Abstract
Introduction
Neuroimaging in seizures associated with nonketotic hyperglycemia (NKH) is considered normal. We report magnetic resonance imaging (MRI) abnormalities in four patients with NKH and seizures.
Methods
We prospectively evaluated clinical and radiological abnormalities in four patients with NKH during the period March 2004 to December 2005.
Results
All patients presented with seizures, either simple or complex partial seizures or epilepsia partialis continua. Two of them had transient hemianopia. MRI showed subcortical T2 hypointensity in the occipital white matter and in or around the central sulcus (two patients each), T2 hyperintensity of the overlying cortex (two patients), focal overlying cortical enhancement (three patients) and bilateral striatal hyperintensity (one patient). Diffusion-weighted imaging (DWI) performed in three patients showed restricted diffusion. The ictal semiology and electroencephalographic (EEG) findings correlated with the MRI abnormalities. On clinical recovery, the subcortical T2 hypointensity and striatal hyperintensity reversed in all patients. The initial cortical change evolved to FLAIR hyperintensity suggestive of focal cortical gliosis. The radiological differential diagnosis considered initially included encephalitis, malignancy and hemorrhagic infarct rendering a diagnostic dilemma.
Conclusion
We identified subcortical T2 hypointensity rather than hyperintensity as a characteristic feature of seizures associated with NKH. Only very few similar reports exist in literature. Reversible bilateral striatal T2 hyperintensity in NKH has not been reported to the best of our knowledge.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Seizures are a common presenting manifestation in patients in a nonketotic hyperglycemic (NKH) hyperosmolar state [1]. Several transient changes in magnetic resonance imaging (MRI) have been described following seizures [2, 3]. These brain parenchymal changes are typically hyperintense on T2-weighted (T2-W) and fluid attenuated inversion recovery (FLAIR) images. Previous MRI studies in seizures secondary to NKH are unremarkable [4, 5]. But a few cases have been reported recently with focal changes [6–8]. We report focal neuronal loss and reversible focal subcortical T2 hypointensity on MRI in four patients with NKH and seizures. We also attempted to characterize the pathogenesis of this unusual finding.
Materials and methods
Patients
This study was carried out in Sree Chitra Tirunal Institute for Medical Sciences and Technology, which is a tertiary referral center for neurological disorders in South India. During a study of seizure-related reversible MRI changes we identified one patient with NKH with seizures and subcortical T2 hypointensity on MRI. Following this we identified three patients with diabetic NKH and seizures from among all hospitalizations for seizures during the period 2004–2005 in this Institute. All of them had characteristic MRI changes. Their clinical presentation, and seizure characteristics at entry and follow-up were studied in detail (Table 1).
Imaging
All patients were imaged using a 1.5-T magnet (Signa GE, Milwaukee, Wis., on initial presentation in patient 1, and Avanto Magnetom, Siemens, Germany, in all the others) and were reviewed by a radiologist with special interest in epilepsy (K.D.). The T1-weighted (T1-W), T2-W, proton density (PD), FLAIR sequences and intravenous (0.1 mmol/kg) gadolinium-enhanced studies were available in all patients. Diffusion-weighted imaging (DWI) was performed in three of the patients (Table 2).
Other investigations
A complete biochemical profile (serum blood sugar, serum sodium and potassium, blood urea nitrogen and calculated serum osmolality), hemogram, peripheral smear, renal and liver function tests, urine ketone body estimation and cerebrospinal fluid study were done in all patients. Visual acuity was also tested and visual field charting (Goldman’s perimetry) done in all patients.
Assessment of seizures
Two trained epileptologists (R.A and S.V.T) involved in the study examined the patients to identify the type of seizure(s) and the seizure burden. All the patients were subjected to a 16-channel scalp electroencephalography (SEEG) during the active phase and follow-up, a scalp-video EEG (S-VEEG) was done to note the ictal onset as and when required. The lobar localization of interictal epileptiform abnormalities was noted and the ictal onset zone in S-VEEG was also noted.
Follow-up
All four patients were followed-up until their symptoms abated and/or their MRI abnormality resolved. A minimum of 1 year follow-up was available for all the patients. Their diabetic status were reviewed and monitored during follow-up. Treatment received during follow-up was also ascertained.
Results
Demographic and clinical characteristics
Clinical and demographic features and various laboratory parameters are listed in Table 1. Of the four patients, two were male and two female. Their ages ranged from 42 to 60 years (mean 50.5±8.4 years). All of them had seizures as the initial manifestation except patient 1, in whom seizure occurred 3 days following headache, vomiting, visual hallucination and acute right hemifield visual loss. In addition to patient 1, another patient (patient 4) also had hemianopia, which resolved completely later. Seizures were simple/complex partial in all, with epilepsia partialis continua in one patient (patient 2). Scalp and V-EEG findings are shown in Table 1. All patients had longstanding diabetes mellitus type 2. The associated metabolic and biochemical abnormalities are also shown in Table 1. The calculated serum osmolality ranged from 303–317 mOsm/l. None of the patients had ketonuria.
Imaging characteristics
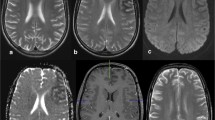
The various imaging abnormalities during the acute phase and follow-up are shown in Table 2 and are detailed below. The MRI abnormalities observed acutely were focal subcortical hypointensities on T2-W, PD, FLAIR and gradient sequences in all patients (Figs. 1, 2, 3 and 4). Overlying cortical gyral hyperintensities were noted in three patients (Figs. 2, 3 and 4). T1-W sequences were normal. Focal overlying cortical enhancement was observed in three patients (Figs. 2, 3 and 4). DWI studies performed acutely in three patients showed focal areas of restricted diffusion in all (Figs. 2 and 4). On follow-up the subcortical hypointensity disappeared in all patients. Subtle focal volume loss at the site of initial subcortical hypointensity was noted in one patient (Fig. 1). Focal overlying cortical changes evolved to FLAIR hyperintensity suggestive of focal gliosis in all three with this abnormality (Figs. 3 and 4). Bilateral striatal hyperintensity was observed acutely in one patient (patient 1) that had completely resolved on follow-up (Fig. 1).
Axial brain MRI in patient 1 during the acute phase shows a normal T1-W image after contrast agent administration (a) with subcortical hypointensity in the left temporoparietooccipital region on the PD image (b) and T2-W image (c, d). Bilateral striatal hyperintensities are also well seen (b). At the 3-year follow-up, T2-W (e) and FLAIR images (f) show resolution with mild left peritrigonal white matter volume loss
Axial brain MRI in patient 2 during ictus (a–e) shows focal subcortical T2 hypointensity around the left central sulcus on the T2-W and FLAIR images (a, b) along with focal contrast enhancement of the overlying cortex (arrow) (c). DWI and apparent diffusion coefficient (ADC) map (d, e) show an area of restricted diffusion in the corresponding regions (arrow). At the 25-day follow-up, the T2-W image shows near total resolution of the subcortical hypointensity (f)
Axial brain MRI in patient 3, 2 days after a seizure shows subtle subcortical hypointensity on T2-W, PD and gradient images (arrow) (a–c) and overlying cortical enhancement on the TI-W image (d) in the right motor area. At the 21-day follow-up the T2-W image shows resolution of the subcortical hypointensity (e). The FLAIR image (f) shows residual focal cortical hyperintensity in the same area of earlier contrast enhancement suggestive of gliosis (arrow)
Axial brain MRI in patient 4 shows subcortical hypointensity in the left parietooccipital region on T2-W and FLAIR images (a, b). The T1-W image after contrast agent administration (c) shows enhancement of the cortical region (arrow) that is seen as hyperintense on the FLAIR image (arrow). DWI and ADC map (d, e) show restricted diffusion in the corresponding area. The FLAIR image (f) 6 weeks later shows resolution of the subcortical hypointensity with an area of residual cortical hyperintensity at the site of the initial cortical enhancement (arrow)
Follow-up
Seizures were controlled in all patients with adequate hydration and treatment of hyperglycemia. Only in one patient (patient 1) were antiepileptic drugs initiated during hospitalization, which was stopped at discharge. Hemianopia reversed completely in both the patients (patients 1 and 4).
Discussion
NKH is a relatively common complication of diabetes mellitus type 2, especially in patients above 50 years of age. The seizures are refractory to antiepileptic drugs and occur in 15–40% of patients with NKH, and focal motor seizures and epilepsia partialis continua are particularly common in these patients [9, 10]. All four patients in this series presented with focal seizures. Two of them had transient hemianopia, which reversed completely. A reversible field defect is another important clinical marker of NKH as recently observed by various authors [7, 11, 12].
Previous imaging studies utilizing MRI in NKH have been unremarkable [4, 5, 11–13]. There are several reports of T2 hyperintensity following seizures [2, 3]. However, all our patients had T2 hypointensity which was focal and showed good clinicoelectrographic correlation. A few recent studies have also shown subcortical T2 hypointensity in seizures with NKH [6–8]. However, these studies were retrospective, unlike the present prospective study. Other clinical conditions which could have evoked T2 hypointensities on MRI (see Table 3), such as viral encephalitis, meningitis, leptomeningeal metastasis, hemorrhagic infarct and hypoxia, were appropriately ruled out in our series [14]. All these conditions are usually associated with leptomeningeal enhancement on MRI, which was lacking in our patients. Subcortical hypointensity can also occur in chronic neurological diseases such as multiple sclerosis, Sturge-Weber syndrome, tuberous sclerosis [15], hemimegalencephaly [16] and moyamoya disease [17], but it seldom reverses in a short time span. The other associated abnormalities noted along with focal subcortical T2 hypointensity were cortical gyral swelling (two patients) and striatal hyperintensity (one patient), which also reversed completely.
The precise pathogenesis of this transient subcortical hypointensity remains uncertain. There was a close temporal relationship between seizures and MRI changes. The complete resolution of the changes suggests a transient physiological response to the seizures.
The DWI in three patients in this series showed restricted diffusion that suggested ‘cytotoxic edema’. The focal cytotoxic edema could be related to seizures, focal ischemia or hyperviscosity [18]. Restricted diffusion has been recently reported in seizures with NKH [7] and diabetic ketoacidosis [19]. Animal studies have indicated subcortical T2 hypointensity as a feature of early cortical ischemia [20]. The occurrence of hypointensity on gradient images suggests free radical accumulation as also indicated by animal studies [21]. These subcortical changes were transient, suggesting a reversible pathology. However, the occurrence of residual FLAIR hyperintensity in the cortex in these patients suggests a primary cortical pathology with the subcortical changes reflecting a secondary abnormality. One of the possible mechanisms could be subcortical transient free radical accumulation due to excitotoxic axonal damage during seizures.
The clinical features and the MRI changes were restricted to occipital and adjoining areas in two of our patients. Other recent studies have also shown a similar predilection [22]. Vasogenic edema and changes in posterior leukoencephalopathy (PLE) also predominantly involves the occipital lobes [23]. Several factors associated with longstanding diabetes leads to altered cerebrovascular reactivity predisposing to the risk of development of PLE [24]. These factors include sympathetic dysautonomia, endothelial dysfunction and endothelial damage. Increased free radical release has also been shown with PLE in diabetes.
One of our patients, in addition had symmetrical reversible striatal T2 hyperintensities. Other conditions that cause symmetrical basal ganglia lucencies such as dyselectrolytemia, coagulopathies, hypertensive encephalopathy, hypoxia, toxins, and hepatic and renal dysfunction, were excluded in this patient [25]. Unilateral basal ganglia T2 hyperintensities in NKH have been reported previously [18], but no case of symmetrical reversible T2 hyperintense in NKH has been reported to the best of our knowledge.
Conclusion
Patients with seizures in NKH may have transient MRI abnormalities that are characterized by subcortical T2 hypointensity in addition to other more widely recognized changes such as T2 hyperintensity and enhancement. Recognition of this radiological abnormality in NKH, especially in those patients with subtle clinical features, is important in restricting unwarranted investigations and to institute early therapy. These patients in general have a good prognosis.
References
Schomer DL (1993) Focal status epilepticus and epilepsia partialis continua in adults and children. Epilepsia 34 [Suppl 1]:S29–S36
Senn P, Lovblad KO, Zutter D, et al (2003) Changes on diffusion-weighted MRI with focal motor status epilepticus: case report. Neuroradiology 45:246–249
Flacke S, Wullner U, Keller E, Hamzei F, Urbach H (2000) Reversible changes in echo planar perfusion and diffusion-weighted MRI in status epilepticus. Neuroradiology 42:92–95
Cokar O, Aydin B, Ozer F (2004) Non-ketotic hyperglycaemia presenting as epilepsia partialis continua. Seizure 13:264–269
Lammouchi T, Zoghlami F, Ben Slamia F, Grira M, Harzallah MS, Benammou S (2004) Epileptic seizures in non-ketotic hyperglycemia. Neurophysiol Clin 34:183–187
Seo DW, Na DG, Na DL, Moon SY, Hong SB (2003) Subcortical hypointensity in partial status epilepticus associated with nonketotic hyperglycemia. J Neuroimaging 13:259–263
Lavin PJ (2005) Hyperglycemic hemianopia: a reversible complication of non-ketotic hyperglycemia. Neurology 65:616–619
Wang CP, Hsieh PF, Chen CC, et al (2005) Hyperglycemia with occipital seizures: images and visual evoked potentials. Epilepsia 46:1140–1144
Maccario M, Messis CP, Vastola EF (1965) Focal seizures as a manifestation of hyperglycemia without ketoacidosis. A report of seven cases with review of the literature. Neurology 15:195–206
Cochin JP, Hannequin D, Delangre T, Guegan-Massardier E, Augustin P (1994) Continuous partial epilepsy disclosing diabetes mellitus. Rev Neurol (Paris) 150:239–241
Brazis PW, Lee AG, Graff-Radford N, Desai NP, Eggenberger ER (2000) Homonymous visual field defects in patients without corresponding structural lesions on neuroimaging. J Neuroophthalmol 20:92–96
Freedman KA, Polepalle S (2004) Transient homonymous hemianopia and positive visual phenomena in nonketotic hyperglycemic patients. Am J Ophthalmol 137:1122–1124
Ozer F, Mutlu A, Ozkayran T (2003) Reflex epilepsy and non-ketotic hyperglycemia. Epileptic Disord 5:165–168
Lee JH, Na DG, Choi KH, et al (2002) Subcortical low intensity on MR images of meningitis, viral encephalitis, and leptomeningeal metastasis. AJNR Am J Neuroradiol 23:535–542
Stricker T, Zuerrer M, Martin E, Boesch C (1991) MRI of two infants with tuberous sclerosis. Neuroradiology 33:175–177
Salamon N, Andres M, Chute DJ, et al (2006) Contralateral hemimicrencephaly and clinical-pathological correlations in children with hemimegalencephaly. Brain 129:352–365
Chabbert V, Ranjeva JP, Sevely A, Boetto S, Berry I, Manelfe C (1998) Diffusion- and magnetisation transfer-weighted MRI in childhood moya-moya. Neuroradiology 40:267–271
Chu K, Kang DW, Kim DE, Park SH, Roh JK (2002) Diffusion-weighted and gradient echo magnetic resonance findings of hemichorea-hemiballismus associated with diabetic hyperglycemia: a hyperviscosity syndrome? Arch Neurol 59:448–452
Placidi F, Floris R, Bozzao A, et al (2001) Ketotic hyperglycemia and epilepsia partialis continua. Neurology 57:534–537
Ida M, Mizunuma K, Hata Y, Tada S (1994) Subcortical low intensity in early cortical ischemia. AJNR Am J Neuroradiol 15:1387–1393
Benjelloun N, Renolleau S, Represa A, Ben-Ari Y, Charriaut-Marlangue C (1999) Inflammatory responses in the cerebral cortex after ischemia in the P7 neonatal rat. Stroke 30:1916–1924
Sabitha KM, Girija AS, Vargese KS (2001) Seizures in hyperglycemic patients. J Assoc Physicians India 49:723–726
Rangi PS, Partridge WJ, Newlands ES, Waldman AD (2005) Posterior reversible encephalopathy syndrome: a possible late interaction between cytotoxic agents and general anaesthesia. Neuroradiology 47:586–590
Cotton F, Kamoun S, Rety-Jacob F, Tran-Minh VA, Nighoghossian N, Hermier M (2005) Acute hypertensive encephalopathy with widespread small-vessel disease at MRI in a diabetic patient: pathogenetic hypotheses. Neuroradiology 47:599–603
Finelli PF, DiMario FJ Jr (2003) Diagnostic approach in patients with symmetric imaging lesions of the deep gray nuclei. Neurologist 9:250–261
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Raghavendra, S., Ashalatha, R., Thomas, S.V. et al. Focal neuronal loss, reversible subcortical focal T2 hypointensity in seizures with a nonketotic hyperglycemic hyperosmolar state. Neuroradiology 49, 299–305 (2007). https://doi.org/10.1007/s00234-006-0189-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-006-0189-6