Abstract
Parkinson’s disease (PD) is the second most common age-related neurodegenerative disorder. PD is characterized by progressive loss of dopamine-producing neurons in the substantia nigra (SN) region of brain tissue followed by the α-synuclein-based Lewy bodies’ formation. These conditions are manifested by various motor and non-motor symptoms such as resting tremor, limb rigidity, bradykinesia and posture instability, cognitive impairment, sleep disorders, and emotional and memory dysfunctions. Long non-coding RNAs (lncRNAs) are closely related to protein-coding genes and are involved in various biological processes. Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) lncRNA is involved in different pathways, including alternative splicing, transcriptional regulation, and post-transcriptional regulation, and also interacts with RNAs as a miRNA sponge. MALAT1 is highly expressed in brain tissues and several lines of evidence suggested it is probably involved in synapse generation and other neurophysiological pathways. This narrative review discussed all aspects of MALAT1-associated mechanisms involved in the PD pathogenesis, i.e., perturbed α-synuclein homeostasis, apoptosis and autophagy, and neuro-inflammation. Lastly, the possible applications of MALAT1 as a diagnostic biomarker and its importance to developing therapeutic strategies were highlighted. The literature search was conducted using neurodegeneration, neurodegenerative disorders, Parkinson’s disease, lncRNA, and MALAT1 as search items in Google Scholar, Web of Knowledge, PubMed, and Scopus up to December 2021.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is the second most common chronic neurodegenerative disorder among the elderly, followed by Alzheimer’s disease (AD). It affects 2–3% of people aged 65 and over worldwide. Approximately 8–18 out of every 100,000 people are diagnosed with new cases of PD every year. The number of patients is expected to double between 2005 and 2030, putting a heavy burden on society [1,2,3,4].
The main characteristic of PD is programmed cell death and progressive degradation of dopaminergic neurons in the substantia nigra (SN) region, followed by the α-synuclein-based Lewy bodies, leading to striatal dopamine deficiency and motor dysfunction [5,6,7]. Extensive loss of dopamine-producing neurons reduces the dopamine levels in the brain and leads to motor clinical manifestations including resting tremor, limb rigidity, akinesia, bradykinesia, posture instability, and ankyloses arthritis [8,9,10]. After the dopaminergic loss, various non-dopaminergic neurons degrade and result in non-motor or dopamine-resistant manifestations, i.e., cognitive impairment, autonomic nervous dysfunction, sleep disorders, depression, anxiety, olfactory dysfunction, and emotional and memory disorders [2, 11, 12].
For decades, the exact pathophysiology of PD was unknown. At the same time, different experimental and epidemiologic studies reported that both genetic predisposition and environmental factors play a crucial role in the pathogenesis of this brain disorder. Familial PD has resulted from dominant and/or recessive mutations in several genes, including alpha-synuclein (SNCA), ligase parkin, leucine-rich repeat kinase 2 (LRRK2), eglycase DJ-1 (or PD protein 7), and 5-hydroxytryptamine receptor 2A (HTR2A) [13, 14], while environmental factors (exogenous neurotoxins, age, diet, and lifestyle) also cause sporadic PD [15]. Old age is the leading risk factor for PD. Although Parkinson’s disease is rare under 50, its prevalence increases 5 to 10 times in the sixth to ninth decades of life [10, 16]. It has been observed that these factors induce several changes in various cellular and molecular events that initiate neurodegeneration and neuroinflammation [2]. Events include mitochondrial dysfunction, autophagy, apoptosis, oxidative stress, calcium hemostasis, axonal transport, and neuro-inflammation. Altogether suggest that the progression and development of PD is a systemic and comprehensive process, and all these factors work together to eventually cause the death of dopaminergic neurons in substantia nigra [17,18,19].
Current diagnostic methods for PD are mainly based on clinical manifestations and can only be confirmed by autopsy [20]. Furthermore, the rate of primary degradation of motor function is rapid [21]. In addition, there is no effective treatment for PD, and further research is needed to identify the pathogenesis of the disease and improve early detection methods and targeted treatments in the future.
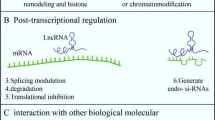
Recent progress in genome-wide transcriptome analysis revealed that only 2% of human transcripts are protein-coding genes, and a vast majority of transcripts are non-protein-coding [22, 23]. Generally, non-coding RNAs (ncRNAs) are categorized into small ncRNA (sncRNA) that are less than 200 bp in length (e.g., piRNA, siRNA, snoRNA, snRNA, miRNA, and rRNA); and long ncRNA (lncRNA), which are 200 bp to 100 kb in length. To date, more than 50,000 lncRNA has been identified in the human genome [24, 25]. These lncRNAs are located both in the nucleus and cytoplasm. They have a potentially crucial role in regulating protein-coding gene expressions in different levels, including epigenetic regulation, transcription regulation, and post-translation control, while they do not code any protein themselves [26,27,28].
Also, several lines of evidence showed that lncRNAs are involved in different biological processes, including organogenesis, cell proliferation and differentiation, survival, dosage compensation, genome imprinting, and chromatin remodeling [29,30,31,32,33]. The function of lncRNAs is associated with their interactions with DNA, RNA, and proteins [34]. Also, their complicated secondary and higher-order structures make them suitable and flexible to recognize proteins and other targets [35, 36]. It is noteworthy that their unique secondary structures make them tissue specific even at a level greater than protein-coding RNAs [37]. In the central nervous system (CNS), lncRNAs are highly expressed and regulated by several neuro-biologic processes, including neuron plasticity, neurogenesis, brain development, etc., via histone modifications, mRNA degradation, and alternative splicing [38,39,40,41]. Abnormal expression of lncRNAs and genetic variations and epigenetics dysregulation in them are associated with human neurologic disorders such as PD, AD, Huntington’s disease (HD), and schizophrenia [42,43,44].
Alterations in the expression of lncRNAs in the brain of PD patients suggested that lncRNA impairment may occur in the early stages of the disease and highlighted its importance as a biomarker for early diagnosis of PD [10]. Also, the expression of lncRNAs altered during aging is highly associated with PD development [45, 46]. Ni et al. reported that 87 different lncRNAs expressed in the substantia nigra of PD patients [47]. Also, 13 lncRNAs had an altered expression in the peripheral blood leukocytes of PD patients [48]. High expression in the brain tissues and variated expression in different neuronal regions during neuropathological conditions support the idea that MALAT1 plays a crucial role in the pathogenesis of PD. Furthermore, microarray analysis revealed that MALAT1 is among the specific regulatory agents that are associated with the formation of neuronal synapses [49,50,51].
The current narrative review summarized the role of MALAT1 lncRNA in the pathogenesis of PD and highlighted its importance as a biomarker that could be implicated for diagnostic, prognostic, and therapeutic approaches. For this purpose, neurodegeneration, neurodegenerative disorders, Parkinson’s disease, lncRNA, and MALAT1 were considered search items. A comprehensive literature search was conducted using items in form of alone or combined in Google Scholar, Web of Knowledge, PubMed, and Scopus up to December 2021. Also, the references of main articles were searched to find further relevant studies.
MALAT1 (Metastasis-Associated Lung Adenocarcinoma Transcript 1)
MALAT1 is a widely investigated lncRNA also identified as nuclear-enriched abundant transcript 2 (NEAT2), HCN, LINC00047, NCRN00047, PRO02853 [52]. The first time, it was identified in a screening for transcripts associated with metastasis and survival in patients with non-small-cell lung cancer (NSCLC) as a prognostic marker [22]. Evidence implied that MALAT1 is upregulated in various cancers and drives tumorigenesis via inducing tumor cell proliferation [51, 53,54,55]. MALAT1 gene primary sequence contains more than 8000 bp and showed high levels of conservation among 33 mammal species. MALAT1 is mainly located at the nucleus and expressed ubiquitously in approximately all tissues of humans, including skin, brain, bone marrow and immune cells, vascular endothelial cells, adipose, liver, lungs, pancreas, and bladder, and the highest expression is in the pancreas and lungs [54, 56, 57].
MALAT1 Biogenesis
MALAT1 gene is located on human 11q13 and mouse 19q1 chromosomes [58]. RNA polymerase II transcribes it, and the initial transcript is about 7–8 kb in humans and 6.7 kb in mice. Two RNase P and RNase Z act on the primary transcript to produce a 6.7-bp larger piece, and a smaller component contains 61 nucleotides known as MALAT1-associated small cytoplasmic RNA (mascRNA) [49, 52]. Unlike the typical cleavage and polyadenylation process, MALAT1 lacks a poly-A tail in the 3ʹ end and forms a triple-helix structure that protects the 3ʹ end against 3ʹ–5ʹ exonuclease. The regulation of MALAT1 turnover is not thoroughly investigated. A recent study introduced the Drosha-DGCR8 complex (part of the microprocessor involved in the biogenesis of miRNA) to be involved in degradation by interacting with the 5ʹ end [59,60,61].
Nervous System (NS)-Associated Physiologic Function of MALAT1
The ubiquitous presence and evolutionary conservation of MALAT1 may indicate its essential functions, while MALAT1 knockdown showed no phenotypic effects in mice. An acceptable explanation for this could be that MALAT1 functionally activates under stress and is not a normal physiologic condition. Another possibility is that other vital lncRNAs compensate for the altered function of MALAT1 [56, 62]. MALAT1 can regulate the transcription of genes acting as a molecular scaffold on inter-chromatin granule clusters. These effects are due to interference in alternative splicing, transcriptional regulation, and post-transcriptional regulation. Also, MALAT1 could interact with RNAs and act as a miRNA sponge via binding to the RNA response elements [56, 63].
MALAT1 is highly expressed in brain tissues and especially in highly active human neo-cortex regions [50]. DNA microarray analysis showed that following the MALAT1 depletion, the expression profile of specific genes significantly associated with synapse and dendrite development of cultured neuron cells was altered. Moreover, in mice hippocampus and Purkinje cells, MALAT1 was first detected between post-natal day 0 (P0) and P7 and finally reached the peak at P28. These results suggest that MALAT1 is probably involved in synapse generation from the early post-natal weeks. Further in vitro studies showed that genetic depletion of MALAT1 reduces synaptic density. In contrast, MALAT1 over-expression leads to a cell-autonomous elevation in the synaptic density in mice primary hippocampus neuron cell culture. It should be noted that this process is accomplished by regulating neuroligin1 (NLGN1) and synaptic cell adhesion molecule1 (SynCAM1) that are involved in synapse formation [49].
MALAT1 also expressed in the cerebrospinal fluid (CSF) and neuro-pathological changes affect its levels. CSF analysis in AD patients compared to the healthy controls showed that MALAT1 levels were decreased due to neurodegenerative consequences [64]. Recent studies showed that MALAT1 levels are different in various regions of the brain and pathologic conditions occurred, while the expression of MALAT1 altered to an abnormal level due to exogenous or endogenous inducers. For instance, it was reported that increased expression of MALAT1 in the brain of human alcoholic is limited to the hippocampus, brain stem, and cerebellum regions and MALAT1 expression is normal in frontal and motor cortices. Also, in alcohol-subjected rats, MALAT1 overexpression was observed in the cortex [65]. MALAT1 is expressed in different types of neurons in the brain. Since it plays an essential role in the normal development of the brain and its physiological activities, it is not surprising that dysregulated MALAT1 is associated with CNS disorders [22].
MALAT1 and Parkinson’s Disease
Abnormal expression of MALAT1 was demonstrated in PD. It was reported that MALAT1 was up-regulated in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP)-induced mice model of PD and methyl-4-phenylpyridinium (MPP +)-induced cells [66,67,68]. Theo et al. conducted a comprehensive analysis on 90 well-annotated lncRNA in the brain of PD patients compared to healthy controls. They proved that several lncRNAs, including TncRNA, SNGH1, MALAT1, and lincRNA-p21, were significantly up-regulated in patients. In this case, the MALAT1 expression was elevated threefold of the normal levels, and most alterations occurred in the primary stages even before the disease course [66]. MALAT1 dysregulation in PD resulted in pathologic conditions and affected multiple pathways, including perturbed α-synuclein hemostasis, apoptosis and autophagy, and neuro-inflammation. Here, we categorized studies conducted in association with MALAT-1 and PD (Table 1).
Perturbed α-Synuclein Hemostasis
The α-synuclein (SNCA) is a presynaptic neuronal protein associated with neurodegenerative disorders. Aberrant oligomeric conformational structures of α-synuclein lead to Lewy bodies’ formation and involve the cellular hemostasis disturbance and neuronal death [69, 70]. Abnormal aggregation of this protein is associated with PD, Lewy body dementia, and multisystem atrophy [71] and leads to abnormal deposition of other proteins in brain tissue [72]. Intra-neuronal aggregation of α-synuclein as a result of risk SNCA variants was reported in PD patients [73, 74] (Fig. 1).
Schematic of various pathways that are affected by MALAT1 dysregulation in PD. In case of perturbed SNCA hemostasis, MALAT1 dysregulation is associated with SNCA aggregation and Lewy bodies’ formations. Resveratrol and beta-asarone are two candidates that inhibit SNCA aggregation in a MALAT1/miR-129/SNCA-dependent manner. While they inhibit MALAT1 expression, dramatic improvement in the TH + neurons viability and miR-129 overexpression leads to inhibition of SNCA aggregation. In case of apoptosis and autophagy, MALAT1 overexpression inhibits the expression of different miRNAs including miR-124, miR-124-3p, miR-205-5p, and miR-135b that leads to elevation in PTEN, DAPK1, LRRK2, and GPNMP elements and reduces cell viability and increases apoptosis. In case of neuro-inflammation, a correlation between MALAT1 levels and IFN-γ, TNF-α- IL-1β, and IL-6 inflammatory cytokines was observed. Also, it was shown that MALAT1 suppressed NRF2 and leads to NLRP3 and ROS production. Abbreviations. DAPK1, death-associated protein kinase; GPNMB, glycoprotein non-metastatic melanoma protein B; IFN-γ, interferon-γ; IL-6, interleukin-6; IL-1β, interleukin-1β; LRRK2, leucine-rich repeat kinase 2; MALAT1, metastasis-associated lung adenocarcinoma transcript 1; NLRP3, NOD-like receptor (NLR) family pyrin domain containing 3; NRF2, nuclear factor erythroid 2-related factor; PD, Parkinson’s disease; PTEN, to phosphatase and tensin homolog; ROS, reactive oxygen species; SNCA, alpha-synuclein; TH + , tyrosine hydroxylase positive; TNF-α, tumor necrosis factor-α
Zhang et al. showed that MALAT1 was upregulated in the midbrain tissue of the MPTP neurotoxin-induced PD mice model and resulted in reeling gait, slow motion, and less activity as well as perturbed α-synuclein hemostasis. In this case, they investigated the neuroprotective effects of cis-2, 4, 5-trimethoxy-1-allyl phenyl (β-asarone), the main component of Acorus tatarinowii Schott; both in a murine model of PD and MPP + -exposed SH-SY5Y cells. The PD mice model was induced by intraperitoneal injection of MPP + (20 mg/kg) in a total of four doses over an 8-h period. They showed that intra-gastric administration of β-asarone (10 mg/kg) for 28 days dramatically improves cell line viability and significantly increases the number of tyrosine hydroxylase positive (TH +) neurons in animals, which results in MALAT1 inhibition followed by α-synuclein regulation [67].
In another study, Xia et al. reported that MALAT1 binds to miR-129 (a negative regulator of SNCA) and inhibits its expression. In this case, MALAT1 induces SNCA overexpression and α-synuclein aggregation that develops neuronal apoptosis in the murine model of PD. The PD mice model was established using MPTP (20 mg/kg) in four doses at 8-h periods and a 3-week period observation. Also, they demonstrated that resveratrol (50 mg/kg/day, intra-gastric, for 3 weeks) increases the number of TH + cells and the expression of miR-129 due to MALAT1 inhibition. It seems that the inhibitory effect of resveratrol occurs through a blockage in the transcription of the MALAT1 promoter. Thus, modulation of MALAT1/miR-129/SNCA and inhibition of α-synuclein dysregulation could be considered the therapeutic effects of resveratrol in PD [68].
Apoptosis and Autophagy
Autophagy and apoptosis are essential contributors to developing neurodegenerative disorders, e.g., PD [75, 76]. Increasing evidence implied that autophagy plays a crucial role in disease pathogenesis; likewise, apoptosis is considered an important signal for the degradation of dopaminergic neurons [77, 78] (Fig. 1).
In PD, miR-124 regulates cell apoptosis and autophagy processes in dopamine-producing neurons. It protects them via modulation of adenosine monophosphate (AMP)-activated protein kinase (AMPK)/mechanistic target of rapamycin (mTOR) pathway [79]. MALAT1 can directly bind and inhibits miR-124 expression (51). Liu et al. reported that MALAT1 interacts with and negatively regulates miR-124 expression in a murine model of PD and SH-SY5Y cells. The murine model was induced using 30 mg/kg/day MPTP for 4 days. miR-124 downregulation leads to phosphatase and tensin homolog (PTEN)-induced kinase1 protein stability, and dopaminergic neuron apoptosis was accelerated [80]. In another study, Lu et al. reported that MALAT1/mir-124-3p/death-associated protein kinase1 (DAPK1) signaling cascade mediates cellular apoptosis and could be considered a therapeutic approach for PD. DAPK1 is a critical factor in neuronal cell dysfunction and was initially discovered in progression of interferon-γ (IFN-γ)-induced programmed cell death. They showed that DAPK1 expression is associated with MALAT1 levels and significantly upregulated in MPTP-induced mice (30 mg/kg/day for 5 consecutive days) and MPP+-induced SH-SY5Y cell models of PD, while was negatively correlated with miR-124-3p levels. In other words, miR-124-3p mimic could efficiently inhibit the expression of DAPK1 and alleviates cell apoptosis, while MALAT1 knockdown improved behavioral changes and reduced apoptosis in mice via miR-124-3p upregulation and DAPK1 downregulation. Also, it should be noted that following MPTP administration, behavioral tests were significantly impaired, with slower average speed and shorter total distance. Surprisingly, MALAT1 knockdown attenuated behavioral deficits [81].
Chen et al. introduced the MALAT1/miR-205-5p/LRRK2 axis as another pathway that MALAT1 is involved in PD pathogenesis. The PD mice model was induced by intraperitoneal administration of 20 mg/kg/dose MPTP in four doses at 2-h intervals. They showed that MALAT1 sponges miR-205-5p in PD murine models’ midbrain, leading to elevated LRRK2 levels. Also, they reported that LRRK2 overexpression reduces viability and promotes apoptosis in MPP+-induced MN9D cells (dopaminergic neuron cell line) [82].
Glycoprotein non-metastatic melanoma protein B (GPNMB) is a glycoprotein associated with tissue injury and inflammation and directly targeted by miR-135b-5p. GPNMB increased selectively in PD patients, while miR-135b has a protective effect against PD pathogenesis via inducing pyroptosis [83,84,85]. Lv et al. investigated the regulatory effects of MALAT1 on the proliferation and apoptosis in MPP+-treated SK-N-SH and SK-N-BE cells as PD in vitro model. They showed that MALAT1 targeted miR-135b-5p/GPNMB axis. As MALAT1 is increasingly expressed in the treated cells, its downregulation accelerates proliferation and inhibits cell apoptosis [86].
Neuro-inflammation
Neuroinflammation and neurodegeneration are closely associated with neurologic disorders. Inflammation is considered an essential contributor to PD pathogenesis [87,88,89]. The expression of inflammasome was demonstrated in the brain tissue of PD patients and seems to accelerate PD development [90, 91]. Besides, studies proved that various lncRNAs, i.e., LincRA-Cox-2, lncRNA THRIL, and lncRNA NEAT1, are widely involved in the differentiation of immune cells and regulation of immune responses [10, 92]. MALAT1 interacts with serum amyloid A3 (SAA3) and accelerates the secretion of inflammatory mediators, i.e., tumor necrosis factor α (TNF-α) and interleukin (IL)-6 from high glucose-exposed endothelial cells [93]. Also, it was shown that MALAT1 knockdown inhibits severe inflammation in lipopolysaccharide (LPS)-induced mice model of septic via upregulation in the expression of miR-146a and reduced NFκB (p65) phosphorylation [94] (Fig. 1).
In the case of PD, Cai et al. investigated the role of MALAT1 in neuro-inflammatory processes involved in the MPTP-induced mice model of PD and cell models, including LPS/ATP-induced neuro 2A (N2a) human cell line and BV2 murine microglial cells. The PD mice model was induced using 20 mg/kg MPTP, 3 times each day. They reported that MALAT1 is highly expressed both in vivo and in vitro models of PD. Also, several factors, i.e., nuclear factor erythroid 2-related factor 2 (NRF2), NOD-like receptor (NLR) family pyrin domain containing 3 (NLRP3), enhancer of zeste homolog 2 (EZH2), and a catalytic subunit of polycomb repressive complex 2 (PRC2), are involved in MALAT1-associated neuroinflammation. They proved that MALAT1 interacted with EZH2 in NRF2 gene promoter loci and repressed the NRF2 transcription, which results in reactive oxygen species (ROS) overexpression and subsequent activation of NLRP3 inflammasome [95].
In another study, Yang investigated the serum expression of MALAT1 and analyzed the MALAT1 single-nucleotide polymorphisms (SNPs), i.e., rs3200401, rs11227209, rs4102217, rs591291, rs619586, and rs664589, in serum of sporadic PD patients compared to healthy controls. They showed that higher serum levels of MALAT1 are significantly associated with lower mini-mental state examination (MMSE) scores and higher IFN-γ, TNF-α, IL-6, and IL-1β serum levels. Also, mutant alleles in rs3200401 (C > T) and rs4102217 (G > C) SNPs are dramatically associated with susceptibility to PD and facilitated the production of inflammatory cytokines, compared to wild-type alleles. In the other part of the study, the inflammation cell model was developed using PC12 cells treated with LPS, and the cytokine production was measured in pcDNA3.1-MALAT1/si-MALAT1-transfected PC 12 cells. In this case, they reported higher secretion of inflammatory cytokines in the pcDNA3.1-MALAT1 groups compared to the Mock group [96].
Concluding Remarks
Following an increase in the elderly population, the number of PD patients increases year by year and widely influences the quality of life, and puts a heavy burden on society. In clinical cases, the diagnosis of PD is based on tissue pathology, and existing strategies have only limited effectiveness in the early stages of the disease. Therefore, finding early diagnostic and effective treatment strategies is urgently needed.
Increasing results demonstrated that MALAT1 lncRNA is closely associated with PD pathogenesis, and while the symptoms were manifested, the expression of MALAT1 alters in PD patients. The cerebrospinal fluid (CSF) is closely associated with the leading site of PD pathology, and CSF contents could significantly reflect the molecular alterations on the brain tissue. In this case, CSF is an optimal source for diagnostic biomarkers. Also, various studies reported that the expression of lncRNAs in the leukocyte samples of PD patients is significantly associated with disease progression. Since the expression level of MALAT1 can be considered a diagnostic and prognostic biomarker.
On the other hand, therapeutically targeting MALAT1 in different ways seems to be a potential approach to regulate its expression. Multiple studies reported that MALAT1-specific antisense oligonucleotide (ASO) efficiently represses MALAT1 expression and inhibits tumor progression and metastasis [97, 98]. Likewise, application of MALAT1-inhibiting siRNA could be implicated in the case of PD to reduce neuroinflammation, apoptosis and autophagy, and α-synuclein aggregation as consecutive results of MALAT1 overexpression in PD. It is noteworthy that MALAT1 is highly expressed in almost all human tissues and is involved in crucial physiologic mechanisms, including synapse formation, skeletal myogenesis, and vascular growth. Also, the pathogenesis of PD is complicated, and further investigations are needed to clearly explain the exact cellular and molecular mechanisms that MALAT1 and other factors involved in PD. So, MALAT1 targeting in pathologic conditions is more complex than a simple silencing to be an efficient treatment procedure.
Change history
23 July 2022
A Correction to this paper has been published: https://doi.org/10.1007/s12035-022-02961-w
Abbreviations
- AD:
-
Alzheimer’s disease
- AMP:
-
Adenosine monophosphate
- ASO:
-
Specific antisense oligonucleotide
- CSF:
-
Cerebro-spinal fluid
- CNS:
-
Central nervous system
- DAPK1:
-
Death-associated protein kinase1
- EZH2:
-
Enhancer of zeste homolog 2
- GPNMB:
-
Glycoprotein non-metastatic melanoma protein B
- HD:
-
Huntington’s disease
- HTR2A:
-
5-Hydroxytryptamine receptor 2A
- IFN-γ:
-
Interferon-γ
- IL:
-
Interleukin
- lncRNA:
-
Long ncRNA
- LPS:
-
Lipopolysaccharide
- LRRK2:
-
Leucine-rich repeat kinase 2
- MALAT1:
-
Metastasis-associated lung adenocarcinoma transcript 1
- mascRNA:
-
MALAT1-associated small cytoplasmic RNA
- MPP+ :
-
Methyl-4-phenylpyridinium
- MPTP:
-
1-Methyl–4-phenyl-1, 2, 3, 6-tetrahydropyridine
- N2a:
-
Neuro 2A
- nc-RNAs:
-
Non-coding RNAs
- NEAT2:
-
Nuclear-enriched abundant transcript 2
- NRF2:
-
Nuclear factor erythroid 2-related factor 2
- NLGN1:
-
Neuroligin1
- NLR:
-
NOD-like receptor
- NLRP3:
-
NLR family pyrin domain containing 3
- NS:
-
Nervous system
- NSCLC:
-
Non-small-cell lung cancer
- PD:
-
Parkinson’s disease
- PRC2:
-
Polycomb repressive complex 2
- PTEN:
-
Phosphatase and tensin homolog
- ROS:
-
Reactive oxygen species
- SAA3:
-
Serum amyloid A3
- SN:
-
Substantia nigra
- SNCA:
-
Alpha-synuclein
- sncRNA:
-
Small ncRNA
- SNP:
-
Single nucleotide polymorphism
- SynCAM1:
-
Synaptic cell adhesion molecule1
- TH+ :
-
Tyrosine hydroxylase positive
- TNF-α:
-
Tumor necrosis factor-α
References
Xin C, Liu J (2021) Long Non-coding RNAs in Parkinson’s disease. Neurochem Res 1–12
Rasheed M, Liang J, Wang C, Deng Y, Chen Z (2021) Epigenetic regulation of neuroinflammation in Parkinson’s disease. Int J Mol Sci 22(9):4956
Taghizadeh E, Gheibihayat SM, Taheri F, Afshani SM, Farahani N, Saberi A (2021) LncRNAs as putative biomarkers and therapeutic targets for Parkinson’s disease. Neurol Sci 42(10):4007–4015
Tang Y, Meng L, Wan C-M, Liu Z-H, Liao W-H, Yan X-X et al (2017) Identifying the presence of Parkinson’s disease using low-frequency fluctuations in BOLD signals. Neurosci Lett 645:1–6
Hirsch L, Jette N, Frolkis A, Steeves T, Pringsheim T (2016) The incidence of Parkinson’s disease: a systematic review and meta-analysis. Neuroepidemiology 46(4):292–300
Rezaei O, Nateghinia S, Estiar MA, Taheri M, Ghafouri-Fard S (2021) Assessment of the role of non-coding RNAs in the pathophysiology of Parkinson’s disease. Eur J Pharmacol 173914
Recasens A, Perier C, Sue CM (2016) Role of microRNAs in the regulation of α-synuclein expression: a systematic review. Front Mol Neurosci 9:128
Beitz JM (2014) Parkinson’s disease: a review. Front Biosci (Schol Ed) 6(6):65–74
Devos D, Moreau C, Dujardin K, Cabantchik I, Defebvre L, Bordet R (2013) New pharmacological options for treating advanced Parkinson’s disease. Clin Ther 35(10):1640–1652
Lv Q, Wang Z, Zhong Z (2020) Huang W (2020) Role of long noncoding RNAs in Parkinson’s disease: putative biomarkers and therapeutic targets. Parkinson’s Disease 2020
Lim SY, Lang AE (2010) The nonmotor symptoms of Parkinson’s disease—an overview. Mov Disord 25(S1):S123–S130
Wood LD, Neumiller JJ, Setter SM, Dobbins EK (2010) Clinical review of treatment options for select nonmotor symptoms of Parkinson’s disease. Am J Geriatr Pharmacother 8(4):294–315
Bonifati V (2014) Genetics of Parkinson’s disease–state of the art, 2013. Parkinsonism Relat Disord 20:S23–S28
Renani PG, Taheri F, Rostami D, Farahani N, Abdolkarimi H, Abdollahi E et al (2019) Involvement of aberrant regulation of epigenetic mechanisms in the pathogenesis of Parkinson’s disease and epigenetic-based therapies. J Cell Physiol 234(11):19307–19319
Koros C, Simitsi A, Stefanis L (2017) Genetics of Parkinson’s disease: genotype–phenotype correlations. Int Rev Neurobiol 132:197–231
Hipp MS, Kasturi P, Hartl FU (2019) The proteostasis network and its decline in ageing. Nat Rev Mol Cell Biol 20(7):421–435
Nair VD, Ge Y (2016) Alterations of miRNAs reveal a dysregulated molecular regulatory network in Parkinson’s disease striatum. Neurosci Lett 629:99–104
Lyu Y, Bai L, Qin C (2019) Long noncoding RNAs in neurodevelopment and Parkinson’s disease. Animal models and experimental medicine 2(4):239–251
Andican G, Konukoglu D, Bozluolcay M, Bayülkem K, Firtiına S, Burcak G (2012) Plasma oxidative and inflammatory markers in patients with idiopathic Parkinson’s disease. Acta Neurol Belg 112(2):155–159
Capurro A, Bodea L-G, Schaefer P, Luthi-Carter R, Perreau VM (2015) Computational deconvolution of genome wide expression data from Parkinson’s and Huntington’s disease brain tissues using population-specific expression analysis. Front Neurosci 8:441
Schapira AH (2013) Recent developments in biomarkers in Parkinson disease. Curr Opin Neurol 26(4):395
Zhang X, Hamblin MH, Yin K-J (2017) The long noncoding RNA Malat1: its physiological and pathophysiological functions. RNA Biol 14(12):1705–1714
Yin K-J, Hamblin M, Chen YE (2014) Non-coding RNAs in cerebral endothelial pathophysiology: emerging roles in stroke. Neurochem Int 77:9–16
Iyer MK, Niknafs YS, Malik R, Singhal U, Sahu A, Hosono Y et al (2015) The landscape of long noncoding RNAs in the human transcriptome. Nat Genet 47(3):199–208
Barres BA (2008) The mystery and magic of glia: a perspective on their roles in health and disease. Neuron 60(3):430–440
Mercer TR, Dinger ME, Mattick JS (2009) Long non-coding RNAs: insights into functions. Nat Rev Genet 10(3):155–159
Xie C, Yuan J, Li H, Li M, Zhao G, Bu D et al (2014) NONCODEv4: exploring the world of long non-coding RNA genes. Nucleic Acids Res 42(D1):D98–D103
Fatica A, Bozzoni I (2014) Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet 15(1):7–21
Shen J, Siegel AB, Remotti H, Wang Q, Shen Y, Santella RM (2015) Exploration of deregulated long non-coding RNAs in association with hepatocarcinogenesis and survival. Cancers 7(3):1847–1862
Cesana M, Cacchiarelli D, Legnini I, Santini T, Sthandier O, Chinappi M et al (2011) A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell 147(2):358–369
Grote P, Herrmann BG (2015) Long noncoding RNAs in organogenesis: making the difference. Trends Genet 31(6):329–335
Menon DU, Meller VH (2015) Identification of the Drosophila X chromosome: the long and short of it. RNA Biol 12(10):1088–1093
Kanduri C (2016) Long noncoding RNAs: lessons from genomic imprinting. Biochim Biophys Acta Gene Regul Mech 1859(1):102–11
Bhartiya D, Kapoor S, Jalali S, Sati S, Kaushik K, Sachidanandan C et al (2012) Conceptual approaches for lncRNA drug discovery and future strategies. Expert Opin Drug Discov 7(6):503–513
Brown JA, Kinzig CG, DeGregorio SJ, Steitz JA (2016) Methyltransferase-like protein 16 binds the 3′-terminal triple helix of MALAT1 long noncoding RNA. Proc Natl Acad Sci 113(49):14013–14018
Brown JA, Kinzig CG, DeGregorio SJ, Steitz JA (2016) Hoogsteen-position pyrimidines promote the stability and function of the MALAT1 RNA triple helix. RNA 22(5):743–749
Yang Y, Li Y, Yang H, Guo J, Li N (2021) Circulating microRNAs and long non-coding RNAs as potential diagnostic biomarkers for Parkinson’s disease. Front Mol Neurosci 14
Mercer TR, Dinger ME, Sunkin SM, Mehler MF, Mattick JS (2008) Specific expression of long noncoding RNAs in the mouse brain. Proc Natl Acad Sci 105(2):716–721
Khorkova O, Hsiao J, Wahlestedt C (2015) Basic biology and therapeutic implications of lncRNA. Adv Drug Deliv Rev 87:15–24
Rinn JL, Chang HY (2012) Genome regulation by long noncoding RNAs. Annu Rev Biochem 81:145–166
Majidinia M, Mihanfar A, Rahbarghazi R, Nourazarian A, Bagca B, Avci ÇB (2016) The roles of non-coding RNAs in Parkinson’s disease. Mol Biol Rep 43(11):1193–1204
Soreq L, Guffanti A, Salomonis N, Simchovitz A, Israel Z, Bergman H et al (2014) Long non-coding RNA and alternative splicing modulations in Parkinson’s leukocytes identified by RNA sequencing. PLoS Comput Biol 10(3):e1003517
Zhou Y, Gu C, Li J, Zhu L, Huang G, Dai J et al (2018) Aberrantly expressed long noncoding RNAs and genes in Parkinson’s disease. Neuropsychiatr Dis Treat 14:3219
Qureshi IA, Mehler MF (2012) Emerging roles of non-coding RNAs in brain evolution, development, plasticity and disease. Nat Rev Neurosci 13(8):528–541
Chakrabarti S, Mohanakumar KP (2016) Aging and neurodegeneration: a tangle of models and mechanisms. Aging Dis 7(2):111
Ghanam A, Xu Q, Ke S, Azhar M, Cheng Q, Song X (2017) Shining the light on senescence associated LncRNAs. Aging Dis 8(2):149
Ni Y, Huang H, Chen Y, Cao M, Zhou H, Zhang Y (2017) Investigation of long non-coding RNA expression profiles in the substantia nigra of Parkinson’s disease. Cell Mol Neurobiol 37(2):329–338
Soreq L, Salomonis N, Guffanti A, Bergman H, Israel Z, Soreq H (2015) Whole transcriptome RNA sequencing data from blood leukocytes derived from Parkinson’s disease patients prior to and following deep brain stimulation treatment. Genomics Data 3:57–60
Bernard D, Prasanth KV, Tripathi V, Colasse S, Nakamura T, Xuan Z et al (2010) A long nuclear-retained non-coding RNA regulates synaptogenesis by modulating gene expression. EMBO J 29(18):3082–3093
Lipovich L, Dachet F, Cai J, Bagla S, Balan K, Jia H et al (2012) Activity-dependent human brain coding/noncoding gene regulatory networks. Genetics 192(3):1133–1148
Zhang T-H, Liang L-Z, Liu X-L, Wu J-N, Su K, Chen J-Y et al (2017) Long non-coding RNA MALAT1 interacts with miR-124 and modulates tongue cancer growth by targeting JAG1 retraction in/10.3892/or.2018.6688. Oncol Rep 37(4):2087–2094
Hutchinson JN, Ensminger AW, Clemson CM, Lynch CR, Lawrence JB, Chess A (2007) A screen for nuclear transcripts identifies two linked noncoding RNAs associated with SC35 splicing domains. BMC Genomics 8(1):1–16
Tripathi V, Shen Z, Chakraborty A, Giri S, Freier SM, Wu X et al (2013) Long noncoding RNA MALAT1 controls cell cycle progression by regulating the expression of oncogenic transcription factor B-MYB. PLoS Genet 9(3):e1003368
Michalik KM, You X, Manavski Y, Doddaballapur A, Zörnig M, Braun T et al (2014) Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ Res 114(9):1389–1397
Schmidt LH, Spieker T, Koschmieder S, Humberg J, Jungen D, Bulk E et al (2011) The long noncoding MALAT-1 RNA indicates a poor prognosis in non-small cell lung cancer and induces migration and tumor growth. J Thorac Oncol 6(12):1984–1992
Goyal B, Yadav SRM, Awasthee N, Gupta S, Kunnumakkara AB, Gupta SC (2021) Diagnostic, prognostic, and therapeutic significance of long non-coding RNA MALAT1 in cancer. Biochim Biophys Acta Rev Cancer 188502
Tano K, Mizuno R, Okada T, Rakwal R, Shibato J, Masuo Y et al (2010) MALAT-1 enhances cell motility of lung adenocarcinoma cells by influencing the expression of motility-related genes. FEBS Lett 584(22):4575–4580
Wilusz JE (2016) Long noncoding RNAs: re-writing dogmas of RNA processing and stability. Biochim Biophys Acta Gene Regul Mech 1859(1):128–138
Wilusz JE, Freier SM, Spector DL (2008) 3′ end processing of a long nuclear-retained noncoding RNA yields a tRNA-like cytoplasmic RNA. Cell 135(5):919–932
Macias S, Plass M, Stajuda A, Michlewski G, Eyras E, Cáceres JF (2012) DGCR8 HITS-CLIP reveals novel functions for the microprocessor. Nat Struct Mol Biol 19(8):760–766
Yu B, Shan G (2016) Functions of long noncoding RNAs in the nucleus. Nucleus 7(2):155–166
Eißmann M, Gutschner T, Hämmerle M, Günther S, Caudron-Herger M, Groß M et al (2012) Loss of the abundant nuclear non-coding RNA MALAT1 is compatible with life and development. RNA Biol 9(8):1076–1087
Sun Q, Hao Q, Prasanth KV (2018) Nuclear long noncoding RNAs: key regulators of gene expression. Trends Genet 34(2):142–157
Yao J, Wang XQ, Li YJ, Shan K, Yang H, Wang YNZ et al (2022) Long non-coding RNA MALAT1 regulates retinal neurodegeneration through CREB signaling. EMBO Mol Med 14(3):e15623
Kryger R, Fan L, Wilce PA, Jaquet V (2012) MALAT-1, a non protein-coding RNA is upregulated in the cerebellum, hippocampus and brain stem of human alcoholics. Alcohol 46(7):629–634
Theo F, Kraus J, Haider M, Spanner J, Steinmaurer M, Dietinger V et al (2017) Altered long noncoding RNA expression precedes the course of Parkinson’s disease–a preliminary report. Mol Neurobiol 54(4):2869
Zhang Q-S, Wang Z-H, Zhang J-L, Duan Y-L, Li G-F, Zheng D-L (2016) Beta-asarone protects against MPTP-induced Parkinson’s disease via regulating long non-coding RNA MALAT1 and inhibiting α-synuclein protein expression. Biomed Pharmacother 83:153–159
Xia D, Sui R, Zhang Z (2019) Administration of resveratrol improved Parkinson’s disease-like phenotype by suppressing apoptosis of neurons via modulating the MALAT1/miR-129/SNCA signaling pathway. J Cell Biochem 120(4):4942–4951
Bennett MC (2005) The role of α-synuclein in neurodegenerative diseases. Pharmacol Ther 105(3):311–331
Dehay B, Bourdenx M, Gorry P, Przedborski S, Vila M, Hunot S et al (2015) Targeting α-synuclein for treatment of Parkinson’s disease: mechanistic and therapeutic considerations. Lancet Neurol 14(8):855–866
Robinson JL, Lee EB, Xie SX, Rennert L, Suh E, Bredenberg C et al (2018) Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain 141(7):2181–2193
Bengoa-Vergniory N, Roberts RF, Wade-Martins R, Alegre-Abarrategui J (2017) Alpha-synuclein oligomers: a new hope. Acta Neuropathol 134(6):819–838
Vekrellis K, Xilouri M, Emmanouilidou E, Rideout HJ, Stefanis L (2011) Pathological roles of α-synuclein in neurological disorders. Lancet Neurol 10(11):1015–1025
Nalls MA, Pankratz N, Lill CM, Do CB, Hernandez DG, Saad M et al (2014) Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat Genet 46(9):989–993
Ghavami S, Shojaei S, Yeganeh B, Ande SR, Jangamreddy JR, Mehrpour M et al (2014) Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog Neurobiol 112:24–49
Xiong N, Xiong J, Jia M, Liu L, Zhang X, Chen Z et al (2013) The role of autophagy in Parkinson’s disease: rotenone-based modeling. Behav Brain Funct 9(1):1–12
Lynch-Day MA, Mao K, Wang K, Zhao M, Klionsky DJ (2012) The role of autophagy in Parkinson’s disease. Cold Spring Harb Perspect Med 2(4):a009357
Zhang L, Dong Y, Xu X, Xu Z (2012) The role of autophagy in Parkinson’s disease. Neural Regen Res 7(2):141
Gong X, Wang H, Ye Y, Shu Y, Deng Y, He X et al (2016) miR-124 regulates cell apoptosis and autophagy in dopaminergic neurons and protects them by regulating AMPK/mTOR pathway in Parkinson’s disease. Am J Transl Res 8(5):2127
Liu W, Zhang Q, Zhang J, Pan W, Zhao J, Xu Y (2017) Long non-coding RNA MALAT1 contributes to cell apoptosis by sponging miR-124 in Parkinson disease. Cell Biosci 7(1):1–9
Lu Y, Gong Z, Jin X, Zhao P, Zhang Y, Wang Z (2020) LncRNA MALAT1 targeting miR-124-3p regulates DAPK1 expression contributes to cell apoptosis in Parkinson’s disease. J Cell Biochem 121(12):4838–4848
Chen Q, Huang X, Li R (2018) lncRNA MALAT1/miR-205-5p axis regulates MPP+-induced cell apoptosis in MN9D cells by directly targeting LRRK2. Am J Transl Res 10(2):563
Zeng R, Luo D-X, Li H-P, Zhang Q-S, Lei S-S, Chen J-H (2019) MicroRNA-135b alleviates MPP+-mediated Parkinson’s disease in in vitro model through suppressing FoxO1-induced NLRP3 inflammasome and pyroptosis. J Clin Neurosci 65:125–133
Moloney EB, Moskites A, Ferrari EJ, Isacson O, Hallett PJ (2018) The glycoprotein GPNMB is selectively elevated in the substantia nigra of Parkinson’s disease patients and increases after lysosomal stress. Neurobiol Dis 120:1–11
Murthy MN, Blauwendraat C, Guelfi S, Hardy J, Lewis PA, Trabzuni D (2017) Increased brain expression of GPNMB is associated with genome wide significant risk for Parkinson’s disease on chromosome 7p15. 3. Neurogenetics 18(3):121–33
Lv K, Liu Y, Zheng Y, Dai S, Yin P, Miao H (2021) Long non-coding RNA MALAT1 regulates cell proliferation and apoptosis via miR-135b-5p/GPNMB axis in Parkinson’s disease cell model. Biol Res 54
Ransohoff RM (2016) How neuroinflammation contributes to neurodegeneration. Science 353(6301):777–783
Joshi N, Singh S (2018) Updates on immunity and inflammation in Parkinson disease pathology. J Neurosci Res 96(3):379–390
Kabra A, Sharma R, Kabra R, Baghel US (2018) Emerging and alternative therapies for Parkinson disease: an updated review. Curr Pharm Des 24(22):2573–2582
He Q, Wang Q, Yuan C, Wang Y (2017) Downregulation of miR-7116-5p in microglia by MPP+ sensitizes TNF-α production to induce dopaminergic neuron damage. Glia 65(8):1251–1263
Wang S, Yuan Y-H, Chen N-H, Wang H-B (2019) The mechanisms of NLRP3 inflammasome/pyroptosis activation and their role in Parkinson’s disease. Int Immunopharmacol 67:458–464
Heward JA, Lindsay MA (2014) Long non-coding RNAs in the regulation of the immune response. Trends Immunol 35(9):408–419
Puthanveetil P, Chen S, Feng B, Gautam A, Chakrabarti S (2015) Long non-coding RNA MALAT 1 regulates hyperglycaemia induced inflammatory process in the endothelial cells. J Cell Mol Med 19(6):1418–1425
Ding Y, Guo F, Zhu T, Li J, Gu D, Jiang W et al (2018) Mechanism of long non-coding RNA MALAT1 in lipopolysaccharide-induced acute kidney injury is mediated by the miR-146a/NF-κB signaling pathway. Int J Mol Med 41(1):446–454
Cai L-J, Tu L, Huang X-M, Huang J, Qiu N, Xie G-H et al (2020) LncRNA MALAT1 facilitates inflammasome activation via epigenetic suppression of Nrf2 in Parkinson’s disease. Mol Brain 13(1):1–15
Yang H (2021) LncRNA MALAT1 potentiates inflammation disorder in Parkinson’s disease. Int J Immunogenet 48(5):419–428
Yoon J-H, Abdelmohsen K, Gorospe M (2013) Posttranscriptional gene regulation by long noncoding RNA. J Mol Biol 425(19):3723–3730
Arun G, Diermeier S, Akerman M, Chang K-C, Wilkinson JE, Hearn S et al (2016) Differentiation of mammary tumors and reduction in metastasis upon Malat1 lncRNA loss. Genes Dev 30(1):34–51
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design, drafting the article or revising it critically for important intellectual content, and approval of the final version. MA contributed in preparing table and writing the manuscript. MJ designed and contributed to the preparation of the figure and manuscript. MR revised the article.
Corresponding author
Ethics declarations
Ethics Approval
This is a review article. There is not ethical approval applicable.
Consent to Participate
Not applicated.
Consent for Publication
This is a review article. There is no consent to publish applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abrishamdar, M., Jalali, M.S. & Rashno, M. MALAT1 lncRNA and Parkinson’s Disease: The role in the Pathophysiology and Significance for Diagnostic and Therapeutic Approaches. Mol Neurobiol 59, 5253–5262 (2022). https://doi.org/10.1007/s12035-022-02899-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-02899-z