Abstract
Studies suggest that adjuvant chemotherapy for early stage breast cancer (BC) is associated with cognitive impairment related to attention, memory, and visuospatial functioning. However, other studies have failed to confirm that relationship. We report one of the first longitudinal, controlled studies of cognitive effects of chemotherapy in older post-menopausal women. Sixty-one post-menopausal women with non-metastatic BC were administered neuropsychological tests before adjuvant therapy (Time1), six months after treatment (Time2), and at a final 6-month follow-up (Time3). Thirty women were treated with chemotherapy; thirty-one women who received no chemotherapy were controls. Cognitive domains measured included motor, language, attention/concentration/working memory, visuospatial, and memory (verbal and visual). Time-by-treatment interaction was significant in the motor domain (P = 0.007) with poorer performance in women treated with chemotherapy. For the other domains, scores did not significantly vary over time by group. In post-menopausal women, chemotherapy was not associated with changes in cognitive function in areas reported by BC survivors: attention, memory, and information processing. Motor slowing in women treated with chemotherapy could be secondary to peripheral neuropathy rather than an indication of more general declines in cognitive processing. Future studies should control for the independent effects of slowed motor functioning when looking to study possible chemotherapy related cognitive processing deficits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past decade, there has been increasing concern about the cognitive effects of chemotherapy treatment. However, several published studies claim that the literature has not demonstrated a clear causal relationship between adjuvant chemotherapy and subsequent cognitive difficulties [1]. A number of cross-sectional studies of women with breast cancer (BC) previously treated with chemotherapy documented a prevalence of cognitive impairment (28–75%) [2–6]. Interestingly, one study [7] found pre-chemotherapy rates of cognitive impairment in BC patients similar to post-treatment rates. These findings illustrate the importance of using longitudinal designs to clarify the relationship between chemotherapy and cognitive functioning.
Findings from the few published longitudinal studies have been mixed. One study of 85 women with early stage BC found no significant differences in cognitive change over time between groups treated with chemotherapy or not [8]. Two studies found no significant mean group decline in women receiving chemotherapy but reported that a subset of the women demonstrated a decline in cognitive function [7, 9]. One recent study of 61 post-menopausal women found a subtle negative influence of chemotherapy on cognitive function as compared with women receiving adjuvant hormonal therapy [10]. Inferences about possible causal effects of chemotherapy on cognition in these studies are methodologically limited. Hurria et al. [9] did not include a control group to control for aging or practice effects. Two of the studies [7, 8] included both pre-and post-menopausal women, raising the possibility that differential effects of estrogen level on cognitive function may have confounded the chemotherapy findings. The research to date has not definitively shown a clear causal relationship between chemotherapy and cognitive difficulties. Some of the confounding factors included menopausal status as well as not having a control group. The current study attempts to address the limited generalizability of the existing literature by controlling for the confounding variables of menopausal status and by including a control group.
The purpose of the current study was to examine, prospectively, the cognitive effects of chemotherapy treatment in post-menopausal women treated for early stage BC as compared with women diagnosed with DCIS who are not treated with chemotherapy. We hypothesized that women with BC treated with surgery and chemotherapy will exhibit greater cognitive impairment immediately post-treatment and 6 months following treatment when compared to women with BC treated with surgery alone.
Methods
Participants
Participants were 61 women (mean age 60.7; range 46.95–70.96) diagnosed with non-metastatic BC [ductal carcinoma in situ (DCIS), Stage I, II, or IIIa] receiving treatment at Columbia University Medical Center (CUMC). Eligibility criteria were: ages 45–70, curative breast surgery, and post-menopausal (no menstrual period for ≥12 months or surgical menopause). Exclusion criteria were: prior primary malignancy; prior exposure to chemotherapy or craniospinal radiation; neoadjuvant chemotherapy; neurological, significant psychiatric or medical comorbidities that might affect ability to participate; or minimal English fluency. We included women on SSRI’s who met all other requirements.
Seventy-four women signed consent. Four were later excluded from participation due to exclusion criteria. Seventy eligible women completed a baseline evaluation; data presented here include the 61 women who returned to complete a second, six-month evaluation as well as the 52 women who completed the third six-month follow-up.
Women treated with a 3–6-month adjuvant chemotherapy regimen comprised the experimental group (CT). The no-chemotherapy comparison group (No-CT) included women diagnosed with BC who were treated with surgery and, in many cases, other adjuvant therapies per standard medical care (e.g., radiation, hormonal therapy).
All eligible patients who were screened and signed consent were enrolled in the study. The study was approved by the IRB of the New York State Psychiatric Institute and CUMC.
Procedures
Participants completed a battery of standardized neuropsychological tests and self-rated questionnaires at three times. The Time1 (T1) evaluation was conducted post-surgery but before any treatment. The same evaluation took place approximately 6 months after T1 or within a month after completing chemotherapy for the CT group (T2) and 6 months after the second evaluation (T3).
Measures
Neuropsychological tests
The neuropsychological tests utilized for this study assess five cognitive domains: motor speed, language, attention/concentration, visuospatial, and memory. The tests are: standardized, valid, and reliable measures of the different cognitive domains, and have been used in other studies of women with BC [11]. When available, alternate forms (shown with an asterisk) of the same test were used to minimize practice effects; order of alternate forms was counterbalanced.
Motor
-
Finger Tapper [14] is a test of fine motor speed.
Language
-
Controlled Oral Word Association Test (COWAT)* [15] is a test of verbal fluency.
-
Boston Naming Test [16] is a measure of word-finding abilities.
Attention/concentration/working memory
-
Trailmaking Test* [17] is a test of speed for visual search, attention, mental flexibility, and motor function.
-
WAIS-III Digit Symbol [18] is a test of speed of processing.
-
WAIS-III Digit Span [18] assesses working memory and mental manipulation.
-
WAIS-III Number/Letter [18] is a more complex test of working memory and mental manipulation.
-
WAIS-III Arithmetic [18] is a mental arithmetic test.
Visuospatial
Memory
-
Verbal: Buschke Selective Reminding Test* [21] is a measure of verbal memory through a list learning procedure.
-
Visual: Benton Visual Retention Test* [22] is a measure of visual perception and visual memory that comprises 2 scores: number correct and errors.
A cognitive domain-specific score was used. Five scores were computed as the mean of z-scores on the tests representing each domain. These domain z-scores were used as continuous variables in analyses.
Self-reported cognitive problems
-
At each evaluation, women were asked to rate their perceived memory abilities on a five-point Likert scale, which was dichotomized for analyses such that ratings >2 were coded as memory problems.
Psychological distress
-
Depression and anxiety: The Beck Depression Inventory II (BDI) [23, 24] assesses general depression. The Zung Self-rating Anxiety Scale (ZAS) [25, 26] assesses anxiety symptoms. Scale scores on the BDI and ZAS were used as continuous measures in analyses.
Data analysis
Statistical analyses were performed using SAS 9.2. For comparison of baseline characteristics between treatment groups, we used t tests for continuous measures and Chi-square tests for categorical measures. Pearson’s two-tailed correlation test was used to evaluate redundancy among the 15 neuropsychological tests. For any particular pair of tests, a correlation of |0.9| or higher suggests that the pair of tests measures the same construct.
To test whether the relationship between time (T1–T2 and T1–T3) and cognitive domain scores differed as a function of treatment group, the interaction effect of group and time was estimated in multi-level modeling (PROC MIXED; SAS 9.2), adjusted for age, diagnosis, number of tests in each domain, and hormone replacement therapy status. Mixed effect models have several advantages over conventional regression analyses, some of which are the abilities to handle repeated measurements within subjects of both predictors and outcome variables, to model autocorrelation effects, and to handle missing data. Maximum likelihood methods were used to obtain estimates for the models. Statistical significance was two-tailed and set at 5% for all tests.
Results
Demographics
Demographic information is presented in Table 1. The chemotherapy regimens seen here are comparable to those in similar studies. Thirty-six women (60%) were on some form of endocrine therapy at the T2 evaluation (Tamoxifen: 53%; Arimidex: 47%), with significantly more of these women (22) in the No-CT group. By T3, 41 women (80%) were on endocrine therapy (No-CT: 20, CT: 21) with no significant difference between treatment groups. At T1, there were seven women (four in the No-CT group and three in the CT group) taking psychiatric medication (all SSRI’s). These women were not significantly different than the rest of the group on T1 neuropsychological measures. The percentage of participants with even mild symptoms of depression (BDI ≥ 10; 28.3%) or anxiety (ZAI ≥ 45; 18.6%) was small.
The nine women who withdrew without completing the T2 evaluation, as compared with those who continued in the study, were not significantly different in age, diagnosis, chemotherapy regimen, history of taking HRT, and T1 depression and anxiety. They were more likely to receive chemotherapy (trend, P < 0.10) and had significantly fewer years of education and lower estimated IQ. These women also showed poorer T1 performance in several cognitive domains: language (P = 0.0001), motor (P = 0.003), attention (P = 0.010), and verbal memory (P = 0.04). Another nine women withdrew between the T2 and T3 evaluations. This group did not differ significantly from those who completed T3 in key demographic or cancer-related characteristics. They did show poorer T1 cognitive performance in motor (P = 0.003), visual memory (P = 0.01) and verbal memory (P < 0.07, trend). Specific reasons for withdrawal in almost cases are unknown. Of the 18 women who did not complete the study, we excluded two, one for chronic alcohol abuse, and one for very low T1 cognitive scores.
The treatment groups were well balanced on most variables, but they significantly differed by diagnosis. Use of chemotherapy was associated with higher stage cancer. The treatment groups also were unbalanced in their history of taking hormone replacement therapy (HRT). Women who received chemotherapy were less likely to have ever taken HRT than those not receiving chemotherapy. Thus, diagnosis and HRT status were included in the model.
Pearson’s two-tailed correlation test was used to evaluate redundancy among the 15 neuropsychological tests. A 0.93 correlation was found between BVRT-Correct and BVRT-Error scores. Thus, only the BVRT-Correct score is presented and used in the model. In addition, as the visual memory and verbal memory domains were each comprised of only one test score, they were combined to form a single memory domain, similar to other studies [3, 27].
The intervals between assessments were not significantly different for the treatment groups: Surgery-T1: [CT (29.17 ± 16.13 days), No-CT (33.52 ± 26.15 days); T1–T2: CT (218 ± 7.81 days), No-CT (210 ± 7.08 days); T2–T3: CT (219 ± 36.28), No-CT (232 ± 43.27)]. Thus, testing interval was not used as a covariate in the model.
T1 Neuropsychological performance
T1 cognitive and psychological functioning is presented in Table 2. The treatment groups did not significantly differ on any test or domain of functioning with the exception of memory. Before any treatment, the CT group had lower verbal memory scores on the Buschke (Total Score) as compared to the No-CT group.
T1 BDI depression and Zung anxiety scores were uncorrelated with cognitive performance and did not differ between treatment groups.
Mixed model
A main effect of time was seen in the language and visuospatial domains with a tendency for women to do better over time. There was no main effect of chemotherapy treatment.
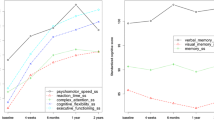
Four of the five domain scores used in the model showed no significantly different within-group changes between T1–T2 and T1–T3 (Table 3). However, time-by-treatment group was significant in the motor domain (F = 5.01; P = 0.007; Fig. 1). Women in the No-CT group showed significantly better (faster) performance on motor tasks between T1 and T3 (t = 2.73; P = 0.007). Women in the CT group showed a non-significant, but borderline, decline in motor performance between T1 and T3 (t = −1.76; P = 0.08) (Table 4).
Adding diagnosis and HRT status to the model was not found to significantly impact performance in any of the cognitive domains. However, when age was added into the model for the visuospatial domain, it was found to be significant (F = 9.4; P = 0.003). An increase in 1 year of age was significantly associated with an increase of 0.1562 standard deviations in the visuospatial domain. Age was not significant in any of the other domains.
Self-reported cognitive problems
Women reported memory problems at all three time points: T1, 30% (18/61); T2, 39% (24/61); and T3, 38% (19/50). Self-reported memory problems were not associated with treatment type, nor were they related to domain z-scores at any of the time points. However, the percentage of women who complained of memory problems in the CT group tended to go up over time (T1 = 27%, T2 = 43%, T3 = 46%), whereas the percentage of women in the No-CT group who complained of memory problems tended to remain the same over time (T1 = 32%, T2 = 35%, T3 = 31%). Self-reported memory problems were significantly related to mean depression scores at T1 [t(55) = −2.48, P = 0.016] and T3 [t(47) = −2.3, P = 0.04], and a trend at T2 [t(57) = −1.79, P = 0.078]. Self-reported memory scores were significantly related to anxiety at T2 [t(57) = −3.124, P = 0.003].
Discussion
In the current longitudinal study, we found a significant relationship between time and treatment in the motor domain of functioning. Women who were not treated with chemotherapy improved more on motor tasks as compared with women receiving chemotherapy who showed a non-significant decline on motor performance 6 months following treatment. Women in the No-CT group are likely exhibiting a practice effect that women in the CT group are not showing. Practice effects refer to increases on tests that happen when an individual retakes the same or a similar test. This is due to experience with the test and testing procedures and is especially relevant when the time between evaluations is relatively short. The lack of enhanced performance on some tasks after repeated testing may, in fact, be an indicator of pathology [28]. Thus, our current finding of a “lack of practice effect” can actually be viewed as a deficit and needs to be accounted for [29]. Time and treatment were not significantly related to any of the other cognitive domains. Although women reported memory problems at all three time points and the treatment groups significantly differed at baseline, there was no relationship between time and treatment in the memory domain.
This motor effect has generally not been associated with “chemobrain”-by-women treated for BC. Anecdotal concerns tend to focus on memory and attention problems. The most common areas of cognitive difficulties seen in the BC literature are: working memory, visual memory, verbal memory, executive functioning, information processing speed, attention, learning, and visuospatial functioning [11, 27, 30–33].
Motor functioning, however, could be affected by both surgical and non-surgical treatments of BC. With the advent of increasingly neurotoxic drugs and multiple chemotherapy regimens, the incident of chemotherapy-induced peripheral neuropathy in cancer treatment, in general, is increasing [34]. The prevalence of chronic neuropathic pain following BC surgery may exceed 50% [35]. In addition, neuropathy is the dose limiting toxicity of paclitaxel, a common chemotherapeutic agent to treat BC. Although the natural history of neuropathy tends to be improved over time, some patients may be left with residual deficits [36].
Peripheral neuropathy has been linked to decreased motor functioning in diabetes [37], HIV infection [38], and pediatric cancers. Peripheral neuropathy due to vincristine treatment is a fairly common effect seen in children treated for all and is manifested as impaired fine and gross motor function both during treatment [39] as well as after treatment and may be related to radiation therapy or chemotherapy [40, 41]. In one study, pediatric all survivors were found to show decreased motor nerve conduction in the peripheral nerves but not within the CNS 5 years after treatment [42]. Thus, decreased motor functioning may be independent of CNS functioning.
Differentiating the independent effects of simple motor slowing from more generalized declines in cognitive processing can be difficult. This is due to the fact that most neuropsychological measures measure multiple areas of functioning and are often unable to assess only one realm of functioning [43]. Statistically controlling for other influences, such as peripheral neuropathy as well as utilizing measures of processing that are free from motor functioning could help identify the presence of CNS processing problems as has been shown in HIV-1 disease [38]. HIV-1 infection was found to compromise CNS-mediated cognitive processes in symptomatic patients even after controlling for simple motor functioning, peripheral neuropathy, age education, and mood. Simple motor speed was controlled for statistically but, in addition, the Stroop Interference Task, a measure relatively unaffected by peripheral motor slowing, was used as a measure of complex cognitive processing. This methodology has generally not been used in BC studies and could possibly account for some of the mixed findings linking chemotherapy to declines in cognitive functioning.
Our findings, along with the findings in two recent longitudinal studies [8, 44], do not support the widely held belief in a direct causal effect of adjuvant chemotherapy on general cognitive dysfunction in areas such as memory, attention, and information processing. Women who go onto receive chemotherapy start out with lower memory scores as compared to the women who are not treated with chemotherapy which may be related to the cancer itself, past hormonal treatment, or other unknown variables. These scores, though, do not significantly change after chemotherapy. However, we did observe that women receiving chemotherapy were less likely to show what we believe is a practice effect on tests of motor functioning, which can be viewed as impairment. This impairment, however, may be secondary to peripheral neuropathy associated with certain chemotherapy agents and may not, in fact, be reflective of a change or decline in CNS cognitive processing.
Similar to others, we found no relationship in BC survivors between neuropsychologically assessed cognitive impairment and self-rated cognitive difficulties [3–5, 45]. A poor correlation between subjective and objective ratings of cognitive functioning is common in many populations: cancer [46], HIV [47], epilepsy [48], and mild head injury [49]. Similar to other studies, our data show a relationship between self-rated cognitive difficulties and depression and anxiety [45].
Limitations of this study include a relatively small sample size, which may have prevented detection of more subtle chemotherapy effects in domains of cognitive processing. In addition, in order to enroll enough subjects, we included women taking different chemotherapeutic agents for a varied amount of time, which may have affected the results. It is also possible that the tests used are not sensitive enough to detect subtle cognitive problems in those areas [50]. In addition, women who withdrew from the study were more likely than women who continued in the study to receive chemotherapy and be impaired at baseline in several cognitive domains. Their continued participation might have had an impact on the results. We tried to limit attrition by offering to conduct follow-up assessments in women’s homes. While such attrition is not uncommon, better methods for limiting attrition in this type of research are needed.
Further, we evaluated cognitive function soon after women completed chemotherapy and then 6 months later, but a longer follow-up may be needed to detect later cognitive changes. We used a post-surgery baseline which may have inflated the baseline rates of impairment. Change in cognitive function could be affected by surgery and anesthesia, the stress of receiving a cancer diagnosis, or other factors related to the cancer disease process. A post-test only, nonrandomized study design provides little validity for inferring a chemotherapy effect [51]. Thus, we argue that using of a post-diagnosis baseline, while not ideal, is preferable to no baseline cognitive assessment.
Some investigators have transformed cognitive change scores using Heaton’s Reliable Change Index (RCI) to account for practice effects. A recent review paper [52] reported that a comparative study in neurologically stable patients found that regression models were better able to predict outcomes than the use of the RCI and produced narrower confidence intervals [53–56]. Vardy et al. recommended either a regression model or the RCI to analyze longitudinal data in this area of study. We used a mixed model, which have several advantages over conventional regression analyses, including the abilities to handle repeated measurements within subjects of both predictors and outcome variables, to model autocorrelation effects, and to handle missing data.
The control group was BC patients, rather than women with no BC. Although we recognize the benefits of a healthy control group to establish normative data, we chose a BC control group to provide a more stringent test of chemotherapy effects. All participants experienced the emotional and cognitive toll of receiving a cancer diagnosis and undergoing surgery. Any group differences, therefore, infer more specifically a chemotherapy effect than comparison to a healthy group, in which group differences could be attributed to nonspecific effects of many factors associated with cancer diagnosis and treatment. Finally, some of the women were treated with endocrine therapy by the second and third evaluations. Although this treatment may affect cognitive functioning, a recent prospective study of post-menopausal women showed that anastrozole does not appear to impair any aspect of cognitive function. They suggested that estrogen depletion, once a woman is post-menopausal, does not notably interfere with memory and attention [57]. However, this may not hold true for women receiving both chemotherapy and endocrine treatment, who may be more vulnerable to cognitive compromise.
Despite the limitations, this study contributes to the small but growing number of controlled longitudinal neuropsychological studies examining cognitive effects of chemotherapy. It is one of the larger studies in this body of research and one of the first studies to control for menopausal status in addition to the psychological and medical challenge of receiving a cancer diagnosis and surgery.
In conclusion, our overall findings provide no evidence that adjuvant chemotherapy treatment for early stage BC produces significant cognitive decline in post-menopausal women in the areas of most complaints: memory, attention, and information processing. There is evidence, however, that BC patients receiving chemotherapy exhibit motor slowing as compared to BC patients who do not receive chemotherapy. This motor slowing is likely related to peripheral symptoms associated with certain chemotherapy regimens. This hypothesis needs to be explored further by controlling for diagnosed peripheral neuropathy as well as symptoms of neuropathy and utilizing relatively motor-free measures in neuropsychological studies of BC survivors.
References
Raffa RB, Duong PV, Finney J, Garber DA, Lam LM, Mathew SS, Patel NN, Plaskett KC, Shah M, Jen Weng HF (2006) Is ‘chemo-fog’/’chemo-brain’ caused by cancer chemotherapy? J Clin Pharm Ther 31(2):129–138
Ahles TA, Saykin AJ, Furstenberg CT, Cole B, Mott LA, Skalla K, Whedon MB, Bivens S, Mitchell T, Greenberg ER et al (2002) Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J Clin Oncol 20(2):485–493
Brezden CB, Phillips KA, Abdollel MTB, Tannock IF (2000) Cognitive function in breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol 18(14):2695–2701
Schagen SB, Van Dam FS, Muller MJ, Boogerd W, Lindeboom J, Bruning PF (1999) Cognitive deficits after postoperative adjuvant chemotherapy for breast carcinoma. Cancer 85(3):640–650
van Dam F, Schagen SB, Muller MJ, Boogerd W, vd Wall E, Fortuyn MED, Rodenhuis S (1998) Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: High dose versus standard-dose chemotherapy. J Natl Cancer Inst 90(3):210–218
Wieneke MH, Dienst ER (1995) Neuropsychological assessment of cognitive functioning following chemotherapy for breast cancer. Psychooncology 4(1):61–66
Wefel JS, Lenzi R, Theriault RL, Buzdar AU, Cruickshank S, Meyers CA (2004) “Chemobrain” in breast carcinoma? A prologue. Cancer 101(3):466–475
Jenkins V, Shilling V, Deutsch G, Bloomfield D, Morris R, Allan S, Bishop H, Hodson N, Mitra S, Sadler G et al (2006) A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. Br J Cancer 94(6):828–834
Hurria A, Rosen C, Hudis C, Zuckerman E, Panageas KS, Lachs MS, Witmer M, van Gorp WG, Fornier M, D’Andrea G et al (2006) Cognitive function of older patients receiving adjuvant chemotherapy for breast cancer: a pilot prospective longitudinal study. J Am Geriatr Soc 54(6):927–931
Stewart A, Collins B, Mackenzie J, Tomiak E, Verma S, Bielajew C (2008) The cognitive effects of adjuvant chemotherapy in early stage breast cancer: a prospective study. Psychooncology 17:122–130
Jansen CE, Miaskowski C, Dodd M, Dowling G, Kramer J (2005) A metaanalysis of studies of the effects of cancer chemotherapy on various domains of cognitive function. Cancer 104(10):2222–2233
Klove H (1963) Clinical neuropsychology. In: Forester F (ed) Medical clinics of North America. Saunders, New York City, NY
Matthews C, Klove H (1964) Instructional manual for the adult neuropsychological tests battery. University of Wisconsin Medical School, Madison, WI
Bornstein RA (1985) Normative data on selected neuropsychological measures from a non-clinical sample. J Clin Psychol 41(5):651–659
Benton AL, Hamsher K, Sivan AB (1983) Multilingual aphasia examination, 3rd edn. AJA Associates, Iowa City
Tombaugh TN, Kozak J, Rees L (1999) Normative data stratified by age and education for two measures of verbal fluency. Arch Clin Neuropsychol 14(2):167–177
Tombaugh TN, Rees L, McIntyre N (1998) Normative data for the trail making test. In: Spreen O, Strauss E (eds) A compendium of neuropsychological tests: administration. Oxford University Press, New York
Wechsler D (1997) Wechsler adult intelligence scale, 3rd revision (WAIS-III). Psychological Corporation, San Antonio
Kolb B, Whishaw IQ (1995) Fundamentals of human neuropsychology. Freeman Press, New York
Spreen O, Strauss E (1998) A compendium of neuropsychological tests: administration, norms and commentary, 2nd edn. Oxford University Press, New York
Buschke H, Fuld P (1974) Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology 24:1019–1025
Sivan A (1992) Benton visual retention test. Psychological Corporation, New York
Beck AT, Beamesderfer A (1974) Assessment of depression: the depression inventory. Mod Probl Pharmacopsychiatr 7:151–169
Beck AT, Steer RA, Garbin MG (1988) Psychometric properties of the beck depression inventory: twenty-five years of evaluation. Clin Psychol Rev 8(1):77–100
Zung WW (1973) The differentiation of anxiety and depressive disorders: a psychopharmacological approach. Psychosomatics 14(6):362–366
Bystritsky A, Linn LS, Ware JE (1990) Development of a multidimensional scale of anxiety. J Anxiety Disord 4(2):99–115
Wefel JS, Lenzi R, Theriault RL, Davis RN, Meyers CA (2004) The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: results of a prospective, randomized, longitudinal trial. Cancer 100(11):2292–2299
Zehnder AE, Blasi S, Berres M, Spiegel R, Monsch AU (2007) Lack of practice effects on neuropsychological tests as early cognitive markers of Alzheimer Disease? Am J Alzheimers Dis Other Demen 22:416–426
Vardy J, Wefel JS, Ahles T, Tannock IF, Schagen SB (2008) Cancer and cancer-therapy related cognitive dysfunction: an international perspective from the Venice cognitive workshop. Ann Oncol 19(4):623–629
Eberhardt B, Dilger S, Musial F, Wedding U, Weiss ET, Wolfgang HR, Miltner (2006) Short-term monitoring of cognitive functions before and during the first course of treatment. J Cancer Res Clin Oncol 132(4):234–240
Meyers CA (2008) How chemotherapy damages the central nervous system. J Biol 7(4):11
Stewart A, Collins B, Mackenzie J, Tomiak E, Verma S, Bielajew C (2008) The cognitive effects of adjuvant chemotherapy in early stage breast cancer: a prospective study. Psychooncology 17(2):122–130
Castellon SA, Ganz PA, Bower JE, Petersen L, Abraham L, Greendale GA (2004) Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. J Clin Exp Neuropsychol 26(7):955–969
Wickham R (2007) Chemotherapy-induced peripheral neuropathy: a review and implications for oncology nursing practice. Clin J Oncol Nurs 11(3):361–376
Jung BF, Ahrendt GM, Oaklander AL, Dworkin RH (2003) Neuropathic pain following breast cancer surgery: proposed classification and research update. Pain 104(1–2):1–13
Jung BF, Herrmann D, Griggs J, Oaklander AL, Dworkin RH (2005) Neuropathic pain associated with non-surgical treatment of breast cancer. Pain 118(1–2):10–14
Ratner DP, Adams KM, Levin NW, Rourke BP (1983) Effects of hemodialysis on the cognitive and sensory-motor functioning of the adult chronic hemodialysis patient. J Behav Med 6(3):291–311
Llorente AM, Miller EN, D’Elia LF, Selnes OA, Wesch J, Becker JT, Satz P (1998) Slowed information processing in HIV-1 disease. The Multicenter AIDS Cohort Study (MACS). J Clin Exp Neuropsychol 20(1):60–72
Vainionpaa L (1993) Clinical neurological findings of children with acute lymphoblastic leukaemia at diagnosis and during treatment. Eur J Pediatr 152(2):115–119
Reinders-Messelink HA, Schoemaker MM, Hofte M, Goeken LN, Kingma A, van den Briel MM, Kamps WA (1996) Fine motor and handwriting problems after treatment for childhood acute lymphoblastic leukemia. Med Pediatr Oncol 27(6):551–555
Copeland DR, Dowell RE Jr, Fletcher JM, Sullivan MP, Jaffe N, Cangir A, Frankel LS, Judd BW (1988) Neuropsychological test performance of pediatric cancer patients at diagnosis and one year later. J Pediatr Psychol 13(2):183–196
Lehtinen S (2003) Neurotoxicity in children after treatment for acute lymphoblastic leukaemia and methotrexate neurotoxicity in a controlled animal model. Oulu University Press, Oulu, Finland
Lezak MD (1995) Neuropsychological assessment, 3rd edn. Oxford University Press, New York
Hermelink K, Untch M, Lux M, Kreienberg R, Beck T, Bauerfeind I, Münzel K (2007) Cognitive function during neoadjuvant chemotherapy for breast cancer: results of a prospective, multicenter, longitudinal study. Cancer 109(9):1905–1913
Shilling V, Jenkins V (2007) Self-reported cognitive problems in women receiving adjuvant therapy for breast cancer. Eur J Oncol Nurs 11(1):6–15
Klepstad P, Hilton P, Moen J, Fougner B, Borchgrevink P, Kaasa S (2002) Self reports are not related to objective assessments of cognitive function and sedation in patients with cancer pain admitted to a palliative care unit. Palliat Med 16:513–519
Saykin AJ, Janssen RS, Sprehn GC, Kaplan JE, Spira TJ, O’Connor B (1991) Longitudinal evaluation of neuropsychological function in homosexual men with HIV infection: 18-month follow-up. J Neuropsychiatry Clin Neurosci 3(3):286–298
Deutsch G, Saykin AJ, Sperling MR (1996) Metamemory in temporal lobe epilepsy. Assessment 3(3):255–263
McAllister TW, Flashman LA, Sparling MB, Saykin AJ, Ferguson R, Yanofsky N (1999) Relationship of psychopathology to post-concussive symptoms one month after mild traumatic brain injury (MTBI). J Neuropsychiatry Clin Neurosci 11(1):150–151
Jansen CE, Miaskowski CA, Dodd MJ, Dowling GA (2007) A meta-analysis of the sensitivity of various neuropsychological tests used to detect chemotherapy-induced cognitive impairment in patients with breast cancer. Oncol Nurs Forum 34(5):997–1005
Campbell D, Stanley J (1963) Experimental and quasi-experimental designs for research. Houghton Mifflin, Boston
Vardy J, Rourke S, Tannock IF (2007) Evaluation of cognitive function associated with chemotherapy: a review of published studies and recommendations for future research. J Clin Oncol 25:2455–2463
Temkin NR, Heaton RK, Grant I, Dikmen SS (1999) Detecting significant change in neuropsychological test performance: a comparison of four models. J Int Neuropsychol Soc 5:357–369
Heaton RK, Temkin N, Dikmen S, Avitable N, Taylor MJ, Marcotte TD, Grant I (2001) Detecting change: a comparison of three neuropsychological methods, using normal and clinical samples. Arch Clin Neuropsychol 16(1):75–91
Erlanger D, Feldman D, Kutner K, Kaushik T, Kroger H, Festa J, Barth J, Freeman J, Broshek D (2003) Development and validation of a web-based neuropsychological test protocol for sports-related return-to-play decision-making. Arch Clin Neuropsychol 18(3):293–316
Ouimet LA, Stewart A, Collins B, Schindler D, Bielajew C (2008) Measuring neuropsychological change following breast cancer treatment: an analysis of statistical models. J Clin Exp Neuropsychol 31(1):73–89
Jenkins VA, Ambroisine LM, Atkins L, Cuzick J, Howell A, Fallowfield L (2008) Effects of anastrozole on cognitive performance in postmenopausal women: a randomised, double-blind chemoprevention trial (IBIS II). Lancet Oncol 9:953–961
Acknowledgments
Dr. Tager is the recipient of a grant from the NCI (RO3-CA96422) and a research grant from the Avon Products Foundation. Dr. Hershman is the recipient of an American Society of Clinical Oncology Advanced Clinical Research Award and a K07 Award from the NCI (CA95597).
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s10549-009-0684-7
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tager, F.A., McKinley, P.S., Schnabel, F.R. et al. The cognitive effects of chemotherapy in post-menopausal breast cancer patients: a controlled longitudinal study. Breast Cancer Res Treat 123, 25–34 (2010). https://doi.org/10.1007/s10549-009-0606-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-009-0606-8