Abstract
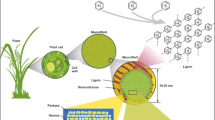
Oxidative pretreatment of lignocellulosic biomass improves the efficacy of plant cell wall polysaccharides in biochemical conversion processes. In presence of oxidants, recalcitrant lignins are oxidized and dissolved to enhance the access of cellulose and hemicellulose to pretreatment chemicals and hydrolytic enzymes. This chapter focuses on the common types of oxidative pretreatments, their applications in biomass conversion, and the physiochemical changes in biomass structure during the pretreatment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Pretreatment
- Ozone
- Hydrogen peroxide
- Catalysis
- Biofuel
- Enzymatic hydrolysis
- Lignin
- Oxidation
- Delignification
- Oxidoreductase
13.1 Oxidative Pretreatment: An Overview of Processes
The application of oxidation in the processing of lignocellulosic materials has a long history, with the earliest industrial use of gaseous chlorine in bleaching dating back to the eighteenth century [1]. Oxidation of lignocellulosic materials alters the physiochemical properties of plant polymers, resulting in fractionation and modification of plant cell wall components. Oxidative bleaching of plant pulp, in particular, has been the primary industrial application of lignocellulose oxidation.
Oxidation reactions have also been studied as an approach to improving the accessibility of polysaccharides during biochemical conversion of lignocellulosic biomass, and to extracting lignins from biomass for further valorization. Since these reactions precede enzymatic saccharification of plant polysaccharides, they are colloquially referred to as oxidative pretreatment. The use of oxidants in pretreatment has been demonstrated to increase the yield of sugars from enzymatic digestion of pretreated biomass, and to result in physiochemical changes in plant cell wall components. This section reviews the processing characteristics of various oxidative pretreatments, and the following section focuses on the effects of oxidative pretreatment on plant cell wall components.
13.1.1 Pulp Bleaching Processes
Pulp bleaching is a sequence of unit operations (also referred to as stages) where wood pulp is treated with oxidants in order to whiten the pulp. A detailed overview of the process can be found in the writings of Sjöström [2], Biermann [3], and Bajpai [4]. Most pulp bleaching sequences use oxidation reactions to remove residual lignins and to convert naturally occurring wood chromophores to products that absorb little or no light in the visible range. During these reactions , color-bearing lignins and aromatic extractives are removed from wood pulp, leaving behind bleached product that is primarily cellulose. Because of the economic impacts of cellulose depolymerization during pulp bleaching, the oxidants used in pulp bleaching.
The oxidants used in pulp bleaching has evolved from early options of chlorine [5] and hypochlorite to more environmentally friendly chlorine-free oxidants including molecular oxygen, hydrogen peroxide, and ozone [6].
13.1.2 Oxidative Pretreatment for Biochemical Conversion of Lignocellulose
Biochemical conversion of lignocellulose offers potential in delivering fuel, chemicals, and materials with reduced life-cycle of greenhouse gas emissions compared to petroleum refining [7]. Pretreatment of lignocellulose disrupts the plant cell wall structure in biomass, leading to increased yields in enzymatic saccharification and sugar bioconversion. As a result, a pretreatment stage is indispensable in any economically viable biochemical biomass conversion process.
Oxidative pretreatment is a category of pretreatment methods that involves the use of oxidants as a means of disrupting and fractionating lignocellulose. Compared to other pretreatment methods, oxidative pretreatment is unique in its ability to modify and remove lignin from lignocellulosic biomass [8]. The type and extent of chemical changes to lignin, as well as other plant cell wall components, vary depending on the type of oxidant used, operation conditions, and the characteristics of biomass being pretreated.
13.1.2.1 Wet Oxidation
Wet oxidation is a pretreatment method that employs molecular oxygen or air in a pressurized reaction loaded with biomass and an alkaline aqueous solution (e.g., Na2CO3, NaOH). Biomass suspended in water is treated under pressurized (5–20 MPa) air or oxygen, and elevated temperature (150–350 °C). This pretreatment method has been demonstrated to be effective on various biomass feedstock including wheat straw [9, 10], reed [11], rice husk [12], rape straw [13], sugarcane bagasse [14], and wood pulp waste [15].
During wet oxidation of herbaceous biomass, lignin is converted to carbon dioxide (CO2) as well as water-soluble carboxylic acids including acetic, formic, succinic, oxalic, and glutaconic acids. Hemicellulose is also solubilized during wet oxidation, with the extent of dissolution and degradation positively correlated with temperature [9]. Compared to steam explosion pretreatment, wet oxidation is advantageous due to lower processing temperature (<200 °C) and reduced generation of sugar dehydration products (e.g., furfural, 5-hydroxyformaldehyde) known to inhibit sugar-fermenting organisms [10].
13.1.2.2 Alkaline Hydrogen Peroxide
Hydrogen peroxide (H2O2) has a higher standard oxidation potential (Table 13.1) than molecular oxygen, and demonstrates effectiveness in biomass pretreatment under ambient conditions. Under alkaline conditions (pH 11–12), hydrogen peroxide dissociates and evolves into potently oxidative radicals (•OH, •OOH). These radicals oxidize lignins, resulting in change in lignin dissolution and removal from the plant cell wall matrix. The presence of alkali also promotes solubilization of hemicellulose, which further increases the accessibility of cellulose to hydrolytic enzymes.
As a pretreatment method, alkaline hydrogen peroxide (AHP) pretreatment is effective in removing both hemicellulose and lignins from the plant cell wall matrix, and thus increasing the accessibility of cellulose to hydrolytic enzymes [16, 17]. AHP has been demonstrated as an effective standalone pretreatment for a number of herbaceous feedstocks including wheat straw [18], rice hulls [19], corn stover [20, 21], bamboo [22], and agave bagasse [23], while the pretreatment is not effective on cactus pear [24] and gooseweed [25]. The efficacy of AHP pretreatment under elevated temperature (150–180 °C) on woody biomass has also been reported for Douglas fir [26, 27] and hybrid poplar [21].
AHP pretreatment has also been reported as a secondary post-treatment used in tandem with other pretreatment methods including wet oxidation [12], alkali pretreatment [28, 29], steam explosion [30], and microwave [31,32,33]. As part of a multi-stage pretreatment process, the delignifying AHP step reduces the cell wall recalcitrance synergistically with other pretreatments that are effective in hemicellulose removal [34]. The amount of hydrogen peroxide used in AHP post-treatment is reduced as compared to using AHP as a standalone pretreatment process, and may lead to an improvement in the economic feasibility of the entire pretreatment process [29].
13.1.2.3 Catalytic Hydrogen Peroxide Pretreatment
The application of metal catalysts in AHP pretreatment has been reported as an approach to improving both pretreatment efficacy and reducing oxidant use. Examples of effective catalysts include manganese acetate [35], copper-diimine complexes [36], magnetite nanoparticles [37], and titanium dioxide [38]. The effects of catalysts include accelerated oxidation reactions, reduced time needed for effective pretreatment, and improved efficacy of recalcitrance reduction. In presence of metal catalysts, oxidation reactions between hydrogen peroxide and plant cell wall components can be accelerated, or go through pathways different from those under catalyst-free conditions. Although the specific reaction mechanisms of catalytic biomass oxidation by hydrogen peroxide is not fully understood, a number of possible reaction schemes have been proposed. Transitional metal catalysts including manganese, copper, and iron can catalyze Fenton reactions that generate hydroxyl (•OH) and superoxide (O2•–) radicals, which in turn oxidizes lignins and other aromatic structures in biomass [39].
13.1.2.4 Ozone
Ozone is an environmentally friendly oxidant that generates hydroxyl radicals while dissolved in water and thus has potential applications in lignin oxidation and biomass pretreatment. Recent developments in ozone pretreatment of biomass have been detailed in a recent review by Travaini and coauthors [40]. The efficacy of ozone pretreatment is associated with the moisture content of biomass during pretreatment process, as dissolved ozone is more effective in lignin oxidation compared to gaseous ozone [41]. Currently, the high energy and financial cost of ozone generation has limited the use of ozone in biomass pretreatment as part of cellulosic biofuel production . To overcome these limitations, ozone pretreatment with low severity may be used in tandem with other synergistic pretreatment methods [42].
13.1.2.5 Enzymatic Pretreatment
Oxidoreductases have been associated with both the plant biosynthesis and fungal biodegradation of lignins. Laccase and peroxidases have been shown to affect lignin synthesis and accumulation in Arabidopsis [43,44,45], and their use in biomass pretreatment has also been reported. Deng et al. independently reported a Trametes versicolor laccase pretreatment mediated with 1-hydroxybenzotriazole that reduces non-productive enzymatic binding on lignin during wheat straw hydrolysis, and the pretreatment also results in a 26% increase in the yield of reducing sugars during enzymatic saccharification [46]. Heap et al. used T. versicolor laccase pretreatment in tandem with alkaline peroxide pretreatment to further improve saccharification yield from wheat straw [47]. Enhanced saccharification yield was also observed in Eucalyptus [48] and bamboo [49] pretreated with a laccase-mediator system.
13.2 Effects of Oxidative Pretreatment Processes on Plant Cell Wall Components
Physical and chemical changes to plant cell wall components, as well as an increase in enzymatic digestibility, have been associated with oxidation reactions during oxidative pretreatment. The extent of these changes is associated with the type of the pretreatment, as well as pretreatment process parameters including pH temperature, oxygen partial pressure, residence time, and oxidant–biomass stoichiometry. Some examples of pretreatment effects on enzymatic conversion are summarized in Table 13.2.
Via cleavage of covalent intramolecular linkages [39] and oxidation of lignin side chains [50], oxidative pretreatment is effective in hemicellulose solubilization and lignin removal. Redistribution and removal of recalcitrant cell wall polymer is associated with improved accessibility of hydrolytic enzymes. Wu et al. reported the reduced binding affinity of oxidized lignins to cellulases, which leads to improved enzymatic hydrolysis rate of cellulose [51]. The surface morphology of plant cell wall matrix is also affected by oxidative pretreatment, with an increase in surface roughness [52], pore volume [16], and pore surface area [22, 53]. Oxidative pretreatment also increases the water retention value [54], which is an indicator of enzymatic digestibility of pretreated biomass [55].
13.3 Knowledge Gaps
Oxidative pretreatment has demonstrated potential in reducing plant cell recalcitrance to biochemical conversion, although additional research is still needed in this area to better understand the physiological changes in biomass during oxidative pretreatment. Specifically, the oxidation chemistry during depolymerization and repolymerization of lignin polymers is not yet fully understood, and the effect of catalysts during oxidative pretreatment has not been well studied. The role of naturally occurring, cell wall–associated transition metals in biomass has been reported [61], which reveals the potential of breeding biomass crops with metal profiles suitable to oxidative pretreatment. In addition, more research is warranted in identifying strategies in reducing costs related to pretreatment chemical consumption and product separations during oxidative pretreatment [29].
References
Torén, K., & Blanc, P. D. (1997). The history of pulp and paper bleaching: Respiratory-health effects. The Lancet, 349, 1316–1318.
Sjöström, E. (1993). Chapter 8 – Pulp bleaching. In E. Sjöström (Ed.), Wood Chem (2nd ed., pp. 165–203). San Diego: Academic Press.
Biermann, C. J. (1996). Chapter 5 – Pulp bleaching. In: C. J. Biermann (Ed.), Biermann’s handbook of pulp and paper: Raw material and pulp making (2nd ed., pp. 123–136). San Diego: Academic.
Bajpai, P. (2018). Chapter 19 – Pulp bleaching. In: P. Bajpai (Ed.), Biermann’s handbook of pulp and paper: Raw material and pulp making (3rd ed., pp. 465–491). San Diego: Elsevier.
Koda, K., Shintani, H., Matsumoto, Y., & Meshitsuka, G. (1999). Evaluation of the extent of the oxidation reaction during chlorine bleaching of pulp. Journal of Wood Science, 45, 149–153.
Kaur, D., Bhardwaj, N. K., & Lohchab, R. K. (2019). Effect of incorporation of ozone prior to ECF bleaching on pulp, paper and effluent quality. Journal of Environmental Management, 236, 134–145.
Cai, H., Han, J., Wang, M., Davis, R., Biddy, M., & Tan, E. (2018). Life-cycle analysis of integrated biorefineries with co-production of biofuels and bio-based chemicals: Co-product handling methods and implications. Biofuels, Bioproducts and Biorefining, 12, 815–833.
Chaturvedi, V., & Verma, P. (2013). An overview of key pretreatment processes employed for bioconversion of lignocellulosic biomass into biofuels and value added products. 3 Biotech, 3, 415–431.
Schmidt, A. S., & Thomsen, A. B. (1998). Optimization of wet oxidation pretreatment of wheat straw. Bioresource Technology, 64, 139–151.
Bjerre, A. B., Olesen, A. B., Fernqvist, T., Plöger, A., & Schmidt, A. S. (1996). Pretreatment of wheat straw using combined wet oxidation and alkaline hydrolysis resulting in convertible cellulose and hemicellulose. Biotechnology and Bioengineering, 49, 568–577.
Szijarto, N., Kadar, Z., Varga, E., Thomsen, A. B., Costa-Ferreira, M., & Reczey, K. (2009). Pretreatment of reed by wet oxidation and subsequent utilization of the pretreated fibers for ethanol production (Report). Applied Biochemistry and Biotechnology, 157, 83.
Banerjee, S., Sen, R., Pandey, R. A., Chakrabarti, T., Satpute, D., Giri, B. S., & Mudliar, S. (2009). Evaluation of wet air oxidation as a pretreatment strategy for bioethanol production from rice husk and process optimization. Biomass and Bioenergy, 33, 1680–1686.
Arvaniti, E., Bjerre, A. B., & Schmidt, J. E. (2012). Wet oxidation pretreatment of rape straw for ethanol production. Biomass and Bioenergy, 39, 94–105.
Martín, C., Marcet, M., & Thomsen, A. B. (2008). Comparison between wet oxidation and steam explosion as pretreatment methods for enzymatic hydrolysis of sugarcane bagasse. BioResources, 3, 670–683.
Ji, X., Liu, S., Wang, Q., Yang, G., Chen, J., & Fang, G. (2015). Wet oxidation pretreatment of wood pulp waste for enhancing enzymatic saccharification. BioResources, 10, 2184.
Li, J., Lu, M., Guo, X., Zhang, H., Li, Y., & Han, L. (2018). Insights into the improvement of alkaline hydrogen peroxide (AHP) pretreatment on the enzymatic hydrolysis of corn stover: Chemical and microstructural analyses. Bioresource Technology, 265, 1–7.
Ho, M. C., Ong, V. Z., & Wu, T. Y. (2019). Potential use of alkaline hydrogen peroxide in lignocellulosic biomass pretreatment and valorization – A review. Renewable and Sustainable Energy Reviews, 112, 75–86.
Saha, B. C., & Cotta, M. A. (2006). Ethanol production from alkaline peroxide pretreated enzymatically saccharified wheat straw. Biotechnology Progress, 22, 449–453.
Saha, B. C., & Cotta, M. A. (2007). Enzymatic saccharification and fermentation of alkaline peroxide pretreated rice hulls to ethanol. Enzyme and Microbial Technology, 41, 528–532.
Mittal, A., Katahira, R., Donohoe, B. S., Black, B. A., Pattathil, S., Stringer, J. M., & Beckham, G. T. (2017). Alkaline peroxide delignification of corn stover. ACS Sustainable Chemistry & Engineering, 5, 6310–6321.
Gupta, R., & Lee, Y. Y. (2010). Pretreatment of corn stover and hybrid poplar by sodium hydroxide and hydrogen peroxide. Biotechnology Progress, 26, 1180–1186.
Song, X., Jiang, Y., Rong, X., Wei, W., Wang, S., & Nie, S. (2016). Surface characterization and chemical analysis of bamboo substrates pretreated by alkali hydrogen peroxide. Bioresource Technology, 216, 1098–1101.
Perez-Pimienta, J. A., Poggi-Varaldo, H. M., Ponce-Noyola, T., Ramos-Valdivia, A. C., Chavez-Carvayar, J. A., Stavila, V., & Simmons, B. A. (2016). Fractional pretreatment of raw and calcium oxalate-extracted agave bagasse using ionic liquid and alkaline hydrogen peroxide. Biomass and Bioenergy, 91, 48–55.
de Souza Filho, P. F., Ribeiro, V. T., dos Santos, E. S., & de Macedo, G. R. (2016). Simultaneous saccharification and fermentation of cactus pear biomass – Evaluation of using different pretreatments. Industrial Crops and Products, 89, 425–433.
Vu, P. T., Unpaprom, Y., & Ramaraj, R. (2018). Impact and significance of alkaline-oxidant pretreatment on the enzymatic digestibility of Sphenoclea zeylanica for bioethanol production. Bioresource Technology, 247, 125–130.
Alvarez-Vasco, C., & Zhang, X. (2013). Alkaline hydrogen peroxide pretreatment of softwood: Hemicellulose degradation pathways. Bioresource Technology, 150, 321–327.
Alvarez-Vasco, C., & Zhang, X. (2017). Alkaline hydrogen peroxide (AHP) pretreatment of softwood: Enhanced enzymatic hydrolysability at low peroxide loadings. Biomass and Bioenergy, 96, 96–102.
Liu, T., Williams, D. L., Pattathil, S., Li, M., Hahn, M. G., & Hodge, D. B. (2014). Coupling alkaline pre-extraction with alkaline-oxidative post-treatment of corn stover to enhance enzymatic hydrolysis and fermentability. Biotechnology for Biofuels, 7, 48.
Stoklosa, R. J., del Pilar Orjuela, A., da Costa Sousa, L., Uppugundla, N., Williams, D. L., Dale, B. E., Hodge, D. B., & Balan, V. (2017). Techno-economic comparison of centralized versus decentralized biorefineries for two alkaline pretreatment processes. Bioresource Technology, 226, 9–17.
Yang, B., Boussaid, A., Mansfield, S. D., Gregg, D. J., & Saddler, J. N. (2002). Fast and efficient alkaline peroxide treatment to enhance the enzymatic digestibility of steam-exploded softwood substrates. Biotechnology and Bioengineering, 77, 678–684.
Wu, C. J., Zhao, C. S., Li, J., & Chen, K. F. (2011). The effect of microwave treatment on the hydrogen peroxide bleaching of soda-AQ wheat straw pulp. Advances in Materials Research, 236–238, 1307–1312.
Ayyasamy, S., Venkatachalam, S., Sangeetha, V., & Priyenka, D. (2016). Improving enzymatic saccharification of cassava stem using peroxide and microwave assisted pre-treatment techniques. Chemical Industry and Chemical Engineering Quarterly, 23, 50–50.
Huang, X., De Hoop, C. F., Li, F., Xie, J., Hse, C.-Y., Qi, J., Jiang, Y., & Chen, Y. (2017). Dilute alkali and hydrogen peroxide treatment of microwave liquefied rape straw residue for the extraction of cellulose nanocrystals. Journal of Nanomaterials, 2017, 4049061.
Zhu, M.-Q., Wen, J.-L., Wang, Z.-W., Su, Y.-Q., Wei, Q., & Sun, R.-C. (2015). Structural changes in lignin during integrated process of steam explosion followed by alkaline hydrogen peroxide of Eucommia ulmoides Oliver and its effect on enzymatic hydrolysis. Applied Energy, 158, 233–242.
Lucas, M., Hanson, S. K., Wagner, G. L., Kimball, D. B., & Rector, K. D. (2012). Evidence for room temperature delignification of wood using hydrogen peroxide and manganese acetate as a catalyst. Bioresource Technology, 119, 174–180.
Bhalla, A., Bansal, N., Pattathil, S., et al. (2018). Engineered lignin in poplar biomass facilitates cu-catalyzed alkaline-oxidative pretreatment. ACS Sustainable Chemistry & Engineering, 6, 2932–2941.
Koo, H., Salunke, B., Iskandarani, B., Oh, W.-G., & Kim, B. (2017). Improved degradation of lignocellulosic biomass pretreated by Fenton-like reaction using Fe 3 O 4 magnetic nanoparticles. Biotechnology and Bioprocess Engineering, 22, 597–603.
Kuznetsov, B., Kuznetsova, S., Danilov, V., & Yatsenkova, O. (2009). Influence of UV pretreatment on the abies wood catalytic delignification in the medium “acetic acid–hydrogen peroxide–TiO 2”. Reaction Kinetics and Catalysis Letters, 97, 295–300.
Gierer, J. (1982). The chemistry of delignification – A general concept – Part II. Holzforschung, 36, 55–64.
Travaini, R., Martín-Juárez, J., Lorenzo-Hernando, A., & Bolado-Rodríguez, S. (2016). Ozonolysis: An advantageous pretreatment for lignocellulosic biomass revisited. Pretreat Biomass, 199, 2–12.
Den, W., Sharma, V. K., Lee, M., Nadadur, G., & Varma, R. S. (2018). Lignocellulosic biomass transformations via greener oxidative pretreatment processes: Access to energy and value-added chemicals. Frontiers in Chemistry, 6, 141.
Mulakhudair, A. R., Hanotu, J., & Zimmerman, W. (2017). Exploiting ozonolysis-microbe synergy for biomass processing: Application in lignocellulosic biomass pretreatment. Biomass and Bioenergy, 105, 147–154.
Berthet, S., Demont-Caulet, N., Pollet, B., et al. (2011). Disruption of LACCASE4 and 17 results in tissue-specific alterations to lignification of Arabidopsis thaliana stems. Plant Cell, 23, 1124.
Shigeto, J., Itoh, Y., Hirao, S., Ohira, K., Fujita, K., & Tsutsumi, Y. (2015). Simultaneously disrupting AtPrx2, AtPrx25 and AtPrx71 alters lignin content and structure in Arabidopsis stem. Journal of Integrative Plant Biology, 57, 349–356.
Cosio, C., Ranocha, P., Francoz, E., Burlat, V., Zheng, Y., Perry, S. E., Ripoll, J.-J., Yanofsky, M., & Dunand, C. (2017). The class III peroxidase PRX17 is a direct target of the MADS-box transcription factor AGAMOUS-LIKE15 (AGL15) and participates in lignified tissue formation. The New Phytologist, 213, 250–263.
Deng, Z., Xia, A., Liao, Q., Zhu, X., Huang, Y., & Fu, Q. (2019). Laccase pretreatment of wheat straw: Effects of the physicochemical characteristics and the kinetics of enzymatic hydrolysis. Biotechnology for Biofuels, 12, 159.
Heap, L., Green, A., Brown, D., van Dongen, B., & Turner, N. (2014). Role of laccase as an enzymatic pretreatment method to improve lignocellulosic saccharification. Catalysis Science & Technology, 4, 2251–2259.
Rico, A., Rencoret, J., del Río, J. C., Martínez, A. T., & Gutiérrez, A. (2014). Pretreatment with laccase and a phenolic mediator degrades lignin and enhances saccharification of Eucalyptus feedstock. Biotechnology for Biofuels, 7, 6.
Gaikwad, A., & Meshram, A. (2019). Effect of particle size and mixing on the laccase-mediated pretreatment of lignocellulosic biomass for enhanced saccharification of cellulose. Chemical Engineering Communications, 207, 1–11.
Gould, J. M. (1985). Studies on the mechanism of alkaline peroxide delignification of agricultural residues. Biotechnology and Bioengineering, 27, 225–231.
Wu, K., Ying, W., Shi, Z., Yang, H., Zheng, Z., Zhang, J., & Yang, J. (2018). Fenton reaction-oxidized bamboo lignin surface and structural modification to reduce nonproductive cellulase binding and improve enzyme digestion of cellulose. ACS Sustainable Chemistry & Engineering, 6, 3853–3861.
Selig, M. J., Vinzant, T. B., Himmel, M. E., & Decker, S. R. (2009). The effect of lignin removal by alkaline peroxide pretreatment on the susceptibility of corn stover to purified cellulolytic and xylanolytic enzymes. Applied Biochemistry and Biotechnology, 155, 94–103.
Shimizu, F. L., de Azevedo, G. O., Coelho, L. F., Pagnocca, F. C., & Brienzo, M. (2020). Minimum lignin and xylan removal to improve cellulose accessibility. Bioenergy Research, 13, 775–785.
Bhalla, A., Bansal, N., Stoklosa, R. J., Fountain, M., Ralph, J., Hodge, D. B., & Hegg, E. L. (2016). Effective alkaline metal-catalyzed oxidative delignification of hybrid poplar. Biotechnology for Biofuels, 9, 34.
Weiss, N. D., Felby, C., & Thygesen, L. G. (2018). Water retention value predicts biomass recalcitrance for pretreated lignocellulosic materials across feedstocks and pretreatment methods. Cellulose, 25, 3423–3434.
Szijártó, N., Kádár, Z., Varga, E., Thomsen, A. B., Costa-Ferreira, M., & Réczey, K. (2009). Pretreatment of reed by wet oxidation and subsequent utilization of the pretreated fibers for ethanol production. Applied Biochemistry and Biotechnology, 155, 83–93.
Palonen, H., Thomsen, A. B., Tenkanen, M., Schmidt, A. S., & Viikari, L. (2004). Evaluation of wet oxidation pretreatment for enzymatic hydrolysis of softwood. Applied Biochemistry and Biotechnology, 117, 1–17.
Martin, C., Marcet, M., & Thomsen, A. B. (2008). Comparison between wet oxidation and steam explosion as pretreatment methods for enzymatic hydrolysis of sugarcane bagasse. BioResources, 3, 670–683.
Correia, J. A. d. C., Júnior, J. E. M., Gonçalves, L. R. B., & Rocha, M. V. P. (2013). Alkaline hydrogen peroxide pretreatment of cashew apple bagasse for ethanol production: Study of parameters. Bioresource Technology, 139, 249–256.
Bhalla, A., Fasahati, P., Particka, C. A., Assad, A. E., Stoklosa, R. J., Bansal, N., Semaan, R., Saffron, C. M., Hodge, D. B., & Hegg, E. L. (2018). Integrated experimental and technoeconomic evaluation of two-stage Cu-catalyzed alkaline–oxidative pretreatment of hybrid poplar. Biotechnology for Biofuels, 11, 143.
Bansal, N., Bhalla, A., Pattathil, S., Adelman, S. L., Hahn, M. G., Hodge, D. B., & Hegg, E. L. (2016). Cell wall-associated transition metals improve alkaline-oxidative pretreatment in diverse hardwoods. Green Chemistry, 18, 1405–1415.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Lim, Y.J., Li, Z. (2021). Understanding Fundamental and Applied Aspects of Oxidative Pretreatment for Lignocellulosic Biomass and Lignin Valorization. In: Liu, ZH., Ragauskas, A. (eds) Emerging Technologies for Biorefineries, Biofuels, and Value-Added Commodities. Springer, Cham. https://doi.org/10.1007/978-3-030-65584-6_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-65584-6_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-65583-9
Online ISBN: 978-3-030-65584-6
eBook Packages: EnergyEnergy (R0)