Abstract
Purpose
We performed a cross-sectional study of neurocognitive function in non-brain cancer patients treated with long-term bevacizumab.
Methods/patients
From 2015 to 2017, we included patients with different types of cancer treated with bevacizumab with or without chemotherapy (BEV; N = 20) or only chemotherapy (ChT; N = 19) for at least 34 weeks, patients who received non-brain radiotherapy (RxT; N = 19), and healthy controls (HC; N = 19) were assessed once at week 34 of treatment (BEV and ChT) or at completion of radiotherapy. Neurocognition was evaluated with the Hopkins Verbal Learning Test-Revised (HVLT-R) total and delayed recall, the Trail Making Test A and B, and the Controlled Oral Word Association Test in the four groups. Non-parametric tests were used to assess differences between groups.
Results
The BEV, ChT, and RxT groups scored significantly lower than the HC group on all tests and especially on the HVLT-R total recall. In no case were the mean scores of the BEV group significantly lower than those of the ChT or RxT groups.
Conclusions
Neurocognitive impairment was seen even in patients treated with local non-brain radiotherapy. Treatment with bevacizumab for a long period of time does not seem to worsen neurocognitive function to a greater extent than chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bevacizumab is a recombinant humanized monoclonal IgG1 antibody that binds to vascular endothelial growth factor (VEGF) to inhibit angiogenesis, resulting in the inhibition and regression of newly formed vasculature and the alteration of vascular function and tumour blood flow [1]. It is approved by the US Food and Drug Administration for the treatment of glioblastoma in the recurrent setting and of colorectal, lung, kidney, cervical, and ovarian cancers in several settings. VEGF-A is a key regulator of tumour-associated angiogenesis in glioblastoma, which is a highly vascularised tumour where the presence of abnormal new vessels is a marker for histological diagnosis. The accelerated FDA approval of bevacizumab for the treatment of recurrent glioblastoma was based on the results of two phase II studies that showed an improvement in 6-month progression-free survival (PFS) and objective radiographic responses higher than those described in historic controls [2, 3]. In contrast, neither of the two phase III randomized trials in the first-line setting (AVAGLIO [4] and RTOG 0825 [5]) comparing standard treatment (radiotherapy with concurrent and adjuvant temozolomide) with or without the addition of bevacizumab showed an improvement in overall survival for patients treated with bevacizumab [4, 5]. Several other well-designed phase II and III trials in the first-line and recurrent settings showed similar results: an increase in PFS without a consistent impact on overall survival [6, 7]. Nonetheless, bevacizumab is currently used to treat glioblastoma in the recurrent setting and is included as a useful treatment in the guidelines of both the European Association of Neurooncology [8] and the National Comprehensive Cancer Network (https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf).
A net clinical benefit substudy in the RTOG 0825 trial [5] found a deterioration of neurocognitive function in patients treated with bevacizumab that was first seen at week 22 and was statistically worse at week 34 of treatment. These findings led to a certain degree of mistrust of bevacizumab as a first-line treatment of glioblastoma, as it seemed that the 3–4 months of PFS benefit conferred by bevacizumab were obtained at the cost of neurocognitive decline. Neurocognitive function is clearly a crucial factor in patients with brain tumours, many of whom present with neurocognitive deficits that tend to worsen over the course of the disease [9].
There are several possible explanations for the neurocognitive deterioration reported in the RTOG 0825 trial. It may have been partly due to an underestimation of the infiltrative progression pattern, since FLAIR sequences, which evaluate this infiltrative progression, were not part of the response criteria in the trial. Thus, patients could have been erroneously considered progression-free when they were in fact progressing and accumulating neurocognitive impairments. Alternatively, the deterioration could have been the result of the combinatory effect of bevacizumab and radiotherapy, rather than that of bevacizumab alone. Finally, bevacizumab may have been the sole cause of the deterioration, which may have been detected in this trial and not in others simply because neurocognitive assessments are not generally included in trials of non-brain cancers.
To shed light on this third alternative, we have assessed neurocognitive function in non-brain cancer patients without previous neurological disease or brain metastases who were treated with bevacizumab for at least 34 weeks. We compared the bevacizumab-treated patients with healthy controls and with cancer patients who received chemotherapy or radiotherapy without bevacizumab.
Materials and methods
Patients
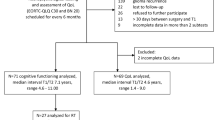
This study was designed to include non-brain cancer patients treated at the Medical and Radiation Oncology Services of the Catalan Institute of Oncology at Hospital Germans Trias i Pujol between 2015 and 2017. We designed a four-arm prospective study to enrol non-brain cancer patients in four types of individuals: the BEV group included patients with cancer who were treated with bevacizumab, with or without chemotherapy, for at least 34 weeks; the ChT group included patients with cancer who were treated with chemotherapy alone for at least 34 weeks; the RxT group were patients with cancer who were treated with radiotherapy alone; and the HC group included randomly selected persons without cancer working at the hospital in different capacities. For each patient included in the BEV arm, we included one patient in each of the other arms, starting with the ChT group, followed by the RxT group, and finally the HC group. Individuals with previous or current neurological disease, brain metastases or primary central nervous system tumors were excluded from the study. Patients should be at least 18 years old and sign the Informed Consent form before participating in the study.
Participants were asked to provide information about their socio-demographic background in an interview and clinical information was obtained from patient medical records. We recorded information about age, sex, handedness, educational level, Eastern Cooperative Oncology Group performance status (ECOG PS), disease characteristics, cancer treatments, and concomitant medications.
Neurocognitive assessments
The study was designed as a cross-sectional study to assess the neurocognitive status of a patient at a given time. Therefore, only one full neurocognitive assessment was done for each individual. The assessment was performed at week 34 in the BEV and ChT groups, after completion of radiotherapy in the RxT group, and at an unspecified time in the HC group.
Short- and long-term memory were assessed with the Hopkins Verbal Test-Revised (HVLT-R), processing speed with the Trail Making Test A (TMT_A), executive function with the Trail Making Test B (TMT_B), and verbal fluency with the Controlled Oral Word Association Test (COWAT).
The HVLT-R is a verbal learning and memory test in which an individual is asked to learn and recall a list of 12 nouns. The test includes three parts: total recall (TR), 20-min delayed recall (DR), and yes/no delayed recognition (RECOG). The RECOG test was not included in the present study. The HVLT-R is administered by reading the list of words aloud and then asking the individual to repeat the words—immediately and after 20 min. The result of the TR and DR is between 0 and 12, according to the mean number of words recalled in both parts of the test.
The TMT_A and the TMT_B consist of 25 circles distributed over a sheet of paper. In the TMT_A, the circles are numbered 1–25 and the individual is asked to draw lines to connect the numbers in ascending order. In the TMT_B, the circles include numbers (1–13) and letters (A–L). The patient is asked to connect the circles in ascending order alternating numbers and letters (1-A-2-B-3-C, etc.). The patient is instructed to connect the circles as quickly as possible, and scores are based on the number of seconds needed to complete the task. We adjusted the number of seconds according to the age and educational level of our subjects [10], obtaining percentile scores, where < 26 was considered below average.
The COWAT is a lexical fluency test that measures spontaneous verbal production of words beginning with a specific letter of the alphabet (on three tasks with a total of three letters) in 1 min. The score is calculated as a z value based on the mean number of words produced with all three letters (mean), the standard deviation (SD), and the normative mean for the healthy population (x), according to the following formula:
Z < − 0.66 were considered below average.
On all tests, a higher result indicates a better performance. The results of the tests were communicated to patients but in no case did test results cause a change in their treatment.
Statistical analysis
Demographic characteristics were described by frequencies and distribution differences were assessed with the χ2 test or Fisher’s exact test, as appropriate. The Kruskal–Wallis test was used for comparison of test score distributions between groups. When statistically significant differences were detected, we performed pairwise comparisons between groups with the Mann–Whitney U test, controlling for multiple tests using the false discovery rate (FDR) procedure. All statistical analyses were performed with the Statistical Package for Social Science (SPSS), version 24.0.
Results
Seventy-seven individuals were included in the study: 20 in the BEV group, 19 in the ChT group, 19 in the RxT group, and 19 in the HC group. Each participant was given the battery of tests by a neurocognitive investigator (CP), and a neurologist (GL). The complete battery of tests took around 30 min for each participant.
Patient characteristics
Table 1 shows the demographic and clinical characteristics of the patients and controls. There were more females than males in all the groups. All individuals had ECOG PS 0–1 and a similar exposure to antidepressants and anxiolytics. The number of patients who received a chemotherapy regimen associated with neurocognitive impairment was similar in the BEV and the ChT groups (p = 0.13). When only the three groups of cancer patients were included in the analysis, in the RxT group, more patients had a lower level of education (p = 0.054) and a better ECOG PS (p = 0.05). In addition, all the patients in the RxT group had local disease, while those in the BEV and ChT groups had mainly advanced disease (p < 0.001).
Test results
Table 2 and Fig. 1 show the mean results for each test and group. The HC group had significantly better scores in all tests than the other three groups: HVLT-R TR (p = 0.002), HVLT-R DR (p = 0.001), TMT_A (p = 0.002), TMT_B (p = 0.003), COWAT (p = 0.01).
Table 3 displays the Mann–Whitney p values for the comparison of mean test scores between groups. Significant differences were observed in mean scores on the HVLT-R TR between the BEV and HC groups (p = 0.001), between the ChT and HC groups (p = 0.003), and between the RxT and the HC groups (p < 0.001). Significant differences were observed in mean scores on the HVLT-R DR between the ChT and the HC groups (p = 0.005) and between the RxT and HC groups (p = 0.002). On the TMT_A, differences were significant only between the RxT and HC groups (p = 0.007), and between the BEV and HC groups (p = 0.011). Differences on the TMT_B were significant for the comparison between the BEV and HC groups (p = 0.005) and between the RxT and HC groups (p = 0.003). Finally, significant differences on the COWAT were observed only between the RxT and HC groups (p = 0.006). No other significant differences between groups were observed on any of the tests, including when comparing only the three groups of cancer patients (BEV vs. ChT, BEV vs. RxT, ChT vs. RxT).
Discussion
Bevacizumab has been associated with neurocognitive deterioration in patients with glioblastoma [5, 11], but it is not clear whether bevacizumab is the sole cause of this deterioration or if it acts together with other characteristics unique to glioblastoma and its treatment. To remove these possible confounding factors, we have examined the effect of bevacizumab in non-brain cancer patients who were treated with chemotherapy with or without bevacizumab or with radiotherapy alone and in healthy controls. We have found that bevacizumab was not associated with a worsening of neurocognitive function compared to other treatments.
A significant decline in cognitive function, particularly in processing speed, as measured by the TMT_A test, and in executive function, measured by the TMT_B test, was observed in patients receiving bevacizumab in the RTOG 0825 clinical trial at week 34 [11]. In addition, a later analysis showed further deterioration on the COWAT [5]. Other studies have also linked bevacizumab to undesirable neurocognitive effects, including brain atrophy after prolonged treatment [12]. VEGF inhibition has a special role in modulating both vascular and neuronal behaviour in the central nervous system. VEGF inhibition led to a cytotoxic effect on neurons and a decrease in the dendritic length of hippocampal neurons in rats [1]. A reduction in the synaptic plasticity of the hippocampus, which could lead to an impairment of short-term memory and learning, was observed in glioblastoma patients after bevacizumab treatment [13]. Neurocognitive impairment attributed to dysregulation of VEGF receptor signalling has also been reported after treatment with sunitinib malate, an inhibitor of the VEGF receptor 2 [14].
Chemotherapy has also been associated with neurocognitive decline, and post-chemotherapy cognitive impairment (PCCI), known colloquially as “chemobrain”, has been reported in patients with breast cancer, where a large number of long-term survivors receive adjuvant therapies [15]. Several studies exploring the pathophysiological mechanisms underlying the cognitive decline associated with chemotherapy [16] have suggested that cytostatic agents can disrupt neurobiological processes by impairing hippocampal neurogenesis or by producing neuroinflammation and a change in myelination [3, 17,18,19]. Furthermore, neuroimaging studies have found that chemotherapy produces structural alterations and changes in gray matter [20, 21].
Several drugs have also been associated with neurocognitive deterioration, including 5-fluorouracil, doxorubicin, cyclophosphamide, taxanes, methotrexate, bis-chloroethylnitrosurea carmustine (BCNI), fludarabine, cytarabine, and platinates [22]. There are many patients who, after having received these treatments, report cognitive disorders associated with a decline in attention, memory, information processing speed, and executive functions. In our series, however, neurocognitive function did not differ between the BEV and the ChT groups among patients treated with these drugs. In fact, while alterations in these patients were as expected, the addition of bevacizumab did not worsen neurological impairment. Conversely, alterations in patients treated with radiotherapy for local disease were unexpected in our series since patients with prior brain radiotherapy or neurological disease were excluded from the study. Patients treated with local radiotherapy alone were diagnosed of gynaecological, breast or prostate cancer with a supposed 5 year survival rate above 90%. Thus, their cognitive status should apparently be that of the general population of that age-group. As we did not find differences in age, socio-economic status, and education levels compared to the HC, we think that the cognitive impairment detected in RxT should be taken into consideration in further studies.
In fact, to the best of our knowledge, the effect of non-brain radiotherapy on neurocognitive function has not previously been explored and our findings indicate that it merits further study.
Other factors could also have affected neurocognitive function in our patients, such as the impact of the diagnosis itself, surgery and anaesthesia, menopause, anxiety, depression, fatigue, genetic predisposition, comorbid medical conditions, pain severity, and general quality of life. Nor can we rule out an effect on neurocognitive function produced by the cancer itself [23, 24]. In fact, cognitive impairment has been reported in breast cancer even before the administration of chemotherapy [25]. These factors were not explored in the present study due to the low number of patients but we recommend their inclusion in future studies.
Our study has several limitations in addition to the relatively small number of patients included—a consequence of the small number of patients who were progression-free after at least 34 weeks of bevacizumab treatment, which was the time frame in which neurocognitive alterations had been found in previous studies of glioblastoma patients receiving bevacizumab [5, 11, 26]. In the BEV arm, patients received chemotherapy and bevacizumab as maintenance therapy, although the ideal comparison would have been with patients receiving bevacizumab alone. However, bevacizumab is never indicated as the sole treatment in any disease; rather, it is always combined with chemotherapy. Additionally, our results are based only on a single evaluation of cognitive functions at one given time and could well have changed if the tests had been administered at different intervals or if we had tested at baseline before starting treatment. However, such a study would need to include a very large number of patients initially since many of those assessed at baseline could well have changed their treatment or progressed before week 34. We point out this aspect as the main limitation of our study. In fact we designed this transversal study to explore the neurocognitive impairments after having received bevacizumab for a long time, as they have been detected in long-term-treated glioblastoma patients, in the setting of a clinical trial (RTOG 0825). Our results justify a prospective study which includes data about the basal neurocognitive status of patients before any oncological treatment. Finally, we did not take into account the possible effect of anxiety, depression, or stress on test results, although the use of antidepressants and anxiolytics was similar across the groups, including HC.
It is unclear to what extent baseline cognitive impairment is related to anxiety and depression. Certainly, learning of a cancer diagnosis can affect mood, which may in turn affect sleep and other physiological functions. Most data on this issue proceed from breast cancer patients. In a small study of breast cancer patients who were to receive either chemotherapy or radiation treatment, pre-treatment worry was associated with alterations in brain function, as measured by functional magnetic resonance imaging, and with subjective and objective measures of cognitive function in both treatment groups, with the pre-chemotherapy group reporting significantly more worry [27]. However, in several studies of women undergoing adjuvant or neoadjuvant chemotherapy for breast cancer, the authors reported that anxiety, distress, or poor quality of life correlated with self-perceived cognitive concerns but not with neuropsychological test results [28,29,30]. In another study of breast cancer patients, cognitive impairment observed prior to systemic treatment was associated with certain comorbidities but not with anxiety, depression, or type of surgery [31].
To the best of our knowledge, this is the first study to investigate the neurocognitive effects of bevacizumab in patients with non-brain cancers. Our findings suggest that bevacizumab in and of itself may not worsen neurocognitive function to a greater extent than chemotherapy. In addition, the fact that patients receiving local non-brain radiotherapy had worse neurocognitive function than healthy controls suggests that the term “cancer-related cognitive impairment (CRCI)” may be more apt than PCCI. Our findings lend support to the premise that a deeper understanding of the nature, causes, incidence, and prevalence of CRCI would be of interest for the management of both brain and non-brain cancer patients and indicate that neurocognitive function should be assessed both at baseline and after treatment in all cancers.
Data availability
Test results and database are available at request.
References
Latzer P, Schlegel U, Theiss C. Morphological changes of cortical and hippocampal neurons after treatment with VEGF and bevacizumab. CNS Neurosci Ther. 2016;22(6):440–50. https://doi.org/10.1111/cns.12516.
Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–5.
Seigers R, Fardell JE. Neurobiological basis of chemotherapy-induced cognitive impairment: a review of rodent research. Neurosci Biobehav Rev. 2011;35(3):729–41. https://doi.org/10.1016/j.neubiorev.2010.09.006.
Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–22. https://doi.org/10.1056/NEJMoa1308345.
Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. https://doi.org/10.1056/NEJMoa1308573.
Herrlinger U, Schafer N, Steinbach JP, Weyerbrock A, Hau P, Goldbrunner R, et al. Bevacizumab plus irinotecan versus temozolomide in newly diagnosed O6-methylguanine-DNA methyltransferase nonmethylated glioblastoma: the randomized GLARIUS trial. J Clin Oncol. 2016;34(14):1611–9. https://doi.org/10.1200/JCO.2015.63.4691.
Wick W, Gorlia T, Bendszus M, Taphoorn M, Sahm F, Harting I, et al. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377(20):1954–63. https://doi.org/10.1056/NEJMoa1707358.
Weller M, van den Bent M, Tonn JC, Stupp R, Preusser M, Cohen-Jonathan-Moyal E, et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017;18(6):e315–e329329. https://doi.org/10.1016/S1470-2045(17)30194-8.
Bosma I, Vos MJ, Heimans JJ, Taphoorn MJ, Aaronson NK, Postma TJ, et al. The course of neurocognitive functioning in high-grade glioma patients. Neuro Oncol. 2007;9(1):53–62.
Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19(2):203–14.
Wefel JS, Pugh SL, Armstrong TS, Gilbert MR, Won M, Wendland MM, et al. Neurocognitive function (NCF) outcomes in patients with glioblastoma (GBM) enrolled in RTOG 0825. J Clin Oncol. 2013;31(15 _suppl):2004. https://doi.org/10.1200/jco.2013.31.15_suppl.2004.
Bag AK, Kim H, Gao Y, Bolding M, Warren PP, Fathallah-Shaykh HM, et al. Prolonged treatment with bevacizumab is associated with brain atrophy: a pilot study in patients with high-grade gliomas. J Neurooncol. 2015;122(3):585–93. https://doi.org/10.1007/s11060-015-1751-z.
Fathpour P, Obad N, Espedal H, Stieber D, Keunen O, Sakariassen PO et al. (2014) Bevacizumab treatment for human glioblastoma. Can it induce cognitive impairment? Neuro Oncol 16(5), 754–6. doi: 10.1093/neuonc/nou013.
Abdel-Aziz AK, Mantawy EM, Said RS, Helwa R. The tyrosine kinase inhibitor, sunitinib malate, induces cognitive impairment in vivo via dysregulating VEGFR signaling, apoptotic and autophagic machineries. Exp Neurol. 2016;283(Pt A):129–41. https://doi.org/10.1016/j.expneurol.2016.06.004.
Selamat MH, Loh SY, Mackenzie L, Vardy J. Chemobrain experienced by breast cancer survivors: a meta-ethnography study investigating research and care implications. PLoS ONE. 2014;9(9):e108002. https://doi.org/10.1371/journal.pone.0108002.
Kaiser J, Bledowski C, Dietrich J. Neural correlates of chemotherapy-related cognitive impairment. Cortex A J Devoted Study Nervous Syst Behav. 2014;54:33–50. https://doi.org/10.1016/j.cortex.2014.01.010.
Seigers R, Schagen SB, Van Tellingen O, Dietrich J. Chemotherapy-related cognitive dysfunction: current animal studies and future directions. Brain Imaging Behav. 2013;7(4):453–9. https://doi.org/10.1007/s11682-013-9250-3.
Briones TL, Woods J. Dysregulation in myelination mediated by persistent neuroinflammation: possible mechanisms in chemotherapy-related cognitive impairment. Brain Behav Immun. 2014;35:23–322. https://doi.org/10.1016/j.bbi.2013.07.175.
Christie LA, Acharya MM, Parihar VK, Nguyen A, Martirosian V, Limoli CL. Impaired cognitive function and hippocampal neurogenesis following cancer chemotherapy. Clin Cancer Res. 2012;18(7):1954–65. https://doi.org/10.1158/1078-0432.CCR-11-2000.
Simo M, Rifa-Ros X, Rodriguez-Fornells A, Bruna J. Chemobrain: a systematic review of structural and functional neuroimaging studies. Neurosci Biobehav Rev. 2013;37(8):1311–21. https://doi.org/10.1016/j.neubiorev.2013.04.015.
Deprez S, Amant F, Smeets A, Peeters R, Leemans A, Van Hecke W, et al. Longitudinal assessment of chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning. J Clin Oncol. 2012;30(3):274–81. https://doi.org/10.1200/JCO.2011.36.8571.
Wefel JS, Kesler SR, Noll KR, Schagen SB. Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA Can J Clin. 2015;65(2):123–38. https://doi.org/10.3322/caac.21258.
Hurria A, Somlo G, Ahles T. Renaming, "chemobrain". Cancer Invest. 2007;25(6):373–7. https://doi.org/10.1080/07357900701506672.
Bompaire F, Durand T, Leger-Hardy I, Psimaras D, Ricard D. Chemotherapy-related cognitive impairment or %3c%3c chemobrain %3e%3e: concept and state of art. Geriatrie et psychologie neuropsychiatrie du vieillissement. 2017;15(1):89–988. https://doi.org/10.1684/pnv.2017.0659.
Wefel JS, Lenzi R, Theriault R, Buzdar AU, Cruickshank S, Meyers CA. 'Chemobrain' in breast carcinoma?: a prologue. Cancer. 2004;101(3):466–75. https://doi.org/10.1002/cncr.20393.
Vannorsdall TD. Cognitive changes related to cancer therapy. Med Clin N Am. 2017;101(6):1115–34. https://doi.org/10.1016/j.mcna.2017.06.006.
Berman MG, Askren MK, Jung M, Therrien B, Peltier S, Noll DC, et al. Pretreatment worry and neurocognitive responses in women with breast cancer. Health Psychol. 2014;33(3):222–31. https://doi.org/10.1037/a0033425.
Jansen CE, Cooper BA, Dodd MJ, Miaskowski CA. A prospective longitudinal study of chemotherapy-induced cognitive changes in breast cancer patients. Support Care Cancer. 2011;19(10):1647–56. https://doi.org/10.1007/s00520-010-0997-4.
Tager FA, McKinley PS, Schnabel FR, El-Tamer M, Cheung YK, Fang Y, et al. The cognitive effects of chemotherapy in post-menopausal breast cancer patients: a controlled longitudinal study. Breast Cancer Res Treat. 2010;123(1):25–34. https://doi.org/10.1007/s10549-009-0606-8.
Biglia N, Bounous VE, Malabaila A, Palmisano D, Torta DM, D'Alonzo M, et al. Objective and self-reported cognitive dysfunction in breast cancer women treated with chemotherapy: a prospective study. Eur J Cancer Care (Engl). 2012;21(4):485–92. https://doi.org/10.1111/j.1365-2354.2011.01320.x.
Mandelblatt JS, Stern RA, Luta G, McGuckin M, Clapp JD, Hurria A, et al. Cognitive impairment in older patients with breast cancer before systemic therapy: is there an interaction between cancer and comorbidity? J Clin Oncol. 2014;32(18):1909–18. https://doi.org/10.1200/JCO.2013.54.2050.
Funding
No funding was required for this study.
Author information
Authors and Affiliations
Contributions
CP and CB designed the study and drafted the manuscript. CP and GL administered the test to the patients. JMV and AME performed statistical analysis. LV, EC, VQ, JCP, MR, AE, JLM, BP, AM, VT, SV identified patients and administered informed consent to patients. All authors read and accepted the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Carmen Balana, Margarita Romeo, Jose Luis Manzano, and Enric Carcereny declare an advisory role for Roche. Claudia Panciroli has travel and accommodations paid by Servier and BMS to disclose. Giuseppe Lucente has travel and accommodations paid by Eisai, Bial and Krka to disclose. Vanesa Quiroga has travel and accommodations paid by Roche to disclose. Juan Carlos Pardo has travel and accommodations paid by BMS and Merck to disclose. Anabel Mañes has travel and accommodations paid by Bayern to disclose. Jose Luis Manzano declares an advisory role for Novartis, Amgen, Merck and BMS, and travel and accommodations paid by BMS and Novartis. Enric Carcereny declares an advisory role for MSD and BMS. Laura Vidal has an employment with Novartis to disclose. Anna Estival has travel and accommodations paid by MSD, Pharmamar, and Novartis to disclose. The other authors declare no conflict of interest.
Ethics approval
The study was approved by the Ethics Committee of Hospital Germans Trias i Pujol. The study was performed in accordance with the Declaration of Helsinki.
Informed consent
All patients gave their signed informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Panciroli, C., Lucente, G., Vidal, L. et al. Assessment of neurocognitive decline in cancer patients, except brain cancer, under long-term treatment with bevacizumab. Clin Transl Oncol 22, 411–419 (2020). https://doi.org/10.1007/s12094-019-02143-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-019-02143-6