Abstract
Non-ketotic hyperglycinemia (NKH) is a severe neurological disorder caused by defects in glycine (GLY) catabolism and characterized by a high cerebrospinal fluid/plasma GLY ratio. Treatment is often ineffective and limited to the control of symptoms and detoxification of GLY. In the present work, we investigated the in vivo effects of GLY intracerebroventricular administration on oxidative stress parameters in rat striatum, cerebral cortex, and hippocampus. In vitro effects of GLY were also evaluated in striatum. The effects of bezafibrate (BEZ), a potential neuroprotective agent, on the possible alterations caused by GLY administration were further evaluated. Our in vivo results showed that GLY increased the activities of the antioxidant enzymes superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione reductase (GR), and glucose-6-phosphate dehydrogenase (G6PDH) in striatum. Furthermore, GLY decreased the concentrations of total glutathione and reduced glutathione (GSH), as well as GSH/oxidized glutathione ratio in vivo in hippocampus. In vitro data also showed that GLY induced lipid peroxidation and decreased GSH in striatum. Regarding the effects of BEZ, we found that GLY-induced increase of GPx, SOD, and GR activities was attenuated or prevented by this compound. However, BEZ did not alter GLY-induced decrease of GSH in hippocampus. We hypothesize that GLY-induced increase of the activities of antioxidant enzymes in striatum occurs as a mechanism to avoid accumulation of reactive oxygen species and consequent oxidative damage. Furthermore, since BEZ prevented GLY-induced alterations, it might be considered as an adjuvant therapy for NKH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-ketotic hyperglycinemia (NKH) is an inborn error of glycine (GLY) catabolism caused by a defect in the GLY cleavage system (GCS), a mitochondrial multienzymatic complex present in the brain, liver, kidney, and placenta [1]. Mutations in two GCS-encoding genes have been associated with NKH: the AMT and the GLDC genes, the latter accounting for over 80% of the genetic defects observed in patients [2]. The worldwide incidence of NKH is unknown, but it is estimated to be 1:55,000 in Finland, and 1:63,000 newborns in British Columbia, Canada [3, 4]. Symptoms usually start early in life and are predominantly neurological, including delayed development, hypotonia, and seizures, which may lead to premature death. Hiccups and apnea are also commonly observed [3, 4]. Brain MRI scans show hypoplasia of the corpus callosum, decreased myelination, and enlargement of the ventricles, among other morphological abnormalities [5]. The main biochemical characteristic of NKH is GLY accumulation in cerebrospinal fluid (CSF) and plasma, as well as in other tissues, reaching concentrations as high as 7.3 mM in the brain [1, 6]. Diagnosis is based on the CSF/plasma GLY ratio, since elevation of GLY levels in urine or plasma may occur in several organic acidurias. In this context, a ratio above 0.08 is indicative of NKH, but any value above 0.04 deserves enzymatic and mutational analysis in order to confirm the diagnosis [4].
Treatment of NKH focuses on two main strategies: GLY level reduction and seizure control [1]. Reports suggest that the most efficient treatment consists of combining a GLY scavenger, usually sodium benzoate, and N-methyl-d-aspartate (NMDA) receptor antagonists, such as ketamine or dextromethorphan [3, 7]. Seizures may persist, especially when treatment cannot be initiated in the neonatal period. In these cases, an adjuvant therapy with antiepileptic drugs or a ketogenic diet is necessary [1, 8]. However, given that a correlation between GLY concentrations and disease severity has not yet been established, and most patients do not respond satisfactorily to anticonvulsant therapy, few options remain. Therefore, it is interesting to investigate alternatives to the traditional therapeutic strategies.
Bezafibrate (BEZ) is a drug from the group of fibrates that are widely used to treat dyslipidemia [9]. BEZ is a pan-agonist of peroxisome proliferator-activated receptors (PPAR), whose activation sets in motion signaling pathways and transcription factors responsible for the expression of genes involved in lipid homeostasis, energy metabolism, antioxidant defenses, and mitochondrial biogenesis [10]. Besides its use for dyslipidemias, BEZ has recently been considered for the treatment of common neurological disorders, such as Huntington’s and Parkinson’s diseases [11]. In this context, studies with animal models for these disorders indicate that the upregulation of antioxidant genes is a beneficial effect induced by BEZ [12].
Although respiratory dysfunction and seizures observed in patients with NKH have been attributed to GLY, and its role as both inhibitory and excitatory neurotransmitter in the brainstem and the cerebral cortex, respectively, are well known, the exact mechanisms responsible for brain abnormalities in this disorder are still unclear [4]. GLY is a co-agonist of NMDA receptor so that it is suggested that elevated levels of this amino acid induce excitotoxicity [13]. Furthermore, in vitro and ex vivo studies have shown that GLY induces oxidative damage, mitochondrial dysfunction, and glial reactivity in rat cerebral cortex and striatum [14,15,16,17]. It was also demonstrated that mice with deficient GCS are predisposed to neural tube defects, growth retardation, and abnormal brain morphology [18].
Since GLY has been shown to induce neurotoxicity in different animal models, we decided to investigate the effects of an intracerebroventricular administration of GLY on oxidative stress parameters in brain structures of rats, specifically striatum, hippocampus, and cerebral cortex. The in vitro effects of GLY were also evaluated in rat striatum. Moreover, considering that BEZ is an emerging drug in the treatment of neurological disorders, we evaluated the potential benefits of a BEZ pre-treatment on the possible deleterious in vivo effects caused by GLY.
Material and Methods
Animals
Twenty-three- or thirty-day-old Wistar rats, obtained from the Central Animal House of the Department of Biochemistry, ICBS, Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brazil, were used. The animals were maintained on a 12:12-h light/dark cycle (lights on 07:00–19:00 h) in air-conditioned constant temperature (22 ± 1 °C) colony room, with free access to water and 20% (w/w) protein commercial chow (SUPRA, Porto Alegre, RS, Brazil). The National Animal Rights Regulation (Law 11.794/2008) and the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication 85-23, revised 1996) were followed, and all efforts were directed towards minimizing the number of animals used, as well as their stress and suffering.
Bezafibrate Pre-treatment
Rats were divided into three groups: vehicle + NaCl (control), vehicle + GLY and BEZ + GLY. The latter group received BEZ (prepared in corn oil) by gavage at a dose of 30 mg/kg/day for 7 days [19, 20] while the other groups received corn oil (vehicle). The treatment occurred once a day at the same time (11:00 am–1:00 pm) and was performed by trained personnel. Experiments performed in our laboratory showed that BEZ per se, at the dose of 30 mg/kg/day, does not alter the biochemical parameters evaluated in the present study [19].
Glycine Administration
After BEZ treatment, the animals were anesthetized with isoflurane (4%). Anesthesia was induced by placing the animals in a simple lidded jar and exposing them to a cotton ball embedded with isoflurane. After confirming that the rats were anesthetized, they were placed on a stereotaxic apparatus, two small holes were drilled in the skull and 5 μmol of either NaCl or GLY (2 μL of a 2.5 M solution, pH 7.4) were injected bilaterally into the ventricles (0.6 mm posterior to the bregma, 1.1 mm lateral to the midline, and 3.2 mm ventral from dura [21]. The solutions were injected over 2 min (1 μL/min) via a needle connected by a polyethylene tube to a 10-μL Hamilton syringe, and the needle was left in place for another minute before being carefully removed. The experimental protocol was based on a previous work [14] and approved by the Ethics Committee for Animal Research of the Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil, and followed the NIH Guide for the Care and Use of Laboratory Animals (NIH publication 85-23, revised 1985). All efforts were made to minimize the number of animals used and their suffering.
Thirty minutes after GLY injection, the animals were euthanized by decapitation and had their hippocampi, striata, and cerebral cortices dissected on an ice-cold Petri dish. In some experiments, the animals were euthanized 15 min after GLY injection and hippocampi were dissected. The brain structures were then homogenized (1:10, w/v) in a 20 mM sodium phosphate buffer containing 140 mM KCl, pH 7.4, and centrifuged at 750g for 10 min. The supernatant was collected and used for measurement of oxidative stress parameters.
Striatum Preparation and Incubation
In vitro experiments were performed in striatum supernatants. On the day of the experiments, the rats were euthanized by decapitation without anesthesia and the brain was rapidly excised on a Petri dish placed on ice and the blood and external vessels were carefully removed. The striatum was dissected, weighed and separately homogenized in 12 volumes (1:12, w/v) of 20 mM sodium phosphate buffer, pH 7.4, containing 140 mM KCl. The homogenates of the brain structure were centrifuged at 750g for 10 min at 4 °C to discard nuclei and cell debris [22]. The pellet was discarded and cortical supernatants, corresponding to a suspension of mixed and preserved organelles, including mitochondria, were separated and incubated at 37 °C for 20 min with 10 mM GLY. Controls did not contain the amino acid in the incubation medium. Immediately after incubation, aliquots were taken to measure biochemical parameters.
Superoxide Dismutase Activity
Superoxide dismutase (SOD) activity was determined based on the autooxidation of pyrogallol mediated by superoxide anion [23]. The reaction occurred at 25 °C in a medium containing 50 mM Tris buffer with 1 mM EDTA, pH 8.2, 80 U/mL catalase, 0.4 mM pyrogallol, and approximately 45 μg of protein. A calibration curve with commercial SOD was performed. The oxidation of pyrogallol leads to the generation of a yellow product that is measured at 420 nm on a spectrophotometer. Results were expressed as units per milligram protein.
Glutathione Peroxidase Activity
Glutathione peroxidase (GPx) activity was determined by measuring NADPH consumption at 25 °C using tert-butyl hydroperoxide as substrate [24]. The reaction medium contained 100 mM phosphate buffer/1 mM EDTA, pH 7.7, 2 mM GSH, 0.1 U/mL glutathione reductase, 0.4 mM azide, 0.5 mM tert-butyl hydroperoxide, 0.1 mM NADPH, and approximately 75 μg of protein. Absorbance was monitored on a spectrophotometer at 340 nm. Results were expressed as units per milligram protein.
Glutathione Reductase Activity
Glutathione reductase (GR) activity was measured at 25 °C in a medium containing oxidized glutathione (GSSG,1 mM) and NADPH (0.1 mM) as substrates, as well as 200 mM sodium phosphate/6.3 mM EDTA buffer, pH 7.5, and approximately 0.1 mg of protein [25]. NADPH consumption was monitored on a spectrophotometer at 340 nm. Results were expressed as units per milligram protein.
Glutathione S-transferase Activity
Glutathione S-transferase (GST) activity was determined by monitoring the rate of formation of dinitrophenyl-S-glutathione [26]. The reaction medium consisted of 50 mM potassium phosphate, pH 6.5, 1 mM GSH, 1 mM 1-chloro-2,4-dinitrobenzene (CDNB) as substrate, and tissue supernatants (approximately 60 μg of protein). The assay was performed at 25 °C and the absorbance determined on a spectrophotometer at 340 nm. Results were expressed as units per milligram protein.
Glucose-6-phosphate Dehydrogenase Activity
Glucose-6-phosphate dehydrogenase (G6PDH) activity was assayed at 25 °C in a medium containing 100 mM Tris-HCl buffer, pH 7.5, 10 mM MgCl2, 0.5 mM NADP+, and approximately 45 μg of protein [27]. The formation of NADPH was monitored on a spectrophotometer at 340 nm and results were expressed as units per milligram of protein.
Catalase Activity
CAT activity was assayed according to Aebi [28], following the decrease of absorbance at 25 °C at 240 nm due to H2O2 decomposition. The reaction medium contained tissue supernatants (approximately 3 μg of protein), 10 mM potassium phosphate buffer, pH 7.0, 0.1% Triton X-100, and 20 mM H2O2.
Malondialdehyde Levels
Malondialdehyde (MDA) levels were determined as previously described by Esterbauer, Cheeseman [29], with slight modifications. One hundred microliters of tissue supernatant (0.3 mg of protein), 200 μL of 10% trichloroacetic acid, and 300 μL of 0.67% thiobarbituric acid were mixed and incubated for 2 h in a boiling bath. After the incubation, the tubes were left for 5 min at room temperature to cool down. Two hundred microliters were then transferred to a 96-well plate. The resulting pink product was measured at 532 nm. A calibration curve was performed using 1,1,3,3-tetramethoxypropane. MDA values were expressed as nanomoles per milligram of protein.
Total, Reduced, and Oxidized Glutathione Concentrations
Non-enzymatic antioxidant defenses were evaluated by measuring total (GS), reduced (GSH), and oxidized (GSSG) glutathione concentrations. GSH concentrations were determined according to the assay described by Browne and Armstrong [30]. One hundred fifty microliters of samples (0.3 mg of protein) were initially treated with 5% metaphosphoric acid (1:1, v/v) and centrifuged at 7000g for 10 min. The resulting supernatant was transferred to a 96-well plate (30 μL/well) and incubated with 185 μL of 100 mM sodium phosphate containing 5 mM EDTA buffer, pH 8.0, and 15 μL of o-phthaldialdehyde (1 mg / mL) for 15 min at room temperature in the dark. After the incubation, the fluorescence was measured at 350 and 420 nm as excitation and emission wavelengths, respectively. GSH concentrations were calculated using a calibration curve prepared with a commercial GSH solution and results were expressed as nanomoles per milligram of protein.
tGS and GSSG concentrations were determined by following the enzymatic recycling method described by Teare et al. [31]. Briefly, the tissue was homogenized in four volumes (w/v) of a mixture (1:1 ratio) of 5.5% sulfosalicylic acid and 0.11% Triton X-100. After a 5-min incubation at 4 °C, the samples were centrifuged at 10,000g for 10 min at 4 °C, and the supernatant was collected for analysis of glutathione levels. For GSSG measurement, 30 μL of this supernatant was added to 91.6 μL of a GSH masking reagent (74 mM sodium carbonate and 1.3% 2-vinylpyridine in the reaction medium) and incubated for 1 h at room temperature. For the measurement of tGS, 10 μL of the same supernatant was diluted in 190 μL of 100 mM sodium phosphate 6 mM EDTA buffer, pH 7.4, and kept on ice until the GSSG samples were ready. The samples prepared for tGS and GSSG quantification were subjected to enzymatic recycling analysis by adding 210 μL of 300 μM NADPH, 30 μL of 6 mM DTNB and 30 μL of 450 U/mL GR. The linear increase in absorbance was monitored at 412 nm using a microplate reader (Spectramax M5, Molecular Devices, CA, US). A standard curve was built with known amounts of GSSG (1–100 μM). Results are expressed as picomoles per milligram of tissue.
Glutamate-Cysteine Ligase Activity
Glutamate-cysteine ligase (GCL) activity was measured based on the reaction of naphthalene-2,3dicarboxialdehyde with the γ-glutamylcysteine. The assay contained 40 mM ATP, 20 mM glutamic acid, 2 mM cysteine, and hippocampus supernatants with approximately 0.1-mg protein. The product formed was measured at 472-nm excitation and 528-nm emission in spectrofluorometer according to White et al. [32]. One unit of GCL is defined as micromoles of GSH formed per minute and specific activity is represented as units per milligram protein.
2′,7′-Dichlorofluorescin Oxidation
2′,7′-Dichlorofluorescin (DCFH) oxidation was determined according to the method of LeBel et al. [33], with slight modifications, to examine reactive oxygen species generation. Twenty microliters of tissue supernatants, 30 μL of 20 mM sodium phosphate buffer, pH 7.4, containing 140 mM KCl, and 200 μL of DCF-DA (10 mM, prepared in 1.25% methanol) were pipetted on a 96-well plate and incubated for 15 min at 37 °C in the dark. DCF-DA is permeable to the cell membrane and is deacetylated by esterases to DCFH in the intracellular medium. This non-fluorescent product is converted by reactive species into the highly fluorescent product dichlorofluorescein (DCF). After incubation, the resulting fluorescence was read on a fluorescence microplate reader at excitation and emission wavelengths of 480 and 535 nm, respectively. Results were calculated based on a standard curve carried out with a DCF solution, and results were expressed as micromoles per milligram of protein.
Sulfhydryl Group Content
Total sulfhydryl group content was performed by adding 50 μL of tissue supernatants to 980 μL of PBS and 30 μL of 10 mM 5,5-dithio-bis (2-nitrobenzoic acid) (DTNB), as previously described by Aksenov and Markesbery [34]. DTNB is reduced by sulfhydryl groups generating a yellow product (TNB), which is measured at 412 nm on spectrophotometer. Results were calculated based on TNB formation and expressed as nanomoles per milligram of protein.
Protein Carbonyl Formation
Estimation of protein carbonyl formation was performed according to Reznick and Packer [35], slightly modified. Sample supernatants (0.6 mg of protein) were incubated with 10 mM 2,4-dinitrophenylhidrazine (DNPH; prepared in 2.5 N HCl) (1:2, v/v) for 1 h in the dark. Proteins were then precipitated with 20% trichloroacetic acid (1:1, v/v) on ice for 10 min and, after centrifugation at 10,000g for 5 min, the resulting pellet was washed with 500 μL of ethyl acetate:ethanol mixture (1:1, v/v). After washing, the pellet was suspended in 550 μL of 6 M guanidine. Carbonyl content was determined by the difference between absorbances of DNPH-treated and blank samples measured at 365 nm. The results were calculated using the extinction coefficient of 22,000 × 106 nmol/mL for aliphatic hydrazones and expressed as nanomoles per milligram of protein.
Protein Determination
Protein levels were measured by the method of Lowry et al. [36] using bovine serum albumin as standard.
Statistical Analysis
Experiments were performed in triplicates and results were presented as mean ± standard deviation. Data were analyzed using Student’s t test for unpaired samples or one-way analysis of variance (ANOVA) followed by Duncan multiple range test when the F value was significant. Both tests were run in a compatible PC computer using the Statistical Package for the Social Sciences (SPSS) software. Differences between groups were considered significant at P < 0.05.
Results
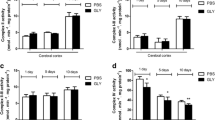
We initially tested the effects of an intracerebroventricular administration of GLY (5 μmol) on the activities of important antioxidant enzymes, namely SOD, CAT, GPx, GR, GST, and G6PDH, in striatum, cerebral cortex, and hippocampus supernatants 30 min after administration. Figure 1 shows that GLY increased the activities of SOD [F(2,9) = 4.311; P < 0.05], GPx [F(2,6) = 30.558; P < 0.001], GR [F(2,10) = 11.762; P < 0.01], and G6PDH [F(2,10) = 4.661; P < 0.05], without altering GST [t(8) = − 0.721] and CAT [t(9) = 0.196] activities in rat striatum. No significant changes were observed on any of these enzyme activities in cerebral cortex [SOD: t(8) = − 0.72, GPx: t(8) = − 0.721, GR: t(7) = − 1.043, G6PDH: t(5) = − 1.228, GST: t(7) = 0.532, CAT: t(6) = 1.253] and hippocampus [SOD: t(7) = 0.64, GPx: t(7) = 1.781, GR: t(7) = −2.064, G6PDH: t(7) = 1.18, GST: t(6) = 0.211, CAT: t(8) = 2.744] (Fig. 1).
Effect of intracerebroventricular administration of glycine (GLY, 5 μmol) on the activity of superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione reductase (GR), glucose-6-phosphate dehydrogenase (G6PDH), and glutathione S-transferase (GST) in rat striatum (a), hippocampus (b), and cerebral cortex (c). A group of animals received bezafibrate (BEZ, 30 mg/kg/day) by gavage for 7 days before GLY administration. Results are presented as mean ± standard deviation for three to five independent experiments (animals). Statistical analysis was performed using Student’s t test for unpaired samples or ANOVA followed by Duncan test. *P < 0.05, **P < 0.01, ***P < 0.001, compared to controls; #P < 0.05, ##P < 0.01, ###P < 0.001, compared to GLY
The effects of BEZ on GLY-induced alterations on the activities of antioxidant enzymes in striatum were also evaluated. Our results demonstrate that BEZ fully prevented the increase of SOD [F(2,9) = 4.311; P < 0.05] and GR [F(2,10) = 11.762; P < 0.01] activities, and attenuated the increase of GPx activity [F(2,6) = 30.558; P < 0.001] caused by GLY administration. On the other hand, BEZ had no effect on GLY-induced increase of G6PDH activity [F(2,10) = 4.661; P < 0.05] (Fig. 1).
Regarding parameters of oxidative damage, we determined the influence of GLY on MDA levels, GSH concentrations, GCL activity, DCFH oxidation, sulfhydryl content, and carbonyl formation. GLY significantly decreased GSH concentrations 30 or 15 min after its injection [30 min: F(2,9) = 7.682; P < 0.05; 15 min: t(6) = 2.97; P < 0.05] in hippocampus, whereas tGS [t(6) = 0.198; P < 0.05] concentrations and GSH/GSSG ratio [t(6) = 0.403; P < 0.05] were also decreased in this structure 15 min after injection (supplementary figure). In contrast, the other parameters were not altered in hippocampus [GCL activity: t(6) = − 0.979] (supplementary figure), [MDA levels: t(6) = − 0.892, DCFH oxidation: t(7) = 0.25, sulfhydryl content: t(9) = − 0.914, carbonyl formation: t(7) = − 0.31] (Fig. 4). No changes on these parameters were also observed in striatum [MDA levels: t(7) = 1.769, GSH levels: t(9) = 1.278, DCFH oxidation: t(7) = − 0.3, sulfhydryl content: t(9) = − 0.3, carbonyl formation: t(9) = − 1.813] (Fig. 2) and cerebral cortex [MDA levels: t(6) = − 0.12, GSH levels: t(7) = − 0.741, DCFH oxidation: t(7) = 0.195, sulfhydryl content: t(6) = − 0.578, carbonyl formation: t(7) = − 1.189] (Fig. 3). We further verified that BEZ was not able to prevent the decrease of GSH induced by GLY in hippocampus 30 min after GLY administration [F(2,9) = 7.682; P < 0.05] (Fig. 4).
Effect of intracerebroventricular administration of glycine (GLY, 5 μmol) on malondialdehyde (MDA) levels (a), reduced glutathione (GSH) concentrations (b), 2′,7′-dichlorofluorescin (DCFH) oxidation (c), sulfhydryl group content (d), and carbonyl formation (e) in rat striatum. Results are presented as mean ± standard deviation for three to five independent experiments (animals). Statistical analysis was performed using Student’s t test for unpaired samples. No significant differences were found between groups
Effect of intracerebroventricular administration of glycine (GLY, 5 μmol) on malondialdehyde (MDA) levels (a), reduced glutathione (GSH) concentrations (b), 2′,7′-dichlorofluorescin (DCFH) oxidation (c), sulfhydryl group content (d), and carbonyl formation (e) in rat cerebral cortex. Results are presented as mean ± standard deviation for three to five independent experiments (animals). Statistical analysis was performed using Student’s t test for unpaired samples. No significant differences were found between groups
Effect of intracerebroventricular administration of glycine (GLY, 5 μmol) on malondialdehyde (MDA) levels (a), reduced glutathione (GSH) concentrations (b), 2′,7′-dichlorofluorescin (DCFH) oxidation (c), sulfhydryl group content (d), and carbonyl formation (e) in rat hippocampus. Some animals received bezafibrate (BEZ, 30 mg/kg/day) by gavage for 7 days before GLY administration. Results are presented as mean ± standard deviation for three to five independent experiments (animals). Statistical analysis was performed using Student’s t test for unpaired samples or ANOVA followed by Duncan test. *P < 0.05, compared to controls
In the next set of experiments, we evaluated the in vitro effects of GLY on parameters of oxidative stress in striatum of rats in order to investigate whether this amino acid could exert neurotoxic effects at a shorter period of time (20-min incubation). We verified that GLY significantly increased MDA levels in striatum [t(5) = 1.053], indicating lipid peroxidation, and decreased GSH levels [t(5) = 0.034] (Fig. 5). Overall, these data suggest that GLY induces oxidative stress in rat striatum.
In vitro effect of glycine (GLY, 10 mM) on malondialdehyde (MDA) levels (a) and reduced glutathione (GSH) concentrations (b) in rat striatum. Striatum supernatants were exposed to GLY during 20 min before measuring the parameters. Results are presented as mean ± standard deviation for three to five independent experiments (animals). Statistical analysis was performed using Student’s t test. **P < 0.01, ***P < 0.001, compared to controls
Discussion
NKH is a severe neurological condition characterized by persistent seizures and brain abnormalities along with elevated GLY levels in CSF and plasma of patients [1, 2]. Considering that a growing body of evidence has suggested that GLY accumulation is involved in the onset of symptoms of NKH and that the exact pathomechanisms of this disorder are not fully established, we investigated the effects of an intracerebroventricular administration of GLY on parameters of oxidative stress in rat striatum, hippocampus, and cerebral cortex.
We show here that GLY (5 μmol) administration increased the activity of SOD, GPx, GR, and G6PDH in striatum 30 min after injection, corroborating a previous study showing that the intrastriatal injection of this amino acid (4 μmol) also increases the activity of SOD, GPx, and GR in the same brain region [16]. In this regard, it is well established that the concerted action of these enzymes plays an important role in the antioxidant system once they neutralize superoxide anion and peroxides, and maintains GSH pool at normal levels [37]. So, it may be suggested that the enhancement of the activity of these enzymes occurs to cope with GLY-induced generation of reactive oxygen species (ROS).
On the other hand, we did not observe alterations on parameters of lipoperoxidation (MDA levels), protein oxidative damage (carbonyl formation and sulfhydryl content) and ROS production (DCFH oxidation) evaluated in striatum 30 min after GLY administration. Although these findings seem contradictory with the fact that GLY modulated antioxidant enzyme activities, it is possible that the upregulation of these enzymes was induced to remove the exceeding ROS so that oxidative damage did not occur. This hypothesis implies that GLY could provoke oxidative stress at shorter periods leading to the activation of mechanisms involved in the upregulation of antioxidant enzymes. We indeed found that GLY induced lipid peroxidation and decreased GSH levels in vitro after a 20-min exposition of striatal supernatants to this amino acid.
It should be also considered that our present findings demonstrating that GLY intracerebroventricular administration did not induce oxidative damage in vivo in striatum is in disagreement with previous data showing that intrastriatal administration of GLY caused lipid and protein oxidative damage in this brain structure [16]. These apparently controversial findings may be explained by the different doses of GLY and via of administration used in each study. While in our present work we administered 5 μmol into the brain ventricule, Seminotti et al. [16] injected 4 μmol into the striatum of rats. Although a lower GLY dose was used in the previous study [16], GLY was administered directly into the striatum so that it may be speculated that the neurotoxicity of GLY in this approach was more pronounced. In addition, it should be noted that we evaluated the parameters 30 min after the administration of GLY, whereas Seminotti et al. [16] observed GLY-induced oxidative damage 2 h after the administration.
GLY did not induce significant alterations in cerebral cortex and hippocampus, except for alterations in GSH metabolism in hippocampus. We found a decrease of tGS and GSH concentrations, and of GSH/GSSG ratio. Although the activity of GCL, the first rate-limiting enzyme of GSH synthesis, was not altered by GLY, it is conceivable that other enzymes of GSH synthesis pathway or the cysteine transporter xCT are impaired. In this regard, other studies previously observed disturbances in GSH synthesis in different pathological conditions caused by low cysteine availability due to decreased xCT activity [38]. On the other hand, it is also difficult to explain why GLY exerted differential effects in striatum and hippocampus, but it is tempting to speculate that the variable NMDA receptor subunit composition in these brain structures might play an important role in these effects, since the differential expression of these subunits may confer distinct reactivity of this receptor to GLY [39]. In this particular, previous data evidenced differential in vivo effects for GLY on different energy metabolism enzymes in cerebral cortex and striatum of young rats [14].
The approaches for NKH treatment are limited and ineffective so that there is a critical need for the discovery of new adjuvant therapies to improve the patients’ condition. Since BEZ has been evaluated as a prospective drug in the treatment of neurological disorders [12], we performed a pre-treatment with BEZ attempting to prevent the effects of GLY in striatum and hippocampus. Our results show that BEZ attenuated GLY-induced increase of GPx activity, while the increase of the activities of SOD and GR induced by GLY was fully prevented in striatum, being restored to control values. In contrast, BEZ did not modify G6PDH activity augment in striatum and GSH decrease in hippocampus caused by GLY. These data showing that GLY-induced increase of antioxidant enzyme activities was prevented or attenuated by BEZ are apparently controversial since BEZ is known to induce expression of antioxidant enzymes [40]. Nevertheless, it may be speculated that in our experimental conditions, BEZ exerts its beneficial effects by inducing mitochondrial biogenesis and overexpression of respiratory complex subunits [41]. Therefore, we hypothesize that BEZ is able to prevent an initial ROS build-up induced by GLY, thus avoiding the need for a compensatory increase of antioxidant enzyme activities.
Our study suggests for the first time that striatal antioxidant enzymatic system is more vulnerable than hippocampal and cortical system to the toxic effects exerted by GLY intracerebroventricular administration. Furthermore, the present data showing that oxidative damage parameters were mildly altered by GLY allied to previous findings demonstrating that GLY markedly impairs citric acid cycle and mitochondrial respiratory chain in cerebral cortex and striatum of young rats [14] suggest that GLY-elicited deleterious effects are mainly mediated by bioenergetics disruption. On the other hand, BEZ was able to prevent most alterations elicited by GLY in striatum possibly by induction of mitochondrial biogenesis and overexpression of respiratory complex subunits [42], suggesting that BEZ may be considered for further evaluation as adjuvant therapy for NKH.
References
Hamosh A, Johnston MV (2001) Non-ketotic hyperglycinemia. In: Scriver CR, Beaudet A, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease, vol Editors. 8th edn. McGraw-Hill, New York, pp 2065–2078
Applegarth DA, Toone JR (2001) Nonketotic hyperglycinemia (glycine encephalopathy): laboratory diagnosis. Mol Genet Metab 74(1–2):139–146. https://doi.org/10.1006/mgme.2001.3224
Iqbal M, Prasad M, Mordekar SR (2015) Nonketotic hyperglycinemia case series. J Pediatr Neurosci 10(4):355–358. https://doi.org/10.4103/1817-1745.174445
Hoover-Fong JE, Shah S, Van Hove JL, Applegarth D, Toone J, Hamosh A (2004) Natural history of nonketotic hyperglycinemia in 65 patients. Neurology 63(10):1847–1853
Raghavendra S, Ashalatha R, Thomas SV, Kesavadas C (2007) Focal neuronal loss, reversible subcortical focal T2 hypointensity in seizures with a nonketotic hyperglycemic hyperosmolar state. Neuroradiology 49(4):299–305. https://doi.org/10.1007/s00234-006-0189-6
Heindel W, Kugel H, Roth B (1993) Noninvasive detection of increased glycine content by proton MR spectroscopy in the brains of two infants with nonketotic hyperglycinemia. AJNR Am J Neuroradiol 14(3):629–635
Bjoraker KJ, Swanson MA, Coughlin CR 2nd, Christodoulou J, Tan ES, Fergeson M, Dyack S, Ahmad A et al (2016) Neurodevelopmental outcome and treatment efficacy of benzoate and dextromethorphan in siblings with attenuated nonketotic hyperglycinemia. J Pediatr 170:234–239. https://doi.org/10.1016/j.jpeds.2015.12.027
Cusmai R, Martinelli D, Moavero R, Dionisi Vici C, Vigevano F, Castana C, Elia M, Bernabei S et al (2012) Ketogenic diet in early myoclonic encephalopathy due to non ketotic hyperglycinemia. Eur J Paediat Neurol : EJPN : Off J Eur Paediatr Neurol Soc 16(5):509–513. https://doi.org/10.1016/j.ejpn.2011.12.015
Zafrir B, Jain M (2014) Lipid-lowering therapies, glucose control and incident diabetes: evidence, mechanisms and clinical implications. Cardiovasc Drugs Ther 28(4):361–377. https://doi.org/10.1007/s10557-014-6534-9
Corona JC, Duchen MR (2015) PPARgamma and PGC-1alpha as therapeutic targets in Parkinson’s. Neurochem Res 40(2):308–316. https://doi.org/10.1007/s11064-014-1377-0
Procaccio V, Bris C, Chao de la Barca JM, Oca F, Chevrollier A, Amati-Bonneau P, Bonneau D, Reynier P (2014) Perspectives of drug-based neuroprotection targeting mitochondria. Rev Neurol 170(5):390–400. https://doi.org/10.1016/j.neurol.2014.03.005
Johri A, Calingasan NY, Hennessey TM, Sharma A, Yang L, Wille E, Chandra A, Beal MF (2012) Pharmacologic activation of mitochondrial biogenesis exerts widespread beneficial effects in a transgenic mouse model of Huntington’s disease. Hum Mol Genet 21(5):1124–1137. https://doi.org/10.1093/hmg/ddr541
Kono Y, Shigetomi E, Inoue K, Kato F (2007) Facilitation of spontaneous glycine release by anoxia potentiates NMDA receptor current in the hypoglossal motor neurons of the rat. Eur J Neurosci 25(6):1748–1756. https://doi.org/10.1111/j.1460-9568.2007.05426.x
Moura AP, Grings M, Dos Santos Parmeggiani B, Marcowich GF, Tonin AM, Viegas CM, Zanatta A, Ribeiro CA et al (2013) Glycine intracerebroventricular administration disrupts mitochondrial energy homeostasis in cerebral cortex and striatum of young rats. Neurotox Res 24(4):502–511. https://doi.org/10.1007/s12640-013-9396-1
Moura AP, Parmeggiani B, Grings M, Alvorcem LM, Boldrini RM, Bumbel AP, Motta MM, Seminotti B et al (2015) Intracerebral glycine administration impairs energy and redox homeostasis and induces glial reactivity in cerebral cortex of newborn rats. Mol Neurobiol 53:5864–5875. https://doi.org/10.1007/s12035-015-9493-7
Seminotti B, Knebel LA, Fernandes CG, Amaral AU, da Rosa MS, Eichler P, Leipnitz G, Wajner M (2011) Glycine intrastriatal administration induces lipid and protein oxidative damage and alters the enzymatic antioxidant defenses in rat brain. Life Sci 89 (7–8):276–281. doi:https://doi.org/10.1016/j.lfs.2011.06.013
Moura AP, Grings M, Marcowich GF, Bumbel AP, Parmeggiani B, de Moura Alvorcem L, Wajner M, Leipnitz G (2014) Evidence that glycine induces lipid peroxidation and decreases glutathione concentrations in rat cerebellum. Mol Cell Biochem 395(1–2):125–134. https://doi.org/10.1007/s11010-014-2118-z
Pai YJ, Leung KY, Savery D, Hutchin T, Prunty H, Heales S, Brosnan ME, Brosnan JT et al (2015) Glycine decarboxylase deficiency causes neural tube defects and features of non-ketotic hyperglycinemia in mice. Nat Commun 6:6388. https://doi.org/10.1038/ncomms7388
Grings M, Moura AP, Parmeggiani B, Pletsch JT, Cardoso GMF, August PM, Matte C, Wyse ATS et al (2017) Bezafibrate prevents mitochondrial dysfunction, antioxidant system disturbance, glial reactivity and neuronal damage induced by sulfite administration in striatum of rats: implications for a possible therapeutic strategy for sulfite oxidase deficiency. Biochim Biophys Acta 1863(9):2135–2148. https://doi.org/10.1016/j.bbadis.2017.05.019
Nakajima T, Tanaka N, Kanbe H, Hara A, Kamijo Y, Zhang X, Gonzalez FJ, Aoyama T (2009) Bezafibrate at clinically relevant doses decreases serum/liver triglycerides via down-regulation of sterol regulatory element-binding protein-1c in mice: a novel peroxisome proliferator-activated receptor alpha-independent mechanism. Mol Pharmacol 75(4):782–792. https://doi.org/10.1124/mol.108.052928
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates. Academic Press, San Diego
Evelson P, Travacio M, Repetto M, Escobar J, Llesuy S, Lissi EA (2001) Evaluation of total reactive antioxidant potential (TRAP) of tissue homogenates and their cytosols. Arch Biochem Biophys 388(2):261–266. https://doi.org/10.1006/abbi.2001.2292
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47(3):469–474
Wendel A (1981) Glutathione peroxidase. Methods Enzymol 77:325–333
Carlberg I, Mannervik B (1985) Glutathione reductase. Methods Enzymol 113:484–490
Mannervik B, Guthenberg C (1981) Glutathione transferase (human placenta). Methods Enzymol 77:231–235
Leong SF, Clark JB (1984) Regional enzyme development in rat brain. Enzymes associated with glucose utilization. Biochem J 218(1):131–138
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Esterbauer H, Cheeseman KH (1990) Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol 186:407–421
Browne RW, Armstrong D (1998) Reduced glutathione and glutathione disulfide. Methods Mol Biol 108:347–352. https://doi.org/10.1385/0-89603-472-0:347
Teare JP, Punchard NA, Powell JJ, Lumb PJ, Mitchell WD, Thompson RP (1993) Automated spectrophotometric method for determining oxidized and reduced glutathione in liver. Clin Chem 39(4):686–689
White CC, Viernes H, Krejsa CM, Botta D, Kavanagh TJ (2003) Fluorescence-based microtiter plate assay for glutamate-cysteine ligase activity. Anal Biochem 318(2):175–180
LeBel CP, Ischiropoulos H, Bondy SC (1992) Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5(2):227–231
Aksenov MY, Markesbery WR (2001) Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci Lett 302(2–3):141–145
Reznick AZ, Packer L (1994) Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol 233:357–363
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Bannister JV (1986) Free radicals in biology and medicine. Barry Halliwell, John M. C. Gutteridge. Q Rev Biol 61 (3):440–441. doi:https://doi.org/10.1086/415130
Kritis AA, Stamoula EG, Paniskaki KA, Vavilis TD (2015) Researching glutamate-induced cytotoxicity in different cell lines: a comparative/collective analysis/study. Front Cell Neurosci 9:91. https://doi.org/10.3389/fncel.2015.00091
Goebel DJ, Poosch MS (1999) NMDA receptor subunit gene expression in the rat brain: a quantitative analysis of endogenous mRNA levels of NR1Com, NR2A, NR2B, NR2C, NR2D and NR3A. Mol Brain Res 69(2):164–170
Chaturvedi RK, Beal MF (2013) Mitochondria targeted therapeutic approaches in Parkinson’s and Huntington’s diseases. Mol Cell Neurosci 55:101–114. https://doi.org/10.1016/j.mcn.2012.11.011
Valero T (2014) Mitochondrial biogenesis: pharmacological approaches. Curr Pharm Des 20(35):5507–5509
Schmitt K, Grimm A, Kazmierczak A, Strosznajder JB, Gotz J, Eckert A (2012) Insights into mitochondrial dysfunction: aging, amyloid-beta, and tau-A deleterious trio. Antioxid Redox Signal 16(12):1456–1466. https://doi.org/10.1089/ars.2011.4400
Funding
This work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Programa de Apoio a Núcleos de Excelência (PRONEX II), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS), Pró-Reitoria de Pesquisa/Universidade Federal do Rio Grande do Sul (PROPESQ/UFRGS), Financiadora de estudos e projetos (FINEP), Rede Instituto Brasileiro de Neurociência (IBN-Net) # 01.06.0842-00, and Instituto Nacional de Ciência e Tecnologia em Excitotoxicidade e Neuroproteção (INCT-EN).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
Supplementary figure
Effect of intracerebroventricular administration of glycine (GLY, 5 μmol) on total (GS) (a), reduced (GSH) (b) and oxidized glutathione (GSSG) (c) concentrations, GSH/GSSG ratio (d) and γ-glutamate-cysteine ligase (GCL) activity (e) in rat hippocampus 15 min after the administration. Results are presented as mean ± standard deviation for three to five independent experiments (animals). Statistical analysis was performed using Student’s t test for unpaired samples. *P < 0.05, **P < 0.01, compared to controls. (GIF 45 kb)
Rights and permissions
About this article
Cite this article
Parmeggiani, B., Grings, M., da Rosa-Junior, N.T. et al. Bezafibrate Prevents Glycine-Induced Increase of Antioxidant Enzyme Activities in Rat Striatum. Mol Neurobiol 56, 29–38 (2019). https://doi.org/10.1007/s12035-018-1074-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1074-0