Abstract
Thyroid hormone (TH) is essential for the proper development of mammalian central nervous system. TH deficiency during the critical period of brain development results in permanent cognitive and neurological impairments. Members of the protein kinase C (PKC) family play a key role in the regulation of cellular functions in the nervous system. Alteration of PKC can be involved in the pathogenesis of neuronal disorders. This review details recent progress made in determining the roles played by PKC isoforms in developing hypothyroid rat brain. Evidence indicates that hippocampus down-regulation of PKCβ and PKCγ may be related to impaired learning and memory observed in perinatal hypothyroid rats. Enhanced PKCα activity in neonatal hypothyroid brain may bring about oxidative stress and cause brain damage. The activated pro-apoptotic PKCs including PKCδ can cause extensive apoptosis in the hypothyroid rat brain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thyroid hormone (TH) is essential for the proper development and function of the mammalian central nervous system (CNS). TH deficiency during critical period of brain development results in permanent and profound influences on neurological functions that contribute to severe cognitive and neurological impairments [1, 2]. Protein kinase C (PKC) isoforms play an important functional role in controlling memory-relevant signaling process. The brain, especially in the vital neural structures that are involved in cognition and mood regulation, contains the highest concentration of PKC in the body [3]. PKC inhibition and functional impairment have been consistently found to significantly impair performance of cognitive tasks. We and others have found oxidative stress in the developing hypothyroid rat brain, and we also found enhanced PKC activity in hypothyroid brain [4–6]. Previous studies in hypothyroid rats during brain development have also shown an enhanced apoptosis in hippocampus [7]. Is there any relationship between the PKC, the oxidative stress and extensive apoptosis in the perinatal hypothyroid rat brain? The purpose of this article is to review recent information regarding the PKC isoforms involved in impaired memory and learning ability, oxidative stress and apoptosis in developing hypothyroid rat brain.
The PKC family
The Ser/Thr PKC family comprises ~2 % of the human kinome and they are broadly conserved in eukaryotes. Several decades of research have documented that members of the PKC family are key signaling molecules involved in diverse cellular functions. According to structural features and activation requirements, the PKC family is divided into three groups. The conventional PKCs (cPKCs; PKCα, PKCβI, PKCβII, and PKCγ) require diacylglycerol and calcium for activation. The novel PKCs (nPKCs), which include PKCδ, PKCε, PKCθ and PKCη, are similarly activated by diacylglycerol and phospholipids, but they do not respond directly to calcium. And the third group atypical PKCs (aPKCs), which include PKCι/λ and PKCζ, can be activated in the absence of diacyglycerol and calcium [8, 9].
Protein kinase C isoforms are important signaling molecules, mediating various extracellular signals into the cell and triggering intracellular signaling events [10]. They are ubiquitously expressed in the CNS and are activated by either Ca2+, phospholipids and diacylglycerol, phorbol-esters, or other agents [11]. The PKC signaling pathway plays an important and regulatory role in a wide range of vital biological functions and processes, such as proliferation, altered gene expression, synaptic plasticity, neuronal injury, synaptic remodeling/repairing and synaptogenesis, differentiation, cell growth and apoptosis, and oncogenesis. Protein kinase C inhibition and functional deficits or abnormally activation will lead to a variety of brain function impairment [12–15].
Protein kinase C isoforms are involved in impaired memory and learning ability in developing hypothyroid brain
Protein kinase C isozymes are important signaling molecules in learning and memory [16–18], as they play critical roles in neurotransmitter release and synaptic plasticity, including long-term potentiation (LTP) and long-term depression (LTD), and deletion of specific PKC genes results in deficits in learning. Conversely, genetic activation of PKC pathways in small groups of hippocampal neurons enhances learning in specific paradigms [19]. The brain, especially the hippocampus and related vital neural structures that are involved in cognition and mood regulation, contains the highest concentration of PKC in the body [3]. Neural events and activated inputs that occur in learning and memory activate PKC and associative learning produces translocation of PKC activity from the cytosolic to the membrane compartment of the CA1 region of the hippocampus [20]. Protein kinase C activation with bryostatin-1 has been found to enhance spatial learning and memory in rats [21]. Protein kinase C inhibition and functional deficits impair cognition. PKCβ is predominantly expressed in area CA1 of the hippocampus [12]. In mice with a deficit in PKCβ, learning of both cued and contextual fear conditioning is impaired [22]. PKCβ1 is one of behaviorally relevant PKC isoforms that was demonstrated to participate in the early synaptic events responsible for the acquisition and consolidation of an inhibitory avoidance learning [23], and bilateral microinjection of a selective inhibitor of PKCβ1 isozyme into the CA1 of the dorsal hippocampus produced amnesia [24]. PKCγ, solely in the brain [25], is believed to play a vital role in LTP and spatial memory formation [26]. It is reported that a null mutation of the PKCγ gene causes moderate impairments in spatial learning [27]. Other studies have shown that training in a spatial discrimination task increases PKCγ immunoreactivity in hippocampal neurons [28]. PKCγ signaling cascade is involved in the consolidation of previously acquired information that is crucial for spatial navigation [29].
Accumulating researches have pointed out important roles of various isoforms of PKC in the formation of memory traces and of their functional insufficiency in pathogenesis of memory disorders. Hypothyroidism is one of the diseases with impaired memory and learning ability. The hippocampus has an important role in many types of learning and memory, whereas impaired hippocampus leads to impaired performance on a variety of behavioral learning ability. As research reported the hippocampus is one of the brain regions particularly vulnerable to the disruption of the TH [30]. Though TH receptor expression is widespread in the brain, some research demonstrated selective sensitivity of the hippocampus to postnatal TH deficiency [31]. Developing hypothyroidism could cause reduced cell numbers and granular layer area in the dentate gyrus (DG) of the hippocampus [32], and decreased branching points numbers of the apical and basal dendritic trees of the pyramidal cells in the cornu ammonis (CA) [33]. Our group has found that TH deficiency during rat brain development will cause hippocampal down-regulation of PKCβ and PKCγ, and this down-regulation may be related to impaired learning and memory observed in perinatal hypothyroid rats [34].
PKC activation is involved in oxidative stress in developing hypothyroid brain
Members of the PKC family play a key role in the regulation of cellular functions in the nervous system. Besides the physiologic regulatory functions, researches indicate that alteration of PKC can be involved in the pathogenesis of neuronal degeneration, such as observed in excitotoxic damage [35], ischemia [36]. When cortical cultures were exposed to zinc, the membrane PKC activity of cultured cortical cells was increased, resulting in excess free radical generation and cell death. The increased PKC activation may be a key step linking zinc influx to subsequent oxidative neuronal injury. As oxidative stress has been implicated as an important injury mechanism in a number of pathological conditions in the CNS, it is tempting to speculate that zinc influx may be a key trigger for oxidative stress through activation of membrane PKC [37].
In 6-n-propylthiouracil (PTU)-induced perinatal hypothyroidism, significantly elevated levels of H2O2 and lipid peroxidation were observed in developing rat cerebellum [38]. Our group also found that as compared to age matched controls, protein carbonyl contents and thiobarbital acid reactive substances in developing hypothyroid rat brain were increased significantly [39]. In addition, our previous researches have showed that perinatal hypothyroidism can enhance Goα mRNA levels in the temporal cortex, sensorimotor cortex, piriform cortex, amygdala, hippocampal CA1-4 subfields, DG, arcuate nucleus (AR) and ventromedial hypothalamic nucleus (VMH) of hypothalamus [40]. Goα is a guanine nucleotide-binding regulatory protein α subunit which is mainly distributed in the CNS and is the most abundant α subunit of G protein in the brain of mammalian animals [41, 42]. It is suggested that activation of Goα can subsequently lead to increased activity of PKC [43]. Sustained activity of PKC can result in depletion of cellular energy stores, alter the balance between phosphorylated and non-phosphorylated states, disrupt cellular homeostasis leading to cell damage and death in the brain [44]. In this regard, we further investigated the activity of PKC in developing hypothyroid rat brain. And our results showed increased PKC activity in the brain of hypothyroid rats. As compared to age matched controls, a very large increase was noticed in brain PKC activity in hypothyroid pups both in cytosol and membrane fractions. The change of membrane PKC activity was more marked than that of cytosol fractions, and hypothyroidism led to a higher ratio of membrane/cytosol PKC activity [5].
Following cytotoxic PKC activation, it has not been clear what downstream events would mediate the oxidative stress. Possibilities include destabilization of intracellular calcium homeostasis, activation of proteases or lipases, and excessive phosphorylation of regulatory proteins [45–48]. All of these may nonspecifically result in excess free radical generation and cell death. Another possibility is that PKC may directly activate enzymes linked to free radical generation. For example, in vascular tissues of diabetes, it has been shown that PKC activation can phosphorylate NADPH oxidase, which in turn induce the increase in reactive oxygen species (ROS) production [49]. In the context of this, we hypothesize that during rat brain development, TH deficiency can cause elevated PKC activity, which subsequently bring about oxidative stress and cause brain damage.
PKC isoforms are involved in enhanced apoptosis in developing hypothyroid brain
TH plays an important role in the modulation of apoptosis during brain development. One research shows that neonatal hypothyroidism causes extensive apoptosis in the internal granular layer of the cerebellum [50–53]. Another research shows that perinatal hypothyroidism enhances apoptosis in the developing rat cerebral cortex [54]. Previous studies in hypothyroid rats during brain development have also shown an enhanced apoptosis in hippocampus [7, 31, 55, 56]. Apoptosis involves activation of a caspase cascade that directly causes disassembly of cellular structures [57, 58]. Cerebral cortices in the hypothyroid group exhibited significantly increased activation of caspase-3 and -7, decreased levels of anti-apoptotic proteins Bcl-2 and Bcl-xL, and increased levels of pro-apoptotic protein Bax [54]. Congenital hypothyroidism increases not only the extent but also the duration of apoptosis by down-regulation of the anti-apoptotic gene Bcl-2 and maintaining a high level of the pro-apoptotic gene Bax. The expression of caspase-3 in the cytosol of hypothyroid pups was significantly higher as compared with that of the age-matched controls [55, 56].
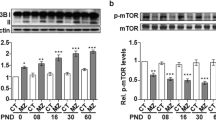
Apoptosis is the physiological process of cell suicide that occurs normally during development or as a stress response, for example to agents that damage DNA beyond the capacity of repair mechanisms [59]. Early studies with tumour-promoting phorbol esters highlighted a major role for PKC in regulating apoptosis. More recently, the roles played by specific members of the PKC family have begun to emerge [60]. PKCs exhibit both direct and indirect effects on the extrinsic and intrinsic apoptotic pathway machinery, being either pro-apoptotic or anti-apoptotic depending on PKC isozyme and cell type [61, 62]. With the discovery and development of more isoform-specific activators and inhibitors, increased precision in the targeting of PKC isoforms has become possible [63]. The results of studies employing such agents have led to a generalized consensus over the roles of PKC isoforms as regulators of apoptosis. Conventional and atypical PKCs are generally considered to be predominantly anti-apoptotic, being principally involved in promoting cell survival and proliferation. The novel PKCs, however, generally have a tumour suppressor function and are regarded as pro-apoptotic proteins. PKCα and PKCδ are two of the PKCs better characterized with respect to their important functions in preventing and promoting apoptosis, respectively. It is interesting that these two protein kinases have been demonstrated as mediating key roles in apoptosis, as these isoforms are present in the majority of cells [64], including neurons [65]. PKC isoforms most predominantly associated with apoptosis promotion are PKCδ. PKCδ, a member of the novel PKC subfamily, is actively involved in cell apoptosis in a stimulus and tissue specific manner; it both regulates the expression and function of apoptotic related proteins and is itself a target for caspases [66]. PKCδ is a substrate for the effector caspase, caspase-3, whose active catalytic domain is thought to be essential for apoptosis [60]. Some studies suggest that activation of PKCδ and caspase-3 occurs rapidly in response to apoptotic stimuli and that both proteins accumulate in the nucleus under these conditions [67]. In our studies, we have found increased PKCδ expression in both cortex and hippocampus of developing hypothyroid rat brain (As shown in Fig. 1), this may be related to enhanced apoptosis in hypothyroid rats during brain development. Although the majority of published work suggests a suppressive role for PKCα in apoptosis, conflicting data indicating a pro-apoptotic function have still been observed. In human prostate cancer cell lines, the presence of PKCα in the mitochondrial membrane was associated with apoptosis [68]. This pro-apoptotic role was in agreement with results from epithelial cell lines of the tonsil [69]. In a research of ours, we found developing hypothyroid pups treated with bisindolylmaleimide XI (a selective PKC inhibitor for PKCα) exhibit some extent of improved performance in the Morris water maze test. And we also found increased PKCα expression and enhanced PKCα activity in hypothyroid rat brain [5]. So we are tempting to speculate that PKC α is involved in impaired brain development observed in perinatal hypothyroid rat brain through pro-apoptotic activity. But this presumption needs to be verified in future studies.
Conclusions
Thyroid hormones (triiodothyronine, T3 and thyroxine, T4) exert well-defined effects on the development and function of the CNS. TH deficiency during the perinatal period causes neurological abnormalities, such as a reduction in dendritic elaborations, neurite outgrowth, synaptogenesis, and myelination as well as delayed cell differentiation and migration [70–72]. But how TH deficiency influences brain development is not clear. PKCs play a key role in the regulation of cellular functions in the nervous system. Besides the physiologic regulatory functions, alteration of PKC can be involved in the pathogenesis of neuronal disorders. Down-regulation of certain PKC isoforms such as PKCβ and PKCγ is involved in impaired memory and learning ability. Enhanced activity of PKCs in the hypothyroid brain can bring about overproduction of free radicals and oxidative stress can cause brain damage. Oxidative stress can also induce neuron apoptosis by activating pro-apoptotic PKC isoforms including PKCδ and also PKCα. In conclusion, PKCs are involved in impaired brain development observed in perinatal hypothyroid rat through different ways. This will throw light on the understandings of how thyroid hormones influence brain development. But all these are presumptions that need to be verified in future studies.
References
Bernal J, Guada o-Ferraz A, Morte B (2003) Perspectives in the study of thyroid hormone action on brain development and function. Thyroid 13(11):1005–1012
Tremont G, Stern RA, Westervelt HJ, Bishop CL, Davis JD (2003) Neurobehavioral functioning in thyroid disorders. Med Health R I 86(10):318–322
Saito N, Kikkawa U, Nishizuka Y, Tanaka C (1988) Distribution of protein kinase C-like immunoreactive neurons in rat brain. J Neurosci 8(2):369
H-m ZHANG, SU Q, LUO M (2007) Effect of hypothyroidism on oxidative stress status in developing rat brain. J Shanghai Jiaotong Univ (Med Sci) 3:015
Zhang HM, Lin N, Dong Y, Su Q, Luo M (2013) Protein kinase cα is involved in impaired perinatal hypothyroid rat brain development. J Neurosci Res 91(2):211–219
Liu W, Dong J, Wang Y, Xi Q, Chen J (2010) Developmental iodine deficiency and hypothyroidism impaired in vivo synaptic plasticity and altered PKC activity and GAP-43 expression in rat hippocampus. Nutr Neurosci 13(5):213–221
Huang X, Yin H, Ji C, Qin Y, Yang R, Zhao Z (2008) Effects of perinatal hypothyroidism on rat behavior and its relation with apoptosis of hippocampus neurons. J Endocrinol Invest 31(1):8–15
Mellor H, Parker PJ (1998) The extended protein kinase C superfamily. Biochem J 332:281–292
Rosse C, Linch M, Kermorgant S, Cameron AJM, Boeckeler K, Parker PJ (2010) PKC and the control of localized signal dynamics. Nat Rev Mol Cell Biol 11(2):103–112
Tanaka C, Nishizuka Y (1994) The protein kinase C family for neuronal signaling. Annu Rev Neurosci 17(1):551–567
Takai Y, Kishimoto A, Inoue M, Nishizuka Y (1977) Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. I. Purification and characterization of an active enzyme from bovine cerebellum. J Biol Chem 252(21):7603
Sun MK, Alkon DL (2009) Protein kinase C activators as synaptogenic and memory therapeutics. Arch Pharm 342(12):689–698
Choi BH, Chae HD, Park TJ, Oh J, Lim J, Kang SS et al (2004) Protein kinase C regulates the activity and stability of serotonin N-acetyltransferase. J Neurochem 90(2):442–454
Poole AW, Pula G, Hers I, Crosby D, Jones ML (2004) PKC-interacting proteins: from function to pharmacology. Trends Pharmacol Sci 25(10):528–535
Young KW, Garro MA, Challiss R, Nahorski SR (2004) NMDA-receptor regulation of muscarinic-receptor stimulated inositol 1, 4, 5-trisphosphate production and protein kinase C activation in single cerebellar granule neurons. J Neurochem 89(6):1537–1546
Bank B, Deweer A, Kuzirian AM, Rasmussen H, Alkon DL (1988) Classical conditioning induces long-term translocation of protein kinase C in rabbit hippocampal CA1 cells. Proc Natl Acad Sci 85(6):1988–1992
Amadio M, Battaini F, Pascale A (2006) The different facets of protein kinases C: old and new players in neuronal signal transduction pathways. Pharmacol Res 54(5):317–325
Sacktor TC (2008) PKMζ, LTP maintenance, and the dynamic molecular biology of memory storage. Prog Brain Res 169:27–40
Zhang G, Liu M, Cao H, Kong L, Wang X, O’Brien JA et al (2009) Improved spatial learning in aged rats by genetic activation of protein kinase C in small groups of hippocampal neurons. Hippocampus 19(5):413–423
Olds JL, Anderson ML, McPhie DL, Staten LD, Alkon DL (1989) Imaging of memory-specific changes in the distribution of protein kinase C in the hippocampus. Science 245(4920):866
Sun MK, Alkon DL (2005) Dual effects of bryostatin-1 on spatial memory and depression. Eur J Pharmacol 512(1):43–51
Weeber EJ, Atkins CM, Selcher JC, Varga AW, Mirnikjoo B, Paylor R et al (2000) A role for the β isoform of protein kinase C in fear conditioning. J Neurosci 20(16):5906
Paratcha G, Furman M, Bevilaqua L, Cammarota M, Vianna M, de Stein ML et al (2000) Involvement of hippocampal PKC [beta] I isoform in the early phase of memory formation of an inhibitory avoidance learning. Brain Res 855(2):199–205
Wu J, Song TB, Li YJ, He KS, Ge L, Wang LR (2007) Prenatal restraint stress impairs learning and memory and hippocampal PKCbeta1 expression and translocation in offspring rats. Brain Res 1141:205–213
Purkayastha S, Fernando SS, Diallo S, Cohen L, Ranasinghe B, Levano K et al (2009) Regulation of protein kinase C isozymes during early postnatal hippocampal development. Brain Res 1288:29–41
Colombo PJ, Wetsel WC, Gallagher M (1997) Spatial memory is related to hippocampal subcellular concentrations of calcium-dependent protein kinase C isoforms in young and aged rats. Proc Natl Acad Sci USA 94(25):14195
Abeliovich A, Paylor R, Chen C, Kim JJ, Wehner JM, Tonegawa S (1993) PKC [gamma] mutant mice exhibit mild deficits in spatial and contextual learning. Cell 75(7):1263–1271
Van der Zee E, Compaan J, De Boer M, Luiten P (1992) Changes in PKC gamma immunoreactivity in mouse hippocampus induced by spatial discrimination learning. J Neurosci 12(12):4808
Alvarez-Jaimes L, Betancourt E, Centeno-Gonzá¢lez M, Feliciano-Rivera MZ, Rodriguez D, Ortiz SP et al (2004) Spatial learning in rats is impaired by microinfusions of protein kinase C-[gamma] antisense oligodeoxynucleotide within the nucleus accumbens. Neurobiol Learn Mem 81(2):120–136
Madeira M, Sousa N, Lima-Andrade M, Calheiros F, Cadete-Leite A, Paula-Barbosa M (1992) Selective vulnerability of the hippocampal pyramidal neurons to hypothyroidism in male and female rats. J Comp Neurol 322(4):501–518
Zhang L, Blomgren K, Kuhn HG, Cooper-Kuhn CM (2009) Effects of postnatal thyroid hormone deficiency on neurogenesis in the juvenile and adult rat. Neurobiol dis 34(2):366–374
Rami A, Rabie A, Patel A (1986) Thyroid hormone and development of the rat hippocampus: cell acquisition in the dentate gyrus. Neuroscience 19(4):1207–1216
Rami A, Patel A, Rabie A (1986) Thyroid hormone and development of the rat hippocampus: morphological alterations in granule and pyramidal cells. Neuroscience 19(4):1217–1226
Zhang HM, Lin N, Dong Y, Su Q, Luo M (2011) Effect of perinatal thyroid hormone deficiency on expression of rat hippocampal conventional protein kinase C isozymes. Mol Cell Biochem 353(1–2):65–71
Felipo V (1993) Inhibitors of protein kinase C prevent the toxicity of glutamate in primary neuronal cultures. Brain Res 604(1–2):192–196
Louis JC, Magal E, Yavin E (1988) Protein kinase C alterations in the fetal rat brain after global ischemia. J Biol Chem 263(36):19282
Noh KM, Kim YH, Koh JY (1999) Mediation by membrane protein kinase C of zinc-induced oxidative neuronal injury in mouse cortical cultures. J Neurochem 72(4):1609–1616
Bhanja S, Chainy G (2010) PTU-induced hypothyroidism modulates antioxidant defence status in the developing cerebellum. Int J Dev Neurosci 28(3):251–262
Zhang HM, Su Q, Luo M (2007) Effect of hypothyroidism on oxidative stress status in developing rat brain. J Shanghai Jiaotong univ (Med Sci) 27(3):286–288
Cai DS, Su Q, Chen Y, Luo M (2000) Effect of thyroid hormone deficiency on developmental expression of Go [alpha] gene in the brain of neonatal rats by competitive RT-PCR and in situ hybridization histochemistry. Brain Res 864(2):195–204
Brabet P, Dumuis A, Sebben M, Pantaloni C, Bockaert J, Homburger V (1988) Immunocytochemical localization of the guanine nucleotide-binding protein Go in primary cultures of neuronal and glial cells. J Neurosci 8(2):701
Trevor Young L, Warsh JJ, Li PP, Siu KP, Becker L, Gilbert J et al (1991) Maturational and aging effects on guanine nucleotide binding protein immunoreactivity in human brain. Dev Brain Res 61(2):243–248
Worley PF, Baraban JM, Van Dop C, Neer EJ, Snyder SH (1986) Go, a guanine nucleotide-binding protein: immunohistochemical localization in rat brain resembles distribution of second messenger systems. Proc Natl Acad Sci USA 83(12):4561
Pavlakovic G, Eyer CL, Isom GE (1995) Neuroprotective effects of PKC inhibition against chemical hypoxia. Brain Res 676(1):205–211
Siesjö BK (1990) Calcium in the brain under physiological and pathological conditions. Eur Neurol 30(Suppl. 2):3–9
Mattson MP (1991) Evidence for the involvement of protein kinase C in neurodegenerative changes in cultured human cortical neurons. Exp Neurol 112(1):95–103
Trump BF, Berezesky IK (1992) The role of cytosolic Ca2+ in cell injury, necrosis and apoptosis. Curr Opin Cell Biol 4(2):227–232
Orrenius S, McConkey DJ, Nicotera P (1991) Role of calcium in toxic and programmed cell death. Adv Exp Med Biol 283:419–425
Inoguchi T, Sonta T, Tsubouchi H, Etoh T, Kakimoto M, Sonoda N et al (2003) Protein kinase C-dependent increase in reactive oxygen species (ROS) production in vascular tissues of diabetes: role of vascular NAD (P) H oxidase. J Am Soc Nephrol 14(suppl 3):S227
Neveu I, Arenas E (1996) Neurotrophins promote the survival and development of neurons in the cerebellum of hypothyroid rats in vivo. J Cell Bio 133(3):631
Xiao Q, Nikodem VM (1998) Apoptosis in the developing cerebellum of the thyroid hormone deficient rat. Front Biosci 3:a52–a57
Singh R, Upadhyay G, Kumar S, Kapoor A, Kumar A, Tiwari M et al (2003) Hypothyroidism alters the expression of Bcl-2 family genes to induce enhanced apoptosis in the developing cerebellum. J Endocrinol 176(1):39
Singh R, Upadhyay G, Godbole M (2003) Hypothyroidism alters mitochondrial morphology and induces release of apoptogenic proteins during rat cerebellar development. J Endocrinol 176(3):321
Kumar A, Sinha RA, Tiwari M, Pal L, Shrivastava A, Singh R et al (2006) Increased pro-nerve growth factor and p75 neurotrophin receptor levels in developing hypothyroid rat cerebral cortex are associated with enhanced apoptosis. Endocrinology 147(10):4893
Huang X, Yang R, Zhao Z, Ji C, Yang R (2005) Mechanism for apoptosis of hippocampus neuron induced by hypothyroidism in perinatal rats. Zhejiang Da Xue Xue Bao Yi Xue Ban 34(4):298–303
Huang X, Zhao Z, Ji C (2005) Effects of hypothyroidism on apoptosis and the expression of Bcl-2 and Bax gene in the neonatal rat hippocampus neurons. Zhonghua Er Ke Za Zhi 43(1):48–52
Yuan J, Yankner BA (2000) Apoptosis in the nervous system. Nature 407(6805):802–809
Thornberry NA, Lazebnik Y (1998) Caspases: enemies within. Science 281(5381):1312
Meier P, Finch A, Evan G (2000) Apoptosis in development. Nature 407(6805):796–801
Gutcher I, Webb P, Anderson N (2003) The isoform-specific regulation of apoptosis by protein kinase C. Cell Mol Life Sci 60(6):1061–1070
Martelli AM, Evangelisti C, Nyakern M, Manzoli FA et al (2006) Nuclear protein kinase C. Biochim Biophys Acta 1761(5–6):542–551
Reyland ME, Bradford AP (2010) “PKC and the control of apoptosis”. Protein Kinase C Cancer Signal Ther 189–222
Dempsey EC, Newton AC, Mochly-Rosen D, Fields AP, Reyland ME, Insel PA et al (2000) Protein kinase C isozymes and the regulation of diverse cell responses. Am J Physiol Lung Cell Mol Physiol 279(3):L429
Cross TG, Scheel-Toellner D, Henriquez NV, Deacon E, Salmon M, Lord JM (2000) Serine/threonine protein kinases and apoptosis. Exp Cell Res 256(1):34–41
Maher P (2001) How protein kinase C activation protects nerve cells from oxidative stress-induced cell death. J Neurosci 21(9):2929
Brodie C, Blumberg P (2003) Regulation of cell apoptosis by protein kinase C δ. Apoptosis 8(1):19–27
DeVries-Seimon TA, Ohm AM, Humphries MJ, Reyland ME (2007) Induction of apoptosis is driven by nuclear retention of protein kinase C δ. J Biol Chem 282(31):22307
Powell C, Brittis N, Stec D, Hug H, Heston W, Fair W (1996) Persistent membrane translocation of protein kinase C alpha during 12-0-tetradecanoylphorbol-13-acetate-induced apoptosis of LNCaP human prostate cancer cells. Cell Growth Differ: Mol Bio J Am Assoc Cancer Res 7(4):419
Knox KA, Johnson GD, Gordon J (1993) A study of protein kinase C isozyme distribution in relation to Bcl-2 expression during apoptosis of epithelial cells in vivo. Exp Cell Res 207(1):68–73
Bernal J, Nunez J (1995) Thyroid hormones and brain development. Eur J Endocrinol 133(4):390–398
Koibuchi N, Chin WW (2000) Thyroid hormone action and brain development. Trends Endocr Metab 11(4):123–128
Porterfield SP, Hendrich CE (1993) The role of thyroid hormones in prenatal and neonatal neurological development—current perspectives. Endocr Rev 14(1):94–106
Acknowledgments
This study was supported by a Grant from the National Natural Science Foundation (81300642). We are most grateful to the editor and to the reviewers for the precious comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, HM., Su, Q. PKC in developmental hypothyroid rat brain. Neurol Sci 35, 1161–1166 (2014). https://doi.org/10.1007/s10072-014-1716-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-014-1716-6