Abstract
Ferroptosis is a recently identified, iron-regulated, non-apoptotic form of cell death. It is characterized by cellular accumulation of lipid reactive oxygen species that ultimately leads to oxidative stress and cell death. Although first identified in cancer cells, ferroptosis has been shown to have significant implications in several neurologic diseases, such as ischemic and hemorrhagic stroke, Alzheimer’s disease, and Parkinson’s disease. This review summarizes current research on ferroptosis, its underlying mechanisms, and its role in the progression of different neurologic diseases. Understanding the role of ferroptosis could provide valuable information regarding treatment and prevention of these devastating diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
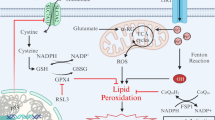
Ferroptosis, first described by Dixon et al. in 2012, is a form of cell death characterized by accumulation of intracellular iron [1]. It is defined by depletion of plasma membrane unsaturated fatty acids and accumulation of iron-induced lipid reactive oxygen species (ROS) [2]. The over-accumulation of lipid ROS leads to an oxidative stress response in cells that causes lethal damage to proteins, nucleic acids, and lipids [3] and eventually to cell death. Thus, ferroptosis requires the coincident depletion of glutathione (GSH) or inactivation of glutathione-dependent antioxidant enzyme glutathione peroxidase 4 (GPX4) and the incorporation of oxidizable polyunsaturated fatty acids into phospholipids [4].
Ferroptosis differs from apoptosis, necrosis, and autophagy morphologically, biochemically, and genetically [1]. Under electron microscopy, ferroptotic cells exhibit shrunken mitochondria, whereas mitochondria are usually swollen in other forms of cell death [1]. Initially, Dixon et al. identified a distinct set of genes that regulate the ferroptotic mechanism, including ribosomal protein L8 (RPL8), iron response element binding protein 2 (IREB2), and ATP synthase F0 complex subunit C3 (ATP5G3) [1]. Later studies showed that numerous genes/proteins participate in this unique cell death process, including cyclooxygenase-2 (PTGS2) [5], p53 [6], nuclear factor E2-related factor 2 (Nrf2) [7], PEBP1 [8], and more. In addition to those key regulators, ferroptosis can be induced by excessive glutamate, intracellular iron accumulation, or treatment with small molecules—for example, erastin, RSL3, and others listed in Table 1. The first ferroptosis-inducing compounds, erastin (which inhibits system xc−, the glutamate/cystine antiporter) and Ras selective lethal 3 (RSL3, which directly inhibits GPX4), were discovered several years before identification of the ferroptosis concept [65, 66]. Stockwell’s group was surprised to find that cells treated with those compounds were neither apoptotic nor necroptotic [1, 57, 65, 66], and that cell death could be inhibited by lipophilic antioxidants (α-tocopherol, butylated hydroxytoluene, and β-carotene), indicating that lipoxygenase activity and lipophilic ROS were involved in this cell death process [61, 67, 68].
Known inducers of ferroptosis can be divided into several categories: system xc− inhibitors (glutamate, erastin, sulfasalazine, and sorafenib), GSH depletion compounds (buthioninesulfoximine and acetaminophen), and GPX4 direct inhibitors (RSL3 and FIN56) [1, 68, 69]. Additionally, several molecules have been identified as inhibitors of ferroptosis, including ferrostatin-1 (Fer-1, which inhibits lipid ROS) [1], deferoxamine (DFO, which chelates iron) [1], zileuton (which inhibits 5-lipoxygenase) [53], and recently identified FINO2 (which oxidizes iron) [70], depending on the mechanism of ferroptosis. In this review, we focus on ferroptosis in different brain diseases and summarize the primary inducers, regulators, and inhibitors of ferroptosis-associated brain disorders, as shown in Table 1.
Ferroptosis has been identified in various cancer cells, including breast [71], lung [1], lymphoma [72], kidney [73], and brain [10]. Strikingly, the inducers of ferroptosis have been shown to target and kill cancerous cells [74]. In 2015, Jiang et al. reported that ferroptosis also contributes to embryo development and that p53 plays a vital role in ferroptosis regulation [6]. More importantly, research in organotypic hippocampal slice cultures has shown that ferroptosis also contributes to neuronal death [1]. Indeed, we and others have shown a connection between ferroptosis and neurodegeneration in experimental intracerebral hemorrhage [35, 75], Parkinson’s disease [15], and periventricular leukomalacia [18]. In this article, we will systematically review the role of ferroptosis in different brain diseases, discuss our current understanding of the underlying mechanism, and describe the possible therapeutic strategies (Figs. 1 and 2).
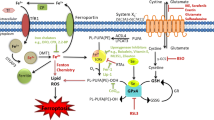
Induction and inhibition of ferroptosis. Ferroptosis is induced by lethal lipid peroxidation in the central nervous system. Dysregulation of intracellular iron metabolism and/or glutathione peroxidation pathways leads to accumulation of lipid reactive oxygen species (ROS) and eventually causes cell death. Various inducers and inhibitors are shown. Arrows indicate promotion; blunt-ended lines indicate inhibition. ATF4, activating transcription factor 4; DFO, deferoxamine; FINO2, 1, 2-dioxolane; FINs, ferroptosis-inducing agents; FtMt, mitochondrial ferritin; GPX4, glutathione peroxidase 4; GSH, glutathione; HMOX1, heme oxygenase-1; HpETE, hydroperoxyeicosatetraenoic acid; KEAP1, Kelch-like ECH-associated protein 1; LOX, lipoxygenase; PE, phosphatidylethanolamine; PEBP1, phosphatidylethanolamine-binding protein 1; PUFA, polyunsaturated fatty acid; RSL3, Ras-selective lethal 3; TF, transferrin; TFR, transferrin receptor; VDAC2/3, voltage-dependent anion channel 2/3; WA, withaferin A
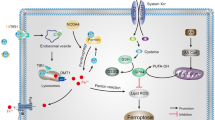
The role of ferroptosis in diverse brain diseases. Various inducers and inhibitors in different brain diseases are shown. AD, Alzheimer’s disease; CoQ10, coenzyme Q10; DFO, deferoxamine; DPI, diphenylene iodonium; Fer-1, ferrostatin-1; Flt3, FMS-like tyrosine kinase-3; PD, Parkinson’s disease; PI3Kα, phosphatidylinositol 3-kinase α; PVL, periventricular leukomalacia; RSL3, Ras-selective lethal 3; WA, withaferin A
Ferroptosis in Stroke
Stroke ranks number 5 among all causes of death, behind diseases of the heart, cancer, chronic lower respiratory disease, and unintentional injuries/accidents [76]. Each year, approximately 795,000 people experience a new or recurrent stroke, 87% of which are ischemic strokes [76]. An ischemic stroke occurs when the blood supply to certain parts of the brain is restricted secondary to occlusion of the internal carotid, middle cerebral, or vertebral/basilar arteries [77]. The resulting depletion of oxygen and nutrients may cause cells to activate the ischemic cascade, which results in oxidative stress, mitochondrial impairment, and, ultimately, cell death [78]. Before ferroptosis was identified, it was already known that iron accumulation exaggerates neuronal damage during reperfusion both clinically and in animal models of ischemic stroke [79,80,81,82,83,84,85]. It had been shown that iron chelation reduces reperfusion damage in animals after an ischemic event [86,87,88,89]. In 2013, Speer et al. hypothesized that ferroptosis might contribute to neuronal death induced by cerebral ischemia and that hypoxia-inducible factor (HIF) prolyl hydroxylases might serve as a target for the beneficial effects of metal chelators [90]. They further postulated that the beneficial effects of iron chelators in preventing ferroptosis were due to inhibition of 2-oxoglutarate, oxygen-dependent dioxygenases, and the HIF prolyl hydroxylases, but not to direct inhibition of Fenton chemistry or ROS formation [90]. Then, in 2017, a study showed that ferroptosis inhibition protected mice against ischemia-reperfusion injury in a middle cerebral artery occlusion (MCAO) model, indicating that ferroptosis contributes to neuronal death after ischemic stroke [34]. Interestingly, the authors found that tau knockout mice were protected from ferroptotic cell death after ischemia-reperfusion injury and introduced the tau–iron interaction as a pleiotropic modulator of ferroptosis and ischemic stroke outcome [34].
Intracerebral hemorrhage (ICH) accounts for 10–30% of all stroke cases and is associated with higher rates of mortality and morbidity than is ischemic stroke [76, 91]. Until recently, apoptosis, necrosis, and autophagy were thought to be the only contributors to neuronal death after ICH [92,93,94,95,96,97]. However, solid data have shown the presence of neuronal ferroptosis after ICH in vitro and in vivo [35, 50, 98]. In 2014, we found that (−)-epicatechin, a brain-permeable flavanol, reduced early brain injury after ICH, in part by decreasing brain iron deposition and ferroptosis-related gene expression [75]. In 2017, we found that Fer-1 prevented hemoglobin-induced neuronal death and reduced GPX4 activity deficiency in brain slice cultures, and it rescued ferroptotic neurons and reduced cyclooxygenase-2 (Cox-2) expression in collagenase- and blood injection-induced ICH mouse models [35]. In addition, using transmission electron microscopy, we showed that ferroptosis co-exists with necrosis and autophagy in vivo and that using a combination of inhibitors to target these different forms of cell death rescued neurons from hemoglobin-induced toxicity better than any inhibitor alone [35, 98]. At the same time, Zille et al. found that a number of ferroptosis inhibitors, including Fer-1, DFO, N-acetylcysteine (which inhibits ROS and reactive lipid species), and Trolox (a vitamin E analog that targets reactive lipid species), were able to rescue mouse primary cortical neurons from hemin- and hemoglobin-induced death in vitro [50]. Additionally, they found that elevated phospho-ERK1/2 levels were associated with enhanced neuronal ferroptosis and that U0126, an MEK inhibitor, inhibited this cell death mechanism [50]. Notably, in erastin- or amino acid starvation-induced ferroptosis in cancer cells, the more selective and potent MEK inhibitor PD0325901 failed to block cell death [99]. The authors claimed that U0126 had off-target effects and that the MEK-ERK1/2 signaling pathway was not involved in the ferroptotic mechanism [99]. Interestingly, Zille et al. found that necroptosis inhibitor necrostatin-1 also reduced hemin-induced cell death and that the treated cells exhibited a necrotic phenotype with loss of plasma membrane integrity and disintegration of organelles in vitro, indicating that ferroptosis may be an early stage of necrosis [50]. Recently, Zhang et al. showed that GPX4 expression level was dramatically reduced during the acute phase of ICH and that increasing GPX4 level was able to rescue neurons from secondary ferroptotic death and improve ICH outcomes in rats [100].
Ferroptosis in Parkinson’s Disease
Parkinson’s disease (PD) is typified by death of neurons in the substantia nigra pars compacta (SNpc), which regulates motor function. PD causes rigidity, tremor, and other motor symptoms [101, 102]. Apoptosis is known to be a major contributor to cell death during the progression of the disease [103]. Evidence also has shown that iron and dopamine levels in the SNpc are elevated in patients with PD [104,105,106]. Notably, GSH depletion, lipid peroxidation, and elevated ROS levels, which are commonly observed in patients with PD, are also features of ferroptosis [107,108,109]. Consistent with these findings, Ayton et al. reported that mice with a genetic deletion for ceruloplasmin (an iron-export ferroxidase) developed parkinsonism that was rescued by iron chelation [110]. Additionally, iron chelators have been shown to improve motor symptoms in animal models [106, 110,111,112] and in a clinical trial [46].
Recently, ferroptosis has also been linked to PD [15]. Researchers found that ferroptosis is a key cell death pathway for dopaminergic neurons and that Fer-1 administration reduces neuronal death in vitro (SH-SY5Y cell line and differentiated Lund human mesencephalic [LUHMES] cells), ex vivo (organotypic slice cultures), and in vivo (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine [MPTP] mouse model) [10, 15]. Importantly, Do Van et al. claimed that ferroptosis induced by erastin in LUHMES cells was initiated by activation of MEK in a RAS-independent manner [15]. This mechanism differed from findings in other cell lines, in which the calcium chelator BAPTA and PKC inhibitors (the bisindolylmaleimide analog Bis-III and siRNA) were very effective at counteracting erastin-induced cell death [15, 113]. The authors explained that the distinctive mechanisms were due to the unique metabolic feature of dopaminergic neurons [15]. A year later, Gouel et al. found that human platelet lysates protected the LUHMES cell line from erastin-induced ferroptosis [114]. However, they found that AKT, but not MEK or RAS, participated in erastin-induced cell death when they used U0126 and manumycin A to inhibit MEK and RAS, respectively [114]. These controversial results [15, 114] in erastin-treated LUHMES cells may stem from the off-target effect of MEK inhibitor U0126. Thus, whether erastin-induced ferroptosis is RAS-dependent needs further investigation.
On the other hand, some studies have focused on how astrocytes protect against neuronal ferroptotic cell death. Astrocytes have a high capacity to store iron and prevent iron overload in neurons [115]. Astrocytes provide neurons with glutathione S-transferase Mu 2 and other antioxidants to protect them from oxidative damage. Therefore, dysregulation of astrocyte-neuron interactions and inadequate Nrf2 activation in astrocytes may lead to ferroptosis-like cell death in neurons, especially dopaminergic neurons [47, 116].
Ferroptosis in Alzheimer’s Disease
Alzheimer’s disease (AD) is caused by the degeneration of neurons required for learning and memory [16]. Postmortem analysis of brains from AD patients shows evidence of apoptosis, which is likely responsible for a large amount of the neurodegeneration [117, 118]. However, new forms of cell death are now considered to contribute to the neuronal destruction of AD because several of the degenerating processes cannot be explained by apoptosis alone and drugs targeting apoptosis are largely ineffective [119,120,121,122].
Lipid peroxidation and iron dysregulation, which are hallmarks of ferroptosis, have long been noted in AD brains [123, 124]. In a recent study, mice with specific cerebral cortex and hippocampal neuronal GPX4 knockout (GPX4 brain inducible knockout, Gpx4BIKO) exhibited marked cognitive impairment in a water maze test, as well as degeneration of hippocampal neurons [16]. The authors suggested that the degenerating neurons might be undergoing ferroptosis because the level of neurodegeneration was reduced when the mice were fed a high-vitamin E diet or administered the ferroptosis inhibitor liproxstatin-1 [16]. Another study published earlier this year showed that overexpression and hyperphosphorylation of Tau induced ferroptotic neuronal death and that α-lipoic acid administration rescued neurons by downregulating iron transferrin receptor, decreasing phospho-P38 level, and upregulating xCT and GPX4 expression [125]. These studies suggest that ferroptosis can potentially affect neurons important for learning and memory. As an extension, the findings also indicate that ferroptosis may play an important role in neuronal death during AD progression.
Ferroptosis in Huntington’s Disease
Huntington’s Disease (HD) is yet another progressive neurodegenerative disorder that leads to rapid involuntary movements and dementia [126]. Oxidative damage [127], lipid oxidation [128], iron accumulation [17], dysregulation of GSH [41], and decreased GPX activity [24] have been noted in experimental HD animal models and in patients with HD. Delivery of iron chelators has been shown to improve cognitive function in an HD mouse [17].
To date, only one study has used a cellular model of HD (overexpression of HD-causing gene, huntingtin [htt] exon 1) to examine whether ferroptosis inhibitor Fer-1 could prevent cell death [18]. The data indicated a probable role for ferroptosis in the progressive neurodegeneration of HD. In vivo studies are needed to validate the role of ferroptosis in the progression of HD and its disease-specific pathologic mechanisms.
Ferroptosis in Periventricular Leukomalacia
Periventricular leukomalacia (PVL) is a form of cerebral white matter injury that affects premature infants. It is characterized by the death of developing oligodendrocytes [129]. Several studies have indicated an important role for iron in oligodendrocyte death, and some have found elevated levels of ROS biomarkers [130,131,132,133]. In addition, an abundance of lipid oxidation products typical of ferroptosis have been found in the cerebrospinal fluid of infants with white matter injuries [131]. Another study found that GSH depletion in rat oligodendrocytes induced cell death that could be prevented with vitamin E (known to act as a ferroptosis inhibitor) [134]. All evidence indicates that ferroptosis may play a role in PVL.
To mimic PVL in vitro, Skouta et al. cultured oligodendrocytes in cystine-free medium, which depletes GSH and causes cell death [18]. Fer-1 and SRS11-92 (15-fold more potent than the parent Fer-1) fully protected oligodendrocytes from cystine deprivation [18]. These data suggest that ferroptosis is a likely mechanism oligodendrocyte death in PVL.
Ferroptosis in General Neurotoxicity and Aging
Neurotoxicity is defined by the Environmental Protection Agency as “an adverse change in the structure or function of the central and/or peripheral nervous system following exposure to a chemical, physical, or biological agent” [135]. In their initial classification and identification of ferroptosis, Dixon and colleagues found that using Fer-1 to inhibit ferroptosis protected rat hippocampal slice cultures from glutamate-induced neurotoxicity, suggesting a role for lipid ROS-induced cell death and most likely ferroptosis in neurotoxicity [1]. One study from Sanford-Burnham Medical Research Institute identified two potential ferroptosis inhibitors with distinct molecular mechanisms: the PI3Kα inhibitor protects neuronal cells by inducing partial restoration of depleted GSH levels and accumulation of intracellular amino acids, whereas the Flt3 inhibitor prevents lipid peroxidation, a key mechanism of glutamate-mediated toxicity [51]. Another study showed that inhibition of HIF prolyl hydroxylases prevents oxidative stress-induced ferroptosis in vitro [90].
Levels of iron have long been known to increase with aging [136]. Additionally, iron and intra-cell iron retention have been associated with aging in diverse cell types, including neurons [137]. During the process of aging, the distribution of iron molecules changes between neurons and glial cells [138]. Iron accumulation in aged glial cells has been shown to damage neurons by increasing proinflammatory cytokines and establishing neuroinflammation [139, 140]. It has been reported recently that iron retention in neurons promotes premature aging via induction of DNA damage [141]. Intracellular iron retention also has been linked to damage in the epigenome through hypomethylation and transposable elements [142, 143]. The acceleration of aging via DNA damage has recently been named ferrosenescence [137].
Research into senescent cells has revealed an increase in iron accumulation, but impaired ferritinophagy, a lysosomal process that promotes ferritin degradation and ferroptosis [36]. Impaired ferritin degradation leads to a phenotype of senescent cells with elevated iron accumulation, whereby iron is effectively trapped in ferritin, creating a perceived cellular deficiency [36]. Thus, senescent cells are highly resistant to ferroptosis [36].
Ferroptosis in Brain Tumors
Ferroptosis was first identified in the non-small cell lung cancer cell line HT-1080 [1]. Although research has been conducted in relation to ferroptosis in many forms of cancer, little work has examined the role of ferroptosis in brain cancers. Nevertheless, Fer-1 recently was shown to have a neuroprotective role in the dopaminergic neuroblastoma cell line SH-SY5Y under conditions of rotenone-induced oxidative stress [10]. Fer-1 was able to decrease ROS/reactive nitrogen species generated under rotenone insult, mitigate rotenone-induced α-synuclein aggregation, and even quench the stable radical from 2,2-diphenyl-1-picrylhydrazyl (DPPH) [10]. Other investigators reported that mitochondrial ferritin (FtMt) overexpression in SH-SY5Y cells significantly inhibited erastin-induced ferroptosis [144]. They also found that FtMt inhibited ferroptosis by regulating iron homeostasis, in particular by repressing cellular labile iron pool overload and altering iron-related proteins [144].
Relatively more ferroptosis-related studies have pertained to glioblastoma than to neuroblastoma. Shortly after ferroptosis was discovered, a group in Russia transplanted glioma-35 cells into mouse and found that administering iron-containing water to tumor-bearing mice before radiation therapy reduced the supercoiled DNA index on days 1 and 21 after irradiation. Additionally, it dramatically decreased the tumor volume compared with that of control on day 21 [19]. The same group repeated their study in a rat model and found consistent results that iron-containing water promoted radiation-induced tumor cell apoptosis and ferroptosis [145]. Injection of DFO into the tumor-bearing rats reduced the efficiency of this treatment but had no effect on the efficiency of radiotherapy alone [145].
From 2016 to 2018, the Savaskan group published five papers discussing the role of glutamate exchanger xCT (SLC7a11 gene coding protein, a subunit of system xc−) in temozolomide (Temodal/Temcad®, TMZ)-treated glioma cells [60, 63, 146,147,148]. They reported that xCT expression correlated with the malignancy grade of brain tumor and that xCT inhibition disrupted the neurodegenerative and microenvironment-toxifying activity of gliomas [146]. TMZ efficacy can be potentiated when combined with erastin (which inhibits system xc−), and gliomas with high xCT expression are more vulnerable to combination treatment with erastin-TMZ [146]. In the same year, they found that high concentrations (> 200 μM) of sulfasalazine, a system xc− inhibitor that inhibits glioma growth [149], reduced glioma tumor volume by mechanistically inducing ferroptotic cell death in glioma cells in vitro [60]. Importantly, neurons and normal brain tissue barely responded to sulfasalazine, and isolated astrocytes were less sensitive than glioma cells to sulfasalazine toxicity [60]. Sulfasalazine treatment did not affect experimental tumor growth, but it did reduce glioma-derived edema in vivo [60]. Later, they revealed that activating transcription factor 4 (ATF4) was a vital step in elevating cellular xCT and that ATF4 knockdown rendered glioma cells susceptible to erastin, sorafenib, and RSL3-induced ferroptosis [63]. Therefore, they confirmed the previous results [150] that inhibition of ATF4 may be an option for reducing glioma tumor growth and angiogenesis [63], overcoming chemo-resistance from TMZ, and promoting drug efficacy in human gliomas [148]. Fan and colleagues found that, in addition to AFT4, Nrf2 overexpressed in glioma and negatively correlated with patient survival [147]. Consistent with studies in a lung carcinoma cell line [7], bladder carcinoma cells [151], and other cancer cell lines [152], they found that Nrf2 upregulated xCT expression and that activation of Nrf2 signaling promoted resistance to ferroptosis in glioma cell lines [147]. However, Berghe’s group found that withaferin A induced ferroptotic cell death in high-risk neuroblastoma cells by binding to KEAP1 [21]. Consequently, it increased Nrf2 protein level and activated heme oxygenase-1 (HO-1). Elevated HO-1 induced accumulation of Fe2+ and subsequently induced lipid ROS and ferroptosis [21]. Moreover, withaferin A decreased GPX4 expression and induced ferroptosis [21]. Although most researchers believe that activation of the Nrf2 signaling pathway inhibits ferroptosis [153], these results suggest that Nrf2 may play a role in promoting ferroptosis under certain conditions.
Conclusions, Limitations, and Further Directions
The goal of this review was to discuss the role of ferroptosis, a newly identified form of cell death, in various brain disease processes, including neurologic disorders and brain tumors. Although it was first identified in cancer cells [1], ferroptosis has been shown to play an important role in the progression and toxicity of numerous neurologic diseases, including stroke, PD, and HD. As shown in Fig. 2, the common ferroptotic mechanisms in brain diseases result from system xc− blockade, GSH depletion, GPX4 inactivity, lipoxygenase inactivation, and/or intracellular iron accumulation. These mechanisms are consistent with those seen in other disease states (renal disease, ischemic-reperfusion-related disease, and brain tumor) that can be modified by known ferroptotic inducers (glutamate, erastin, and RSL3) and inhibitors (Fer-1, liproxstatin-1, DFO, and vitamin E). However, whether MAPK, PI3K/Akt/mTOR, or KEAP1/Nrf2/HO-1 signaling pathways are common to ferroptosis-related brain diseases, or whether these signaling pathways are disease-specific, remains an open question. In addition, neurons differ from other brain cells (microglia, astrocytes, or oligodendrocytes) and cells from other organs in their metabolism, dividing capacity, nerve impulse function, circuit formation, etc. Thus, when neurons are challenged with ferroptosis inducers, they could exhibit unique mechanisms that have not yet been fully investigated.
Ferroptosis is a unique form of regulated cell death that involves gene sets and signaling pathways distinct from those of apoptosis, necrosis, autophagy, and oxytosis [1, 154]. Apoptotic cells exhibit classic features such as mitochondrial cytochrome c release, caspase activation, and chromatin fragmentation. Additionally, apoptosis can be inhibited by caspase inhibitors, and its main regulators are Bcl-2 and caspase-3 [1, 2]. Cells undergoing necrosis exhibit plasma membrane permeabilization and swollen organelles. Phosphorylation of RIPK1 and RIPK3 play important roles in necrosis, and the necrotic process can be inhibited by necrostatins [1, 2]. Autophagy is characterized by the formation of autophagosomes and autolysosomes, and it can be inhibited by 3-MA [1, 2]. Upregulation of Atg5 and Atg6 plays a vital role in the autophagy pathway [1, 2]. Ferroptosis is induced by iron-dependent lipid ROS [1]. Cells undergoing ferroptosis exhibit shrunken mitochondria and a highly intense mitochondrial membrane [1, 2]. However, emerging evidence shows that ferroptosis might be a regulated necrotic cell death [4, 50]. In addition, studies have found that autophagy and ferroptosis share key regulators (SLC7a11, GPX4, Nrf2, p53, HSPB1, CISD1, FANCD2, and ACSL4) [155]. Some elegant studies showed that autophagy promotes ferroptosis by degradation of ferritin in cancer cells and fibroblasts [156] and that activation of BECN1 (an important regulator in autophagy) promotes ferroptosis by directly blocking system xc− activity in tumor cells [157].
Oxytosis, also known as oxidative glutamate toxicity, is another form of cell death that was identified 30 years ago [158]. It is frequently compared to ferroptosis because the two cell death forms have similar mechanisms of lethality [154]. Oxytosis is induced by depletion of GSH that can result from high concentrations of extracellular glutamate or other reagents that inhibit system xc− [2]. Mitochondrial ROS production, Ca2+ influx, and oxidative stress are the hallmarks of oxytosis [2]. Increasing evidence shows that oxytosis and ferroptosis share many similarities, including inducers (glutamate and RSL3), lethal mechanisms (GSH depletion and lipid ROS), a key regulator (GPX4), metal dependency (iron), and ultrastructural features (mitochondria abnormality) [1, 154, 159, 160]. Some researchers believe that these mechanisms could be one cell death form with two names [154]. The data remain controversial. Dixon et al. claimed that ferroptosis depends on iron only [1], but recent studies showed that ferroptosis may also involve copper and calcium, as oxytosis does [154, 161]. Studies have shown that most ferroptotic cells (erastin-treated MEF cells, erastin-treated BJeLR cells, and RSL3-treated MEF cells) have shrunken mitochondria with increased electron density [1, 6, 154]. In their review, Lewerenz et al. noted that both ferroptotic cells (RSL3-treated MEF cells) and oxytotic cells (glutamate-induced HT4 cells) had swollen mitochondria with outer membranes that ruptured in a time-dependent manner [154]. At this point, more studies are needed to ascertain the relationship and crosstalk between ferroptosis and other forms of cell death and to determine whether ferroptosis belongs to one of those forms of cell death.
This review is generally limited by the amount of research currently being performed on ferroptosis. Given that ferroptosis was only first identified in 2012, relatively few studies have been published on the topic. Iron accumulation that leads to cell death has been shown to contribute to many disease states, but research into the role of ferroptosis in particular is still sparse. Most of the research to date has focused on the contribution of ferroptosis to neurologic processes, but future research should also address the therapeutic benefits of inhibiting ferroptosis in brain cells that exhibit certain neurodegenerative disease characteristics and promoting ferroptosis in brain cancers. Some studies have suggested a role for GSH depletion in the progression of amyotrophic lateral sclerosis (ALS) [43], a neurodegenerative disease that causes muscular atrophy and paralysis [126]. However, conclusions have been mixed, with some researchers indicating no change in GSH levels [162, 163]. At present, the role of ferroptosis in ALS is unclear and requires further research.
Epigenetic modifications are also important regulators of cellular activity and cell death. Although several miRNAs (miR-137 and miR-9) have been linked to ferroptosis [164, 165], studies are needed to investigate how long non-coding RNA or circulating RNA regulates ferroptosis, and how methylation status of the CpG island and modifications of histone tails in the promoter regions of key regulators regulate ferroptosis. Furthermore, little is known about the relationship between ferroptotic cells and circulating immune system reaction. Future research could focus on how ferroptotic cells induce immune cell activation/infiltration or how surrounding immune cells regulate ferroptosis in brain cells.
We believe that ferroptosis is one of the most important cell death forms in brain diseases and that in-depth studies of ferroptosis will provide new opportunities for diagnosis and therapeutic intervention.
References
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ et al (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149(5):1060–1072. https://doi.org/10.1016/j.cell.2012.03.042
Cao JY, Dixon SJ (2016) Mechanisms of ferroptosis. Cell Mol Life Sci 73(11–12):2195–2209. https://doi.org/10.1007/s00018-016-2194-1
Yu H, Guo P, Xie X, Wang Y, Chen G (2017) Ferroptosis, a new form of cell death, and its relationships with tumourous diseases. J Cell Mol Med 21(4):648–657. https://doi.org/10.1111/jcmm.13008
Dixon SJ (2017) Ferroptosis: bug or feature? Immunol Rev 277(1):150–157. https://doi.org/10.1111/imr.12533
Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA et al (2014) Regulation of ferroptotic cancer cell death by GPX4. Cell 156(1–2):317–331. https://doi.org/10.1016/j.cell.2013.12.010
Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H, Baer R, Gu W (2015) Ferroptosis as a p53-mediated activity during tumour suppression. Nature 520(7545):57–62. https://doi.org/10.1038/nature14344
Chen D, Tavana O, Chu B, Erber L, Chen Y, Baer R, Gu W (2017) NRF2 is a major target of ARF in p53-independent tumor suppression. Mol Cell 68(1):224–232 e224. https://doi.org/10.1016/j.molcel.2017.09.009
Wenzel SE, Tyurina YY, Zhao J, St Croix CM, Dar HH, Mao G, Tyurin VA, Anthonymuthu TS et al (2017) PEBP1 wardens ferroptosis by enabling lipoxygenase generation of lipid death signals. Cell 171(3):628–641 e626. https://doi.org/10.1016/j.cell.2017.09.044
Larraufie MH, Yang WS, Jiang E, Thomas AG, Slusher BS, Stockwell BR (2015) Incorporation of metabolically stable ketones into a small molecule probe to increase potency and water solubility. Bioorg Med Chem Lett 25(21):4787–4792. https://doi.org/10.1016/j.bmcl.2015.07.018
Kabiraj P, Valenzuela CA, Marin JE, Ramirez DA, Mendez L, Hwang MS, Varela-Ramirez A, Fenelon K et al (2015) The neuroprotective role of ferrostatin-1 under rotenone-induced oxidative stress in dopaminergic neuroblastoma cells. Protein J 34(5):349–358. https://doi.org/10.1007/s10930-015-9629-7
Dixon SJ, Patel DN, Welsch M, Skouta R, Lee ED, Hayano M, Thomas AG, Gleason CE et al (2014) Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife 3:e02523. https://doi.org/10.7554/eLife.02523
Louandre C, Marcq I, Bouhlal H, Lachaier E, Godin C, Saidak Z, Francois C, Chatelain D et al (2015) The retinoblastoma (Rb) protein regulates ferroptosis induced by sorafenib in human hepatocellular carcinoma cells. Cancer Lett 356(2 Pt B):971–977. https://doi.org/10.1016/j.canlet.2014.11.014
Shaw AT, Winslow MM, Magendantz M, Ouyang C, Dowdle J, Subramanian A, Lewis TA, Maglathin RL et al (2011) Selective killing of K-ras mutant cancer cells by small molecule inducers of oxidative stress. Proc Natl Acad Sci U S A 108(21):8773–8778. https://doi.org/10.1073/pnas.1105941108
Chen L, Li X, Liu L, Yu B, Xue Y, Liu Y (2015) Erastin sensitizes glioblastoma cells to temozolomide by restraining xCT and cystathionine-gamma-lyase function. Oncol Rep 33(3):1465–1474. https://doi.org/10.3892/or.2015.3712
Do Van B, Gouel F, Jonneaux A, Timmerman K, Gele P, Petrault M, Bastide M, Laloux C et al (2016) Ferroptosis, a newly characterized form of cell death in Parkinson’s disease that is regulated by PKC. Neurobiol Dis 94:169–178. https://doi.org/10.1016/j.nbd.2016.05.011
Hambright WS, Fonseca RS, Chen L, Na R, Ran Q (2017) Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol 12:8–17. https://doi.org/10.1016/j.redox.2017.01.021
Chen J, Marks E, Lai B, Zhang Z, Duce JA, Lam LQ, Volitakis I, Bush AI et al (2013) Iron accumulates in Huntington's disease neurons: protection by deferoxamine. PLoS One 8(10):e77023. https://doi.org/10.1371/journal.pone.0077023
Skouta R, Dixon SJ, Wang J, Dunn DE, Orman M, Shimada K, Rosenberg PA, Lo DC et al (2014) Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J Am Chem Soc 136(12):4551–4556. https://doi.org/10.1021/ja411006a
Ivanov SD, Semenov AL, Mikhelson VM, Kovan'ko EG, Iamshanov VA (2013) Effects of iron ion additional introduction in radiation therapy of tumor-bearing animals. Radiats Biol Radioecol 53(3):296–303
Dixon SJ, Winter GE, Musavi LS, Lee ED, Snijder B, Rebsamen M, Superti-Furga G, Stockwell BR (2015) Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death. ACS Chem Biol 10(7):1604–1609. https://doi.org/10.1021/acschembio.5b00245
Hassannia B, Wiernicki B, Ingold I, Qu F, Van Herck S, Tyurina YY, Bayir H, Abhari BA et al (2018) Nano-targeted induction of dual ferroptotic mechanisms eradicates high-risk neuroblastoma. J Clin Invest 128:3341–3355. https://doi.org/10.1172/JCI99032
Bellinger FP, Raman AV, Rueli RH, Bellinger MT, Dewing AS, Seale LA, Andres MA, Uyehara-Lock JH et al (2012) Changes in selenoprotein P in substantia nigra and putamen in Parkinson’s disease. J Parkinsons Dis 2(2):115–126. https://doi.org/10.3233/JPD-2012-11052
Paz-y-Mino C, Carrera C, Lopez-Cortes A, Munoz MJ, Cumbal N, Castro B, Cabrera A, Sanchez ME (2010) Genetic polymorphisms in apolipoprotein E and glutathione peroxidase 1 genes in the Ecuadorian population affected with Alzheimer's disease. Am J Med Sci 340(5):373–377. https://doi.org/10.1097/MAJ.0b013e3181e93475
Chen CM, Wu YR, Cheng ML, Liu JL, Lee YM, Lee PW, Soong BW, Chiu DT (2007) Increased oxidative damage and mitochondrial abnormalities in the peripheral blood of Huntington’s disease patients. Biochem Biophys Res Commun 359(2):335–340. https://doi.org/10.1016/j.bbrc.2007.05.093
Usarek E, Gajewska B, Kazmierczak B, Kuzma M, Dziewulska D, Baranczyk-Kuzma A (2005) A study of glutathione S-transferase pi expression in central nervous system of subjects with amyotrophic lateral sclerosis using RNA extraction from formalin-fixed, paraffin-embedded material. Neurochem Res 30(8):1003–1007. https://doi.org/10.1007/s11064-005-6771-1
Abrams RP, Carroll WL, Woerpel KA (2016) Five-membered ring peroxide selectively initiates ferroptosis in cancer cells. ACS Chem Biol 11(5):1305–1312. https://doi.org/10.1021/acschembio.5b00900
Eling N, Reuter L, Hazin J, Hamacher-Brady A, Brady NR (2015) Identification of artesunate as a specific activator of ferroptosis in pancreatic cancer cells. Oncoscience 2(5):517–532. https://doi.org/10.18632/oncoscience.160
Sultana R, Perluigi M, Butterfield DA (2013) Lipid peroxidation triggers neurodegeneration: a redox proteomics view into the Alzheimer disease brain. Free Radic Biol Med 62:157–169. https://doi.org/10.1016/j.freeradbiomed.2012.09.027
Miller RL, James-Kracke M, Sun GY, Sun AY (2009) Oxidative and inflammatory pathways in Parkinson’s disease. Neurochem Res 34(1):55–65. https://doi.org/10.1007/s11064-008-9656-2
Jin YN, Johnson GV (2010) The interrelationship between mitochondrial dysfunction and transcriptional dysregulation in Huntington disease. J Bioenerg Biomembr 42(3):199–205. https://doi.org/10.1007/s10863-010-9286-7
Goldsteins G, Keksa-Goldsteine V, Ahtoniemi T, Jaronen M, Arens E, Akerman K, Chan PH, Koistinaho J (2008) Deleterious role of superoxide dismutase in the mitochondrial intermembrane space. J Biol Chem 283(13):8446–8452. https://doi.org/10.1074/jbc.M706111200
Armstrong JS, Khdour O, Hecht SM (2010) Does oxidative stress contribute to the pathology of Friedreich's ataxia? A radical question. FASEB J 24(7):2152–2163. https://doi.org/10.1096/fj.09-143222
Wu C, Zhao W, Yu J, Li S, Lin L, Chen X (2018) Induction of ferroptosis and mitochondrial dysfunction by oxidative stress in PC12 cells. Sci Rep 8(1):574. https://doi.org/10.1038/s41598-017-18935-1
Tuo QZ, Lei P, Jackman KA, Li XL, Xiong H, Li XL, Liuyang ZY, Roisman L et al (2017) Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Mol Psychiatry 22(11):1520–1530. https://doi.org/10.1038/mp.2017.171
Li Q, Han X, Lan X, Gao Y, Wan J, Durham F, Cheng T, Yang J et al (2017) Inhibition of neuronal ferroptosis protects hemorrhagic brain. JCI Insight 2(7):e90777. https://doi.org/10.1172/jci.insight.90777
Masaldan S, Clatworthy SAS, Gamell C, Meggyesy PM, Rigopoulos AT, Haupt S, Haupt Y, Denoyer D et al (2018) Iron accumulation in senescent cells is coupled with impaired ferritinophagy and inhibition of ferroptosis. Redox Biol 14:100–115. https://doi.org/10.1016/j.redox.2017.08.015
Rhodes SL, Buchanan DD, Ahmed I, Taylor KD, Loriot MA, Sinsheimer JS, Bronstein JM, Elbaz A et al (2014) Pooled analysis of iron-related genes in Parkinson's disease: association with transferrin. Neurobiol Dis 62:172–178. https://doi.org/10.1016/j.nbd.2013.09.019
Jennis M, Kung CP, Basu S, Budina-Kolomets A, Leu JI, Khaku S, Scott JP, Cai KQ et al (2016) An African-specific polymorphism in the TP53 gene impairs p53 tumor suppressor function in a mouse model. Genes Dev 30(8):918–930. https://doi.org/10.1101/gad.275891.115
Ooko E, Saeed ME, Kadioglu O, Sarvi S, Colak M, Elmasaoudi K, Janah R, Greten HJ et al (2015) Artemisinin derivatives induce iron-dependent cell death (ferroptosis) in tumor cells. Phytomedicine 22(11):1045–1054. https://doi.org/10.1016/j.phymed.2015.08.002
Monti DA, Zabrecky G, Kremens D, Liang TW, Wintering NA, Cai J, Wei X, Bazzan AJ et al (2016) N-acetyl cysteine may support dopamine neurons in Parkinson’s disease: preliminary clinical and cell line data. PLoS One 11(6):e0157602. https://doi.org/10.1371/journal.pone.0157602
Klepac N, Relja M, Klepac R, Hecimovic S, Babic T, Trkulja V (2007) Oxidative stress parameters in plasma of Huntington’s disease patients, asymptomatic Huntington’s disease gene carriers and healthy subjects : a cross-sectional study. J Neurol 254(12):1676–1683. https://doi.org/10.1007/s00415-007-0611-y
Cristalli DO, Arnal N, Marra FA, de Alaniz MJ, Marra CA (2012) Peripheral markers in neurodegenerative patients and their first-degree relatives. J Neurol Sci 314(1–2):48–56. https://doi.org/10.1016/j.jns.2011.11.001
Babu GN, Kumar A, Chandra R, Puri SK, Singh RL, Kalita J, Misra UK (2008) Oxidant-antioxidant imbalance in the erythrocytes of sporadic amyotrophic lateral sclerosis patients correlates with the progression of disease. Neurochem Int 52(6):1284–1289. https://doi.org/10.1016/j.neuint.2008.01.009
Piemonte F, Pastore A, Tozzi G, Tagliacozzi D, Santorelli FM, Carrozzo R, Casali C, Damiano M et al (2001) Glutathione in blood of patients with Friedreich’s ataxia. Eur J Clin Investig 31(11):1007–1011
Eberhard Y, McDermott SP, Wang X, Gronda M, Venugopal A, Wood TE, Hurren R, Datti A et al (2009) Chelation of intracellular iron with the antifungal agent ciclopirox olamine induces cell death in leukemia and myeloma cells. Blood 114(14):3064–3073. https://doi.org/10.1182/blood-2009-03-209965
Devos D, Moreau C, Devedjian JC, Kluza J, Petrault M, Laloux C, Jonneaux A, Ryckewaert G et al (2014) Targeting chelatable iron as a therapeutic modality in Parkinson’s disease. Antioxid Redox Signal 21(2):195–210. https://doi.org/10.1089/ars.2013.5593
Cui Z, Zhong Z, Yang Y, Wang B, Sun Y, Sun Q, Yang GY, Bian L (2016) Ferrous iron induces Nrf2 expression in mouse brain astrocytes to prevent neurotoxicity. J Biochem Mol Toxicol 30(8):396–403. https://doi.org/10.1002/jbt.21803
Linkermann A, Skouta R, Himmerkus N, Mulay SR, Dewitz C, De Zen F, Prokai A, Zuchtriegel G et al (2014) Synchronized renal tubular cell death involves ferroptosis. Proc Natl Acad Sci U S A 111(47):16836–16841. https://doi.org/10.1073/pnas.1415518111
Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M et al (2014) Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol 16(12):1180–1191. https://doi.org/10.1038/ncb3064
Zille M, Karuppagounder SS, Chen Y, Gough PJ, Bertin J, Finger J, Milner TA, Jonas EA et al (2017) Neuronal death after hemorrhagic stroke in vitro and in vivo shares features of ferroptosis and necroptosis. Stroke 48(4):1033–1043. https://doi.org/10.1161/STROKEAHA.116.015609
Kang Y, Tiziani S, Park G, Kaul M, Paternostro G (2014) Cellular protection using Flt3 and PI3Kalpha inhibitors demonstrates multiple mechanisms of oxidative glutamate toxicity. Nat Commun 5:3672. https://doi.org/10.1038/ncomms4672
Lyons SA, Chung WJ, Weaver AK, Ogunrinu T, Sontheimer H (2007) Autocrine glutamate signaling promotes glioma cell invasion. Cancer Res 67(19):9463–9471. https://doi.org/10.1158/0008-5472.CAN-07-2034
Liu Y, Wang W, Li Y, Xiao Y, Cheng J, Jia J (2015) The 5-lipoxygenase inhibitor zileuton confers neuroprotection against glutamate oxidative damage by inhibiting ferroptosis. Biol Pharm Bull 38(8):1234–1239. https://doi.org/10.1248/bpb.b15-00048
Xie Y, Song X, Sun X, Huang J, Zhong M, Lotze MT, Zeh HJR, Kang R et al (2016) Identification of baicalein as a ferroptosis inhibitor by natural product library screening. Biochem Biophys Res Commun 473(4):775–780. https://doi.org/10.1016/j.bbrc.2016.03.052
Probst L, Dachert J, Schenk B, Fulda S (2017) Lipoxygenase inhibitors protect acute lymphoblastic leukemia cells from ferroptotic cell death. Biochem Pharmacol 140:41–52. https://doi.org/10.1016/j.bcp.2017.06.112
Shah R, Shchepinov MS, Pratt DA (2018) Resolving the role of lipoxygenases in the initiation and execution of ferroptosis. ACS Cent Sci 4(3):387–396. https://doi.org/10.1021/acscentsci.7b00589
Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR (2016) Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci U S A 113(34):E4966–E4975. https://doi.org/10.1073/pnas.1603244113
Johannesson T, Kristinsson J, Torsdottir G, Snaedal J (2012) Ceruloplasmin (Cp) and iron in connection with Parkinson’s disease (PD) and Alzheimer’s disease (AD). Laeknabladid 98(10):531–537
Sun X, Ou Z, Xie M, Kang R, Fan Y, Niu X, Wang H, Cao L et al (2015) HSPB1 as a novel regulator of ferroptotic cancer cell death. Oncogene 34(45):5617–5625. https://doi.org/10.1038/onc.2015.32
Sehm T, Fan Z, Ghoochani A, Rauh M, Engelhorn T, Minakaki G, Dorfler A, Klucken J et al (2016) Sulfasalazine impacts on ferroptotic cell death and alleviates the tumor microenvironment and glioma-induced brain edema. Oncotarget 7(24):36021–36033. https://doi.org/10.18632/oncotarget.8651
Yagoda N, von Rechenberg M, Zaganjor E, Bauer AJ, Yang WS, Fridman DJ, Wolpaw AJ, Smukste I et al (2007) RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature 447(7146):864–868. https://doi.org/10.1038/nature05859
Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R, Tang D (2016) Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology 63(1):173–184. https://doi.org/10.1002/hep.28251
Chen D, Fan Z, Rauh M, Buchfelder M, Eyupoglu IY, Savaskan N (2017) ATF4 promotes angiogenesis and neuronal cell death and confers ferroptosis in a xCT-dependent manner. Oncogene 36(40):5593–5608. https://doi.org/10.1038/onc.2017.146
De Jager PL, Srivastava G, Lunnon K, Burgess J, Schalkwyk LC, Yu L, Eaton ML, Keenan BT et al (2014) Alzheimer's disease: early alterations in brain DNA methylation at ANK1, BIN1, RHBDF2 and other loci. Nat Neurosci 17(9):1156–1163. https://doi.org/10.1038/nn.3786
Dolma S, Lessnick SL, Hahn WC, Stockwell BR (2003) Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell 3(3):285–296
Yang WS, Stockwell BR (2008) Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol 15(3):234–245. https://doi.org/10.1016/j.chembiol.2008.02.010
Root DE, Flaherty SP, Kelley BP, Stockwell BR (2003) Biological mechanism profiling using an annotated compound library. Chem Biol 10(9):881–892
Yang WS, Stockwell BR (2016) Ferroptosis: death by lipid peroxidation. Trends Cell Biol 26(3):165–176. https://doi.org/10.1016/j.tcb.2015.10.014
Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, Kang R, Tang D (2016) Ferroptosis: process and function. Cell Death Differ 23(3):369–379. https://doi.org/10.1038/cdd.2015.158
Gaschler MM, Andia AA, Liu H, Csuka JM, Hurlocker B, Vaiana CA, Heindel DW, Zuckerman DS et al (2018) FINO2 initiates ferroptosis through GPX4 inactivation and iron oxidation. Nat Chem Biol 14(5):507–515. https://doi.org/10.1038/s41589-018-0031-6
Ma S, Dielschneider RF, Henson ES, Xiao W, Choquette TR, Blankstein AR, Chen Y, Gibson SB (2017) Ferroptosis and autophagy induced cell death occur independently after siramesine and lapatinib treatment in breast cancer cells. PLoS One 12(8):e0182921. https://doi.org/10.1371/journal.pone.0182921
Kinowaki Y, Kurata M, Ishibashi S, Ikeda M, Tatsuzawa A, Yamamoto M, Miura O, Kitagawa M et al (2018) Glutathione peroxidase 4 overexpression inhibits ROS-induced cell death in diffuse large B-cell lymphoma. Lab Investig 98:609–619. https://doi.org/10.1038/s41374-017-0008-1
Lachaier E, Louandre C, Godin C, Saidak Z, Baert M, Diouf M, Chauffert B, Galmiche A (2014) Sorafenib induces ferroptosis in human cancer cell lines originating from different solid tumors. Anticancer Res 34(11):6417–6422
Kim SE, Zhang L, Ma K, Riegman M, Chen F, Ingold I, Conrad M, Turker MZ et al (2016) Ultrasmall nanoparticles induce ferroptosis in nutrient-deprived cancer cells and suppress tumour growth. Nat Nanotechnol 11(11):977–985. https://doi.org/10.1038/nnano.2016.164
Chang CF, Cho S, Wang J (2014) (−)-Epicatechin protects hemorrhagic brain via synergistic Nrf2 pathways. Ann Clin Transl Neurol 1(4):258–271. https://doi.org/10.1002/acn3.54
Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J et al (2017) Heart Disease and Stroke Statistics-2017 update: a report from the American Heart Association. Circulation 135(10):e146–e603. https://doi.org/10.1161/CIR.0000000000000485
Au A, Griffiths LR, Irene L, Kooi CW, Wei LK (2017) The impact of APOA5, APOB, APOC3 and ABCA1 gene polymorphisms on ischemic stroke: Evidence from a meta-analysis. Atherosclerosis 265:60–70. https://doi.org/10.1016/j.atherosclerosis.2017.08.003
Brouns R, De Deyn PP (2009) The complexity of neurobiological processes in acute ischemic stroke. Clin Neurol Neurosurg 111(6):483–495. https://doi.org/10.1016/j.clineuro.2009.04.001
Dietrich RB, Bradley WG,J (1988) Iron accumulation in the basal ganglia following severe ischemic-anoxic insults in children. Radiology 168(1):203–206. https://doi.org/10.1148/radiology.168.1.3380958
Lipscomb DC, Gorman LG, Traystman RJ, Hurn PD (1998) Low molecular weight iron in cerebral ischemic acidosis in vivo. Stroke 29(2):487–492 discussion 493
Ding H, Yan CZ, Shi H, Zhao YS, Chang SY, Yu P, Wu WS, Zhao CY et al (2011) Hepcidin is involved in iron regulation in the ischemic brain. PLoS One 6(9):e25324. https://doi.org/10.1371/journal.pone.0025324
Park UJ, Lee YA, Won SM, Lee JH, Kang SH, Springer JE, Lee YB, Gwag BJ (2011) Blood-derived iron mediates free radical production and neuronal death in the hippocampal CA1 area following transient forebrain ischemia in rat. Acta Neuropathol 121(4):459–473. https://doi.org/10.1007/s00401-010-0785-8
Fang KM, Cheng FC, Huang YL, Chung SY, Jian ZY, Lin MC (2013) Trace element, antioxidant activity, and lipid peroxidation levels in brain cortex of gerbils after cerebral ischemic injury. Biol Trace Elem Res 152(1):66–74. https://doi.org/10.1007/s12011-012-9596-1
Kondo Y, Asanuma M, Nishibayashi S, Iwata E, Ogawa N (1997) Late-onset lipid peroxidation and neuronal cell death following transient forebrain ischemia in rat brain. Brain Res 772(1–2):37–44
Kondo Y, Ogawa N, Asanuma M, Ota Z, Mori A (1995) Regional differences in late-onset iron deposition, ferritin, transferrin, astrocyte proliferation, and microglial activation after transient forebrain ischemia in rat brain. J Cereb Blood Flow Metab 15(2):216–226. https://doi.org/10.1038/jcbfm.1995.27
Patt A, Horesh IR, Berger EM, Harken AH, Repine JE (1990) Iron depletion or chelation reduces ischemia/reperfusion-induced edema in gerbil brains. J Pediatr Surg 25(2):224–227 discussion 227-228
Davis S, Helfaer MA, Traystman RJ, Hurn PD (1997) Parallel antioxidant and antiexcitotoxic therapy improves outcome after incomplete global cerebral ischemia in dogs. Stroke 28(1):198–204 discussion 204-195
Prass K, Ruscher K, Karsch M, Isaev N, Megow D, Priller J, Scharff A, Dirnagl U et al (2002) Desferrioxamine induces delayed tolerance against cerebral ischemia in vivo and in vitro. J Cereb Blood Flow Metab 22(5):520–525. https://doi.org/10.1097/00004647-200205000-00003
Hanson LR, Roeytenberg A, Martinez PM, Coppes VG, Sweet DC, Rao RJ, Marti DL, Hoekman JD et al (2009) Intranasal deferoxamine provides increased brain exposure and significant protection in rat ischemic stroke. J Pharmacol Exp Ther 330(3):679–686. https://doi.org/10.1124/jpet.108.149807
Speer RE, Karuppagounder SS, Basso M, Sleiman SF, Kumar A, Brand D, Smirnova N, Gazaryan I et al (2013) Hypoxia-inducible factor prolyl hydroxylases as targets for neuroprotection by “antioxidant” metal chelators: from ferroptosis to stroke. Free Radic Biol Med 62:26–36. https://doi.org/10.1016/j.freeradbiomed.2013.01.026
Li Q, Wan J, Lan X, Han X, Wang Z, Wang J (2017) Neuroprotection of brain-permeable iron chelator VK-28 against intracerebral hemorrhage in mice. J Cereb Blood Flow Metab 37:3110–3123. https://doi.org/10.1177/0271678X17709186
Qureshi AI, Suri MF, Ostrow PT, Kim SH, Ali Z, Shatla AA, Guterman LR, Hopkins LN (2003) Apoptosis as a form of cell death in intracerebral hemorrhage. Neurosurgery 52(5):1041–1047 discussion 1047-1048
Hickenbottom SL, Grotta JC, Strong R, Denner LA, Aronowski J (1999) Nuclear factor-kappaB and cell death after experimental intracerebral hemorrhage in rats. Stroke 30(11):2472–2477 discussion 2477-2478
Qureshi AI, Ling GS, Khan J, Suri MF, Miskolczi L, Guterman LR, Hopkins LN (2001) Quantitative analysis of injured, necrotic, and apoptotic cells in a new experimental model of intracerebral hemorrhage. Crit Care Med 29(1):152–157
Wang KY, Wu CH, Zhou LY, Yan XH, Yang RL, Liao LM, Ge XM, Liao YS et al (2015) Ultrastructural changes of brain tissues surrounding hematomas after intracerebral hemorrhage. Eur Neurol 74(1–2):28–35. https://doi.org/10.1159/000434631
Wu H, Wu T, Li M, Wang J (2012) Efficacy of the lipid-soluble iron chelator 2,2′-dipyridyl against hemorrhagic brain injury. Neurobiol Dis 45(1):388–394. https://doi.org/10.1016/j.nbd.2011.08.028
Wu H, Wu T, Xu X, Wang J, Wang J (2011) Iron toxicity in mice with collagenase-induced intracerebral hemorrhage. J Cereb Blood Flow Metab 31(5):1243–1250. https://doi.org/10.1038/jcbfm.2010.209
Li Q, Weiland A, Chen X, Lan X, Han X, Durham F, Liu X, Wan J et al (2018) Ultrastructural characteristics of neuronal death and white matter injury in mouse brain tissues after intracerebral hemorrhage: coexistence of ferroptosis, autophagy, and necrosis. Front Neurol 9:581. https://doi.org/10.3389/fneur.2018.00581
Gao M, Monian P, Quadri N, Ramasamy R, Jiang X (2015) Glutaminolysis and transferrin regulate ferroptosis. Mol Cell 59(2):298–308. https://doi.org/10.1016/j.molcel.2015.06.011
Zhang Z, Wu Y, Yuan S, Zhang P, Zhang J, Li H, Li X, Shen H et al (2018) Glutathione peroxidase 4 participates in secondary brain injury through mediating ferroptosis in a rat model of intracerebral hemorrhage. Brain Res 1701:112–125. https://doi.org/10.1016/j.brainres.2018.09.012
Fearnley JM, Lees AJ (1991) Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain 114(Pt 5):2283–2301
Rinne JO, Rummukainen J, Paljarvi L, Sako E, Molsa P, Rinne UK (1989) Neuronal loss in the substantia nigra in patients with Alzheimer’s disease and Parkinson’s disease in relation to extrapyramidal symptoms and dementia. Prog Clin Biol Res 317:325–332
Schober A (2004) Classic toxin-induced animal models of Parkinson's disease: 6-OHDA and MPTP. Cell Tissue Res 318(1):215–224. https://doi.org/10.1007/s00441-004-0938-y
Pichler I, Del Greco MF, Gogele M, Lill CM, Bertram L, Do CB, Eriksson N, Foroud T, Myers RH, Consortium PG, Nalls M, Keller MF, International Parkinson's Disease Genomics C, Wellcome Trust Case Control C, Benyamin B, Whitfield JB, Genetics of Iron Status C, Pramstaller PP, Hicks AA, Thompson JR, Minelli C (2013) Serum iron levels and the risk of Parkinson disease: a Mendelian randomization study. PLoS Med 10 (6):e1001462. doi:https://doi.org/10.1371/journal.pmed.1001462
Dexter DT, Wells FR, Agid F, Agid Y, Lees AJ, Jenner P, Marsden CD (1987) Increased nigral iron content in postmortem parkinsonian brain. Lancet 2(8569):1219–1220
Lei P, Ayton S, Finkelstein DI, Spoerri L, Ciccotosto GD, Wright DK, Wong BX, Adlard PA et al (2012) Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nat Med 18(2):291–295. https://doi.org/10.1038/nm.2613
Sian J, Dexter DT, Lees AJ, Daniel S, Agid Y, Javoy-Agid F, Jenner P, Marsden CD (1994) Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Ann Neurol 36(3):348–355. https://doi.org/10.1002/ana.410360305
Dexter D, Carter C, Agid F, Agid Y, Lees AJ, Jenner P, Marsden CD (1986) Lipid peroxidation as cause of nigral cell death in Parkinson’s disease. Lancet 2(8507):639–640
Cassarino DS, Fall CP, Swerdlow RH, Smith TS, Halvorsen EM, Miller SW, Parks JP, Parker WD Jr et al (1997) Elevated reactive oxygen species and antioxidant enzyme activities in animal and cellular models of Parkinson’s disease. Biochim Biophys Acta 1362(1):77–86
Ayton S, Lei P, Duce JA, Wong BX, Sedjahtera A, Adlard PA, Bush AI, Finkelstein DI (2013) Ceruloplasmin dysfunction and therapeutic potential for Parkinson disease. Ann Neurol 73(4):554–559. https://doi.org/10.1002/ana.23817
Ayton S, Lei P, Hare DJ, Duce JA, George JL, Adlard PA, McLean C, Rogers JT et al (2015) Parkinson’s disease iron deposition caused by nitric oxide-induced loss of beta-amyloid precursor protein. J Neurosci 35(8):3591–3597. https://doi.org/10.1523/JNEUROSCI.3439-14.2015
Lei P, Ayton S, Appukuttan AT, Volitakis I, Adlard PA, Finkelstein DI, Bush AI (2015) Clioquinol rescues Parkinsonism and dementia phenotypes of the tau knockout mouse. Neurobiol Dis 81:168–175. https://doi.org/10.1016/j.nbd.2015.03.015
Zhang HM, Su Q (2014) PKC in developmental hypothyroid rat brain. Neurol Sci 35(8):1161–1166. https://doi.org/10.1007/s10072-014-1716-6
Gouel F, Do Van B, Chou ML, Jonneaux A, Moreau C, Bordet R, Burnouf T, Devedjian JC et al (2017) The protective effect of human platelet lysate in models of neurodegenerative disease: Involvement of the Akt and MEK pathways. J Tissue Eng Regen Med 11(11):3236–3240. https://doi.org/10.1002/term.2222
Codazzi F, Pelizzoni I, Zacchetti D, Grohovaz F (2015) Iron entry in neurons and astrocytes: a link with synaptic activity. Front Mol Neurosci 8:18. https://doi.org/10.3389/fnmol.2015.00018
Ishii T, Warabi E, Mann GE (2018) Circadian control of BDNF-mediated Nrf2 activation in astrocytes protects dopaminergic neurons from ferroptosis. Free Radic Biol Med. https://doi.org/10.1016/j.freeradbiomed.2018.09.002
Cotman CW, Anderson AJ (1995) A potential role for apoptosis in neurodegeneration and Alzheimer’s disease. Mol Neurobiol 10(1):19–45. https://doi.org/10.1007/BF02740836
Mattson MP (2006) Neuronal life-and-death signaling, apoptosis, and neurodegenerative disorders. Antioxid Redox Signal 8(11–12):1997–2006. https://doi.org/10.1089/ars.2006.8.1997
Khandelwal PJ, Herman AM, Moussa CE (2011) Inflammation in the early stages of neurodegenerative pathology. J Neuroimmunol 238(1–2):1–11. https://doi.org/10.1016/j.jneuroim.2011.07.002
Wang X, Wang W, Li L, Perry G, Lee HG, Zhu X (2014) Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim Biophys Acta 1842(8):1240–1247. https://doi.org/10.1016/j.bbadis.2013.10.015
Gordon PH, Moore DH, Miller RG, Florence JM, Verheijde JL, Doorish C, Hilton JF, Spitalny GM et al (2007) Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trial. Lancet Neurol 6(12):1045–1053. https://doi.org/10.1016/S1474-4422(07)70270-3
Parkinson Study Group PI (2007) Mixed lineage kinase inhibitor CEP-1347 fails to delay disability in early Parkinson disease. Neurology 69(15):1480–1490. https://doi.org/10.1212/01.wnl.0000277648.63931.c0
Pratico D, Sung S (2004) Lipid peroxidation and oxidative imbalance: early functional events in Alzheimer’s disease. J Alzheimers Dis 6(2):171–175
Castellani RJ, Moreira PI, Liu G, Dobson J, Perry G, Smith MA, Zhu X (2007) Iron: the redox-active center of oxidative stress in Alzheimer disease. Neurochem Res 32(10):1640–1645. https://doi.org/10.1007/s11064-007-9360-7
Zhang YH, Wang DW, Xu SF, Zhang S, Fan YG, Yang YY, Guo SQ, Wang S et al (2018) Alpha-lipoic acid improves abnormal behavior by mitigation of oxidative stress, inflammation, ferroptosis, and tauopathy in P301S Tau transgenic mice. Redox Biol 14:535–548. https://doi.org/10.1016/j.redox.2017.11.001
Johnson WM, Wilson-Delfosse AL, Mieyal JJ (2012) Dysregulation of glutathione homeostasis in neurodegenerative diseases. Nutrients 4(10):1399–1440. https://doi.org/10.3390/nu4101399
Browne SE, Ferrante RJ, Beal MF (1999) Oxidative stress in Huntington’s disease. Brain Pathol 9(1):147–163
Lee J, Kosaras B, Del Signore SJ, Cormier K, McKee A, Ratan RR, Kowall NW, Ryu H (2011) Modulation of lipid peroxidation and mitochondrial function improves neuropathology in Huntington’s disease mice. Acta Neuropathol 121(4):487–498. https://doi.org/10.1007/s00401-010-0788-5
Volpe JJ, Kinney HC, Jensen FE, Rosenberg PA (2011) Reprint of “the developing oligodendrocyte: key cellular target in brain injury in the premature infant”. Int J Dev Neurosci 29(6):565–582. https://doi.org/10.1016/j.ijdevneu.2011.07.008
Back SA, Luo NL, Mallinson RA, O'Malley JP, Wallen LD, Frei B, Morrow JD, Petito CK et al (2005) Selective vulnerability of preterm white matter to oxidative damage defined by F2-isoprostanes. Ann Neurol 58(1):108–120. https://doi.org/10.1002/ana.20530
Inder T, Mocatta T, Darlow B, Spencer C, Volpe JJ, Winterbourn C (2002) Elevated free radical products in the cerebrospinal fluid of VLBW infants with cerebral white matter injury. Pediatr Res 52(2):213–218. https://doi.org/10.1203/00006450-200208000-00013
Brault S, Martinez-Bermudez AK, Roberts J 2nd, Cui QL, Fragoso G, Hemdan S, Liu HN, Gobeil F Jr et al (2004) Cytotoxicity of the E(2)-isoprostane 15-E(2t)-IsoP on oligodendrocyte progenitors. Free Radic Biol Med 37(3):358–366. https://doi.org/10.1016/j.freeradbiomed.2004.05.007
Welin AK, Svedin P, Lapatto R, Sultan B, Hagberg H, Gressens P, Kjellmer I, Mallard C (2007) Melatonin reduces inflammation and cell death in white matter in the mid-gestation fetal sheep following umbilical cord occlusion. Pediatr Res 61(2):153–158. https://doi.org/10.1203/01.pdr.0000252546.20451.1a
Back SA, Gan X, Li Y, Rosenberg PA, Volpe JJ (1998) Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced death caused by glutathione depletion. J Neurosci 18(16):6241–6253
Roberts RA, Aschner M, Calligaro D, Guilarte TR, Hanig JP, Herr DW, Hudzik TJ, Jeromin A et al (2015) Translational biomarkers of neurotoxicity: a health and environmental sciences institute perspective on the way forward. Toxicol Sci 148(2):332–340. https://doi.org/10.1093/toxsci/kfv188
Hagemeier J, Geurts JJ, Zivadinov R (2012) Brain iron accumulation in aging and neurodegenerative disorders. Expert Rev Neurother 12(12):1467–1480. https://doi.org/10.1586/ern.12.128
Sfera A, Bullock K, Price A, Inderias L, Osorio C (2017) Ferrosenescence: the iron age of neurodegeneration? Mech Ageing Dev 174:63–75. https://doi.org/10.1016/j.mad.2017.11.012
Zecca L, Gallorini M, Schunemann V, Trautwein AX, Gerlach M, Riederer P, Vezzoni P, Tampellini D (2001) Iron, neuromelanin and ferritin content in the substantia nigra of normal subjects at different ages: consequences for iron storage and neurodegenerative processes. J Neurochem 76(6):1766–1773
Pedini M, De Meo G, Ricci A, Bastianini L, Jacquignon P (1990) New heterocyclic derivatives of benzimidazole with germicidal activity--VII--2-(5′-nitro-2′-furyl or 2′-thienyl) benzimidazoles with different substituents in the 5-position. Farmaco 45(3):303–312
Xu J, Jia Z, Knutson MD, Leeuwenburgh C (2012) Impaired iron status in aging research. Int J Mol Sci 13(2):2368–2386. https://doi.org/10.3390/ijms13022368
Mollet IG, Patel D, Govani FS, Giess A, Paschalaki K, Periyasamy M, Lidington EC, Mason JC et al (2016) Low dose iron treatments induce a DNA damage response in human endothelial cells within minutes. PLoS One 11(2):e0147990. https://doi.org/10.1371/journal.pone.0147990
Sturm A, Ivics Z, Vellai T (2015) The mechanism of ageing: primary role of transposable elements in genome disintegration. Cell Mol Life Sci 72(10):1839–1847. https://doi.org/10.1007/s00018-015-1896-0
Orr WC (2016) Tightening the connection between transposable element mobilization and aging. Proc Natl Acad Sci U S A 113(40):11069–11070. https://doi.org/10.1073/pnas.1613350113
Wang YQ, Chang SY, Wu Q, Gou YJ, Jia L, Cui YM, Yu P, Shi ZH et al (2016) The protective role of mitochondrial ferritin on erastin-induced ferroptosis. Front Aging Neurosci 8:308. https://doi.org/10.3389/fnagi.2016.00308
Ivanov SD, Semenov AL, Kovan'ko EG, Yamshanov VA (2015) Effects of iron ions and iron chelation on the efficiency of experimental radiotherapy of animals with gliomas. Bull Exp Biol Med 158(6):800–803. https://doi.org/10.1007/s10517-015-2865-1
Sehm T, Rauh M, Wiendieck K, Buchfelder M, Eyupoglu IY, Savaskan NE (2016) Temozolomide toxicity operates in a xCT/SLC7a11 dependent manner and is fostered by ferroptosis. Oncotarget 7(46):74630–74647. https://doi.org/10.18632/oncotarget.11858
Fan Z, Wirth AK, Chen D, Wruck CJ, Rauh M, Buchfelder M, Savaskan N (2017) Nrf2-Keap1 pathway promotes cell proliferation and diminishes ferroptosis. Oncogenesis 6(8):e371. https://doi.org/10.1038/oncsis.2017.65
Chen D, Rauh M, Buchfelder M, Eyupoglu IY, Savaskan N (2017) The oxido-metabolic driver ATF4 enhances temozolamide chemo-resistance in human gliomas. Oncotarget 8(31):51164–51176. https://doi.org/10.18632/oncotarget.17737
Chung WJ, Sontheimer H (2009) Sulfasalazine inhibits the growth of primary brain tumors independent of nuclear factor-kappaB. J Neurochem 110(1):182–193. https://doi.org/10.1111/j.1471-4159.2009.06129.x
Lewerenz J, Baxter P, Kassubek R, Albrecht P, Van Liefferinge J, Westhoff MA, Halatsch ME, Karpel-Massler G et al (2014) Phosphoinositide 3-kinases upregulate system xc(−) via eukaryotic initiation factor 2alpha and activating transcription factor 4—a pathway active in glioblastomas and epilepsy. Antioxid Redox Signal 20(18):2907–2922. https://doi.org/10.1089/ars.2013.5455
Ye P, Mimura J, Okada T, Sato H, Liu T, Maruyama A, Ohyama C, Itoh K (2014) Nrf2- and ATF4-dependent upregulation of xCT modulates the sensitivity of T24 bladder carcinoma cells to proteasome inhibition. Mol Cell Biol 34(18):3421–3434. https://doi.org/10.1128/MCB.00221-14
Habib E, Linher-Melville K, Lin HX, Singh G (2015) Expression of xCT and activity of system xc(−) are regulated by NRF2 in human breast cancer cells in response to oxidative stress. Redox Biol 5:33–42. https://doi.org/10.1016/j.redox.2015.03.003
Abdalkader M, Lampinen R, Kanninen KM, Malm TM, Liddell JR (2018) Targeting Nrf2 to suppress ferroptosis and mitochondrial dysfunction in neurodegeneration. Front Neurosci 12:466. https://doi.org/10.3389/fnins.2018.00466
Lewerenz J, Ates G, Methner A, Conrad M, Maher P (2018) Oxytosis/ferroptosis-(re-) emerging roles for oxidative stress-dependent non-apoptotic cell death in diseases of the central nervous system. Front Neurosci 12:214. https://doi.org/10.3389/fnins.2018.00214
Kang R, Tang D (2017) Autophagy and ferroptosis—what’s the connection? Curr Pathobiol Rep 5(2):153–159. https://doi.org/10.1007/s40139-017-0139-5
Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh HJ 3rd, Kang R, Tang D (2016) Autophagy promotes ferroptosis by degradation of ferritin. Autophagy 12:1–4. https://doi.org/10.1080/15548627.2016.1187366
Song X, Zhu S, Chen P, Hou W, Wen Q, Liu J, Xie Y, Liu J et al (2018) AMPK-mediated BECN1 phosphorylation promotes ferroptosis by directly blocking system xc(−) activity. Curr Biol 28(15):2388–2399 e2385. https://doi.org/10.1016/j.cub.2018.05.094
Murphy TH, Malouf AT, Sastre A, Schnaar RL, Coyle JT (1988) Calcium-dependent glutamate cytotoxicity in a neuronal cell line. Brain Res 444(2):325–332
Miyamoto M, Murphy TH, Schnaar RL, Coyle JT (1989) Antioxidants protect against glutamate-induced cytotoxicity in a neuronal cell line. J Pharmacol Exp Ther 250(3):1132–1140
Maher P (2018) Potentiation of glutathione loss and nerve cell death by the transition metals iron and copper: implications for age-related neurodegenerative diseases. Free Radic Biol Med 115:92–104. https://doi.org/10.1016/j.freeradbiomed.2017.11.015
Maher P, van Leyen K, Dey PN, Honrath B, Dolga A, Methner A (2018) The role of Ca(2+) in cell death caused by oxidative glutamate toxicity and ferroptosis. Cell Calcium 70:47–55. https://doi.org/10.1016/j.ceca.2017.05.007
Nissen C, Gratwohl A, Speck B (1991) Management of aplastic anemia. Eur J Haematol 46(4):193–197
Bonnefont-Rousselot D, Lacomblez L, Jaudon M, Lepage S, Salachas F, Bensimon G, Bizard C, Doppler V et al (2000) Blood oxidative stress in amyotrophic lateral sclerosis. J Neurol Sci 178(1):57–62
Luo M, Wu L, Zhang K, Wang H, Zhang T, Gutierrez L, O'Connell D, Zhang P et al (2018) miR-137 regulates ferroptosis by targeting glutamine transporter SLC1A5 in melanoma. Cell Death Differ 25(8):1457–1472. https://doi.org/10.1038/s41418-017-0053-8
Zhang K, Wu L, Zhang P, Luo M, Du J, Gao T, O'Connell D, Wang G et al (2018) miR-9 regulates ferroptosis by targeting glutamic-oxaloacetic transaminase GOT1 in melanoma. Mol Carcinog 57:1566–1576. https://doi.org/10.1002/mc.22878
Acknowledgments
We thank Dr. Fei Yang from Capital Medical University for valuable comments and suggestions. We thank Claire Levine, MS, ELS, for assistance with manuscript editing.
Funding
This review paper was supported by American Heart Association Postdoctoral Fellowship Award 17POST33660191 to XL, American Heart Association Scientist Development Grant 16SDG30980031 to XH, and the Scientific Research Program of Beijing Municipal Commission of Education (grant no. KM201710025005 to YW). JW is supported by the National Institutes of Health (R21NS102899) and a Stimulating and Advancing ACCM Research (StAAR) grant from the Department of Anesthesiology and Critical Care Medicine, John Hopkins University.
Author information
Authors and Affiliations
Contributions
AW, YW, WW, QL, and JW wrote the manuscript; AW, YW, XH, XL, QL, and JW revised the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Weiland, A., Wang, Y., Wu, W. et al. Ferroptosis and Its Role in Diverse Brain Diseases. Mol Neurobiol 56, 4880–4893 (2019). https://doi.org/10.1007/s12035-018-1403-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1403-3