Abstract
Cancer is the most challenging global health crisis. In the recent times, studies on extracellular vesicles (EVs) are adding a new chapter to cancer research and reports on EVs explores cancer in a new dimension. Exosomes are a group of subpopulations of EVs. It originates from the endosomes and carries biologically active molecules to the neighboring cells which in turn transforms the recipient cell activity. In general, it plays a role in cellular communication. The correlation between exosomes and cancer is fascinating. Tumor-derived exosomes (TEXs) play a dynamic role in cancer progression and are associated with uncontrolled cell growth, angiogenesis, immune suppression, and metastasis. Its molecular cargo is an excellent source of cancer biomarkers. Several advanced molecular profiling approaches assist in exploring the TEXs in depth. This paves the way for a strong foundation for identifying and detecting more specific and efficient biomarkers. TEXs are also gaining importance in scientific society for its role in cancer therapy and several clinical trials based on TEXs is a proof of its significance. In this review, we have highlighted the role of TEXs in mediating immune cell reprogramming, cancer development, metastasis, EMT, organ-specific metastasis, and its clinical significance in cancer theranostics. TEXs profiling is an effective method to understand the complications associated with cancer leading to good health and well-being of the individual and society as a whole.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The discovery of exosomes and the innumerable research evidences generated has created a milestone in cancer research [1]. Exosomes are a subpopulation of extracellular vesicles (EVs) and it is secreted from the endosomes and transports DNA, RNA, proteins, and lipids [1, 2]. Recently, many studies have revealed that tumor-derived exosomes (TEXs) are involved in multiple events in cancer development [3,4,5]. The molecular signature of TEXs regulates uncontrolled cell growth, immune suppression, angiogenesis, metastasis (EMT-Epithelial–mesenchymal transition, organ-specific metastasis), cancer stem cell development, and drug and therapeutic resistance development [6,7,8,9]. In cancer, the most challenging stage is the early detection of cancer and TEXs serve as one of the most promising source of cancer biomarkers [10, 11]. Exosomes-based cancer investigation is associated with some complications, such as exosomes heterogeneity, and the discrepancies involved in the isolation protocols [12]. It has been reported that single-exosome profiling approach is a promising intra-disciplinary platform for decoding exosomes diversity at the molecular level [13, 14].
In this review, we have explored TEXs and its association in cancer progression, advanced techniques of TEXs profiling, the clinical importance of TEXs in cancer, TEXs-based research challenges, solutions, and the future directions. This review will encourage a large audience to explore the TEXs and its cancer association and pave way for a better therapeutic solution for cancer which can lead to good health and well-being of the population.
Biogenesis of exosomes
Exosomes are secreted by all cell types and its biogenesis (Fig. 1) involves a more complex process in the cellular system [15]. The exosomes biosynthesis process is initiated from the endosomal compartment where the membrane of an early endosome undergoes a sequence of inward invaginations as it develops into a late endosome, bearing multiple intraluminal vesicles (ILVs). The multivesicular body (MVB) is a mature form of endosome and it carries many exosomes [16,17,18]. Fusion of MVBs with plasma membrane [19] releases the exosomes and each of these exosomes contains the molecular signature of the parental cell [16, 20]. Exosomes and its membranes encompass several molecules which play a vital role in cellular communication. Its biogenesis is classified into two different pathways, which includes endosomal sorting complex needed for transport, namely (ESCRT)-dependent pathways [21, 22], and ESCRT-independent pathways. ESCRT complex is made of several subunits, such as ESCRT-0 (it activates ESCRT-I subunit), ESCRT-I (involved in cargo selection), ESCRT-II (cargo selection with ESCRT-II), and ESCRT-III (supports fusion of MVBs with plasma membrane and exosomes release). ESCRT-independent pathway of exosomes biogenesis involves several cytoplasmic lipids and tetraspanin proteins (CD63, CD9, etc.). In cancers, exosomes biogenesis occurs through the ESCRT-independent pathway [23] and exosomes transport cellular information from the parent cells to the recipient cells [24, 25]. TEXs cargo can transform normal cells into malignant cells [26]. Scientific research also reveals that exosomes release is closely linked to the Rap GTPase (Ras-proximate-1 Guanosine Triphosphatase) proteins that controls the secretory pathways, specifically Rab27a and Rab27b (Ras analog in brain). It has been shown that TEXs are reduced when Rab proteins are knocked down [27, 28].
Exosomes biogenesis and its molecular components. a Exosomes biogenesis (Adapted with permission from Ref. [198]. Copyright @ 2022 American Chemical Society.) and b Molecular component of exosomes. (Created with BioRender.com.)
Property of tumor-derived exosomes (TEXs)
Electron microscopy can be considered a useful tool in determining the shape and structure of TEXs [29]. The observations reveal that they are nothing but round membrane-bound vesicles, each with a diameter of less than 50 nm [30, 31]. TEXs and exosomes derived from non-malignant cells differ significantly due to their molecular cargo [32, 33]. TEXs extracted from tumor cells have been shown to express characteristic protein molecules that can be used as biomarkers in cancer screening [34, 35]. In TEXs, immunosuppressive proteins (Programmed Cell Death Ligand 1(PD-L1), FasL), cytokines (Transforming growth factor (TGF-1), Interleukin-10 (IL-10)), and transmembrane proteins (CD39, CD73) are highly expressed. TEX’s cancer antigen cargo enhances the immune responses, and, on the other hand, its immunosuppressive molecule downregulates the immune responses [36, 37]. This dual nature of TEXs requires a more detailed investigation for clinical applications [38, 39]. Recent scientific research focuses on TEXs enhancing the development of anti-cancer immune responses [40] and TEX-mediated cancer immune modulation has gained the spotlight in the current decade [36, 38, 39].
TEXs and cellular communication
Exosomes are excellent messenger molecules [41]. Their surface is embedded with signaling molecules received from the parent cell and their internal cargo, such as DNA, RNA proteins play a role in cell reprogramming. Communication between TEXs and recipient cells occurs via various methods, such as surface molecules, phagocytosis, and uptake via ligand molecules [38]. A complex molecular cascade involves TEXs and its recipient–cell’s intercommunication [42].
Role of TEXs in the tumor microenvironment
A tumor microenvironment (TME) is a cancer-developing ecosystem. TME-associated cell signaling is a more complex pathway to promote cancer [43, 44]. Exosomes are well suited for this task as they explain how information travels from the tumor to the surrounding tissues [45, 46]. Tumors depend on the stroma to sustain their growth. During tumor progression, fibroblasts change their shape, protein expression, growth pattern, and cytokine profile. TEXs are involved in the reprogramming of Cancer Associated Fibroblasts (CAFs) via the TGF/Smad pathway [47], resulting in the transformation of normal epithelial cells into premalignant cells [48, 49]. In a growing tumor, hypoxic conditions cause malignant cells to produce more exosomes [50, 51]. TEXs-mediated molecules play a vital role in enhancing cancer angiogenesis [52, 53]. Exosomes are a powerful tool for tumor cells communication in cancer [54]. The existence of previously secreted exosomes in the microenvironment has been shown to limit the subsequent release of exosomes, showing that the secretory pathway is controlled by a negative feedback loop [55, 56]. Tumors also absorb exosomes efficiently, suggesting the existence of a positive feedback loop critical to tumor cell activity [57, 58].
TEXs effects on the immune system
TEXs develop strong intercellular communication during cancer development in the tumor microenvironment [59]. They carry multiple immunosuppressing cargoes which play a vital role in immune cell development, maturation, and anti-cancer activity [59].
Macrophages
Macrophage immune cells perform a vital role in antigen presentation and phagocytosis and provide the link between innate and adaptive immunity [60]. TEXs reprogram the macrophages through multiple signaling pathways. One study found that breast cancer originates from TEXs bearing Annexin II (Anx II) that promotes angiogenesis and this event is mediated by activated p38, signal transducer, and activator of transcription 3 (STAT3), and Mitogen-activated protein kinase (MAPK) pathways [61]. It has been reported that TEXs containing T-cell immunoglobulin and mucin-domain containing-3 (Tim-3) amplify the cancer cells in EMT [44]. In addition, exosomes miRNA has been observed to be the most predominant area for understanding human pathological conditions. TEXs-derived miRNAs play a vital role in macrophage function modifications. TEXs release miR-21 and miR-29a to create a pro-metastatic-inflammation signal via Toll-like receptors (TLR) as a result of which tumor grows with aggressive patterns and the cancer cells metastasize [62]. Scientific studies have shown that miRNA-181, miRNA-125, miRNA-222, and miRNA-21 released by TEXs play a role in angiogenesis in the context of Macrophage 2 (M2) polarization [63, 64]. TEX's miRNAs also direct the conversion of macrophages to tumor-motivated cell populations involved in cancer cell proliferation, invasion, and metastasis.
Dendritic cells (DCs)
Dendritic cells are the antigen-presenting cells in the immune system and they form the interlink between innate and adaptive immunity [65]. Some of the research groups observed that TEXs-mediated IL-6 production inhibits DCs proliferation [66]. It was reported that pancreatic cancer-derived exosomal miR-203 cargo when transferred into DCs led to downregulation of TLR, Tumor Necrosis Factor Alpha (TNF-α), and Interleukin-12 (IL-12) [67]. The microRNAs and long non-coding RNA of TEXs are also involved in the escape of cancer cells from DCs [63].
Natural killer (NK) cells
Natural killer (NK) cells and Natural killer T (NKT) cells are lymphocytic subsets whose action play a vital role in anticancer activity [68]. NK cell dysfunctions were observed in multiple hematologic malignancies and in chronic lymphocytic leukemia (CLL) [69]. Activation of the NKG2C, NKG2D, NKP30, and NKP46 receptors play a vital role in NK cell cytotoxicity [70]. The NKG2D receptor actively regulates the cytotoxicity of B cells, NKT cells, CD8 +, and gamma delta T cells. Scientific evidence shows that exosomes downregulate NKG2D as effective immune cells and lose their cytotoxic effect on aging cancer cells [71]. TEX-secreted Tumor Necrosis Factor Beta1 (TGF-β1) led to this immunological cellular function transformation [72]. TEXs carry miR-210 and miR-23a in the hypoxic condition of the tumor microenvironment. TEXs downregulate CD107a (this is the functional marker of NK cells) expression in NK cells [73]. In these multiple TEX-regulated events, NK cells eventually lose its activity.
B cells
Although exosomes activities have been studied on a wide range of leukocytes and other cells in the tumor microenvironment, little has been explored about the communication between tumor-derived exosomes and B cells. The importance of B cells for anti-tumor immunity is controversial, as both pro- and anti-tumorigenic effects have been proposed [74]. TEXs enhance the proliferation of the B-Reg subpopulation of B cells and aids in tumor cell escape from immune system obsequiousness [75]. B-reg cells release anti-inflammatory signals via IL-10 that reduce the antitumor activity of T cells and Regulatory T cells (Tregs) leading to decreased immune response [76]. B10 cells, among the B-regs, have recently been shown to play important immunological regulatory roles in inflammation and tumorigenesis. By generating IL10, induced B10 cells can further transmit the inhibitory phenotype to other immune cells and upregulate the percentages of B-regs and Tregs, creating a positive feedback loop driven by exosomes [77].
T cells
TEXs inhibit the proliferation of cytotoxic T cells (CD8 +) and activate CD8 + apoptosis [78]. A scientific study showed that TEXs suppress the anti-cancer phenomena of CD8 + [79, 80]. The most exciting observed phenomenon is that TEXs downregulate CD27/28, not only in in vitro systems but also in head and neck cancer tissues [81]. In addition, TEXs miRNAs are involved in the development of a functionally modified CD8 + cell population. Tumor cells exclusively express Fas ligand (FasL), a transmembrane protein related to receptor Fas-mediated apoptosis promotion [82]. This immune activity changes in exciting ways when TEXs major histocompatibility complex-I (MHC-I) expression triggers the Fas/FasL signaling pathway of CD8 + cell apoptosis [83,84,85]. Scientific studies have observed that metastatic melanoma cells release abundant exosomes with Programmed Cell Death Ligand 1 (PDL-1) expression regulated by Interferon (IFN) [64]. TEXs with PDL-1 overexpression is a more reliable biomarker compared to blood PDL-1 in tumor biopsy. In the case of melanoma patients, the circulating TEXs PDL-1 may be an interesting early diagnostic tool for treatment [86]. Plasma-derived exosomes cargo contains PDL-1 mRNA, and this was observed in lung cancer and melanoma [87]. TEXs-PDL-1 expression plays the role in CD8 +-based PDL-mediated apoptosis suppression [88]. TEXs contains elements that stimulate immunosuppression in the extracellular environment of the tumor and indirectly attract immunosuppressive T-regulatory (Treg) cells. TEXs also produce molecules that inhibit the development of DCs, T-reg cells, and myeloid suppressor cells (MDSCs) [89]. However, the mechanistic features of the molecular interactions of T cells with TEXs are still unknown [90]. TEXs has been proven to have a suppressive effect on immune cells. TEXs also lead to the alteration of several genes of immune cells at the transcriptional level [91]. Moreover, dynamic molecular charges of TEXs play an important role in the functional modifications of the immune system [20, 92].
Myeloid-derived suppressor cells (MDSCs)
Myeloid-derived suppressor cells (MDSCs) are immature heterogeneous cell groups of granulocytes, macrophages, and DCs [93]. TEXs regulates the development, survival, and immunosuppression of MDSCs [47]. Its membrane carries the Heat shock protein (HSP) ligands that activates MDSCs via TLR2/MyD88 [94]. A group of researchers observed that renal cell carcinoma-derived exosomes have Heat shock protein-70 (HSP70), which triggers the STAT3 via TLR2/MyD88 [95]. Another group of researchers investigated the A8 peptide aptamer, which binds with TEXs membrane containing HSP70, leading to the blocking of HSP70/TLR2-mediated activation of MDSCs [96]. TEXs identically regulate tumor progression via the Prostaglandin E2 (PGE2) and TGF-β molecules [97]. miRNA-expressing profile suggests that TEXs cargo miRNA-342, miRNA-320, miRNA-27, and miRNA-126 support tumor development [98]. Figure 2 summarizes immune modification by TEXs in the tumor microenvironment.
Role of TEXs in cancer development
TEXs play a critical role in reprogramming cellular signaling in the tumor microenvironment via their cargoes [95, 96]. Cellular stress conditions such as hypoxia, hyperacidity, and starvation accelerate exosomes secretion and cancer progression. 3 [99, 100]. TEXs can modulate the ECM via regulation of multiple exosomes surface molecules causing cells to lose their ability to adhere [101,102,103,104]. Several types of integrin have been described in exosomes. TEX’s integrin builds the cancer cell colonies, a pre-metastatic niche, and it is also involved in organ-specific metastasis [105]. Cargoes of exosomes like miRNAs, lncRNAs, and proteins are involved in the aggressive progression of cancer metastasis [106]. Additional TEXs also regulate the transformation of a large population of associated cells of the tumor microenvironment into cancer-associated fibroblasts (CAF) [107, 108]. CAFs derived exosomes miRNAs and several growth factors target tumor microenvironment cells (TME) and alter cellular metabolism by increasing glucose uptake and reducing mitochondrial oxidative phosphorylation [109].
TEXs in angiogenesis
Angiogenesis is a multiple event in cancer, where cancer cells are generated by new blood vessels that promote cancer metastasis [110]. In the case of cancer angiogenesis, cancer growth, and metastasis, Vascular Endothelial Growth Factor (VEGF) / VEGF receptor plays a major role [111, 112]. TEXs are involved in angiogenesis and the secretion of VEGF, Platelet-derived growth factor (PDGF), transforming growth factor β (TGF-β), interleukin-8 (IL-8), fibroblast growth factor (FGF), and tumor necrosis factor α (TNF-α) [113, 114] promoted angiogenesis. Research evidences show that in vivo and in vitro models of TEXs are involved in multiple cancer angiogenesis like glioblastoma and pancreatic and nasopharyngeal carcinomas [90, 115]. In the brain, cancer revascularization initiated via exosomes carry higher expression of levels of miRNA-221, proteoglycans, glypican-1, and syndecan-4 and as a result, generates new blood vessel synthesis [116]. Extracellular vesicles research-updated information suggests that exosomes support cancer angiogenesis via complex cell-to-cell communication.
TEXs in metastasis
A group of investigators observed that HSP90-containing exosomes secreted in breast cancer consequently modulated the ECM more aggressively in breast cancer [117]. Phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) and Mitogen-activated protein kinase (MAPK) signaling played a crucial role in the upregulation of Matrix Metalloproteinases (MMPs) [118]. Bladder cancer TEXs showed multiple HSP expression that induced mesenchymal markers, thus promoting EMT [118]. A scientific team reported that prostate TEXs carrying miRNA-100-5p, miRNA-21-5p, and miRNA-139-5p promoted cancer metastasis through MMP-2 and MMP-9 [119]. The research study reported that higher expression of MMPs and integrins regulated the degradation of ECM (Fig. 3) [117]. In cancer, cellular migration control via TEXs integrins also played an important role in the organ-specific migration of cancer cells and niche formation prior to metastasis [118, 120].
TEXs and cancer metastasis. Tumor-derived exosomes regulate cancer metastasis events, a TEXs regulate uncontrolled cell growth, suppress the immune response, and promote angiogenesis in cancer, b TEXs in extracellular matrix (ECM) remodeling (ECM) c TEXs role in EMT (epithelial–mesenchymal transition) and organ-specific metastasis. (Created with BioRender.com.)
TEXs in EMT and organ-specific metastasis
Epithelial-to-mesenchymal transition (EMT) is one of the most complex event in cancer development. It regulates the formation of new cancer metastatic cell niches and is involved in the migration of cancer cells to distant organs and the formation of secondary tumors [121]. During EMT, TEX’s miRNA cargo promotes this transition [57]. During EMT, cancer cells lose their adhesive properties and express high levels of mesenchymal cell markers (N-cadherin, vimentin, snail, and twist) [122,123,124]. Hypoxia-mediated TEXs express high levels of miR-301a-3p in pancreatic cancer, which induces macrophages in the M2 subpopulation. Activation of the PTEN/PI3K pathway increases EMT via macrophage 2 (M2) polarization [125]. Scientific studies have shown that TEXs matrix metalloproteinase (MMP)-13 cargo was responsible for the development of aggressive metastases of nasopharyngeal cancer (NPC) [126]. The dynamic expression pattern of TEX’s integrin drives the circulating cancer cells to migrate to the specific organs (Fig. 4) [127,128,129]. TEXs integrin includes two different subunits such as α and β, both of which guide blood-circulated tumor cells to reach specific different organs, such as bone, liver, brain, lung, and lymph nodes [128, 129].
TEXs analysis techniques
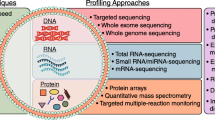
Cancer is one of the deadliest diseases in the world [130], but early detection and treatment can limit cancer development and extend a patient’s life expectancy [131]. Common diagnostic procedures such as biopsies, endoscopies, and magnetic resonance imaging are intrusive and expensive, and the availability of such facilities around the world is uncertain [132]. As a result, the detection of a molecular signature for cancer, also known as a cancer biomarker, is becoming a more effective and promising diagnostic tool for oncologists and researchers [133]. The use of tumor-derived exosomes as cancer biomarkers has the potential to pinpoint the mechanism of tumor initiation and the stages of malignancy. TEXs are released into extracellular fluids in large quantities with excellent stability. Exosomes are crucial for cell signaling and molecular exchanges that contribute to the interactions of cancer cells with the tumor microenvironment [134]. TEXs are involved in both physiological and pathological events and play a role in a variety of cellular activities. The use of TEXs as a biosignature is crucial for the future of non-invasive preclinical malignancy analysis and therapy efficacy analysis. Although the use of TEXs as a prognostic tool for cancer detection is still in its early stages, the study of TEXs for early malignancy initiation and understanding of therapeutic effects has attracted great interest [36]. Some of TEX’s analysis techniques are discussed in Table 1.
Biosensors: point of care (POC) assays
Exosomes in body fluids need to be identified and validated by a timely and efficient method. POC assays, especially biosensors, are attracting great interest from academics and engineers around the world due to its effectiveness in exosomes analysis. Biosensors outperform instrument-dependent technologies in terms of cost, multiplexing potential, fast results, high sensitivity, high accuracy, and ease of use [135, 136]. People in low-income countries who do not have access to sophisticated cancer detection methods can now benefit from using biosensors to detect cancer at an early stage by detecting TEXs, which can help improve cancer therapy strategies [137]. Because malignant antigens are found in abundance on the outside of TEXs, exploiting variations in the surface composition of TEXs is a convenient technique for efficient and easy exosomes detection. The binding energy and fidelity of the bioreceptor and the target determines the selection of the recognition element [137]. The key-lock paradigm of the antigen–antibody interaction is the best-known biological recognition [138].
Colorimetric assay
Colorimetric biosensors used to identify exosomes are of outstanding importance for the empirical POC approach due to their ease of use and uncomplicated interpretation [139]. Colorimetric biosensors can be detected with the naked eye through a color shift that is easily and immediately observed. TEXs are collected by capture elements and detection elements labeled with nanomaterials are subsequently connected to the TEXs, resulting in the color change visible due to the accumulation of nanoparticles. The detection is completed within 15 min [140].
Fluorescence assay
Due to their high sensitivity, fluorescent biosensors offer significant advantages for the detection of exosomes. Fluorescence signal generation often depends on the use of fluorophores. The aptamer sequence is divided into two segments, the capture probe and the detection probe, which are used to capture exosomes and it forms a layered structure that generates luminescence. The exosomes concentration is calculated from the luminescence intensities of the images [141, 142]
Surface plasmon resonance (SPR)
SPR is a label-free, real-time analytical technique that detects the chemical interactions on the surface of a gold layer by measuring changes in the refractive index [139]. SPR is particularly delicate to cellular dockings happening by 200 nm of the gold layer. This gap is quite close to the size of exosomes. As a result, SPR-based biosensors are ideal for studying TEXs. The substratum for previously described SPR biosensors for TEXs identification has been predominantly elicited from gold chips and real-time signal changes, resulting in antibody-exosomes binding which can be recorded [143, 144].
Surface-enhanced Raman scattering (SERS)
Due to their exceptionally increased inquisitive signals, which includes singular molecule-level responsiveness and insensitivity to quenching, SERS-based biosensors are utilized for TEXs recognition [145]. In this process, nanoparticles play a role in capture of TEXs with greater efficiency and sensitivity [146, 147].
Electrochemical biosensors
Electrochemical (EC) biosensors (Fig. 5) can convert the identification of biomolecules into signals, such as current, potential, and impedance [148]. Many labeled tags such as enzymes, ferrocene, interactive electroactive compounds, or nanoparticles are being developed for applications toward electrochemical biosensors for signal generation [149]. Due to advances in microprocessors along with the introduction of novel electrodes, electrochemical biosensors are moving toward miniaturization and portability [150].
Electrochemical biosensor. a Integrated magnetic electrochemical exosomes sensor (iMEX) based on CD 63 antibody protocol. b Image of iMEX. c The functional components of iMEX. (Adapted with permission from Ref. [161]. Copyright @ 2018 American Chemical Society)
Clinical applications of TEXs as cancer biomarkers
TEXs levels in the blood and other body fluids (such as saliva) have been associated with tumor formation [151]. TEXs expression levels in patients have been correlated with advanced tumor stage, positive lymph node status, and metastasis [152]. Exosomes generated from cancer cells include various molecular characteristics that are specific to malignancies [153]. Exosomes have been hypothesized as a vehicle via which cytotoxic T-lymphocyte responses could be boosted by the interaction of tumor exosomes with dendritic cells [154]. TEXs are employed in clinical diagnosis and as a screening factor in cancer patients. A new technique for capturing circulating TEXs, which can be used as tumor markers in customized diagnostics, has been developed [155, 156]. Microfluidics on chip TEXs RNA profiling (Fig. 6) enhances the potentiality of liquid biopsies [156, 157]. If the roles of exosomes and exosomes miRNA in cancer progression are identified, the testing will be more reliable and less difficult. As a result, combining TEXs and circulating tumor cells detection could increase cancer diagnosis precision [158, 159]. TEXs have emerged as a key player in tumor survival, angiogenesis, invasion, metastasis, and immunomodulation by conveying oncogenic molecules, such as miRNAs, DNA, proteins, and lipids [160, 161]. As exosomes-carrying miRNAs can be transported to biofluids, liquid biopsies of plasma, urine, saliva [162], and cerebrospinal fluid is a non-invasive way of getting precise information regarding the tumor environment [163, 164]. For example, in recent years, some laboratories have used urine exosomes miRNA as prostate and bladder cancer biomarkers, so high levels of exosomes miRNA-21 and miRNA-21-5p [165] are deemed as a sign of prostate and bladder cancer development, respectively [166, 167]. TEXs have a great clinical impact on cancer biomarker research (Fig. 7).
Microfluidics on chip EVs RNA profiling. a Magnetic bead and antibody conjugated approach of EVs capture and RNA profiling. b Scanning electronic microscopy (SEM) Image of the magnetic bead after capture of EVs. c Image of the microfluidic immuno-magnetic exosome RNA(iMER) model. (Adapted with permission from Ref. [161]. Copyright @ 2018 American Chemical Society)
Application of TEXs in cancer immunotherapy development
Tumor Exosomes (TEXs) are said to promote tumor progression, survival, and metastasis. These TEXs participate in the tumor microenvironment and regulate the survival of cancer cells, while affecting the healthy/immune cells [58]. TEXs, as they are released by the cancer cells, can be very promising diagnostic markers. The exosomes carry proteins specific to the origin (parent) cells and thus it is easy to locate the site of the tumors [168]. TEXs activate or suppress immunity via tumor-associated immunity as they contain Major Histocompatibility complexes (MHCs) that can play a crucial role in regulating tumor responses [169]. TEXs can be modified or engineered so that they can be used for therapy. Due to immune suppression, the CD8 Cytotoxic T-lymphocytes (CTLs) get exhausted and thus do not function properly [170]. Cancer cells can be eliminated by relieving the CTLs from exhaustion using the two checkpoint regulators PD-L1 and (Cytotoxic T lymphocyte antigen-4) CTAL-4 [171]. PD-1 is a receptor on the effector T cells that binds to the PD-L1 checkpoint regulator on the surface of cancer cells [172]. Scientific research focuses on TEXs surface PD-L1 blocking antibody development to prevent TEX-mediated immune cell apoptosis [145, 173, 174]. This protein blockade strategy has been used for the treatment of gastric cancer [175]. Since exosomes cargoes contain the proteins from the parent cells, they carry the PD-L1 receptor from the tumor cells that they originate from. Thus the exosomes PD-L1 can be used as a very good therapeutic target in a variety of cancer malignancies [176]. Similarly, many other proteins like T cell immunoglobulin and mucin-domain containing-3 (TIM-3) and its ligand Galectin can be used as potential biomarkers as they regulate anti-tumor immune responses [30, 177]. The presence of Tumor-Associated Antigens (TAA) is of great advantage as these antigens can be used to make vaccines against cancer [38]. TEXs loaded with DCs (Dendritic Cells) are more advantageous cancer vaccines as DCs are the naturally occurring antigen-presenting cells [178]. TEXs enriched with miR124 promoted anti-tumor responses in mice with colorectal cancer [179]. Also due to their specificity and stability TEXs can be used for the delivery of therapeutic drugs to the target site (Cancer cells) and thus can eliminate the risk of non-specific targeting of the healthy or immune cells [180, 181]. TEX-related therapeutic approach needs more molecular profiling (because its inner cargo molecule is still under investigation as a therapeutic development domain) [182].
Clinical trials
Exosomes are becoming increasingly recognized as important cell-to-cell communication mediators [183]. They deliver active biomolecules to the target cells, play crucial roles in many physiological and pathological processes, and hold great potential as cutting-edge disease-treating approaches [184, 185]. Clinical trials (Table 2) using exosomes show promise in the treatment of numerous ailments [186]. The understanding of the underlying mechanisms that govern the varied roles of exosomes that have been discovered is still far from comprehensive, and more multidisciplinary study is required to deal with these tiny vesicles. The various forms of approaches have been developed keeping different ailments as targets. Exosomes associated with cancer/tumors (TAEs) have been used as cancer vaccines since they carry tumor antigens. They are combined with the anti-sense molecules to slowly release antigens, allowing the recruitment of immune cells to treat malignant glioma of the brain (NCT01550523). In the case of exosomes derived from immune cells (dendritic cells), they can be loaded with tumor antigens and act as vaccines for conditions, like Non-Small Cell Lung Cancer (NCT01159288). The chimera of exosomes derived from one’s tumor and dendritic cells are also being produced to promote antigen presentation for the immune cells (NCT05559177). Patients with Metastatic Pancreatic cancer with Kras (Kristen Rat Sarcoma Viral oncogene homolog) G12D mutation are being treated using exosomes derived from mesenchymal stromal cells. These exosomes are designed with KrasG12D siRNA to specifically act on a particular type of pancreatic cancer. Similarly, for gastric and colorectal cancer, lipid-tagged exosomes (STAT6 anti-sense oligonucleotide (ASO-STAT6)) are being used (NCT05375604). With the advancement of science, it is now possible to administer exosomes orally in an edible powder form. This helps to prevent oral mucositis which is a known complication seen after radiation therapy for Head and Neck cancers (NCT01668849).
Challenges and future directions
Tumor-derived exosomes (TEXs) are an intriguing chapter in cancer research and additional studies are required to reveal several facts about cancer development in a more detailed manner. It also transforms the cancer liquid biopsies [187]. To fully decipher their molecular interactions and information, further research is essential, but, the heterogeneity of EV’s small sizes presents technical challenges [188]. Exosomes heterogeneity [189, 190] (it is based on exosomes size, origin, and molecular diversity) develops more complications in exosome-based cancer biomarkers and therapeutic development. Currently, scientists are opting for an innovative solution for it, called single-exosome profiling (it is a multidisciplinary approach which reveals the complex secrets about exosomes) (Fig. 8). As a non-invasive biomarker, exosomes could guide effective personalized medicine strategies and a novel treatment for delivering endogenous modulation or exogenous therapeutics [191, 192]. These exosomes play a role in the tumor’s well-being in the tumor microenvironment (TME). Circulating exosomes in cancer patients are different from those in healthy donors in terms of both quantity and diversity [193]. Multiple scientific investigations suggest that TEXs play a vital role in future therapeutic development [192, 194, 195]. This research domain requires dynamic fusion approaches, such as advanced assay [196] omics, nanotechnology, artificial intelligence (AI), and machine learning [189]. Thus, exosomes research is a new horizon that decodes complications in cancer. It can in future become a bright star in the upcoming precision [197] medicine era for good health and well-being of patients.
Single-exosome profiling (Adapted with permission from Ref. [192]. Copyright @ 2022 American Chemical Society) (Created with BioRender.com.)
Conclusion
Tumor-derived exosomes (TEXs) provide us information to understand cancer development in an extensive way. It has a promising role in cancer biomarkers examination, especially in early cancer detection. Still, some of the questions are answerable, like how during cancer development TEXs nature changes and its inter-cargo packaging mechanism gets altered. Therefore, TEXs DNA should be subjected to more examination (global research indicates only miRNA and proteins) and although, several clinical trials mention that TEXs-based therapeutic approach is really complicated because of its dual nature, in future, interdisciplinary research involving exosomes and nanotechnology fusion may develop a better solution for cancer for the good health and well-being of people around the world.
Data availability
All relevant data are provided within the manuscript.
References
Dai J, Su Y, Zhong S, Cong L, Liu B, Yang J, Tao Y, He Z, Chen C, Jiang Y. Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduct Target Ther. 2020;5(1):145. https://doi.org/10.1038/s41392-020-00261-0.
Sun Z, Yang S, Zhou Q, Wang G, Song J, Li Z, Zhang Z, Xu J, Xia K, Chang Y, Liu J, Yuan W. Emerging role of exosome-derived long non-coding RNAs in tumor microenvironment. Mol Cancer. 2018;17(1):82. https://doi.org/10.1186/s12943-018-0831-z.
Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–79.
Zhang L, Yu D. Exosomes in cancer development, metastasis, and immunity. Biochim Biophys Acta Rev Cancer. 2019;1871(2):455–68.
Baig MS, et al. Tumor-derived exosomes in the regulation of macrophage polarization. Inflamm Res. 2020;69(5):435–51.
Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–83.
Ghosh, S., et al., Clinical impact of exosomes in colorectal cancer metastasis. ACS Appl Bio Mater. 2023.
Mukherjee, S., et al., Exosomal miRNAs and breast cancer: a complex theranostics interlink with clinical significance. Biomarkers. 2023. 1–17.
Mathivanan, S., et al., ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res, 2012. 40(Database issue): pp D1241–4.
Wong CH, Chen YC. Clinical significance of exosomes as potential biomarkers in cancer. World J Clin Cases. 2019;7(2):171–90. https://doi.org/10.12998/wjcc.v7.i2.171.
Yu D, Li Y, Wang M, Gu J, Xu W, Cai H, Fang X, Zhang X. Exosomes as a new frontier of cancer liquid biopsy. Mol Cancer. 2022;21(1):56. https://doi.org/10.1186/s12943-022-01509-9.
Dhar R, Devi A, Patil S, Tovani-Palone MR. Exosomes in cancer therapy: advances and current challenges. Electron J Gen Med. 2023;20(5):em524.
Hilton SH, White IM. Advances in the analysis of single extracellular vesicles: a critical review. Sens Actuat Rep. 2021;3:100052. https://doi.org/10.1016/j.snr.2021.100052.
Bordanaba-Florit G, Royo F, Kruglik SG, Falcón-Pérez JM. Using single-vesicle technologies to unravel the heterogeneity of extracellular vesicles. Nat Protoc. 2021;16(7):3163–85. https://doi.org/10.1038/s41596-021-00551-z.
Saleem SN, Abdel-Mageed AB. Tumor-derived exosomes in oncogenic reprogramming and cancer progression. Cell Mol Life Sci. 2015;72(1):1–10.
Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–89.
Rajagopal C, Harikumar KB. The Origin and Functions of Exosomes in Cancer. Front Oncol. 2018;8:66.
Bhattacharya B, et al. Exosome DNA: An untold story of cancer. Clin Transl Disc. 2023;3: e218.
Shivji GG, et al. Role of exosomes and its emerging therapeutic applications in the pathophysiology of non-infectious diseases. Biomarkers. 2022;27(6):534–48.
Abels ER, Breakefield XO. Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cell Mol Neurobiol. 2016;36(3):301–12.
Tricarico C, Clancy J, D’Souza-Schorey C. Biology and biogenesis of shed microvesicles. Small GTPases. 2017;8(4):220–32.
Guo X, Tan W, Wang C. The emerging roles of exosomal circRNAs in diseases. Clin Transl Oncol. 2021;23(6):1020–33.
Dhar R, et al. Exosome and epithelial–mesenchymal transition: a complex secret of cancer progression. J Cell Mol Med. 2023.
Miller IV, Grunewald TG. Tumour-derived exosomes: tiny envelopes for big stories. Biol Cell. 2015;107(9):287–305.
Chaput N, et al. Exosome-based immunotherapy. Cancer Immunol Immunother. 2004;53(3):234–9.
Jabbari N, et al. Tumor-derived extracellular vesicles: insights into bystander effects of exosomes after irradiation. Lasers Med Sci. 2020;35(3):531–45.
Jafari A, et al. Exosomes and cancer: from molecular mechanisms to clinical applications. Med Oncol. 2021;38(4):45.
Gebeyehu A, et al. Role of exosomes for delivery of chemotherapeutic drugs. Crit Rev Ther Drug Carrier Syst. 2021;38(5):53–97.
Meehan K, Vella LJ. The contribution of tumour-derived exosomes to the hallmarks of cancer. Crit Rev Clin Lab Sci. 2016;53(2):121–31.
Yang R, et al. Galectin-9 interacts with PD-1 and TIM-3 to regulate T cell death and is a target for cancer immunotherapy. Nat Commun. 2021;12(1):832.
Graner MW, Schnell S, Olin MR. Tumor-derived exosomes, microRNAs, and cancer immune suppression. Semin Immunopathol. 2018;40(5):505–15.
Fontana S, et al. Contribution of proteomics to understanding the role of tumor-derived exosomes in cancer progression: state of the art and new perspectives. Proteomics. 2013;13(10–11):1581–94.
Rak J, Guha A. Extracellular vesicles–vehicles that spread cancer genes. BioEssays. 2012;34(6):489–97.
Ludwig N, Whiteside TL. Potential roles of tumor-derived exosomes in angiogenesis. Expert Opin Ther Targets. 2018;22(5):409–17.
Niu L, et al. Tumor-derived exosomal proteins as diagnostic biomarkers in non-small cell lung cancer. Cancer Sci. 2019;110(1):433–42.
Sharma S, et al. Tumor-derived exosomes in ovarian cancer - liquid biopsies for early detection and real-time monitoring of cancer progression. Oncotarget. 2017;8(61):104687–703.
Pashoutan Sarvar, D., K. Shamsasenjan, and P. Akbarzadehlaleh, Mesenchymal Stem Cell-Derived Exosomes: New Opportunity in Cell-Free Therapy. Adv Pharm Bull, 2016. 6(3): 293–299.
Whiteside TL. Tumor-derived exosomes and their role in cancer progression. Adv Clin Chem. 2016;74:103–41.
Kharaziha P, et al. Tumor cell-derived exosomes: a message in a bottle. Biochim Biophys Acta. 2012;1826(1):103–11.
Yu S, et al. Tumor-derived exosomes in cancer progression and treatment failure. Oncotarget. 2015;6(35):37151–68.
Mathieu M, et al. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9–17.
Admyre C, et al. Exosomes with immune modulatory features are present in human breast milk. J Immunol. 2007;179(3):1969–78.
Zhou J, et al. Tumor-derived exosomes in colorectal cancer progression and their clinical applications. Oncotarget. 2017;8(59):100781–90.
Cheng H, et al. The tumor microenvironment shapes the molecular characteristics of exhausted CD8(+) T cells. Cancer Lett. 2021;506:55–66.
Whiteside, T.L., B. Diergaarde, and C.S. Hong, Tumor-Derived Exosomes (TEX) and Their Role in Immuno-Oncology. Int J Mol Sci, 2021. 22(12).
Diaz Bessone MI, et al. The tumor microenvironment as a regulator of endocrine resistance in breast cancer. Front Endocrinol (Lausanne). 2019;10:547.
Tian X, et al. Tumor-derived exosomes, myeloid-derived suppressor cells, and tumor microenvironment. J Hematol Oncol. 2019;12(1):84.
Naito Y, et al. How cancer cells dictate their microenvironment: present roles of extracellular vesicles. Cell Mol Life Sci. 2017;74(4):697–713.
Baghban R, et al. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun Signal. 2020;18(1):59.
Malla RR, Shailender G, Kamal MA. Exosomes: critical mediators of tumour microenvironment reprogramming. Curr Med Chem. 2021;28(39):8182–202.
Lan J, et al. M2 macrophage-derived exosomes promote cell migration and invasion in colon cancer. Cancer Res. 2019;79(1):146–58.
Milane L, et al. Exosome mediated communication within the tumor microenvironment. J Control Release. 2015;219:278–94.
Mashouri L, et al. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer. 2019;18(1):75.
Pittet MJ. Behavior of immune players in the tumor microenvironment. Curr Opin Oncol. 2009;21(1):53–9.
Kara-Terki L et al. Critical Roles of Tumor Extracellular Vesicles in the Microenvironment of Thoracic Cancers. Int J Mol Sci, 2020. 21(17).
Locy H, et al. Immunomodulation of the tumor microenvironment: turn foe into friend. Front Immunol. 2018;9:2909.
Desage AL et al. The Immune Microenvironment of Malignant Pleural Mesothelioma: A Literature Review. Cancers (Basel), 2021. 13(13)
Olejarz W, et al. Tumor-derived exosomes in immunosuppression and immunotherapy. J Immunol Res. 2020;2020:6272498.
Whiteside TL. Exosomes in cancer: another mechanism of tumor-induced immune suppression. Adv Exp Med Biol. 2017;1036:81–9.
DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;19(6):369–82.
Maji S, et al. Exosomal Annexin II promotes angiogenesis and breast cancer metastasis. Mol Cancer Res. 2017;15(1):93–105.
Fabbri M, et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci USA. 2012;109(31):E2110–6.
Chen G, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560(7718):382–6.
Ying X, et al. Epithelial ovarian cancer-secreted exosomal miR-222-3p induces polarization of tumor-associated macrophages. Oncotarget. 2016;7(28):43076–87.
Worbs T, Hammerschmidt SI, Forster R. Dendritic cell migration in health and disease. Nat Rev Immunol. 2017;17(1):30–48.
Yu S, et al. Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J Immunol. 2007;178(11):6867–75.
Zhou M, et al. Pancreatic cancer derived exosomes regulate the expression of TLR4 in dendritic cells via miR-203. Cell Immunol. 2014;292(1–2):65–9.
Vivier E, et al. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol. 2012;12(4):239–52.
Reiners KS, et al. Soluble ligands for NK cell receptors promote evasion of chronic lymphocytic leukemia cells from NK cell anti-tumor activity. Blood. 2013;121(18):3658–65.
Garcia-Iglesias T, et al. Low NKp30, NKp46 and NKG2D expression and reduced cytotoxic activity on NK cells in cervical cancer and precursor lesions. BMC Cancer. 2009;9:186.
Clayton A, et al. Human tumor-derived exosomes down-modulate NKG2D expression. J Immunol. 2008;180(11):7249–58.
Szczepanski MJ, et al. Blast-derived microvesicles in sera from patients with acute myeloid leukemia suppress natural killer cell function via membrane-associated transforming growth factor-beta1. Haematologica. 2011;96(9):1302–9.
Berchem G, et al. Hypoxic tumor-derived microvesicles negatively regulate NK cell function by a mechanism involving TGF-beta and miR23a transfer. Oncoimmunology. 2016;5(4): e1062968.
Ye L, et al. Tumor-derived exosomal HMGB1 fosters hepatocellular carcinoma immune evasion by promoting TIM-1(+) regulatory B cell expansion. J Immunother Cancer. 2018;6(1):145.
Schroeder JC et al. Circulating exosomes inhibit b cell proliferation and activity. Cancers (Basel), 2020. 12(8)
Sarvaria A, Madrigal JA, Saudemont A. B cell regulation in cancer and anti-tumor immunity. Cell Mol Immunol. 2017;14(8):662–74.
Mao Y, et al. Circulating exosomes from esophageal squamous cell carcinoma mediate the generation of B10 and PD-1(high) Breg cells. Cancer Sci. 2019;110(9):2700–10.
Wieckowski EU, et al. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. J Immunol. 2009;183(6):3720–30.
Montes CL, et al. Tumor-induced senescent T cells with suppressor function: a potential form of tumor immune evasion. Cancer Res. 2008;68(3):870–9.
Zhang Y, et al. Interleukin-7 inhibits tumor-induced CD27-CD28- suppressor T cells: implications for cancer immunotherapy. Clin Cancer Res. 2011;17(15):4975–86.
Maybruck BT, et al. Tumor-derived exosomes induce CD8(+) T cell suppressors. J Immunother Cancer. 2017;5(1):65.
Siegel RM, et al. The multifaceted role of Fas signaling in immune cell homeostasis and autoimmunity. Nat Immunol. 2000;1(6):469–74.
Kim JW, et al. Fas ligand-positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes. Clin Cancer Res. 2005;11(3):1010–20.
Abusamra AJ, et al. Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis. Blood Cells Mol Dis. 2005;35(2):169–73.
Contini P, et al. Apoptosis of antigen-specific T lymphocytes upon the engagement of CD8 by soluble HLA class I molecules is Fas ligand/Fas mediated: evidence for the involvement of p56lck, calcium calmodulin kinase II, and Calcium-independent protein kinase C signaling pathways and for NF-kappaB and NF-AT nuclear translocation. J Immunol. 2005;175(11):7244–54.
Cordonnier M, et al. Tracking the evolution of circulating exosomal-PD-L1 to monitor melanoma patients. J Extracell Vesicles. 2020;9(1):1710899.
Del Re M, et al. PD-L1 mRNA expression in plasma-derived exosomes is associated with response to anti-PD-1 antibodies in melanoma and NSCLC. Br J Cancer. 2018;118(6):820–4.
Theodoraki MN, et al. Clinical significance of PD-L1(+) exosomes in plasma of head and neck cancer patients. Clin Cancer Res. 2018;24(4):896–905.
Mincheva-Nilsson L, Baranov V. Cancer exosomes and NKG2D receptor-ligand interactions: impairing NKG2D-mediated cytotoxicity and anti-tumour immune surveillance. Semin Cancer Biol. 2014;28:24–30.
Skog J, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–6.
Balaj L, et al. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat Commun. 2011;2:180.
Ye SB, et al. Tumor-derived exosomes promote tumor progression and T-cell dysfunction through the regulation of enriched exosomal microRNAs in human nasopharyngeal carcinoma. Oncotarget. 2014;5(14):5439–52.
Kumar V, et al. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016;37(3):208–20.
Liu Y, et al. Contribution of MyD88 to the tumor exosome-mediated induction of myeloid derived suppressor cells. Am J Pathol. 2010;176(5):2490–9.
Diao J, et al. Exosomal Hsp70 mediates immunosuppressive activity of the myeloid-derived suppressor cells via phosphorylation of Stat3. Med Oncol. 2015;32(2):453.
Gobbo J et al. Restoring anticancer immune response by targeting tumor-derived exosomes with a HSP70 peptide aptamer. J Natl Cancer Inst; 2016. 108(3)
Xiang X, et al. Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer. 2009;124(11):2621–33.
Ridder K, et al. Extracellular vesicle-mediated transfer of functional RNA in the tumor microenvironment. Oncoimmunology. 2015;4(6): e1008371.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74.
Roma-Rodrigues C, et al. Smuggling gold nanoparticles across cell types—a new role for exosomes in gene silencing. Nanomedicine. 2017;13(4):1389–98.
Sung BH, et al. Directional cell movement through tissues is controlled by exosome secretion. Nat Commun. 2015;6:7164.
Koumangoye RB, et al. Detachment of breast tumor cells induces rapid secretion of exosomes which subsequently mediate cellular adhesion and spreading. PLoS ONE. 2011;6(9): e24234.
Mu W, Rana S, Zoller M. Host matrix modulation by tumor exosomes promotes motility and invasiveness. Neoplasia. 2013;15(8):875–87.
Luga V, et al. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151(7):1542–56.
Paolillo M, Schinelli S Integrins and exosomes, a dangerous liaison in cancer progression. Cancers (Basel), 2017. 9(8)
Bai S, et al. Role of tumour-derived exosomes in metastasis. Biomed Pharmacother. 2022;147: 112657.
Webber J, et al. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 2010;70(23):9621–30.
Zhang Q, Peng C. Cancer-associated fibroblasts regulate the biological behavior of cancer cells and stroma in gastric cancer. Oncol Lett. 2018;15(1):691–8.
Zhao H, et al. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. Elife. 2016;5: e10250.
Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23(5):1011–27.
Spannuth WA, Sood AK, Coleman RL. Angiogenesis as a strategic target for ovarian cancer therapy. Nat Clin Pract Oncol. 2008;5(4):194–204.
Momeny M, et al. Anti-tumour activity of tivozanib, a pan-inhibitor of VEGF receptors, in therapy-resistant ovarian carcinoma cells. Sci Rep. 2017;7:45954.
Katoh M. Therapeutics targeting angiogenesis: genetics and epigenetics, extracellular miRNAs and signaling networks (Review). Int J Mol Med. 2013;32(4):763–7.
Nishida N, et al. Angiogenesis in cancer. Vasc Health Risk Manag. 2006;2(3):213–9.
Gesierich S et al. Systemic induction of the angiogenesis switch by the tetraspanin D6.1A/CO-029. Cancer Res, 2006; 66(14): 7083–94.
Monteforte A, et al. Glioblastoma exosomes for therapeutic angiogenesis in peripheral ischemia. Tissue Eng Part A. 2017;23(21–22):1251–61.
Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–8.
Syn N, et al. Exosome-mediated metastasis: from epithelial-mesenchymal transition to escape from immunosurveillance. Trends Pharmacol Sci. 2016;37(7):606–17.
Sanchez CA, et al. Exosomes from bulk and stem cells from human prostate cancer have a differential microRNA content that contributes cooperatively over local and pre-metastatic niche. Oncotarget. 2016;7(4):3993–4008.
Dhar R et al. Exosomal microRNAs (exoMIRs): micromolecules with macro impact in oral cancer. 3 Biotech. 2022(12):155.
Geiger TR, Peeper DS. Metastasis mechanisms. Biochim Biophys Acta. 2009;1796(2):293–308.
Gout S, Huot J. Role of cancer microenvironment in metastasis: focus on colon cancer. Cancer Microenviron. 2008;1(1):69–83.
Mani SA, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–15.
Whiteside TL. The role of tumor-derived exosomes in epithelial mesenchymal transition (EMT). Transl Cancer Res. 2017;6(Suppl 1):S90–2.
Wang X, et al. Hypoxic tumor-derived exosomal miR-301a mediates M2 macrophage polarization via PTEN/PI3Kgamma to promote pancreatic cancer metastasis. Cancer Res. 2018;78(16):4586–98.
You Y, et al. Matrix metalloproteinase 13-containing exosomes promote nasopharyngeal carcinoma metastasis. Cancer Sci. 2015;106(12):1669–77.
Dhar R et al. Interrelation between extracellular vesicles miRNAs with chronic lung diseases. J Cell Physiol, 237(11):4021–4036.
Zhao L, Ma X, Yu J. Exosomes and organ-specific metastasis. Mol Ther Methods Clin Dev. 2021;22:133–47.
Hoshino A, Costa-Silva B, Shen TL, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–35.
Ferlay J et al. Cancer statistics for the year 2020: an overview. Int J Cancer, 2021.
Chen Y, et al. The function of LncRNAs and their role in the prediction, diagnosis, and prognosis of lung cancer. Clin Transl Med. 2021;11(4): e367.
Bi WL, et al. Artificial intelligence in cancer imaging: clinical challenges and applications. CA Cancer J Clin. 2019;69(2):127–57.
Seijo LM, et al. Biomarkers in lung cancer screening: achievements, promises, and challenges. J Thorac Oncol. 2019;14(3):343–57.
Cheng N, et al. Recent advances in biosensors for detecting cancer-derived exosomes. Trends Biotechnol. 2019;37(11):1236–54.
Gowri A, Ashwin Kumar N, Suresh Anand BS, Recent advances in nanomaterials based biosensors for point of care (PoC) diagnosis of Covid-19—a minireview. Trends Analyt Chem, 2021;137: 116205.
Sardini E, Serpelloni M, Tonello S, Printed electrochemical biosensors: opportunities and metrological challenges. Biosensors (Basel), 2020. 10(11).
Moss JL, et al. Persistent poverty and cancer mortality rates: an analysis of county-level poverty designations. Cancer Epidemiol Biomarkers Prev. 2020;29(10):1949–54.
Yoshida K, et al. Exploring designability of electrostatic complementarity at an antigen-antibody interface directed by mutagenesis, biophysical analysis, and molecular dynamics simulations. Sci Rep. 2019;9(1):4482.
Xia Y, et al. A visible and colorimetric aptasensor based on DNA-capped single-walled carbon nanotubes for detection of exosomes. Biosens Bioelectron. 2017;92:8–15.
Oliveira-Rodriguez M, et al. Development of a rapid lateral flow immunoassay test for detection of exosomes previously enriched from cell culture medium and body fluids. J Extracell Vesicles. 2016;5:31803.
Chen X, et al. A paper-supported aptasensor based on upconversion luminescence resonance energy transfer for the accessible determination of exosomes. Biosens Bioelectron. 2018;102:582–8.
He F, et al. Quantification of exosome based on a copper-mediated signal amplification strategy. Anal Chem. 2018;90(13):8072–9.
Zhu L, et al. Label-free quantitative detection of tumor-derived exosomes through surface plasmon resonance imaging. Anal Chem. 2014;86(17):8857–64.
Sina AA, et al. Label-free detection of exosomes using a surface plasmon resonance biosensor. Anal Bioanal Chem. 2019;411(7):1311–8.
Kwizera EA, et al. Molecular detection and analysis of exosomes using surface-enhanced raman scattering gold nanorods and a miniaturized device. Theranostics. 2018;8(10):2722–38.
Zong S, et al. SERS-fluorescence-superresolution triple-mode nanoprobe based on surface enhanced Raman scattering and surface enhanced fluorescence. J Mater Chem B. 2020;8(36):8459–66.
Wang Z, et al. Screening and multiple detection of cancer exosomes using an SERS-based method. Nanoscale. 2018;10(19):9053–62.
Park J, et al. An integrated magneto-electrochemical device for the rapid profiling of tumour extracellular vesicles from blood plasma. Nat Biomed Eng. 2021;5(7):678–89.
Song D, et al. Sandwich-type electrochemical immunosensor for CEA detection using magnetic hollow Ni/C@SiO(2) nanomatrix and boronic acid functionalized CPS@PANI@Au probe. Talanta. 2021;225: 122006.
Wang S, et al. Aptasensor with expanded nucleotide using dna nanotetrahedra for electrochemical detection of cancerous exosomes. ACS Nano. 2017;11(4):3943–9.
Azmi AS, Bao B, Sarkar FH. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev. 2013;32(3–4):623–42.
Rahbarghazi R, et al. Tumor-derived extracellular vesicles: reliable tools for Cancer diagnosis and clinical applications. Cell Commun Signal. 2019;17(1):73.
Logozzi M, et al. High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS ONE. 2009;4(4): e5219.
Filipazzi P, et al. Recent advances on the role of tumor exosomes in immunosuppression and disease progression. Semin Cancer Biol. 2012;22(4):342–9.
Sun Y, Liu J. Potential of cancer cell-derived exosomes in clinical application: a review of recent research advances. Clin Ther. 2014;36(6):863–72.
Langevin S, et al. Comprehensive microRNA-sequencing of exosomes derived from head and neck carcinoma cells in vitro reveals common secretion profiles and potential utility as salivary biomarkers. Oncotarget. 2017;8(47):82459–74.
Shao H, et al. New technologies for analysis of extracellular vesicles. Chem Rev. 2018;118(4):1917–50.
An T, et al. Exosomes serve as tumour markers for personalized diagnostics owing to their important role in cancer metastasis. J Extracell Vesicles. 2015;4:27522.
Cheng G. Circulating miRNAs: roles in cancer diagnosis, prognosis and therapy. Adv Drug Deliv Rev. 2015;81:75–93.
Liao J, et al. Exosome-shuttling microRNA-21 promotes cell migration and invasion-targeting PDCD4 in esophageal cancer. Int J Oncol. 2016;48(6):2567–79.
Salomon C, et al. Exosomal signaling during hypoxia mediates microvascular endothelial cell migration and vasculogenesis. PLoS ONE. 2013;8(7): e68451.
Krishnan A, et al. Salivary exosomes: a theranostics secret of oral cancer—correspondence. Int J Surg. 2022;108: 106990.
Tanaka Y, et al. Clinical impact of serum exosomal microRNA-21 as a clinical biomarker in human esophageal squamous cell carcinoma. Cancer. 2013;119(6):1159–67.
Melo SA, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523(7559):177–82.
Ghorbanmehr N, et al. miR-21-5p, miR-141-3p, and miR-205-5p levels in urine-promising biomarkers for the identification of prostate and bladder cancer. Prostate. 2019;79(1):88–95.
Lin H, et al. Urinary exosomal miRNAs as biomarkers of bladder Cancer and experimental verification of mechanism of miR-93-5p in bladder Cancer. BMC Cancer. 2021;21(1):1293.
Josson S, et al. Stromal fibroblast-derived miR-409 promotes epithelial-to-mesenchymal transition and prostate tumorigenesis. Oncogene. 2015;34(21):2690–9.
Wolfers J, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7(3):297–303.
Verdi J, et al. Development and clinical application of tumor-derived exosomes in patients with cancer. Curr Stem Cell Res Ther. 2022;17(1):91–102.
Farhood B, Najafi M, Mortezaee K. CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: a review. J Cell Physiol. 2019;234(6):8509–21.
Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol. 2016;39(1):98–106.
Alsaab HO, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561.
Cheng L, et al. Exosomes from melatonin treated hepatocellularcarcinoma cells alter the immunosupression status through STAT3 pathway in macrophages. Int J Biol Sci. 2017;13(6):723–34.
Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest. 2015;125(9):3384–91.
Hou W, et al. A novel tetravalent bispecific antibody targeting programmed death 1 and tyrosine-protein kinase Met for treatment of gastric cancer. Invest New Drugs. 2019;37(5):876–89.
Xie F, et al. The role of exosomal PD-L1 in tumor progression and immunotherapy. Mol Cancer. 2019;18(1):146.
Gao J, et al. Expression profiles and clinical value of plasma exosomal Tim-3 and Galectin-9 in non-small cell lung cancer. Biochem Biophys Res Commun. 2018;498(3):409–15.
Gu X, et al. Improved vaccine efficacy of tumor exosome compared to tumor lysate loaded dendritic cells in mice. Int J Cancer. 2015;136(4):E74-84.
Rezaei R, et al. Tumor-derived exosomes enriched by miRNA-124 promote anti-tumor immune response in CT-26 tumor-bearing mice. Front Med (Lausanne). 2021;8: 619939.
Batista IA, Melo SA Exosomes and the future of immunotherapy in pancreatic cancer. Int J Mol Sci, 2019. 20(3)
Seo N, Akiyoshi K, Shiku H. Exosome-mediated regulation of tumor immunology. Cancer Sci. 2018;109(10):2998–3004.
Dhar R, et al. Exosome-based cancer vaccine: a cutting-edge approach—correspondence. Int J Surg. 2022;108: 106993.
Chen YS, et al. Exosomes in clinical trial and their production in compliance with good manufacturing practice. Ci Ji Yi Xue Za Zhi. 2019;32(2):113–20.
Yang F, Wang M, Guan X. Exosomes and mimics as novel delivery platform for cancer therapy. Front Pharmacol. 2022;13:1001417.
Huang Y, et al. Exosomes function in tumor immune microenvironment. Adv Exp Med Biol. 2018;1056:109–22.
Rezaie J, Feghhi M, Etemadi T. A review on exosomes application in clinical trials: perspective, questions, and challenges. Cell Commun Signal. 2022;20(1):145.
Keller S, et al. Body fluid derived exosomes as a novel template for clinical diagnostics. J Transl Med. 2011;9:86.
Brinton LT, et al. Formation and role of exosomes in cancer. Cell Mol Life Sci. 2015;72(4):659–71.
Morales RT, et al. Future of digital assays to resolve clinical heterogeneity of single extracellular vesicles. ACS Nano. 2022;16(8):11619–45.
Dhar R et al. Decoding of exosome heterogeneity for cancer theranostics. Clin Transl Med. 2023;e1288.
Gould SJ, Raposo G As we wait: coping with an imperfect nomenclature for extracellular vesicles. J Extracell Vesicles, 2013. 2
Atay S, Godwin AK. Tumor-derived exosomes: a message delivery system for tumor progression. Commun Integr Biol. 2014;7(1): e28231.
Harding C, et al. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97(2):329–39.
Martins VR, et al. Tumor-cell-derived microvesicles as carriers of molecular information in cancer. Curr Opin Oncol. 2013;25(1):66–75.
Kar R, et al, Exosome-based smart drug delivery tool for cancer theranostics. ACS Biomater Sci Eng. 2023.
Wilson DH, et al. The simoa HD-1 analyzer: a novel fully automated digital immunoassay analyzer with single-molecule sensitivity and multiplexing. J Lab Autom. 2016;21(4):533–47.
Dhar R, et al. Exosome: a megastar of future cancer personalized and precision medicine. Clin Transl Disc. 2023;3: e208.
Huda MN, et al. Potential use of exosomes as diagnostic biomarkers and in targeted drug delivery: progress in clinical and preclinical applications. ACS Biomater Sci Eng. 2021;7(6):2106–49.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, S., Dhar, R., Kumar, L.B.S.S. et al. Theranostic signature of tumor-derived exosomes in cancer. Med Oncol 40, 321 (2023). https://doi.org/10.1007/s12032-023-02176-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02176-6