Summary
Background Redirecting T cells to tumor cells using bispecific antibodies (BsAbs) is emerging as a potent cancer therapy. The main concept of this strategy is to cross-link tumor cells and T cells by simultaneously binding to cell surface tumor-associated antigen (TAA) and the CD3ƹ chain. However, immune checkpoint programmed cell death ligand-1 (PD-L1) on tumor cells or other myeloid cells upreglulated remarkablely after the treatment of CD3-binding BsAbs, leads to the generation of suppressed microenvironment for immune evasion and tumor progression. Although this resistance could be partially reversed by anti-PD-L1 treatment, targeting two pathways through one antibody-based molecule may provide a strategic advantage over the combination of BsAbs and immune checkpoint inhibitors. Methods We developed two novel BsAbs PD-1/c-Met DVD-Ig and IgG-scFv both targeting PD-1 to restore the immune effector function of T cells and engaging them to tumor cells via binding to cellular-mesenchymal to epithelial transition factor (c-Met). Binding activities, T cell activation and proliferation were analyzed by flow cytometry. Cell Cytotoxicity and cytokine release were measured using LDH release assay and ELISA, respectively. Anti-tumor response in vivo was evaluated by generate xenograft models in NOD-SCID mice. Results These bispecific antibodies exhibited effective antitumor activity against high- and low- c-Met-expressing gastric cancer cell lines in vitro and mediated strong tumor growth inhibition in human gastric cancer xenograft models. Conclusion The engagement of the PD-1/PD-L1 blockade to c-Met-overexpressing cancer cells is a promising strategy for the treatment of gastric cancer and potentially other malignancies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is the third leading cause of cancer-related deaths worldwide and is a serious global health problem [1]. Despite improvements in surgical resection of the tumor and adjuvant chemotherapy protocols, which are the main treatment strategies in GC, the prognosis of patients with advanced GC remains grim with a relatively low five-year overall survival (OS) rate [2, 3].
C-Met, a receptor-type tyrosine kinase is activated through its ligand, hepatocyte growth factor (HGF), and promotes a variety of tumorigenic responses including malignant progression, angiogenesis, epithelial-mesenchymal transition (EMT), invasion, and metastasis [4]. As early as 2011, c-Met was reported to be over-expressed in more than 50% of advanced GC tumors [5]. Moreover, c-Met gene amplification and expression levels were considered to be associated with GC aggressiveness [6, 7]. Combining chemotherapy with rilotumumab, a completely harmonized monoclonal antibody that targets HGF and prevents it from binding to c-MET, significantly improved the OS in GC patients, suggesting that c-Met might be a promising target for GC treatment [8].
T cells, the most powerful agents of the immune system, play an important role in killing tumor cells [9]. Naturally IgG antibodies fail to recruit cytotoxic T cells directly as they lack the Fc receptors. Engaging T cells with tumor cells via bispecific antibodies (BsAbs) sis an attractive approach to harness T cells for treating cancers [10, 11]. BsAbs combine tumor-associated antigen binding specificity and T cell binding potential into one molecule. The CD3 complex is conventionally used to trigger T cell activation, since it is widely expressed on T cells, and more importantly, can activate cytotoxic T lymphocytes (CTLs) bypassing the pMHC restriction [12, 13]. Approval of blinatumomab targeting CD19 and CD3 by the US-FDA further exemplifies the therapeutic potential of this T cell engaging strategy [14]. Besides, numerous similar BsAbs are under different stages of clinical development [11].
However, many studies have reported that the expression of immune checkpoint molecule PD-L1 on the tumor cells and myeloid cells is dramatically upregulated due to the secretion of IFN-γ after exposure to CD3-binding T-BsAbs, inducing the formation of an immunosuppressive tumor microenvironment with exhausted tumor-specific T cells [15,16,17]. Chang et al. demonstrated that combining PD-1-blocking antibody with anti-Trop2 or anti-CEACAM5 T-BsAb induced stronger anti-tumor activity than T-BsAb alone both in vitro and in vivo [18]. Several studies have concluded that combining PD-1/PD-L1 checkpoint blockade with T-BsAb treatment could significantly augment anti-tumor responses compared to T-BsAb as a single agent [19, 20].
Since Wu et al. reported that PD-L1 overexpression is found in 42.2% of gastric cancer tissues in 2006 [21], numerous studies have reported high PD-L1 expression in gastric cancers and its association with lymph node metastasis and poor survival [22, 23]. Recent trials with anti-PD-1 (pembrolizumab and nivolumab) and anti-PD-L1 (avelumab) antibodies have shown the potential anti-tumor efficacy of these agents in gastric cancer patients [24]. Among them, pembrolizumab was approved for treatment of patients with advanced or recurrent gastric cancer last year.
Collectively, these notions suggest that more effective GC therapy could be achieved by rational combinatorial therapeutic approaches. In this regard, we developed bispecific antibodies PD-1/c-Met DVD-Ig and IgG-scFv using previously reported technologies with the goal of directing PD-1/PD-L1 checkpoint blockade to c-Met-expressing cells [25, 26]. This novel bispecific technology is able to promote the selective reactivation of anti-tumor T cells, and potentially, may offer a feasible option for the treatment of gastric cancers via exerting the dual function of blocking PD-1 and c-Met.
Materials and methods
Cell lines and reagents
Five GC cell lines including MGC803, BGC823, AGS, MKN28 and HGC27 were kindly provided by professor JianXin Gu from our institute. HEK293E, CHO and GC cell lines MKN45 and IM95 were obtained from American Type Culture Collection. CHO/hPD-1 EXD (CHO cells stably express the extra cellular domain of human PD-1) was generated in our laboratory. HEK293E was cultured in FreeStyle™ 293 Expression Medium (Gibco, Life technologies, Shanghai, China) according to the supplier-recommended conditions. CHO and all GC cell lines were maintained in RPMI-1640 medium (Sigma, Shanghai, China) containing 10% fetal bovine serum (Invitrogen, Carlsbad, CA, USA) and 1% penicillin/streptomycin (Hyclone, Shanghai, China) at 37 °C and 5% CO2. Human peripheral blood mononuclear cells (PBMCs) were isolated from fresh peripheral blood of anonymized healthy volunteer donors after the centrifugation on a density gradient using Ficoll-Paque PLUS (GE Healthcare, Shanghai, China). C-Met specific inhibitor JNJ-38877605 was purchased from Selleck Chemicals (Houston, TX, USA). Recombinant Human HGF was obtained from PeproTech (Rocky Hill, NJ, USA). PHA-L and IL-2, used as the stimulation of human peripheral blood lymphocytes, were purchased from Sigma-Aldrich (Shanghai, China) or ProteinTech (Shanghai, China), respectively.
BsAbs expression and purification
PD-1/c-Met DVD-Ig or IgG-scFv molecules were generated by transient co-transfection of the 2 plasmids encoding the heavy- and light-chain respectively in HEK293E cells. Immediately before transfection, cell density was adjusted to 1× 106 cells/ml, the co-transfection was performed using polyethyleneimine (PEI, Polysciences, Shanghai, China) as the transfection mediator. After 6–8 days of culture in flasks on an orbital shaker platform rotating at 180 rpm at 37 °C, 8% CO2, the supernatants were collected by centrifugation and purified using one-step Protein A chromatography (GE Healthcare, Shanghai, China) following the manufacturer’s protocol. The purified antibodies were analyzed by SDS-PAGE and western blotting under non-reduced or reduced conditions and then pooled, concentrated, and stored in 2.0 mg/ml aliquots at −80 °C.
Flow cytometry
For binding property analysis, human gastric cancer cell lines MKN45 and MGC803, which both express c-Met, were used to determine the binding activity of the BsAbs to c-Met. CHO stably expressed the extra cellular domain of human PD-1 was used to determine the binding activity of the BsAbs to PD-1. The cells were diluted in ice-cold PBS supplemented with 2% FBS before adding DVD-Ig, IgG-scFv or IgG4. After 30 min of incubation at 4 °C, the cells were washed and subsequently incubated with the FITC-labeled goat anti-human IgG Fc (Abcam, Shanghai, China). Subsequently, cells were incubated for 1 h at 4 °C, washed twice with PBS, and analyzed on a BD Biosciences FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA).
In vitro cytotoxicity assays and T cell activation assays
MKN45 and MGC803 target cells were collected and re-suspended in round-bottom 96-well plates (3 × 103 cells/well) and were let to adhere to the bottom overnight. Purified DVD-Ig or IgG-scFv at a concentration of 1 nM–50 nM and freshly isolated PBMCs pre-stimulated with 1 μg/ml of PHA-L for 72 h at an E:T ratio of 16:1 were added to the cells. The recommended controls were set up following the manufacturer’s instruction. An IgG4 antibody was used as a control. After incubating for 48 h at 37 °C, 5% CO2, lactate dehydrogenase (LDH) release was measured using a CytoTox 96® Non-Radioactive Cytotoxicity Assay kit (Promega, Madison, WI, USA). After the cytotoxicity assay, the remaining cells were collected and used for T cell activation assay by staining with a FITC-labeled anti-CD3 antibody and PE-labeled anti CD69 antibody, both from Biolegend (San Diego, CA, USA). The cells were then assayed by flow cytometry and analyzed with FlowJo software (FlowJo, LLC, Ashland, OR, USA).
Analysis of T cell proliferation
Pre-stimulated PBMCs (2 × 105 cells/well) labeled with CFSE using the CellTrace Cell Proliferation Kit (Thermo Fisher Scientific, Shanghai, China) were mixed with target cells (MKN45 or MGC803, 1 × 104 cells/well) and the BsAbs. The mixture was incubated in a 5% CO2 incubator at 37 °C for 5 days. The cells were collected and stained with the APC-labeled anti-CD8 antibody (Biolegend, Shanghai, China). The CFSE fluorescence intensity for CD8-positive T cells was quantitated by flow cytometry.
Enzyme-linked immune-sorbent assay (ELISA)
After pre-incubation with 1 μg/ml PHA-L for 3 days, PBMCs were re-suspended to 6 × 104 per well in round-bottom 96-well plates before co-culturing with MKN45 or MGC803 (3 × 103 per well) at 37 °C. Different concentrations of PD-1/c-Met DVD-Ig, IgG-scFv or IgG4 were added to the plates for each reagent (final titration range of 0.01–10 μg/ml). Supernatants were harvested after 48 h of culture and the concentration of three different cytokines (IFN-γ、Granzyme B and Perforin) were determined using ELISA-based cytokine assay kits (RayBiotech, Norcross, GA, USA) following the manufacturer’s instructions.
Cell proliferation assays
GC cell lines MKN45 and MGC803 were cultured in 96-well plates at a density of 3 × 103/well (100 μl/ well). Treatments (100 μl) comprising IgG4, DVD-Ig, IgG-scFv or JNJ38877605 were added ranging from 0 to 0.5 μM to each well for dose-response studies. Absorbance at 570 nm was measured by spectrophotometry (BioTek, Vermont, USA) 4 h after being incubated at 37 °C with 20 μl of alamarBlue® Cell Viability Assay Reagent (Thermo Fisher Scientific, Shanghai, China).
Cell migration, invasion, and colony formation assays
The cell migratory and invasion abilities were tested using 8-μm transwell inserts in 24-well plates (Corning Life Science, Shanghai, China). For migration assays, MKN45 and MGC803 cells were re-suspended in 200 μl serum-free RPMI 1640 medium (3× 105 cells) and cultured in the upper chamber. 700 μl of fetal bovine serum-conditioned medium (10%)serum-containing medium with (+) or without (−) HGF (100 ng/ml) was plated in the bottom wells. DVD-Ig, IgG-scFv, IgG4 or JNJ38877605 were added in both inserts and wells for each treatment. After 24 h of incubation at 37 °C, the cells on the top of the chamber were removed with cotton swabs. Cells that migrated to the lower side of the inserts were fixed in methanol and stained with 0.5% crystal violet for 20 min, then counted in five random fields. For invasion assays, the inserts were coated with Matrigel basement membrane matrix (50 μl/well) (BD Biosciences, Shanghai, China) before adding the cells, Subsequent steps were performed in the same way as described for cell migration assay.
For the colony formation assay, MKN45 or MGC803 cells were pre-treated with DVD-Ig, IgG-scFv, IgG4 or JNJ38877605 for 24 h. Next, the cells were thoroughly dissociated and re-plated at a density of 500 cells per well. After stimulation with HGF (100 ng/ml) for 30 min, the cells were regularly cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, and 1% penicillin and streptomycin (Invitrogen Life Technologies, Shanghai, China) for 2 weeks. Cell clones generated more than 50 cells were washed twice with PBS, fixed in methanol for 15 min, and stained with 0.1% crystal violet (Sangon Biotech Co., Ltd., Shanghai, China) for 15 min at room temperature. Viable colonies were photographed and counted under a microscope (Olympus, Tokyo, Japan). This procedure was repeated three times.
Western blotting
Cell lysate was prepared in RIPA cell lysis buffer (KangWei, Beijing, China) and then centrifuged at 12000 rpm for 10 min at 4 °C. The concentration of protein was quantified using a bicinchoninic acid protein assay kit (Pierce, Rockford, IL, USA). A total of 20 μg protein was separated on 10% SDS-PAGE gels and transferred to a 0.22-μm PVDF membrane (Millipore, MA, USA). After blocking with 5% skim milk in TBST for 2 h at room temperature, the membrane was incubated with primary antibodies overnight at 4 °C, After the addition of secondary antibodies, the membrane was visualized with Thermo Pierce chemiluminescent (ECL) Western Blotting Substrate (Thermo, Waltham, MA, USA) using a ImageQuant™ LAS 4000 system (GE healthcare, Shanghai, China). Anti-E-cadherin (no. 20874–1-AP), anti-N-cadherin (no. 22018–1-AP), anti-Snail1 (no. 13099–1-AP) and anti-c-Met (25869–1-AP) antibodies were obtained from ProteinTech (Shanghai, China). Anti-pMet (Y1234/6) (no. 3077S), anti-AKT (no.4691S), anti-pAKT(S473) (no. 4060S), Anti-ERK1/2 (no. 4695S), anti-pERK1/2(T202/Y204) (no. 4370S), and HRP-conjugated secondary antibodies (1:5000) were purchased from Cell Signaling Technology. Rabbit anti-human GAPDH (1:5000, CST, China) was used as an endogenous control.
In vivo efficacy
Approximately Six- to eight-week-old NOD/SCID mice (SLAC Co. Ltd., Shanghai, China) were raised and treated under specific pathogen-free (SPF) conditions. Single-cell suspensions of MKN45 or MGC803 were implanted subcutaneously (S.C.) on the right flank at 5 × 106 cells into these mice. When tumor volumes reached an average of 50 mm3, the animals were randomized divided into 3 treatment groups by tumor volume: Vehicle (PBS as a negative control), DVD-Ig (10 mg/kg) and IgG-scFv (10 mg/kg),which all administered intraperitoneally (I.P.) 2 times per week. Freshly isolated PBMCs were expanded using 200 IU/ml of IL-2 and cultured for 3 days before being inoculated intraperitoneally (I.P.) on days 7, 21, and 35 after initiation of the implantation. Tumor volume was monitored 2 times per week by measuring the large and small diameters of each tumor with a caliper, and calculated according to the formula V = (length × width2)/2. At the end of the experiment, the mice were sacrificed for obtaining the tumors.
Immunohistochemical staining
The immunohistochemical detection was performed following the standard laboratory protocols. Briefly, after deparaffinization and rehydration, the tissue sections were incubated with 3% hydrogen peroxide in methanol at room temperature for 10 min to quench endogenous peroxidase. Antigen retrieval was performed by immersing slides in 0.01 M sodium citrate buffer (pH 6.0) for 15 min in a water bath at 95 °C before cooling and rinsing once with PBS, and then blocked for 30 min using bovine serum albumin (1%) at room temperature. The sections were incubated with Anti-Ki67 (ProteinTech, cat#27309–1-AP), anti-VEGF (ProteinTech, cat#19003–1-AP), anti-MMP-9 (Cell SignalingTechnology, cat#13667) overnight at 4 °C. Subsequently, the sections were incubated with the HRP-conjugated secondary antibodies for 60 min, and developed using Steady DAB/Plus (Abcam, Shanghai, China). All images were captured from tumor sections using Olympus BX53 microscope and DP controller software (Olympus, Tokyo, Japan).
Statistical analysis
Student’s t test was performed using GraphPad Prism version 6.0 (GraphPad Software, San Diego, CA) when evaluating the data and differences were considered statistically significant when p < 0.05 (*, P < 0.05; **, P < 0.01), error bars represent standard deviation.
Results
Design, expression, and purification of PD-1/c-Met DVD-Ig and IgG-scFv
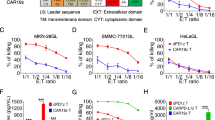
PD-1/c-Met DVD-Ig and IgG-scFv were constructed using standard DNA recombination technologies. For facilitating secretion into cell culture supernatants, all constructs were equipped with an N-terminal signal peptide. Schematic structures of the fusion proteins are shown in Fig. 1a. The novel recombinant BsAbs were purified from cell culture supernatants by Protein A affinity chromatography and analyzed by SDS- PAGE and immunoblotting. As shown in Fig. 1b, under reduced SDS-PAGE conditions, both PD-1/c-Met DVD-Ig and IgG-scFv were observed as two bands at the estimated molecular weight. Under non-reduced conditions, both the BsAbs were observed as a single band around 210 kDa. This was further confirmed by western blot analysis using an anti-IgG Fc antibody.
Generation of PD-1/c-Met DVD-Ig and IgG-scFv bispecific antibodies a Schematic diagram of the BsAbs with PD-1 and c-Met specificities. (Gly4Ser)3 linkers (grey cylinder) were constructed between the PD-1 and c-Met sections. b The BsAbs were expressed in HEK293E cells and then affinity purified with Protein A. Coomassie staining of SDS-PAGE and representative western blot analysis under reduced or non-reduced conditions. c Histogram displaying the relative mRNA expression of c-Met and PD-L1 in seven gastric cancer cell lines (IM95, MKN45, HGC27, MGC803, AGS, BCG823 and MKN28). d The expression level of c-Met protein in gastric cancer cell lines was examined by western blot analysis. e Flow cytometry results showing specific binding of DVD-Ig or IgG-scFv to c-Met in MKN45, MGC803 and to PD-1 in CHO/hPD-1 ECD (CHO cells that overexpress the extracellular domain of human PD-1). A FITC-conjugated anti-human IgG Fc antibody was used to detect the binding signal
Quantitative real-time PCR was performed to evaluate the expression of c-Met and PD-L1 in seven gastric cancer cell lines. The results shown in Fig. 1c indicated that IM95, MKN45, HGC27, MGC803, AGS, BCG823, and MKN28 expressed c-Met (MKN45 > MKN28 > AGS > BCG823 > MGC803 > IM95 > HGC27) and PD-L1 (MKN28 > BCG823 > MKN45 > MGC803 > AGS > IM95 > HGC27). The qPCR results were consistent with the western blot data (Fig. 1d). This suggested that c-Met was highly expressed in GC cell lines and may play a noticeable role in GC progression. After determining the subcutaneous tumorigenicity of the seven GC cell lines in NOD-SCID mice and comparing the expression levels of c-Met and PD-L1, we chose MKN45 and MGC803 cell lines for subsequent in vitro and in vivo assays. Since the two cell lines expressed relatively high levels of PD-L1, but significantly different levels of c-Met, it is feasible to evaluate whether the anti-tumor potency is target-dependent.
Using flow cytometry analysis, we tested the binding potential of each of the two arms of the novel BsAbs to their respective targets. The results revealed that both DVD-Ig and IgG-scFv strongly bound to MKN45 and CHO/hPD-1 ECD (PD-1-transfected CHO cell line), and weakly bound to MGC803 cells, which have a much lower expression of c-Met than MKN45 (Fig. 1e). This result indicated that the binding potency of the BsAbs to c-Met is dependent on the expression level of this target tumor antigen. Collectively, these data suggested that both PD-1/c-Met DVD-Ig and IgG-scFv were able to bind to the corresponding antigens, demonstrating their dual specificities.
PD-1/c-Met DVD-Ig and IgG-scFv mediates T cell cytotoxicity, activation and proliferation in the presence of c-Met-expressing GC cells
PD-1 is expressed mostly on activated T lymphocytes, while the expression is extremely low on resting T cells [16]. Phytohemagglutinin-L (PHA-L) was used for stimulating the isolated PBMCs before they were used as effector cells.
To confirm the ability of PD-1/c-Met DVD-Ig and IgG-scFv to mediate antigen-specific cytotoxic responses in vitro, MKN45, and MGC803 cells were tested as target cells using 16:1 effector: target ratio (E:T) in the presence of increasing concentrations of DVD-Ig, IgG-scFv or IgG4. As shown in Fig. 2a, DVD-Ig and IgG-scFv induced significant cell killing in MKN45, almost 2.5-fold higher than in MGC803 at 50 nM BsAb. Minimal cytotoxic level was observed with IgG4 treatment due to the stimulation of the effector cells, no significant anti-tumor activity was detected because of the presence of PD-1/PD-L1 blockade between T cells and tumor cells or myeloid cells.
Target-dependent cell killing and T cell activation and proliferation induced by PD-1/c-Met DVD-Ig or IgG-scFv a In vitro cytotoxicity mediated by PD-1/c-Met DVD-Ig or IgG-scFv redirected T cells was assessed using LDH release assay. The target cells were MKN45 and MGC803. b T cell activation was assessed by measuring the expression level of CD69 on cell surface of the CD3+ T cells by flow cytometry. c CD8+ T cell proliferation response restored by PD-1/c-Met DVD-Ig or IgG-scFv was detected by measuring CFSE fluorescence intensity dilution
In addition, elevated expression of activation markers CD69 was observed for CD3+T cell subsets upon stimulation with PHA-L. However, T cell activation was limited to a certain range due to the existence of PD-1/PD-L1 blockade between T cells and tumor cells or myeloid cells mentioned above. The expression of CD69 was significantly up-regulated upon the treatment of PD-1/c-Met DVD-Ig or IgG-scFv, suggesting that T cell activation may be partly suppressed by the interaction of PD-1 and PD-L1 after stimulated by PHA-L, and later recovered with the addition of the BsAbs.(Fig. 2b).
We next investigated whether DVD-Ig and IgG-scFv affect T- cell proliferation by co-culturing CFSE-labeled PBMCs, target cells (MKN45 or MGC803), and the BsAbs. As shown in Fig. 2c, DVD-Ig and IgG-scFv in the presence of effector cells and target cells led to multiple populations of diluted CFSE signals measured by flow cytometry, implying several rounds of T cell proliferation. A low level of cell division was also induced with IgG4 treatment or in the absence of BsAbs or target cells, consistent with the low level of activation observed above. This further proves the hypothesis that the BsAbs can reverse the silencing of T cells.
Cytokines released by PD-1/c-Met DVD-Ig and IgG-scFv redirected T cells
Cytokine release was measured using commercial ELISA kits. Different titers of PD-1/c-Met DVD-Ig, IgG-scFv or IgG4 were added to a mixture of pre-stimulated PBMCs and GC cell lines (E:T = 20:1). The levels of IFN-γ, granzyme B, and perforin were measured after 48 h. DVD-Ig induced higher cytokine release from human PBMCs than IgG-scFv in a dose-dependent manner when incubated with c-Met and GC cell lines. Additionally, in the presence of MKN45, the secretion levels of IFN-γ, granzyme B, and perforin were significantly higher than in the presence of MGC803, reflecting a likely correlation between c-Met expression and BsAb potency. DVD-Ig (10 μg/ml) treatment on MKN45), resulted in the increased secretion of IFN-γ (920.11 pg/mL vs 453.56 pg/mL), granzyme B (570.88 pg/mL vs 357.28 pg/mL), perforin (194.89 pg/mL vs 163.82 pg/mL), (2-, 1.6-, and 1.2-fold higher) compared to treatment on MGC803. IgG-scFv treatment also induced a similar cytokine secretion pattern (Fig. 3).
Cytokine release induced by PD-1/c-Met DVD-Ig or IgG-scFv. Soluble IFN-γ, granzyme B, and perforin release were detected from the co-cultured cell supernatants using ELISA kits. Effector PBMC and targets (MKN45 and MGC803) E:T ratio 20:1, time point of 48 h. Mean values of 3 independent data sets are shown
These data collectively demonstrated that the IgG-scFv format is considerably less potent than the DVD-Ig format in inducing cytokine release, and that the potency is somehow dependent on the expression of c-Met.
PD-1/c-Met DVD-Ig and IgG-scFv inhibit GC cell growth via the HGF/c-Met/ERK1/2 way
Given that c-Met is highly expressed in GC cell lines and potentially correlated with GC progression, we determined whether PD-1/c-Met DVD-Ig or IgG-scFv could affect GC cell growth via the HGF/c-Met signaling pathway in the absence of effector cells. Cell viability assay suggested that treatment with the BsAbs significantly inhibited gastric cancer cell growth in a dose-dependent manner, similar to c-Met inhibitor JNJ-38877605 treatment (Fig. 4a). Colony formation assays showed suppressed GC cell growth, validating the cell viability assay results. As shown in Fig. 4b, c, HGF-induced colony formation was dramatically inhibited with BsAbs or JNJ-38877605 treatments in both MKN45 and MGC803 cells.
PD-1/c-Met DVD-Ig or IgG-scFv inhibit proliferation and antagonize c-Met signaling in MET-amplified GC cell lines a Anti-proliferative effects of PD-1/c-Met DVD-Ig or IgG-scFv compared to IgG4 against MKN45 and MGC803 cells. b and c HGF-induced colony formation of MKN45 or MGC803 cells was inhibited by PD-1/c-Met DVD-Ig or IgG-scFv. d The activation of c-Met downstream molecules was suppressed by PD-1/c-Met DVD-Ig or IgG-scFv. JNJ-38877605 used here is a MET kinase inhibitor. Independent experiments were performed at least three times (mean ± s.d.). *P < 0.05, **P < 0.01. The results from a representative experiment are shown
We next assessed the effect of BsAbs on c-Met phosphorylation and HGF/c-Met signaling pathways in these two cell lines. HGF-induced Met phosphorylation at the pY1234/1235 sites was significantly reduced with the treatment of the BsAbs or JNJ38877605 (Fig. 4d). Moreover, the phosphorylation levels of the downstream molecules Akt and Erk1/2 showed a similar pattern of inhibition following the same treatments. These data collectively suggested that PD-1/c-Met DVD-Ig or IgG-scFv exhibit a strong antagonistic effect on HGF-dependent activation of c-Met and the downstream signaling of HGF/Met as anti-c-Met antibodies. Since Akt/Erk1/2 signaling plays a central role in the process of cancer cell growth [27], it may be speculated that the BsAbs inhibit GC cell growth by inactivating the HGF/c-Met/ERK1/2 signaling pathway.
PD-1/c-Met DVD-Ig and IgG-scFv inhibit HGF-induced migration, invasion, and EMT of GC cells
We further determined the effects of DVD-Ig and IgG-scFv on HGF-stimulated gastric cancer cell migration and invasion using trans-well chamber assays. As shown in Fig. 5a, b, 24 h of stimulation with HGF caused a significant increase in cell migration and invasion, and these effects were reverted dramatically in response to treatment with 100 nM DVD-Ig or IgG-scFv. This inhibitory effect was also observed with JNJ-38877605 but not with IgG4.
PD-1/c-Met DVD-Ig or IgG-scFv inhibits HGF-induced migration and invasion of GC cells a and b HGF-stimulated MKN45 or MGC803 migration and invasion were suppressed by PD-1/c-Met DVD-Ig or IgG-scFv. The relative quantitative determinations of migrated and invasive cells were calculated with 5 fields counted per experiment. c and d The effect of PD-1/c-Met DVD-Ig or IgG-scFv on the EMT process of MKN45 or MGC803 cells was analyzed by western blot. The relative expression normalized by GAPDH was analyzed by Image J software and shown as mean ± S.D., *P < 0.05, **P < 0.01
The HGF/c-Met signaling pathway is involved in epithelial-mesenchymal transition (EMT), a developmental process that increases the invasive and metastatic potential of the cells and plays a crucial role in tumor cell migration and invasion [28]. We further investigated essential EMT-related biomarkers including E-cadherin, N-cadherin, and Snail1 by western blot assay. As shown in Fig. 5c, d, epithelial cell marker E-cadherin was down-regulated, and mesenchymal cell markers (N-cadherin, Snail1) were up-regulated after HGF stimulation in both MKN45 and MGC803 cells, demonstrating that HGF could promote EMT transformation in GC cells. In line with the above observations, we found that treatment with DVD-Ig, IgG-scFv, or JNJ38877605 restored E-cadherin expression and attenuated N-cadherin and Snail1 expression, suggesting the EMT phenotype of GC cells was reversed. These findings revealed that DVD-Ig and IgG-scFv could suppress cell migration and invasion by inhibiting the HGF-induced EMT process in human GC.
In vivo evaluation in gastric cancer xenograft model
To evaluate the in vivo efficacy of PD-1/c-Met DVD-Ig and IgG-scFv, the gastric cancer cell lines MKN45 (c-Methigh) and MGC803 (c-Metlow) were used to generate xenograft models in NOD-SCID mice. Treatment schedule of either vehicle (PBS), DVD-Ig or IgG-scFv is shown in Fig. 6a. At days 7, 21 and 35, PBMCs were administered intraperitoneally to the mice at an E:T ratio of 2:1. As shown in Fig. 6b, c, the average weight of the tumors collected from MKN45 or MGC803 xenografts treated by the BsAbs were significantly reduced in comparison with tumors from the vehicle-treated mice.
PD-1/c-Met DVD-Ig or IgG-scFv inhibits tumor growth in c-Met (+) gastric cancer xenograft models a A schematic overview of the treatment schedule. b Representative images of tumors obtained from the NOD/SCID mice injected subcutaneously with MKN45 cells and the quantification of tumor weight in MKN45 xenograft. c Quantification of tumor weights in MGC803 xenograft. d MKN45 or MGC803 xenografts were treated with i.p vehicle (PBS, black), 10 mg/kg DVD-Ig (blue), or 10 mg/kg IgG-scFv (red). Left: Individual mice tumor growth plots versus days post-implantation are shown. Right: Mean tumor volume changes of established MKN45 or MGC803 xenografts from tumor implantation. Error bars represent SEM.*, P < 0.05. **P < 0.01
Additionally, tumor growth was remarkably slower with the administration of DVD-Ig or IgG-scFv compared to the control mice. Complete regression was not observed in MKN45 or MGC803 tumor xenografts (Fig. 6d). This demonstrated that the expression of c-Met did not affect the sensitivity of the BsAbs. Importantly, there were no signs of body weight loss and obvious toxicity in these mice until the end of the experiments, which suggested the safety of PD-1/c-Met DVD-Ig and IgG-scFv for clinical use.
We next investigated the anti-tumor mechanisms of PD-1/c-Met DVD-Ig and IgG-scFv in vivo using Ki67, VEGF-A and MMP-9 antibodies for IHC analysis. Treatment with PD-1/c-Met DVD-Ig or IgG-scFv significantly reduced the percentage of Ki67-positive cells and VEGF-A-positive as well as MMP-9-positive cells compared to IgG4 treatment (Fig. 7a), suggesting that the BsAbs could prevent tumor growth by suppressing cell proliferation, angiogenesis, and metastasis.
IHC staining of tumor sections and schematics showing the mechanism of anti-tumor potent for PD-1/c-Met IgG-scFv a Representative images of Ki67, VEGF-A and MMP-9 expression in human MKN45 gastric tumor xenografts grown in NOD-SCID mice as detected by Immunohistochemistry staining. Magnification: 200×. b Redirecting T cells to tumor cells by binding to tumor-expressed c-Met as well as to PD-1 on the T cells, the cross-linking of the two cells triggers T cell activation, which releases cytotoxic granules including perforin and granzyme to kill tumor cells. Besides, the secretion of IFN-γ upregulates the expression of PD-L1 on tumor cells, yet blockade of the PD-1/PD-L1 interaction by the BsAbs could maintain the activation of T cells. Moreover, blocking PD-1/PD-L1 checkpoint could also reactivate T cells silenced by suppressive myeloid cells such as macrophages and NK cells
Discussion
In this study, we describe a novel class of c-Met-specific BsAbs (PD-1/c-Met DVD-Ig and IgG-scFv) that engage active T cells to c-Met-expressing gastric cancer cells through the PD-1/PD-L1 blockade. The BsAbs exhibited strong binding to the corresponding antigens PD-1 or c-Met and efficient T cell-mediated killing of gastric cancer cells both in vitro and in vivo.
To date, extraordinary clinical benefits of T cell-redirecting BsAb (T-BsAb) have been demonstrated [29]. The common concept of T-BsAb is that it activates T cells bypassing the pMHC restriction by binding to the CD3 complex and redirecting the response to tumor cells by binding to tumor-specific antigens [30]. An obvious obstacle for this activation is the up-regulation of immunosuppressive molecules like PD-L1 in the tumor microenvironment due to the secretion of IFN-γ by T cells, often leading to tumor immune escape [17]. Although combing PD-1/PD-L1 blockade with T-BsAbs is a feasible strategy to mitigate this drawback, targeting two pathways through one antibody-based molecule may offer another rational choice. In this regard, we generated novel bispecific antibodies that redirected activated T cells to tumor cells and block the immunosuppressive PD-1/PD-L1 checkpoint simultaneously. Since PD-L1 was not only expressed on tumor cells, but also on various myeloid cells such as macrophages and DCs [31], the BsAbs could additionally block potential PD-L1-mediated immunosuppressive effects to maximize the anti-tumor efficacy. The interplay of the cells mediated by IgG-scFv (one of our BsAbs) in the tumor microenvironment is illustrated in Fig. 7b. However, the mechanism of how other related factors affect the potency needs to be explored.
Both PD-1/c-Met DVD-Ig and IgG-scFv are designed using the whole IgG4-like skeletal structure owing to its intact binding property to neonatal Fc receptor (FcRn). This allowed increased antibody exposure and longer plasma half-life than non-IgG formats [32]. Furthermore, a recent study reported that FcγR engagement by anti-PD-1 Abs resulted in diminished in vivo activity [33], confirming that IgG4 should be an ideal choice for antibody isotype. In our study, we used Human IgG4 due to its low affinity to FcγR, reducing the risk of off-target FcγR-mediated ADCC and subsequent cytokine storm [34]. Furthermore, the BsAbs are both generated using the appended IgG format. Dual Variable Domain-Ig (DVD-Ig) was engineered by appending the VL and VH domains of c-Met IgG with similar domains from PD-1 IgG while IgG-scFv was constructed by fusing the scFv of c-Met nto the C-terminus of the heavy chain of PD-1 IgG backbone [26, 35]. This dhomodimerized BsAbs are able to match the functional VH/VL pairs accurately and circumvent the mismatched pairing of the heavy chain and light chain, which is a general concern of manufacturing heterodimeric BsAbs [36]. Another potential advantage is that the additional Ag-binding units provide a higher specific binding capacity [37]. In line with this, both the BsAbs in this study could redirect T cells to kill MGC803, which possess a significantly lower expression level of c-Met than MKN45, with a potency similar to or even higher than that of JNJ-38877605 in suppressing cell proliferation, migration, and invasion. Of note, there was a concern about steric hindrance of the two binding sites in DVD-Ig format [26], interestingly, our data showed that DVD-Ig had a stronger binding potency than IgG-scFv, and exhibited more effective anti-tumor activity in in vitro and in vivo studies, thus further intensive studies are needed to explain this mechanism.
In this study, we selected c-Met as the target tumor antigen since c-Met has been regarded as a promising target for cancer therapy by numerous preclinical studies [38, 39]. Despite its strict regulation during normal tissue development, in cancer cells c-Met is highly activated via overexpression, amplification, or mutation, resulting in the activation of different intracellular signaling pathways responsible for promoting proliferation and invasiveness [4, 40]. Our results demonstrate the potent in vitro and in vivo antitumor efficacy of our BsAbs. No obvious toxicity and side effects were observed in the NOD-SCID mice till the end of treatment. C-Met may not be an ideal target for clinical use of T cell redirection since it is wildly expressed in normal tissues increasing the possibility of ‘on-target off-tumor” effects [41]. More importantly, a previous study in our lab suggested that a PD-1 monoclonal antibody displayed stronger tumor growth inhibition than a BsAb constructed in another form but still targeting PD-1 and c-Met. We speculate that the BsAb may mimic HGF and induce a productive homodimerization of c-Met, leading to c-Met signaling pathway activation rather than inhibition. This is similar to other bivalent c-Met-targeted antibodies described by several studies [42, 43]. Based on the notions above, choosing more tumor-specific antigens like CEA or PSMA to generate BsAbs would be an alternative for the treatment of multiple types of solid cancers [44].
Taken together, this study presents the first demonstration of a successful DVD-Ig and IgG-scFv platform to provide a synergy between PD-1/PD-L1 blockade and c-Met-targeted therapy, raising the prospects for extending to other tumors with elevated levels of c-Met or dysregulation of the HGF/c-Met signaling pathway, and to some extent, serving as an alternative to current cancer immunotherapy.
References
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA Cancer J Clin 66(1):7–30. https://doi.org/10.3322/caac.21332
Arrington AK, Nelson R, Patel SS, Luu C, Ko M, Garcia-Aguilar J, Kim J (2013) Timing of chemotherapy and survival in patients with resectable gastric adenocarcinoma. World J Gastrointest Surg 5(12):321–328. https://doi.org/10.4240/wjgs.v5.i12.321
Qiu MZ, Xu RH (2013) The progress of targeted therapy in advanced gastric cancer. Biomark Res 1(1):32. https://doi.org/10.1186/2050-7771-1-32
Organ SL, Tsao MS (2011) An overview of the c-MET signaling pathway. Ther Adv Med Oncol 3(1 Suppl):S7–S19. https://doi.org/10.1177/1758834011422556
Lennerz JK, Kwak EL, Ackerman A, Michael M, Fox SB, Bergethon K, Lauwers GY, Christensen JG, Wilner KD, Haber DA, Salgia R, Bang YJ, Clark JW, Solomon BJ, Iafrate AJ (2011) MET amplification identifies a small and aggressive subgroup of esophagogastric adenocarcinoma with evidence of responsiveness to crizotinib. J Clin Oncol Off J Am Soc Clin Oncol 29(36):4803–4810. https://doi.org/10.1200/JCO.2011.35.4928
Erichsen R, Kelsh MA, Oliner KS, Nielsen KB, Froslev T, Laenkholm AV, Vyberg M, Acquavella J, Sorensen HT (2016) Prognostic impact of tumor MET expression among patients with stage IV gastric cancer: a Danish cohort study. Ann Epidemiol 26(7):500–503. https://doi.org/10.1016/j.annepidem.2016.05.002
Lee HE, Kim MA, Lee HS, Jung EJ, Yang HK, Lee BL, Bang YJ, Kim WH (2012) MET in gastric carcinomas: comparison between protein expression and gene copy number and impact on clinical outcome. Br J Cancer 107(2):325–333. https://doi.org/10.1038/bjc.2012.237
Zhu M, Tang R, Doshi S, Oliner KS, Dubey S, Jiang Y, Donehower RC, Iveson T, Loh EY, Zhang Y (2015) Exposure-response analysis of rilotumumab in gastric cancer: the role of tumour MET expression. Br J Cancer 112(3):429–437. https://doi.org/10.1038/bjc.2014.649
Dreier T, Lorenczewski G, Brandl C, Hoffmann P, Syring U, Hanakam F, Kufer P, Riethmuller G, Bargou R, Baeuerle PA (2002) Extremely potent, rapid and costimulation-independent cytotoxic T-cell response against lymphoma cells catalyzed by a single-chain bispecific antibody. Int J Cancer 100(6):690–697. https://doi.org/10.1002/ijc.10557
Garber K (2014) Bispecific antibodies rise again. Nat Rev Drug Discov 13(11):799–801. https://doi.org/10.1038/nrd4478
Brinkmann U, Kontermann RE (2017) The making of bispecific antibodies. mAbs 9(2):182–212. https://doi.org/10.1080/19420862.2016.1268307
Frankel SR, Baeuerle PA (2013) Targeting T cells to tumor cells using bispecific antibodies. Curr Opin Chem Biol 17(3):385–392. https://doi.org/10.1016/j.cbpa.2013.03.029
Cartellieri M, Arndt C, Feldmann A, von Bonin M, Ewen EM, Koristka S, Michalk I, Stamova S, Berndt N, Gocht A, Bornhauser M, Ehninger G, Schmitz M, Bachmann M (2013) TCR/CD3 activation and co-stimulation combined in one T cell retargeting system improve anti-tumor immunity. Oncoimmunology 2(12):e26770. https://doi.org/10.4161/onci.26770
Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, Noppeney R, Viardot A, Hess G, Schuler M, Einsele H, Brandl C, Wolf A, Kirchinger P, Klappers P, Schmidt M, Riethmuller G, Reinhardt C, Baeuerle PA, Kufer P (2008) Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science 321(5891):974–977. https://doi.org/10.1126/science.1158545
Osada T, Patel SP, Hammond SA, Osada K, Morse MA, Lyerly HK (2015) CEA/CD3-bispecific T cell-engaging (BiTE) antibody-mediated T lymphocyte cytotoxicity maximized by inhibition of both PD1 and PD-L1. Cancer Immunol Immunother: CII 64(6):677–688. https://doi.org/10.1007/s00262-015-1671-y
Zou W, Wolchok JD, Chen L (2016) PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med 8(328):328rv324. https://doi.org/10.1126/scitranslmed.aad7118
Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, Chen L (2012) Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 4(127):127ra137. https://doi.org/10.1126/scitranslmed.3003689
Chang CH, Wang Y, Li R, Rossi DL, Liu D, Rossi EA, Cardillo TM, Goldenberg DM (2017) Combination therapy with bispecific antibodies and PD-1 blockade enhances the antitumor potency of T cells. Cancer Res 77(19):5384–5394. https://doi.org/10.1158/0008-5472.CAN-16-3431
Junttila TT, Li J, Johnston J, Hristopoulos M, Clark R, Ellerman D, Wang BE, Li Y, Mathieu M, Li G, Young J, Luis E, Lewis Phillips G, Stefanich E, Spiess C, Polson A, Irving B, Scheer JM, Junttila MR, Dennis MS, Kelley R, Totpal K, Ebens A (2014) Antitumor efficacy of a bispecific antibody that targets HER2 and activates T cells. Cancer Res 74(19):5561–5571. https://doi.org/10.1158/0008-5472.CAN-13-3622-T
Sun LL, Wang P, Clark R, Hristopoulos M, Ellerman D, Mathieu M, Chu Y-W, Wang H, Totpal K, Ebens AJ, Polson AG, Gould S (2016) Preclinical characterization of combinability and potential synergy of anti-CD20/CD3 T-cell dependent bispecific antibody with chemotherapy and PD-1/PD-L1 blockade. Blood 128(22):4168–4168
Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, Xu N (2006) Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem 108(1):19–24. https://doi.org/10.1016/j.acthis.2006.01.003
Jiang J, Zhu Y, Wu C, Shen Y, Wei W, Chen L, Zheng X, Sun J, Lu B, Zhang X (2010) Tumor expression of B7-H4 predicts poor survival of patients suffering from gastric cancer. Cancer Immunol Immunother: CII 59(11):1707–1714. https://doi.org/10.1007/s00262-010-0900-7
Eto S, Yoshikawa K, Nishi M, Higashijima J, Tokunaga T, Nakao T, Kashihara H, Takasu C, Iwata T, Shimada M (2016) Programmed cell death protein 1 expression is an independent prognostic factor in gastric cancer after curative resection. Gastric Cancer 19(2):466–471. https://doi.org/10.1007/s10120-015-0519-7
Bilgin B, Sendur MA, Bulent Akinci M, Sener Dede D, Yalcin B (2017) Targeting the PD-1 pathway: a new hope for gastrointestinal cancers. Curr Med Res Opin 33(4):749–759. https://doi.org/10.1080/03007995.2017.1279132
Wu C, Ying H, Grinnell C, Bryant S, Miller R, Clabbers A, Bose S, McCarthy D, Zhu RR, Santora L, Davis-Taber R, Kunes Y, Fung E, Schwartz A, Sakorafas P, Gu J, Tarcsa E, Murtaza A, Ghayur T (2007) Simultaneous targeting of multiple disease mediators by a dual-variable-domain immunoglobulin. Nat Biotechnol 25(11):1290–1297. https://doi.org/10.1038/nbt1345
Orcutt KD, Ackerman ME, Cieslewicz M, Quiroz E, Slusarczyk AL, Frangioni JV, Wittrup KD (2010) A modular IgG-scFv bispecific antibody topology. Protein Eng Des Sel: PEDS 23(4):221–228. https://doi.org/10.1093/protein/gzp077
Gan Y, Shi C, Inge L, Hibner M, Balducci J, Huang Y (2010) Differential roles of ERK and Akt pathways in regulation of EGFR-mediated signaling and motility in prostate cancer cells. Oncogene 29(35):4947–4958. https://doi.org/10.1038/onc.2010.240
Nagai T, Arao T, Furuta K, Sakai K, Kudo K, Kaneda H, Tamura D, Aomatsu K, Kimura H, Fujita Y, Matsumoto K, Saijo N, Kudo M, Nishio K (2011) Sorafenib inhibits the hepatocyte growth factor-mediated epithelial mesenchymal transition in hepatocellular carcinoma. Mol Cancer Ther 10(1):169–177. https://doi.org/10.1158/1535-7163.MCT-10-0544
Wu Z, Cheung NV (2018) T cell engaging bispecific antibody (T-BsAb): from technology to therapeutics. Pharmacol Ther 182:161–175. https://doi.org/10.1016/j.pharmthera.2017.08.005
Nagorsen D, Baeuerle PA (2011) Immunomodulatory therapy of cancer with T cell-engaging BiTE antibody blinatumomab. Exp Cell Res 317(9):1255–1260. https://doi.org/10.1016/j.yexcr.2011.03.010
Motz GT, Coukos G (2011) The parallel lives of angiogenesis and immunosuppression: cancer and other tales. Nat Rev Immunol 11(10):702–711. https://doi.org/10.1038/nri3064
Brezski RJ, Georgiou G (2016) Immunoglobulin isotype knowledge and application to fc engineering. Curr Opin Immunol 40:62–69. https://doi.org/10.1016/j.coi.2016.03.002
Dahan R, Sega E, Engelhardt J, Selby M, Korman AJ, Ravetch JV (2015) FcgammaRs modulate the anti-tumor activity of antibodies targeting the PD-1/PD-L1 Axis. Cancer Cell 28(3):285–295. https://doi.org/10.1016/j.ccell.2015.08.004
Linke R, Klein A, Seimetz D (2010) Catumaxomab: clinical development and future directions. mAbs 2(2):129–136
Correia I, Sung J, Burton R, Jakob CG, Carragher B, Ghayur T, Radziejewski C (2013) The structure of dual-variable-domain immunoglobulin molecules alone and bound to antigen. mAbs 5(3):364–372. https://doi.org/10.4161/mabs.24258
Klein C, Sustmann C, Thomas M, Stubenrauch K, Croasdale R, Schanzer J, Brinkmann U, Kettenberger H, Regula JT, Schaefer W (2012) Progress in overcoming the chain association issue in bispecific heterodimeric IgG antibodies. mAbs 4(6):653–663. https://doi.org/10.4161/mabs.21379
Jakob CG, Edalji R, Judge RA, DiGiammarino E, Li Y, Gu J, Ghayur T (2013) Structure reveals function of the dual variable domain immunoglobulin (DVD-Ig) molecule. mAbs 5(3):358–363. https://doi.org/10.4161/mabs.23977
Smyth EC, Sclafani F, Cunningham D (2014) Emerging molecular targets in oncology: clinical potential of MET/hepatocyte growth-factor inhibitors. Oncotargets Ther 7:1001–1014. https://doi.org/10.2147/OTT.S44941
Cecchi F, Rabe DC, Bottaro DP (2012) Targeting the HGF/Met signaling pathway in cancer therapy. Expert Opin Ther Targets 16(6):553–572. https://doi.org/10.1517/14728222.2012.680957
Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G (2012) Targeting MET in cancer: rationale and progress. Nat Rev Cancer 12(2):89–103. https://doi.org/10.1038/nrc3205
Michaud NR, Jani JP, Hillerman S, Tsaparikos KE, Barbacci-Tobin EG, Knauth E, Putz H Jr, Campbell M, Karam GA, Chrunyk B, Gebhard DF, Green LL, Xu JJ, Dunn MC, Coskran TM, Lapointe JM, Cohen BD, Coleman KG, Bedian V, Vincent P, Kajiji S, Steyn SJ, Borzillo GV, Los G (2012) Biochemical and pharmacological characterization of human c-Met neutralizing monoclonal antibody CE-355621. mAbs 4(6):710–723. https://doi.org/10.4161/mabs.22160
Pacchiana G, Chiriaco C, Stella MC, Petronzelli F, De Santis R, Galluzzo M, Carminati P, Comoglio PM, Michieli P, Vigna E (2010) Monovalency unleashes the full therapeutic potential of the DN-30 anti-Met antibody. J Biol Chem 285(46):36149–36157. https://doi.org/10.1074/jbc.M110.134031
Tolbert WD, Daugherty-Holtrop J, Gherardi E, Vande Woude G, Xu HE (2010) Structural basis for agonism and antagonism of hepatocyte growth factor. Proc Natl Acad Sci U S A 107(30):13264–13269. https://doi.org/10.1073/pnas.1005183107
Sanchez-Martin D, Sorensen MD, Lykkemark S, Sanz L, Kristensen P, Ruoslahti E, Alvarez-Vallina L (2015) Selection strategies for anticancer antibody discovery: searching off the beaten path. Trends Biotechnol 33(5):292–301. https://doi.org/10.1016/j.tibtech.2015.02.008
Funding
This work was supported financially by the National Key Research Project Bio-safety Key Technology Development Program 2016YFC1201501.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
None.
Ethical approval
All procedures involving human blood products were in accordance with the 1964 Helsinki declaration and its later amendments. Study involving mice use was carried out according to the protocols approved by Institutional Animal Care and Use Committee of Fudan University.
Informed consent
All individual participants who donated blood samples for analysis signed an informed consent form.
Rights and permissions
About this article
Cite this article
Hou, W., Yuan, Q., Yuan, X. et al. A novel tetravalent bispecific antibody targeting programmed death 1 and tyrosine-protein kinase Met for treatment of gastric cancer. Invest New Drugs 37, 876–889 (2019). https://doi.org/10.1007/s10637-018-0689-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-018-0689-3