [94] reported a decrease (by ~47 %) of lignin number-average molecular weight (M n) after steam explosion of aspen wood, while the weight-average molecular weight (M w) remained almost no change. The steam explosion with SO2 impregnation led to an increase of lignin M w in aspen wood by ~142 %, a decrease of M n by ~42 %, and a broader molar mass distribution, resulting in a more heterogeneous lignin structures. The behaviors of lignin molecular weights under acidic pretreatments appear to depend on the pretreatment type, severity, and biomass species. For aqueous ammonia pretreatment, recent research indicated that the pretreatment did not degrade the lignin macromolecule to a significant extent with limited impact on the lignin molecular weight distribution [100, 104].
Lignin Relocation and Redistribution
In addition to chemical alternations in its structure, lignin is also found to translocate and redistribute in biomass under acidic pretreatment conditions such as DAP, HWP, and SEP, leading to the formation of lignin droplets or agglomerates with various morphologies [62, 105–111]. Selig et al. [105] documented the presence of spherical lignin droplets on the surface of maize stem after dilute acid pretreatment. They proposed that when pretreatment temperature rose above lignin’s phase transition temperature, lignin in biomass became fluidized, coalesced, and had the mobility to move throughout cell wall matrix, becoming trapped within cell walls layers and/or settled out of the bulk liquid phase. Upon cooling after pretreatment, the coalesced and migrated lignin could either precipitate within cell wall layers or settle onto the biomass surface. Similarly, Pingali et al. [107] have suggested that lignin aggregates are present within the bulk of pretreated material prior to the formation of larger lignin droplets at a later stage in pretreatment. Once the pretreatment reaction temperature exceeds the critical glass transition temperature of lignin, such aggregates begin to form. The hydrostatic pressures within cell wall layers then force a fraction of these lignin aggregates to the outer face of biomass which in turn contact with the pretreatment bulk solvent and deposit back onto cell wall surfaces [63, 108]. Using confocal and fluorescence lifetime imaging microscopy, Coletta et al. [112] observed that DAP resulted in a disorder in the arrangement of lignin and its accumulation in the external border of cell walls. Donohoe et al. [106] have proposed that lignin migrating/relocating to a more localized and concentrated distribution in outer cell walls during acidic pretreatments can dramatically open up the structure of cell wall matrix and improve the accessibility of majority of cellulose microfibrils, which enhances the pretreated biomass digestibility. On the other hand, this relocation and redistribution of lignin is more pronounced with higher acid concentration and/or temperature, which overlays the cell wall surfaces and might potentially block enzymes further access to cell wall components [113]. Donaldson et al. [109] investigated the ultrastructures of steam-exploded softwood Pinus radiate and demonstrated that lignin was redistributed within the wood cell walls as a result of melting and contracting into agglomerates due to surface tension effects. The lignin redistribution together with hemicellulose removal during steam explosion led to increased porosity of microfibrillar web which contributed to the enhanced digestibility of steam pretreated wood. Using transmission electron microscope, Kallavus and Gravitis [110] observed lignin redistribution on both the inner and outer surfaces of cell walls as well as inside the cell wall itself after steam explosion treatment of aspen wood.
Recently, Langan et al. [114] combined multiple probes of structure with molecular dynamics simulation to offer some new conceptual understanding of the biomass morphology change during thermochemical pretreatments. According to Langan et al. [114], lignin changes to conformations that result in fewer lignin hemicellulose entanglements as the temperature increases during thermochemical pretreatment. This allows a phase separation with lignin aggregating into crumpled globules and with hemicellulose hydrolyzing via autohydrolysis. As hemicellulose untangles and extrudes itself from the lignin globules, pressure is generated to push apart the lignin globules as cell walls swell, thereby increasing the size of small water-filled voids within the cell wall matrix by a few nanometers. It should be noted that different pretreatment configurations such as flowthrough where the hot water is pumped through a column of stationary biomass particles can result in a different behavior in lignin removal and relocation when compared to the batch pretreatment. Studies demonstrated that there was a linear relationship between xylan and lignin removal during hot water flowthrough pretreatment of corn stover [115]. It has been proposed that as xylan is removed in early part of hot water pretreatment lignin is released to solution due to cleavage of lignin-carbohydrate complex (LCC) linkages. The LCC in solution is then hydrolyzed producing lignin and carbohydrates fragments. The lignin fragment in solution falls back on biomass in batch reactor, whereas in flowthrough reactor the lignin portion can be swept out, leading to its removal from the pretreated biomass.
For AFEX pretreatment, ammonia that penetrates into cell walls can rapidly rush out of the media after pretreatment. This rapid escape of materials was reported to produce large pores (>10 nm in diameter) in the outer secondary cell walls and increase accessible surface area of biomass [116]. In addition, the cleavage of lignin-carbohydrate complexes looses the cross-linked structure and ammonia carries a portion of lignin and hemicellulose to outer layer of the cell wall as well as cell corners [45]. Lee et al. [68] observed that the coastal Bermuda grass fibrils were covered with a lignin coating after pretreatment at 80–100 °C for 30 min, making the fibrils shape smoother and less angular than the untreated fibrils. Ciesielski et al. [117] observed that relocated lignin and hemicelluloses were assembled in abundance as agglomerates of globular material in a disordered and fibrous network located on cell wall surfaces. Similar to DAP and HWP, AFEX also produces lignin aggregation through a separation of phases, but with no significant loss of hemicellulose. Chundawat et al. [45] investigated how liquid-solid loading in AFEX pretreatment influenced the extent of cell wall disruption. High liquid loading AFEX pretreatment resulted in cell wall extractives that were enriched in lignin-derived phenolics depositing on the outer cell walls and inner lumen spaces. On the other hand, low liquid loading AFEX pretreatment led to non-uniformly cell wall surface covered by irregularly shaped, hydrophilic deposits abundant in hemicellulose oligomers, lignin aromatics, and decomposition products which were formed during pretreatment. Furthermore, anhydrous ammonia was observed to severely disrupt the secondary wall, causing it to detach and form globular structures. These globular structures were formed by coalescence of interlamellar lignin packed between adjacent cellulosic fibrils and had similar size to the lignified globular structures reported for dilute acid-pretreated biomass.
Perspectives and Conclusions
It is commonly assumed that lignin presence in untreated biomass restricts enzymatic hydrolysis by impeding the accessibility of cellulase to cellulose and unproductively binding cellulase. While the lignin removal during DAP, HWP, SEP, and AFEX is limited, these pretreatments can reduce the biomass recalcitrance and improve sugar release performance. DAP, SEP, and HWP can disrupt the biomass cell wall matrix with the cleavage of aryl ether linkage in lignin, increasing cellulase accessibility to cellulose. In addition to acidic hydrolysis of hemicellulose, the cleavages of labile linkages between lignin and hemicellulose can facilitate the hemicellulose removal, thus increasing pore volume and available surface area in pretreated biomass which in turn can enhance digestibility of pretreated biomass. During AFEX pretreatment, significant cleavage of esters occurs in biomass as a major cell wall-disrupting reaction. The cleavage of diferulate linkages and lignin–ferulate linkages during AFEX can facilitate delocalization of lignin and hemicelluloses, increasing enzyme accessibility to polysaccharides. Clearly, the lignin-related structural changes in biomass during dilute acid, hot water, steam explosion, and ammonia fiber expansion pretreatments contribute to overcoming biomass recalcitrance. These observations reinforce the results that extensive delignification of biomass during pretreatment is not an indispensable criterion to improve biomass conversion to fermentable sugars. Future advances in pretreatment technology development and optimization to overcome biomass recalcitrance suited for low-cost process are expected to reduce the cost of biomass-derived biofuels.
References
Ragauskas AJ, Williams CK, Davison BH, Britovsek G, Cairney J, Eckert CA et al (2006) Science 311:484–489
Saxena RC, Adhikari DK, Goyal HB (2009) Renew Sust Energ Rev 13:156–167
Himmel M, Ding S, Johnson D, Adney W, Nimlos M, Brady J, Foust T (2007) Science 315:804–807
Davison BH, Parks J, Davis MF, Donohoe BS (2013) Plant cell walls, basics of structure, chemistry, accessibility and the influence on conversion. In: Wyman CE (ed) Aqueous pretreatment of plant biomass for biological and chemical conversion to fuels and chemicals. Wiley, New York, pp 23–38
Yang B, Wyman CE (2008) Biofuels Bioprod Biorefin 2:26–40
Brodeur G, Yau E, Badal K, Collier J, Ramachandran KB, Ramakrishnan S Enzym Res 2011 1–17
Mosier N, Wyman C, Dale B, Elander R, Lee YY, Holtzapple M, Ladisch M (2005) Bioresour Technol 96:673–686
Chandra RP, Bura R, Mabee WE, Berlin A, Pan X, Saddler JN (2007) Adv Biochem Eng Biotechnol 108:67–93
Pu Y, Hu F, Huang F, Davison BH, Ragauskas AJ (2013) Biotechnol Biofuels 6:15–27
Barakat A, Mayer-Laigle C, Solhy A, Arancon RAD, de Vries H, Luque R (2014) RSC Adv 4:48109–48127
Sannigrahi P, Ragauskas AJ (2013) Fundamentals of biomass pretreatment by fractionation. In: Wyman CE (ed) Aqueous pretreatment of plant biomass for biological and chemical conversion to fuels and chemicals. Wiley, New York, pp 201–222
Hallac BB, Sannigrahi P, Pu Y, Ray M, Murphy RJ, Ragauskas AJ (2010) Ind Eng Chem Res 49:1467–1472
Pan X, Gilkes N, Kadla J, Pye K, Saka S, Gregg D, Ehara K, Xie D, Lam D, Saddler J (2006) Biotechnol Bioeng 94:851–861
Nguyen TY, Cai CM, Kumar R, Wyman CE (2015) ChemSusChem. doi:10.1002/cssc.201403045
Davison BH, Drescher SR, Tuskan GA, Davis MF, Nghiem NP (2006) Appl Biochem Biotechnol 129–132:427–435
Chen F, Dixon RA (2007) Nat Biotechnol 25:759–761
Jackson LA, Shadle GL, Zhou R, Nakashima J, Chen F, Dixon RA (2008) Bioenerg Res 1:180–192
Studer MH, DeMartini JD, Davis MF, Sykes RW, Davison B, Keller M, Tuskan GA, Wyman CE (2011) Proc Natl Acad Sci U S A 108:6300–6305
Chang VS, Holtzapple MT (2000) Appl Biochem Biotechnol 84–86:5–37
Varnái A, Siika-Aho M, Viikari L (2010) Enzym Microb Technol 46:185–193
Ding SY, Liu Y-S, Zeng Y, Himmel ME, Baker JO, Bayer EA (2012) Science 338:1055–1060
Lacayo CI, Hwang MS, Ding S-Y, Thelen MP (2013) PLoS One 8:e68266. doi:10.1371/journal.pone.0068266
Kim SB, Um BH, Park SC (2001) Appl Biochem Biotechnol 91–93:81–84
Draude KM, Kurniawan CB, Duff SJB (2001) Bioresour Technol 79:113–120
Ohgren K, Bura R, Saddler J, Zacchi G (2007) Bioresour Technol 98:2503–2510
Ishizawa CI, Jeoh T, Adney WS, Himmel ME, Johnson DK, Davis M (2009) Cellulose 16:677–686
da Costa Sousa L, Chundawat SPS, Balan V, Dale BE (2009) Curr Opin Biotechnol 20:339–347
Lloyd TA, Wyman CE (2005) Bioresour Technol 96:1967–1977
Kim JS, Lee YY, Torget RW (2001) Appl Biochem Biotechnol 91–93:331–340
Sun YE, Cheng JJ (2005) Bioresour Technol 96:1599–1606
Saha BC, Iten LB, Cotta MA, Wu YV (2005) Process Biochem 40:3693–3700
Yu G, Yano S, Inoue H, Inoue S, Endo T, Sawayama S (2010) Appl Biochem Biotechnol 160:539–551
Kobayashi N, Okada N, Hirakawa A, Sato T, Kobayashi J, Hatano S, Itaya Y, Mori S (2009) Ind Eng Chem Res 48:373–379
Kim Y, Mosier NS, Ladisch MR (2009) Biotechnol Prog 25:340–348
Ṕerez JA, Ballesteros I, Ballesteros M, Saez F, Negro MJ, Manzanares P (2008) Fuel 87:3640–3647
Playne MJ (1984) Biotechnol Bioeng 26:426–433
Chen H, Liu L, Yang X, Li Z (2005) Biomass Bioenergy 28:411–417
Ewanick SM, Bura R, Saddler JN (2007) Biotechnol Bioeng 98:737–746
Mosier NS, Hendrickson R, Ho N, Sedlak M, Ladisch MR (2005) Bioresour Technol 96:1986–1993
Balan V, Bals B, Chundawat SPS, Marshall D, Dale BE (2009) Lignocellulosic biomass pretreatment using AFEX. In: Mielenz JR (ed) Biofuels: methods and protocols. Humana, New York, pp 61–77
Chundawat SPS, Venkatesh B, Dale BE (2007) Biotechnol Bioeng 96:219–231
Chundawat SPS, Vismeh R, Sharma LN, Humpula JF, daCosta Sousa L, Chambliss CK, Jones AD, Balan V, Dale BE (2010) Bioresour Technol 101:8429–8438
Teymouri F, Laureano-Perez L, Alizadeh H, Dale BE (2005) Bioresour Technol 96:2014–2018
Bals B, Rogers C, Jin M, Balan V, Dale B (2010) Biotechnol Biofuels 3:1. doi:10.1186/1754-6834-3-1
Chundawat SPS, Donohoe BS, da Costa Sousa L, Elder T, Agarwal UP, Lu F, Ralph J, Himmel ME, Balan V, Dale BE (2011) Energy Environ Sci 4:973–984
Kim KH, Hong J (2001) Bioresour Technol 77:139–144
Alinia R, Zabihi S, Esmaeilzadeh F, Kalajahi JF (2010) Biosyst Eng 107:61–66
Tassinari T, Macy C, Spano L (1980) Biotechnol Bioeng 22:1689–1705
Sidiras D, Koukios E (1989) Biomass 19:289–306
Alvo P, Belkacemi K (1997) Bioresour Technol 61:185–198
Christian V, Shrivastava R, Shukla D, Modi HA, Vyas BRM (2005) Indian J Exp Biol 43:301–312
Xu Z, Huang F (2014) Appl Biochem Biotechnol 174:43–62
Mood SH, Golfeshan AH, Tabatabaei M, Jouzani GS, Najafi GH, Gholami M, Ardjmand M (2013) Renew Sust Energ Rev 27:77–93
Singh R, Shukla A, Tiwari S, Srivastava M (2014) Renew Sust Energ Rev 32:713–728
Ke J, Chen S (2014) Biological pretreatment of biomass in wood-feeding termites. In: RSC Energy and Environment Series, 10 (Biological Conversion of Biomass for Fuels and Chemicals), 177-194
Chen S, Zhang X, Singh D, Yu H, Yang X (2010) Biofuels 1:177–199
Saritha M, Arora A, Lata (2012) Indian J Microbiol 52:122–130
Um BH, Karim MN, Henk LL (2003) Appl Biochem Biotechnol 105:115–125
Tian S, Zhu W, Gleisner R, Pan XJ, Zhu JY (2011) Biotechnol Prog 27:419–427
Huang F, Ragauskas AJ (2012) Ind Biotechnol 8:21–30
Zeng M, Mosier NS, Huang CP, Sherman DM, Ladisch MR (2007) Biotechnol Bioeng 97:265–278
Nitsos CK, Matis KA, Triantafyllidis KS (2013) ChemSusChem 6:110–122
Holopainen-Mantila U, Marjamaa K, Merali Z, Kasper A, de Bot P, Jaaskelainen AS, Waldron K, Kruus K, Tamminen T (2013) Bioresour Technol 138:156–162
Cara C, Ruiz E, Ballesteros I, Negro MJ, Castro E (2006) Process Biochem 41:423–429
Varga E, Réczey K, Zacchi G (2004) Appl Biochem Biotechnol Part A 114:509–523
Ballesteros I, Negro MJ, Oliva JM, Cabañas A, Manzanares P, Ballesteros M (2006) Appl Biochem Biotechnol 130:496–508
Balan V, Sousa LD, Chundawat SPS, Marshall D, Sharma LN, Chambliss CK, Dale BE (2009) Biotechnol Prog 25:365–375
Lee JM, Jameel H, Venditti RA (2010) Bioresour Technol 101:5449–5458
Hu F, Ragauskas AJ (2012) Bionergy Res 5:1043–1066
Jensen JR, Morinelly JE, Gossen KR, Brodeur-Campbell MJ, Shonnard DR (2010) Bioresour Technol 101:2317–2325
Wyman CE, Balan V, Dale BE, Elander RT, Falls M, Hames B, Holtzapple MT, Ladisch MR, Lee YY, Mosier N et al (2011) Bioresour Technol 102:11052–11062
Sannigrahi P, Kim DH, Jung S, Ragauskas AJ (2011) Energy Environ Sci 4:1306–1310
Alvira P, Tomas-Pejo E, Ballesteros M, Negro MJ (2010) Bioresour Technol 101:4851–4861
Mosier NS (2013) Fundamental of aqueous pretreatment of biomass. In: Wyman CE (ed) Aqueous pretreatment of plant biomass for biological and chemical conversion to fuels and chemicals. Wiley, New York, pp 129–140
Ballesteros I, Oliva JM, Negro MJ, Manzanares P, Ballesteros M (2002) Process Biochem 38:187–192
Viola E, Cardinale M, Santarcangelo R, Villone A, Zimbardi F (2008) Biomass Bioenerg 32:613–618
Tengborg C, Stenberg K, Galbe M, Zacchi G, Larsson S, Palmqvist E, Hahn-Hägerdal B (1998) Appl Biochem Biotechnol Part A 70–72:3–15
Vignon MR, Garcia-Jaldon C, Dupeyre D (1995) Int J Biol Macromol 17:395–404
Boussaid A, Cai Y, Robinson J, Gregg DJ, Nguyen Q, Saddler JN (2001) Biotechnol Prog 17:887–892
Stenberg K, Tengborg C, Galbe M, Zacchi G (1998) J Chem Technol Biotechnol 71:299–308
Avellar BK, Glasser WG (1998) Biomass Bioenerg 14:205–218
Samuel R, Pu Y, Jiang N, Fu C, Wang ZY, Ragauskas A (2014) Front Energy Res 1:14
Samuel R, Pu Y, Raman B, Ragauskas AJ (2010) Appl Biochem Biotechnol 162:62–74
Cao S, Pu Y, Studer M, Wyman CL, Ragauskas AJ (2012) RSC Adv 2:10925–10936
Moxley G, Gaspar AR, Higgins D, Xu H (2012) J Ind Microbiol Biotechnol 39:1289–1299
Sannigrahi P, Ragauskas AJ, Miller SJ (2008) Bioenerg Res 1:205–214
Li JB, Gellerstedt G (2008) Ind Crop Prod 27:175–181
El Hage R, Chrusciel L, Desharnais L, Brosse N (2010) Bioresour Technol 101:9321–9329
Pu Y, Cao S, Studer M, Raguaskas AJ, Wyman CE (2010) Chemical characterization of poplar after hot water pretreatment. 32th Symposium on Biotechnology for Fuels and Chemicals, April 19-22, Clearwater Beach, Florida
Samuel R, Cao S, Das BK, Hu F, Pu Y, Ragauskas AJ (2013) RSC Adv 3:5305–5309
Leschinsky M, Zuckerstaetter G, Weber HK, Patt R, Sixta H (2008) Holzforschung 62:653–658
Li JB, Henriksson G, Gellerstedt G (2007) Bioresour Technol 98:3061–3068
Heikkinen H, Elder T, Maaheimo H, Rovio S, Rahikainen J, Kruus K, Tamminen T (2014) J Agric Food Chem 62:10437–10444
Li JB, Gellerstedt G, Toven K (2009) Bioresour Technol 100:2556–2561
Kaparaju P, Felby C (2010) Bioresour Technol 101:3175–3181
Li H, Pu Y, Kumar R, Ragauskas AJ, Wyman CE (2014) Biotechnol Bioeng 111:485–492
Trajano HL, Engle TN, Foston M, Ragauskas A, Tschaplinski T, Wyman C (2013) Biotechnol Biofuels 6:110
Guo X, Zhang L, Shu S, Hao J (2014) Appl Mech Mater 672–674:154–158
Yelle DJ, Kaparaju P, Hunt CG, Hirth K, Kim H, Ralph J, Felby C (2013) Bioenerg Res 6:211–221
Chundawat SPS, Bals B, Campbell T, Sousa L, Gao D, Jin M, Eranki P, Garlock R, Teymouri F, Balan V, Dale BE (2013) Primer on ammoniafiber expansion pretreatment. In: Wyman CE (ed) Aqueous pretreatment of plant biomass for biological and chemical conversion to fuels and chemicals. Wiley, New York, pp 169–200
Liu Z, Padmanabhan S, Cheng K, Schwyter P, Pauly M, Bell AT, Prausnitz JM (2013) Bioresour Technol 135:23–29
Azarpira A, Lu F, Ralph J (2011) Org Biomol Chem 9:6779–6787
Tolbert A, Akinosho H, Khunsupat R, Naskar AK, Ragauskas AJ (2014) Biofuels Bioprod Biorefin 8:836–856
Vanderghem C, Richel A, Jacquet N, Blecker C, Paquot M (2011) Polym Degrad Stab 96:1761–1770
Selig MJ, Viamajala S, Decker SR, Tucker MP, Himmel ME, Vinzant TB (2007) Biotechnol Prog 23:1333–1339
Donohoe BS, Decker SR, Tucker MP, Himmel ME, Vinzant TB (2008) Biotechnol Bioeng 101:913–925
Pingali SV, Urban VS, Heller WT, McGaughey J, O'Neill H, Foston M, Myles DA, Ragauskas A, Evans BR (2010) Biomacromolecules 11:2329–2335
Lima MA, Lavorente GB, da Silva HK, Bragatto J, Rezende CA, Bernardinelli OD, Deazevedo ER, Gomez LD, McQueen-Mason SJ, Labate CA et al (2013) Biotechnol Biofuels 6:75–91
Donaldson LA, Wong KKY, Mackie KL (1988) Wood Sci Technol 22:103–114
Kallavus U, Gravitis J (1995) Holzforschung 49:182–188
Muzamal M, Jedvert K, Theliander H, Rasmuson A (2015) Holzforschung 69:61–66
Coletta VC, Rezende CA, da Conceicao FR, Polikarpov I, Guimaraes FE (2013) Biotechnol Biofuels 6:43–52
Zhang M, Chen G, Kumar R, Xu B (2013) Biotechnol Biofuels 6:147–157
Langan P, Petridis L, O'Neill HM, Pingali SV, Foston M, Nishiyama Y, Schulz R, Lindner B, Hanson BL, Harton S et al (2014) Green Chem 16:63–68
Liu C, Wyman CE (2003) Ind Eng Chem Res 42:5409–5416
Wyman CE, Dale BE, Elander RT, Holtzapple M, Ladisch MR, Lee YY (2005) Bioresour Technol 96:1959–1966
Ciesielski PN, Matthews JF, Tucker MP, Beckham GT, Crowley MF, Himmel ME, Donohoe BS (2013) ACS Nano 7:8011–8019
Acknowledgments
This manuscript has been authored by UT-Battelle, LLC under Contract No. DE-AC05-00OR22725 with the U.S. Department of Energy. The work was supported and performed as part of the BioEnergy Science Center (BESC). The BioEnergy Science Center is a U.S. Department of Energy Bioenergy Research Center supported by the Office of Biological and Environmental Research in the DOE Office of Science.
Author information
Authors and Affiliations
Corresponding author
Additional information
This manuscript has been authored by UT-Battelle, LLC under Contract No. DE-AC05-00OR22725 with the U.S. Department of Energy. The United States Government retains and the publisher, by accepting the article for publication, acknowledges that the United States Government retains a nonexclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this manuscript, or allow others to do so, for United States Government purposes. The Department of Energy will provide public access to these results of federally sponsored research in accordance with the DOE Public Access Plan (http://energy.gov/downloads/doe-public-access-plan).
Rights and permissions
About this article
Cite this article
Pu, Y., Hu, F., Huang, F. et al. Lignin Structural Alterations in Thermochemical Pretreatments with Limited Delignification. Bioenerg. Res. 8, 992–1003 (2015). https://doi.org/10.1007/s12155-015-9655-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-015-9655-5