Abstract
Parkinson’s disease (PD) is a common and severe neurodegenerative disorder associated with a selective loss of dopaminergic neurons in substantia nigra pars compacta. The crucial role of oxidative stress and inflammation in PD onset and progression is evident. It has been proven that garlic extract (GE) protects the cells from oxidative stress, inflammation, mitochondrial dysfunction and apoptosis. That is, we aimed to investigate if GE reveals protective features on the preclinical model of PD. The study has been designed to evaluate both preventive (GE administered before 6-OHDA injection) and therapeutic (GE administered after 6-OHDA injection) effects of GE on the animal model. Forty male Wistar rats were divided into 4 groups including control, lesion, treatment I (received GE before 6-OHDA injection) and treatment II (received GE both before and after 6-OHDA injection). At the end of treatment, hanging, rotarod, open field and passive avoidance tests as well as immunohistochemistry were performed to evaluate the neuroprotective effects of garlic against PD. Our immunohistochemistry analysis revealed that the tyrosine hydroxylase positive cells (TH+) in GE treated groups were significantly higher (p˂0.001) than the lesion group. The motor deficiency significantly improved in hanging, rotarod, open-field and apomorphine-induced rotational tests. We observed an attenuation in memory impairment induced by PD on GE treated group. Therefore, we found that GE protects dopaminergic neurons in 6-OHDA-induced neurotoxicity and ameliorates movement disorders and behavioral deficits.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is an age-related disorder that is characterized by degeneration of dopaminergic neurons in substantia nigra pars compacta (SNpc) (Mohammadipour et al. 2020; Gonzalez-Rodriguez et al. 2020; Ebrahimi et al., 2019). Substantia nigra is located within the midbrain and contains two main sections: pars compacta and pars reticulata (Heidari et al. 2019). Over 80 % of the dopaminergic neurons are located in SNpc and these neurons are the primary source of dopamine in the brain (Heidari et al. 2019). These neurons are connected to the striatum by the medial forebrain bundle (MFB), which acts as the mediator between SNpc and striatum (Wang and Shao 2020). An intense reduction of TH+ cells in the SNpc is one of the critical indicators of PD incidence (Kumari et al. 2018). The factors associated with PD’s onset are still a matter of debate; nonetheless, aging seems to be one of the vital processes involved in it (Pang et al. 2019).
Clinical symptoms of PD can be divided into two main groups: (1) motor dysfunctions such as resting tremors of hands (Mu et al. 2017), bradykinesia (Heidari et al. 2019), muscular rigidity (Ebrahimi et al. 2020; Heidari et al. 2019) and postural instability (Palakurthi and Burugupally 2019), and (2) non-motor dysfunctions including olfactory disorders, rapid eye movement, anxiety, sleepiness, depression and cognitive deficits (Haddadi H et al. 2018). Two types of therapies have currently been considered for PD through dopaminergic and non-dopaminergic pathways (Kumari et al. 2018). To ameliorate PD through the dopaminergic pathway, Levodopa administration is the typical treatment strategy (Kumari et al. 2018). Although this approach is the most effective PD treatment, the significant long-term side effects cannot be excluded (Kumari et al. 2018). Natural products and herbal medicine have shown significant effects in alleviating neurodegenerative disorders (Haeri et al. 2019; Ghasemi et al. 2017; Nillert et al. 2017; Mathew and Biju 2008).
Garlic (Allium sativum) is a dietary plant from onion species and is commonly used worldwide as a spice or seasoning component in cooking. Studies have proven that garlic extract (GE) and garlic-derived compounds could be an effective treatment for pathophysiological conditions, including cancer (Agbana et al. 2020), cardiovascular disorders (Zhu et al. 2018), renal dysfunctions (García Trejo et al. 2017) and neurological disorders (Colín-González et al. 2011).
Interestingly, GE has anti-oxidation and anti-inflammatory effects (Mumtaz et al. 2020; Ghobadi et al. 2019; Lee et al. 2019). Oxidative stress and inflammation are two evident factors involved in PD incidence and progression (Mohammadipour et al. 2020; Heidari et al., 2019). Besides, garlic has been shown to restore disease-induced changes in concentrations of brain monoamine neurotransmitters, including dopamine (Hwang et al. 2019). A previous study reported that garlic administration in mice could increase brain dopamine levels (Hwang et al. 2019). Also, garlic ingredients have been shown to have protective effects against alpha-synuclein, which is strongly involved in PD progression (Wassef et al. 2007). In addition, GE prevents mitochondrial dysfunction in various organs (Orabi et al. 2020; Gao et al. 2019). Since mitochondrial dysfunction is a hallmark of PD and plays a crucial role in the progression of this disease (Mohammadipour et al. 2020); it is logical to think that GE should show a good protective effect against PD. Therefore, to achieve appropriate and natural treatment for PD, this study evaluated GE effects on this neurodegenerative disease at the preclinical level.
Materials and methods
This study was performed at Mashhad University of Medical Sciences, Mashhad, Iran, and the applied experimental protocols of the present study were authorized by the Institutional Animal Care and Use Committee.
Materials
The GE of this study was made in pharmacological laboratory of Mashhad University of Medical Sciences. 6-OHDA, and apomorphine (APO) were purchased from SIGMA.ALDRICH Co. (St. Louis. MO63103 USA). Primary antibody, rabbit anti-tyrosine hydroxylase (TH) (ab191486); secondary antibody, goat anti rabbit (ab97051) and goat serum (ab7481) were purchased from Abcam (Cambridge, UK). Hematoxylin and Eosin (H&E) powder were purchased from Sigma Aldrich.
Animals
Forty healthy adult male Wistar rats (250–270 g) were obtained from the animal facility of Mashhad University of Medical Sciences, Mashhad Iran. The animals were kept in standard laboratory conditions (room temperature: 23 + 2 °C; relative humidity: 60 + 5 %; illumination: 12 h light / dark cycle) and had free access to food and fresh water.
Garlic extract preparation
One kilogram of garlic was peeled and mixed with 70 % alcohol (300 mL of alcohol for every 100 grams of garlic) in a mixture. The obtained solution was then incubated at 40 °C for 72 hours. The solution was filtrated using Whatman filter paper and dried using speedVac (Thermofisher) (Farshbaf-Khalili et al. 2016). Finally, garlic extract was kept in + 4 °C and administrated to animals by gavage.
Study design
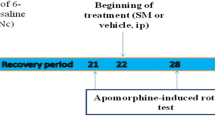
One of the selective neurotoxins commonly used to create the preclinical model is 6-hydroxy dopamine (6-OHDA), which drastically reduces the number of dopaminergic neurons by increasing neuro-inflammation and oxidative stress (Haddadi et al. 2020). In the present study, this neurotoxin was injected into the left MFB in order to create PD.
The animals were divided into four groups (n = 10 for each group):
-
1)
Control: received normal saline by gavage for one week.
-
2)
Lesion: Gavage administration of normal saline for one week + 6-OHDA injection (16 µg/4 µl 0.2 % ascorbate-saline) into the left MFB.
-
3)
Treatment I (preventive): One-week gavage administration of GE (500 mg/kg) + 6-OHDA injection (16 µg/4 µl 0.2 % ascorbate-saline) into the left MFB.
-
4)
Treatment II (therapeutic): One week of GE administration by gavage (500 mg/kg) + 6-OHDA injection (16 µg/4 µl 0.2 % ascorbate-saline) into the left MFB + GE administration for one more week after the injection.
The dose of GE was selected based on previous studies (Hwang et al. 2019; Nillert et al. 2017).
To induce PD in the MFB, animals were anesthetized by Ketamine (75 mg/kg, i.p.) and Xylazine (10 mg/kg, i.p) (Yang et al. 2020) and prepared for the injection of 6-OHDA. Then, they were placed in a stereotaxic device. 6-OHDA was injected into the left MFB [antroposterior: −3.6 mm; mediolateral: +1.8 mm; dorsoventral: −8.2mm] by a Hamilton syringe at a rate of 2 µl/min (Haddadi H et al. 2018). After the procedure, the incision was closed entirely and disinfected. Then, the animals were recovered and allowed to use water and food. All the surgical activities have been done according to aseptic regulation. At the end of the experiment, animals were anesthetized by Ketamine (75 mg/kg, i.p.) and Xylazine (10 mg/kg, i.p) and perfused with 4 % paraformaldehyde solution. The brains were extracted and used for histological studies.
Apomorphine‐induced rotational test
In rat models of PD, apomorphine induced-rotational test (APO) is widely performed to confirm the effects of selective neurotoxin (6-OHDA). The number of rotations caused by apomorphine injection is directly related to the nigrostriatal pathway damage. In this study, APO test was performed at the end of the 2nd and the 6th weeks after the injection. The animals were monitored for their contralateral rotations after inducing APO (2 mg/kg, i.p.). The test time for each rat was 30 min. The animals with 7or ≥ 7 rotations/min were considered the parkinsonian model (Haddadi H et al. 2018; Kumari et al. 2018).
Hanging test
Each rat was put on a wire (with a length of 100 cm and 1 cm in diameter) and supported to hold the wire. Then the wire was inverted to animal hung from it. Hanging time for each rat was recorded and eventually, the data were compared between groups (Haeri et al. 2019; Heidari et al. 2019).
Rotarod test
All the animals were placed on the rotarod (at a speed of 10 rpm) for three days to learn how to walk on it with a kept balance. On the day of the experiment, the animals were placed on the rotary and the time spent on the rotary was recorded for each animal. The maximum time for each rat was considered 600 seconds (Manchanda et al. 2018).
Open field test
This test was performed in order to assess locomotor activity and anxiety-associated behaviors. The open field aperture consists of 16 series of blocks. The outer zone (12 blocks) was considered periphery and the inner zone (4 blocks) was considered centrally. Each animal was put individually in the box’s central area in the 4th week after the injection. Movements of each rat were recorded using a digital camera for 10 min and the following parameters were assessed: (1) the number of crossing in the central zone, (2) the number of crossing in the peripheral zone, (3) the traveled distance in the central zone, (4) the traveled distance in the peripheral zone, (5) the time spent in the central zone, (6) the time spent in the peripheral zone, (7) the total crossing number and (8) the total traveled distance. All the parameters were analyzed using SMART Video Tracking software (Arab et al. 2020).
Passive avoidance test
Six weeks after 6-OHDA injection, the passive avoidance test (PA) was performed to assess the animals’ memory performance. The PA apparatus (is also known as Shuttle box) contains two bright and dark chambers with a grid surface in the floor. A small floating door disconnects the chambers.
On the first day of the experiment, each rat was put in the bright chamber. As soon as the animal entered the dark chamber, the door was closed and the animal received an electric shock (2 mA, 2 seconds). 3, 24 and 48 and 72 hours after the shock, each rat was put into the bright chamber and the time latency for entering the dark chamber was recorded (Rastegar-Moghaddam et al. 2019; Baghishani et al. 2018).
Histology study
Each brain was removed and fixed in normaline solution (10 %) for seven days. The fixed tissues were dehydrated, cleared with the ethanol and xylene passages, and embedded in paraffin. Then, the brains were sectioned in to 5 µm thickness (coronal sections) with 100 µm intervals. The tissue sections were used for Immunohistochemistry (IHC) staining in order to identify Tyrosine hydroxylase positive (TH+) cells (Heidari et al. 2019; Haeri et al. 2019).
For IHC staining, tissue samples were deparaffinized in xylene, rehydrated through descending ethanol concentrations and rinsed in phosphate-buffered saline (PBS). Heat-induced antigen retrieval was done and then, specimens were incubated with bovine serum albumin (BSA) (0.01 gr BSA + 1000 µl PBS + 10 µl Triton X) for 30 min. Next, each tissue section was placed into a hydrogen peroxide solution (H2O2, 0.03 % in PBS) for 15 min in a dark chamber to block endogenous peroxidase. After a wash with PBS, they were incubated with goat serum for 20 min. Finally, the sections were incubated with primary antibody (anti-TH, 1:100, ab191486 Abcam Company) overnight at 4°C. On the next day, the sections were washed three times with PBS for 15 min and were exposed to goat anti-rabbit IgG secondary antibody (ab97051, Abcam Company) for 90 min at room temperature. After three washes with PBS (5 min each), all sections were treated with 3,3′-Diaminobenzidine (DAB) and H2O2 at room temperature for 15 min. The sections washed with tap water and were counterstained with Hematoxylin, dehydrated in increasing graded alcohols, cleared in xylene and mounted with cover slide (Haeri et al. 2019; Heidari et al. 2019; Jalayeri-Darbandi et al. 2018).
Stereology method
First, the sections were photographed using a light microscope attached to the camera (Olympus BX51, Japan). Then, images were transferred to the computer monitor and TH+ cells were counted using a stereological grid. Finally, the mean number of TH+ cells was calculated using the following formula:
In this formula: NA (the mean number of TH+ in the area), “ΣǬ” (the total number of counted cells in each section), “a/f” (frame area), and “ΣP” (the total number of frames on the SNpc) (Heidari et al. 2019; Rastegar-Moghaddam et al. 2019; Haeri et al. 2019).
Statistical analysis
Data from all tests were analyzed using SPSS software (version 20). One-way ANOVA with post hoc Tukey test was used to determine significant differences among groups. p < 0.05 was considered statistically significant otherwise disclosed for the case in the results.
Results
Apomorphine‐induced rotational test
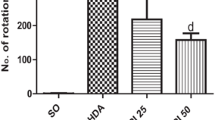
At the end of the second week, the number of rotations in the lesion, treatment I and treatment II groups were significantly higher compared to the control group (p < 0.001). However, the number of rotations, in the treatment I (p < 0.001) and treatment II (p < 0.001) groups were significantly lower than the lesion group. Meanwhile, the number of rotations in the treatment II group was significantly lower than the treatment I group (p < 0.001) (Fig. 1a).
At the end of the 6th week, the number of rotations was significantly increased in the lesion (p < 0.001), treatment I (p < 0.001) and treatment II (p < 0.001) groups compared to the control group. The number of rotations was significantly lower in the treatment I (p < 0.001) and treatment II (p < 0.001) groups than the lesion group. Furthermore, the number of rotations in the treatment II group was significantly lower than the treatment I group (p < 0.01) (Fig. 1b).
Evaluation of hanging test
The hanging test was performed four weeks after the 6-OHDA injection. Our results showed a significant reduction in the time of hanging in the lesion (p < 0.001), the treatment I (p < 0.001) and treatment II (p < 0.001) groups in comparison with the control group. The time of hanging was significantly more in the treatment I (p < 0.001) and treatment II (p < 0.001) groups than the lesion group. This criterion also was significantly more in the treatment II group than the treatment I group (p < 0.01) (Fig. 1c).
Evaluation of rotarod test
The rotarod test was performed four weeks after the injection of 6-OHDA. The results showed a significant flawed performance in the rotarod of the lesion (p < 0.001), the treatment I (p < 0.001) and treatment II (p < 0.001) groups compared to the control group. However, the rotarod duration time in the treatment I and treatment II groups were significantly more than the lesion group (p < 0.001). Also, this time in the treatment II group was significantly more than the treatment I group (p < 0.001) (Fig. 1d).
The comparison of the contralateral rotations at the end of the 2nd (a) and the 6th (b) weeks, the average time of wire hanging (c) and the rotarod performance (d) in all studied groups. ***p < 0.001 refers to the difference between the lesion, treatment I and treatment II groups compared to the control group, +++p < 0.001 shows the differences between treatment groups and the lesion group. $$p < 0.01 and $$$p < 0.001 shows the difference between treatment II and treatment I groups (Mean ± SEM, n = 10)
Evaluation of open‐field test
The results showed that the traveled distance in the lesion (p < 0.001), treatment I (p < 0.001) and treatment II (p < 0.05) groups was significantly lower compared to the control group. The traveled distance was significantly more in the treatment I (p < 0.001) and treatment II (p < 0.001) groups than the lesion group. There were no significant differences in the treatment I and treatment II groups regarding this behavioral test (Fig. 2a).
A significant decrease was observed in the number of central crossing in the lesion group than in the control group (p < 0.001). There was no significant difference between treatment I and treatment II groups in the number of central crossing (Fig. 2b).
Also, the duration spent (time) was significantly lower in the central area for the lesion (P < 0.001), the treatment I (P < 0.001), and treatment II (P < 0.001) groups compared with in the control group. Meanwhile, the animals of the treatment I group spent more time in the central area than the animals of the lesion group (p < 0.01). There was no significant difference between treatment I and treatment II groups (Fig. 2c).
The results also showed that there was a significant decrease in the number of peripheral crossing in the lesion (p < 0.001), treatment I (p < 0.001) and treatment II (p < 0.001) groups compared to the control group. The number of crossing in the peripheral area in the treatment I group was significantly more than the lesion group (p < 0.05). There was no significant difference in the number of peripheral crossing between treatment II and lesion groups. There was also no significant difference in the number of peripheral crossing between treatment I and treatment II groups (Fig. 3b)
Besides, the time spent in the peripheral area was significantly higher in the lesion (p < 0.001), the treatment I (p < 0.001) and treatment II (p < 0.001) groups compared with the control group. The animals of the treatment I group significantly spent less time in the peripheral area in comparison with the lesion group (p < 0.05). There was no significant difference in time spent in the peripheral zone between treatment II and lesion groups. There was also no significant difference in time spent in the peripheral zone in the treatment II group compared with the treatment I group (Fig. 3c).
Our results showed that the total traveled distance was significantly lower in the lesion (p < 0.001), the treatment I (p < 0.001) and treatment II (p < 0.001) groups compared to the control group. There were no significant differences between the treatment groups and the lesion group. We also did not observe significant differences in total traveled distance between treatment I and treatment II groups (Fig. 4a).
The acquired data illustrated that the number of total crossing was significantly lower in the lesion (p < 0.001), the treatment I (p < 0.001) and treatment II (p < 0.001) groups compared to the control group. There was a significant increase in the number of total crossing between treatment I and lesion groups (p < 0.01); but we did not see any significant difference between the treatment II and lesion groups. There was no significant difference between treatment I and treatment II groups in the number of total crossing (Fig. 4b).
Evaluation of passive avoidance memory
Results of this test showed that, three (p < 0.001), twenty-four (p < 0.001), forty-eight (p < 0.01) and seventy-two (p < 0.001) hours post-shock, the time latency for entering the dark chamber in the lesion group was significantly lower in comparison with the control group.
Twenty-four hours after the shock, the treatment I group’s animals had significantly lower time latency to enter the dark chamber than the control group (p < 0.01). The results also showed that, seventy-two hours after the shock, animals of the treatment I (p < 0.001) and treatment II (p < 0.01) groups had significantly lower time latency to enter the dark chamber compared to the control group. Three hours after the shock, animals of the treatment I (p < 0.01) and treatment II (p < 0.01) groups had higher time latency to enter the dark chamber in comparison with the lesion group. Twenty-two hours after receiving the shock, animals of the treatment II group spent more time to enter the dark chamber in comparison with the lesion group (p < 0.001) (Fig. 5).
Histological study
The results of the histological evaluations showed a significant decrease in the mean number of TH+ in the lesion (p < 0.001), the treatment I (p < 0.001) and treatment II (p < 0.05) groups compared to the control group. GE administration significantly increased the mean number of TH+ neurons in the treatment I (p < 0.001) and treatment II (p < 0.001) groups in comparison with the lesion group. We also found that the mean number of TH+ in the treatment II group was significantly more than the treatment I group (p < 0.05) (Fig. 6).
The comparison of the mean number of the TH+ cells in all studied groups (a). The mean number of TH+ cells in the lesion (+++p < 0.001), the treatment I (p < 0.001) and treatment II (+ p < 0.05) groups was lower compared to the control group. The mean number of TH+ increased in treatment I (***p < 0.001) and treatment II (***p < 0.001) groups in comparison with the lesion group. $p < 0.05 shows the difference between treatment I and treatment II groups. (Mean ± SEM, n = 10). The comparison of the mean number of TH+ cells in all studied groups (b). Dopaminergic neurons of the SNpc in the immunohistochemistry staining: a; Control, b; Lesion, c; Treatment I, d; Treatment II. Dopaminergic neurons (TH+) in the control group are more visible than lesion, treatment I and treatment II groups. Arrows show the TH+ cells. Scale bar = 200 µm
Discussion
In this research, the primary purpose was to investigate the positive effects of GE on the depletion of the dopaminergic neurons in SNpc and the motor and non-motor outcomes in the rat model of PD. Among several methods, 6-OHDA injection is frequently applied to induce an animal model of PD in rodents (Fine et al. 2020; Marin et al. 2020; Zhang et al. 2020). 6-OHDA, an optional neurotoxin, reduces dopaminergic neurons and disrupts the nigrostriatal pathway (Barata-Antunes et al. 2020; Sarukhani et al. 2018; Kumari et al. 2018). The present research illustrated that a decrease follows the injection of 6-OHDA into the left MFB in the number of TH+ cells, which confirms the neurotoxic effects of 6-OHDA.
We observed that the lesion group rats had a weak performance in hanging wire and rotarod tests, which is in line with the previous studies that have reported movement disorders following the injection of 6-OHDA (Kumari et al. 2018). We found that the effects of the 6-OHDA injection were not limited to motor disorders and had adverse behavioral effects. Reducing the delay time of entering the dark chamber in the passive avoidance test indicates decreased memory and learning in the lesion group. Also, the animals of the lesion group had a lower crossing number, traveling distance and time duration spent in the central area, which indicates that an anxiety-like behavior occurs in PD. These findings are in line with the previously reported results and various behavioral deficits, including anxiety, depression and apathy, have been observed in PD patients (Wen et al. 2016).
It has been confirmed that oxidative stress and inflammation have a prominent role in PD (Mohammadipour et al. 2020; Heidari et al., 2019; Cenini et al. 2019). On the other hand, GE has been well documented that has a high potential in inhibition of oxidative stress and neuroinflammation (Farzanegi et al. 2020; Ghasemi et al. 2017; Kong et al. 2017). As Farooqui and Farooqui (2018) reported recently, due to various active compounds, garlic exerts antioxidant and anti-inflammatory effects and can be considered a therapeutic agent for neurodegenerative diseases (Farooqui and Farooqui 2018). Therefore, in the present study, we investigated the neuroprotective effects of this extract.
The outcomes obtained from the present research, for the first time, illustrated that GE administration attenuates the depletion of TH+ neurons in the rat midbrain. We also found that the time of administration of the GE, affects its effectiveness on the PD. Even though administration of GE was able to ameliorate the PD symptoms in both treatments I and II groups, but results revealed that the treatment method in the treatment II group (one week before + one week after OHDA-6 injection) was more effective than in the treatment I group (only before OHDA-6). The results of the different tests of this study confirmed that. For example, the apomorphine-induced rotational test showed a decrease in the number of rotations for the animals in the treatment II group compared to the treatment I group.
Thus, according to the present study results and given that the time of onset of the PD is unknown, we suggest that it should be used continuously to get maximum therapeutic effects of the GE.
Our data also revealed that both hanging time in the hanging test and duration time on the rotarod in the treatment groups animals were significantly higher than the lesion group, which proves the beneficial effects of the GE on the motor functions in rat model of PD.
A part of this difference in the motor ability between the lesion and treatment groups could be due to the GE protection of dopaminergic neurons. Dopaminergic neurons are highly vulnerable to oxidative stress and inflammation (Mohammadipour et al. 2020; Heidari et al. 2019; Haeri et al. 2019). On the other hand, GE has very high anti-oxidative and anti-inflammatory effects (Sayed et al. 2019; Song et al. 2019; Javad et al. 2012). There are some organic ingredients in garlic, such as S-allyl-L-cysteine (SAC) or L-deox-yallin and allicin that are the organosulfur (Valentino et al. 2020; Baluchnejadmojarad et al. 2017). Both ingredients can reduce oxidative stress and inflammation biomarkers (Basu et al. 2019; Khajevand-Khazaei et al. 2019). Besides, allicin has been reported that alleviate mitochondrial dysfunction caused by various toxins (Orabi et al. 2020; Gao et al. 2019). Mitochondrial dysfunction is well documented that plays a crucial role in PD’s onset and progression (Mohammadipour et al., 2020). Mitochondrial dysfunction results in decreased ATP production and increased cytochrome c releasing that eventually induces caspase activation and leads to cell death (Mohammadipour et al. 2020; Chen et al. 2019). Since allicin can inhibit ROS production, decrease cytochrome c releasing and increase ATP production (Dai et al. 2020; Liu et al. 2015), it seems to have a high potential to protect dopaminergic cells in neurodegenerative diseases.
Of note, as the previous studies showed, dopaminergic neurons are damaged by alpha-synuclein aggregation in PD and this protein plays a critical role in the progression of PD (Mohammadipour et al. 2020; Guo et al. 2019). Liu et al. (2017) and Guo et al. (2019) reported that alpha-synuclein aggregation could induce inflammation and apoptosis in dopaminergic neurons leading to motor disorders (Guo et al. 2019; Liu et al. 2017). On the other hand, Wassef et al. (2007) found that S-methyl-L-cysteine (found in garlic and cabbage) administration could prevent alpha-synuclein-induced abnormalities (Wassef et al. 2007). Our study shows that GE restores dopaminergic neurons’ loss and improves motor functions, which may be achieved by preventing alpha-synuclein pathogenesis.
Moreover, GE has been reported to increase neurotrophic factors (Jung et al. 2016). The neurotrophic factors have been shown to affect dopamine synthesis in SNpc and striatum (Renko et al. 2018). Renko et al. (2018) showed that striatal injection of neurotrophic factors increases dopamine release in the rat striatum (Renko et al. 2018). Since dopamine depletion plays a critical role in PD-induced motor and behavioral disorders, this critical neurotransmitter elevation can alleviate PD symptoms. Thus, it seems that the improvement of motor and non-motor disabilities observed in the present study would be associated with neurotrophic factors.
Conclusions
In conclusion, the present study demonstrates that GE can protect the dopaminergic neurons in PD. Additionally, our results show that GE could improve PD-induced motor dysfunctions and alleviate non-motor deficits, including memory impairment and anxiety in PD’s preclinical model.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Agbana YL, Ni Y, Zhou M, Zhang Q, Kassegne K, Damintoti Karou S, Kuang Y, Zhu Y (2020) Garlic-derived bioactive compound S-allylcysteine inhibits cancer progression through diverse molecular mechanisms. Nutr Res 73:1–14. https://doi.org/10.1016/j.nutres.2019.11.002
Arab Z, Hosseini M, Mashayekhi F, Anaeigoudari A (2020) Zataria multiflora extract reverses lipopolysaccharide-induced anxiety and depression behaviors in rats. Avicenna J Phytomed 10(1):78–88
Baghishani F, Mohammadipour A, Hosseinzadeh H, Hosseini M, Ebrahimzadeh-Bideskan A (2018) The effects of tramadol administration on hippocampal cell apoptosis, learning and memory in adult rats and neuroprotective effects of crocin. Metab Brain Dis 33(3):907–916. https://doi.org/10.1007/s11011-018-0194-6
Baluchnejadmojarad T, Kiasalari Z, Afshin-Majd S, Ghasemi Z, Roghani M (2017) S-Allyl cysteine ameliorates cognitive deficits in streptozotocin-diabetic rats via suppression of oxidative stress, inflammation, and acetylcholinesterase. Eur J Pharmacol 794:69–76. https://doi.org/10.1016/j.ejphar.2016.11.033
Barata-Antunes S, Teixeira FG, Mendes-Pinheiro B, Domingues AV, Vilaça-Faria H, Marote A, Silva D, Sousa RA, Salgado AJ (2020) Impact of Aging on the 6-OHDA-Induced Rat Model of Parkinson’s Disease. Int J Mol Sci 21(10):3459. https://doi.org/10.3390/ijms21103459
Basu C, Chatterjee A, Bhattacharya S, Dutta N, Sur R (2019) S-allyl Cysteine Inhibits TNF‐α‐induced Inflammation in HaCaT Keratinocytes by Inhibition of NF‐ ΚB‐dependent Gene Expression via Sustained ERK Activation. Exp Dermatol 28(11):1328–1335. https://doi.org/10.1111/exd.14041
Cenini G, Lloret A, Cascella R (2019) Oxidative stress in neurodegenerative diseases: from a mitochondrial point of view. Oxidative Med Cell Longev 2019:2105607. https://doi.org/10.1155/2019/2105607
Chen C, Turnbull DM, Reeve AK (2019) Mitochondrial dysfunction in Parkinson’s disease—cause or consequence? Biology (Basel) 8(2):38. https://doi.org/10.3390/biology8020038
Colín-González AL, Ortiz-Plata A, Villeda-Hernández J, Barrera D, Molina-Jijón E, Pedraza-Chaverrí J, Maldonado PD (2011) Aged garlic extract attenuates cerebral damage and cyclooxygenase-2 induction after ischemia and reperfusion in rats. Plant Foods Hum Nutr 66(4):348–354. https://doi.org/10.1007/s11130-011-0251-3
Dai J, Chen Y, Jiang F (2020) Allicin Reduces Inflammation by regulating ROS/NLRP3 and autophagy in the context of A. Fumigatus infection in mice. Gene 762:145042. https://doi.org/10.1016/j.gene.2020.145042
Ebrahimi V, Aliaghaei A, Piryaei A, Haghir H, Abdollahifar MA, Sadeghi Y (2019) Nigral injection of 6-hydroxydopamine induces changes in spatial arrangement of striatal neuron and glial cells. Front Biosci (Schol Ed) 11:1–8
Ebrahimi V, Eskandarian Boroujeni M, Aliaghaei A, Abdollahifar MA, Piryaei A, Haghir H, Sadeghi Y (2020) Functional dopaminergic neurons derived from human chorionic mesenchymal stem cells ameliorate striatal atrophy and improve behavioral deficits in Parkinsonian rat model. Anat Rec (Hoboken) 303(8):2274–2289. https://doi.org/10.1002/ar.24301
Farooqui T, Farooqui AA (2018) Neuroprotective effects of garlic in model systems of neurodegenerative diseases. Role of the mediterranean diet in the brain and neurodegenerative diseases. Elsevier, Amsterdam, pp 253–269. https://doi.org/10.1016/B978-0-12-811959-4.00016-X
Farshbaf-Khalili A, Mohammadi-Ghalehbin B, Shahnazi M, Asghari S, Javadzadeh Y, Azghani P (2016) Iran Red Crescent Med J 18(12):e29262. https://doi.org/10.5812/ircmj.29262
Farzanegi P, Abbaszadeh H, Farokhi F, Rahmati-Ahmadabad S, Hosseini SA, Ahmad A, Mazandarani MR, Rezaei I, Shokrie M, Vizvari E, Alinejad H, Azarbayjani MA (2020) Attenuated renal and hepatic cells apoptosis following swimming exercise supplemented with garlic extract in old rats. Clin Interv Aging 15:1409–1418. https://doi.org/10.2147/CIA.S250321
Fine JM, Stroebel BM, Faltesek KA, Terai K, Haase L, Knutzen KE, Kosyakovsky J, Bowe TJ, Fuller AK, Frey WH, Hanson LR (2020) Intranasal delivery of low-dose insulin ameliorates motor dysfunction and dopaminergic cell death in a 6-OHDA rat model of Parkinson’s disease. Neurosci Lett 714:134567. https://doi.org/10.1016/j.neulet.2019.134567
Gao W, Wang W, Zhang J, Deng P, Hu J, Yang J, Deng Z (2019) allicin ameliorates obesity comorbid depressive-like behaviors: involvement of the oxidative stress, mitochondrial function, autophagy, insulin resistance and NOX/Nrf2 imbalance in mice. Metab Brain Dis 34(5):1267–1280. https://doi.org/10.1007/s11011-019-00443-y
García Trejo EMÁ, Arellano Buendía AS, Sánchez Reyes O, García Arroyo FE, Arguello García R, Loredo Mendoza ML, Tapia E, Sánchez Lozada LG, Osorio Alonso H (2017) The beneficial effects of allicin in chronic kidney disease are comparable to losartan. Int J Mol Sci 18(9):1980. https://doi.org/10.3390/ijms18091980
Ghasemi S, Hosseini M, Feizpour A, Alipour F, Sadeghi A, Vafaee F, Mohammadpour T, Soukhtanloo M, Ebrahimzadeh Bideskan A, Beheshti F (2017) Beneficial effects of garlic on learning and memory deficits and brain tissue damages induced by lead exposure during juvenile rat growth is comparable to the effect of ascorbic acid. Drug Chem Toxicol 40(2):206–214. https://doi.org/10.1080/01480545.2016.1197238
Ghobadi S, Dastan D, Soleimani M, Nili-Ahmadabadi A (2019) Hepatoprotective potential and antioxidant activity of allium tripedale in acetaminophen-induced oxidative damage. Res Pharm Sci 14(6):488–495. https://doi.org/10.4103/1735-5362.272535
Gonzalez-Rodriguez P, Zampese E, Surmeier DJ (2020) Selective neuronal vulnerability in Parkinson’s disease. Prog Brain Res 252:61–89. https://doi.org/10.1016/bs.pbr.2020.02.005
Guo Y, Wei X, Yan H, Qin Y, Yan S, Liu J, Zhao Y, Jiang F, Lou H (2019) TREM2 deficiency aggravates α-synuclein-induced neurodegeneration and neuroinflammation in Parkinson’s disease models. FASEB J 33(11):12164–12174. https://doi.org/10.1096/fj.201900992R
Haddadi H, Rajaei Z, Alaei H, Shahidani S (2018) Chronic treatment with carvacrol improves passive avoidance memory in a rat model of Parkinson’s disease. Arq Neuro-Psiquiatr 76(2):71–77. https://doi.org/10.1590/0004-282X20170193
Haddadi R, Eyvari-Brooshghalan S, Nayebi AM, Sabahi M, Ahmadi SA (2020) Neuronal degeneration and oxidative stress in the SNc of 6-OHDA intoxicated rats; improving role of silymarin long-term treatment. Naunyn Schmiedeberg's Arch Pharmacol 393(12):2427–2437. https://doi.org/10.1007/s00210-020-01954-7
Haeri P, Mohammadipour A, Heidari Z, Ebrahimzadeh-bideskan A (2019) Neuroprotective effect of crocin on substantia nigra in MPTP-induced parkinson’s disease model of mice. Anat Sci Int 94(1):119–127. https://doi.org/10.1007/s12565-018-0457-7
Heidari Z, Mohammadipour A, Haeri P, Ebrahimzadeh-bideskan A (2019) The effect of titanium dioxide nanoparticles on mice midbrain substantia nigra. Iran J Basic Med Sci 22(7):745–751. https://doi.org/10.22038/ijbms.2019.33611.8018
Hwang KA, Hwang YJ, Hwang IG, Song J, Jun Kim Y (2019) Low temperature-aged garlic extract suppresses psychological stress by modulation of stress hormones and oxidative stress response in brain. J Chin Med Assoc 82(3):191–195. https://doi.org/10.1097/JCMA.0000000000000028
Jalayeri-Darbandi Z, Rajabzadeh A, Hosseini M, Beheshti F, Ebrahimzadeh-Bideskan A (2018) The effect of methamphetamine exposure during pregnancy and lactation on hippocampal doublecortin expression, learning and memory of rat offspring. Anat Sci Int 93(3):351–363. https://doi.org/10.1007/s12565-017-0419-5
Javad H, Seyed-Mostafa HZ, Farhad O, Mehdi M, Ebrahim AO, Nader RG, Ramin GS, Behrooz H (2012) Hepatoprotective effects of hydroalcoholic extract of Allium hirtifolium (Persian shallot) in diabetic rats. J Basic Clin Physiol Pharmacol 23(2):83–87. https://doi.org/10.1515/jbcpp-2012-0017
Jung HY, Lee KY, Yoo DY, Kim JW, Yoo M, Lee S, Yoo KY, Yoon YS, Choi JH, Hwang IK (2016) Essential oils from two allium species exert effects on cell proliferation and neuroblast differentiation in the mouse dentate gyrus by modulating brain-derived neurotrophic factor and acetylcholinesterase. BMC Complement Altern Med 16(1):431. https://doi.org/10.1186/s12906-016-1384-6
Khajevand-Khazaei MR, Azimi S, Sedighnejad L, Salari S, Ghorbanpour A, Baluchnejadmojarad T, Mohseni-Moghaddam P, Khamse S, Roghani M (2019) S-allyl cysteine protects against lipopolysaccharide-induced acute kidney injury in the C57BL/6 mouse strain: involvement of oxidative stress and inflammation. Int Immunopharmacol 69:19–26. https://doi.org/10.1016/j.intimp.2019.01.026
Kong X, Gong S, Su L, Li C, Kong Y (2017) Neuroprotective effects of allicin on ischemia-reperfusion brain injury. Oncotarget 8(61):104492–104507. https://doi.org/10.18632/oncotarget.22355
Kumari N, Agrawal S, Kumari R, Sharma D, Luthra PM (2018) Neuroprotective effect of IDPU (1-(7-Imino-3-Propyl-2,3-Dihydrothiazolo [4,5-d]Pyrimidin-6(7H)-Yl)Urea) in 6-OHDA induced rodent model of Hemiparkinson’s disease. Neurosci Lett 675:74–82. https://doi.org/10.1016/j.neulet.2018.03.040
Lee HJ, Yoon DK, Lee NY, Lee CH (2019) Effect of aged and fermented garlic extracts as natural antioxidants on lipid oxidation in pork patties. Food Sci Anim Resour 39(4):610–622. https://doi.org/10.5851/kosfa.2019.e51
Liu H, Mao P, Wang J, Wang T, Xie CH (2015) Allicin protects PC12 cells against 6-OHDA induced oxidative stress and mitochondrial dysfunction via regulating mitochondrial dynamics. Cell Physiol Biochem 36(3):966–979. https://doi.org/10.1159/000430271
Liu J, Wang X, Lu Y, Duan C, Gao G, Lu L, Yang H (2017) Pink1 interacts with α-synuclein and abrogates α-synuclein-induced neurotoxicity by activating autophagy. Cell Death Dis 8(9):e3056. https://doi.org/10.1038/cddis.2017.427
Manchanda S, Singh H, Kaur T, Kaur G (2018) Low-grade neuroinflammation due to chronic sleep deprivation results in anxiety and learning and memory impairments. Mol Cell Biochem 449(1–2):63–72. https://doi.org/10.1007/s11010-018-3343-7
Marin C, Bonastre M, Fuentes M, Mullol J (2020) Globus pallidus, but not entopeduncular nucleus, 6-OHDA-induced lesion attenuates L-Dopa-induced dyskinesia in the rat model of Parkinson’s disease. Pharmacol Biochem Behav 197:173013. https://doi.org/10.1016/j.pbb.2020.173013
Mathew B, Biju R (2008) Neuroprotective effects of garlic a review. Libyan J Med 3(1):23–33. https://doi.org/10.3402/ljm.v3i1.4747
Mohammadipour A, Haghir H, Ebrahimzadeh-Bideskan A (2020) A link between nanoparticles and Parkinson’s disease. Which nanoparticles are most harmful? Rev Environ Health. 2020 Jul 20. /j/reveh.ahead-of-print/reveh-2020-0043/reveh-2020-0043.xml, https://doi.org/10.1515/reveh-2020-0043
Mu J, Chaudhuri KR, Bielza C, de Pedro-Cuesta J, Larrañaga P, Martinez-Martin P (2017) Parkinson’s disease subtypes identified from cluster analysis of motor and non-motor symptoms. Front Aging Neurosci 9(SEP):301. https://doi.org/10.3389/fnagi.2017.00301
Mumtaz S, Ali S, Khan R, Shakir HA, Tahir HM, Mumtaz S, Andleeb S (2020) Therapeutic role of garlic and vitamins C and E against toxicity induced by lead on various organs. Environ Sci Pollut Res 27(9):8953–8964. https://doi.org/10.1007/s11356-020-07654-2
Nillert N, Pannangrong W, Welbat JU, Chaijaroonkhanarak W, Sripanidkulchai K, Sripanidkulchai B (2017) Neuroprotective effects of aged garlic extract on cognitive dysfunction and neuroinflammation induced by β-amyloid in rats. Nutrients 9(1):24. https://doi.org/10.3390/nu9010024
Orabi SH, Abd Eldaium D, Hassan A, Sabagh HSE, Abd Eldaim MA (2020) Allicin modulates diclofenac sodium induced hepatonephro toxicity in rats via reducing oxidative stress and caspase 3 protein expression. Environ Toxicol Pharmacol 74:103306. https://doi.org/10.1016/j.etap.2019.103306
Palakurthi B, Burugupally SP (2019) Postural instability in Parkinson’s disease: a review. Brain Sciences 9(9):239. https://doi.org/10.3390/brainsci9090239
Pang SY, Ho PW, Liu HF, Leung CT, Li L, Chang EES, Ramsden DB, Ho SL (2019) The interplay of aging, genetics and environmental factors in the pathogenesis of Parkinson’s disease. Transl Neurodegener 8:23. https://doi.org/10.1186/s40035-019-0165-9
Rastegar-Moghaddam SH, Mohammadipour A, Hosseini M, Bargi R, Ebrahimzadeh-Bideskan A (2019) Maternal exposure to atrazine induces the hippocampal cell apoptosis in mice offspring and impairs their learning and spatial memory. Toxin Rev 38(4):298–306. https://doi.org/10.1080/15569543.2018.1466804
Renko JM, Bäck S, Voutilainen MH, Piepponen TP, Reenilä I, Saarma M, Tuominen RK (2018) Mesencephalic astrocyte-derived neurotrophic factor (MANF) elevates stimulus-evoked release of dopamine in freely-moving rats. Mol Neurobiol 55(8):6755–6768. https://doi.org/10.1007/s12035-018-0872-8
Sarukhani M, Haghdoost-Yazdi H, Sarbazi Golezari A, Babayan-Tazehkand A, Dargahi T, Rastgoo N (2018) Evaluation of the antiparkinsonism and neuroprotective effects of hydrogen sulfide in acute 6-hydroxydopamine-induced animal model of Parkinson’s disease: behavioral, histological and biochemical studies. Neurol Res 40(7):523–531. https://doi.org/10.1080/01616412.2017.1390903
Sayed AA, El-Desouky MA, Ibrahim KA (2019) Garlic and allopurinol attenuate hepatic apoptosis induced by fipronil in male albino rats. Regul Toxicol Pharmacol 107:104400. https://doi.org/10.1016/j.yrtph.2019.05.025
Song H, Cui J, Mossine VV, Greenlief CM, Fritsche K, Sun GY, Gu Z (2019) Bioactive components from garlic on brain resiliency against neuroinflammation and neurodegeneration (review). Exp Ther Med 19(2):1554–1559. https://doi.org/10.3892/etm.2019.8389
Valentino H, Campbell AC, Schuermann JP, Sultana N, Nam HG, LeBlanc S, Tanner JJ, Sobrado P (2020) Structure and function of a flavin-dependent S-monooxygenase from garlic (Allium sativum). J Biol Chem 295(32):11042–11055
Wang R, Shao M (2020) L-DOPA-elicited abnormal involuntary movements in the rats damaged severely in substantia nigra by 6-hydroxydopamine. Ann Palliat Med 9(3):947–956. https://doi.org/10.21037/apm.2020.03.32
Wassef R, Haenold R, Hansel A, Brot N, Heinemann SH, Hoshi T (2007) Methionine sulfoxide reductase A and a dietary supplement S-methyl-L-cysteine prevent Parkinson’s-like symptoms. J Neurosci 27(47):12808–12816. https://doi.org/10.1523/JNEUROSCI.0322-07.2007
Wen MC, Chan LL, Tan LC, Tan EK (2016) Depression, anxiety, and apathy in Parkinson’s disease: insights from neuroimaging studies. Eur J Neurol 23(6):1001–1019. https://doi.org/10.1111/ene.13002
Yang SQ, Tian Q, Li D, He SQ, Hu M, Liu SY, Zou W, Chen YJ, Zhang P, Tang XQ (2020) Leptin mediates protection of hydrogen sulfide against 6-hydroxydopamine-induced Parkinson’s disease: involving enhancement in warburg effect. Neurochem Int 135:104692. https://doi.org/10.1016/j.neuint.2020.104692
Zhang S, Tao K, Wang J, Duan Y, Wang B, Liu X (2020) Substantia nigra hyperechogenicity reflects the progression of dopaminergic neurodegeneration in 6-OHDA rat model of Parkinson’s disease. Front Cell Neurosci 14:216. https://doi.org/10.3389/fncel.2020.00216
Zhu Y, Anand R, Geng X, Ding Y (2018) A mini review: garlic extract and vascular diseases. Neurol Res 40(6):421–425. https://doi.org/10.1080/01616412.2018.1451269
Acknowledgements
The data acquired from this research were extracted from MSc student thesis (961863), which was financially supported by the Vice-Chancellor for Research, Mashhad University of Medical Sciences, Mashhad, Iran. Also, the authors would like to thank Mrs. Motejaded for her technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics statement
All procedures were managed following the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals, and approved by the Institutional Laboratory Animal Care and Use Committee of Mashhad University of Medical Sciences, Mashhad, Iran.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bigham, M., Mohammadipour, A., Hosseini, M. et al. Neuroprotective effects of garlic extract on dopaminergic neurons of substantia nigra in a rat model of Parkinson’s disease: motor and non‐motor outcomes. Metab Brain Dis 36, 927–937 (2021). https://doi.org/10.1007/s11011-021-00705-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-021-00705-8