Abstract
Exosomes are the smallest extracellular vesicles (EV) produced under physiological and pathological conditions by all cells and present in all body fluids. They are critical components of the intercellular communication network. Tumor cells release exosomes which are enriched in immunosuppressive molecules as well as biologically-active soluble factors and enzymes. Tumor-derived exosomes (TEX) interact with immune effector cells in the tumor microenvironment and in the circulation, deliver negative signals to these cells and interfere with their anti-tumor functions. By suppressing functions of immune effector cells, TEX promote tumor progression and facilitate tumor escape from the immune system. Thus, TEX can be viewed as immune checkpoint inhibitors. Silencing of TEX-mediated immune inhibition without disrupting the physiologically important cellular communication networks represents a considerable challenge. Current efforts are directed at achieving a better understanding of the role exosomes play in cancer progression and/or outcome and of molecular/genetic mechanisms responsible for immunoinhibitory activity of TEX.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
Tumor-induced immune suppression has been extensively investigated in the last decade. Indeed, escape of tumors from the host immune system has been considered a major barrier for successful immunotherapy of cancer [1]. Recent unprecedented tumor control that can be achieved by targeting immune checkpoint inhibitors (ICIs) clearly emphasizes the importance of the restoration of anti-tumor immune activity in patients with cancer by the use of antagonistic antibodies to CTLA-4, PD-1, PD-L1 or other ICIs [2]. Used alone or in combination with each other and with conventional therapies, check point inhibition can unleash the power of the immune system in many cancer patients whose anti-tumor responses are compromised. While highly promising, ICIs efficiency in restoring anti-tumor responses varies broadly among patients with cancer [3]. It is not clear why some patients respond to ICIs and others do not, but limitations in responses could be explained by the acknowledged existence of multiple ICI pathways in cancer, only some of which are responsive to ICIs being used for therapy. Based on what is known about various immunosuppressive mechanisms operating in the tumor-microenvironment, some general rules can be formulated as follows: (a) these mechanisms are tumor-induced; (b) they involve one or more cellular and molecular pathways that may differ in primary vs. metastatic tumors, are selectively utilized by different tumor types and vary among patients, even those with the same malignancy; and (c) they are not restricted to the tumor microenvironment (TME) but mediate systemic effects leading to the partial or complete inhibition of anti-tumor immune responses in the entire body.
Among many tumor-derived factors or signals that modulate anti-tumor immunity, exosomes, specifically tumor-derived exosomes or TEX, are emerging as a new and so far not widely appreciated mechanism of immune suppression. This chapter will describe how and why exosomes, which are ubiquitously present in all body fluids of patients with cancer, are currently viewed as conveyors of tumor-derived suppression to the immune cells responsible for surveillance and cancer control.
6.2 What Are Exosomes?
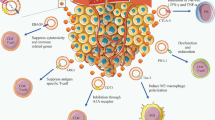
Exosomes are membrane-bound vesicles which are virus size, are produced by all cells and are released by cells under physiological and pathological conditions. They are the smallest of extracellular vesicles (EVs) released by cells, varying in size from 30 to 150 nm, found in supernatants of cultured cells as well as in all body fluids [4]. Although the nomenclature of EVs is still unclear [5], exosomes are a distinct type of vesicles which differ from larger EVs, such microvesicles (MVs, 200–1000 nm) or apoptotic bodies (1000–5000 nm) not only by their size but also by cellular mechanisms used for their secretion, the molecular content and functional properties [6]. MVs are formed by “blebbing” or “pinching off” from the cellular membrane of the parent cell and contain parts of the cytosol more or less randomly enclosed in vesicular “blebs.” Apoptotic bodies are remnants of dead parental cells. The biogenesis of exosomes is unique: they originate from the endocytic compartment and their molecular content reflects, at least in part, that of the parental cell. As tumor cells produce and release masses of exosomes, tumor-derived exosomes (TEX) are ubiquitously present in body fluids of patients with cancer. The ratios of TEX/normal cell-derived exosomes in the plasma of cancer patients varies, but generally TEX represent a substantial proportion of total exosomes recovered from plasma, especially in patients with advanced malignancies [7].
The TEX molecular signature distinguishes them from exosomes derived from normal cells. Further, TEX released by different types of tumor cells have distinct molecular signatures [8]. Exosomes serve as information transfer vehicles, and TEX carry messages from the parent tumor cell to other normal or malignant cells [9]. Upon contacting targeted recipient cells, TEX carrying a cargo consisting of multiple molecular species, including mRNA, miRNA, and DNA, deliver their content to recipient cells and modify functions of these cells [10]. The mechanisms responsible for TEX delivery and processing of their cargo in recipient cells are not entirely understood, but may include the initial ligand-receptor type of binding on the cell surface followed by endocytosis or phagocytosis [11]. Because the TEX cargo is enriched in immunoinhibitory molecules, similar to those present in parental tumor cells, TEX targeting immune cells tend to induce down-stream activation of the inhibitory molecular pathways [12]. It has been shown that TEX isolated from supernatants of cultured tumor cells, which contain only TEX and no other exosomes, effectively mediate suppression of immune cells in ex vivo assays and in vivo in experimental animals. Thus, immunosuppressive TEX are considered to be able to promote tumor growth and to facilitate tumor escape from the host immune system.
6.3 The Immunosuppressive Cargo of TEX
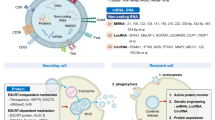
TEX, which originate from the late endosomal compartment of parent tumor cells, acquire their molecular components through the well-defined series of coordinated inward membrane invaginations taking place in late exosomes and multivesicular bodies (MVBs) [13, 14]. Upon fusion of MVBs with the parent cell surface membrane, TEX are released into the extracellular space. TEX formed by this biogenesis process contain elements derived from endosomes (e.g., TSG101, ALIX) as well as from the cell surface membrane and cytosol of a parent cell [6, 15]. Sorting and packaging of TEX for release from the parent cell is executed by the exosomal sorting complex responsible for transport (ESCRT), which might be parent-cell-specific, directing TEX to a pre-defined cellular address.
Upon their release from parental cells, TEX carry a broad variety of molecular species, including membrane-associated proteins, glycoproteins, lipids, and glycolipids as well as a rich vesicular content (reviewed in [10]). The surface membrane of TEX is a lipid-protein bilayer that contains cholesterol, ceramides, sphingomyelins, and phospholipids as well as numerous biologically active proteins such as the major histocompatibility complex (MHC) molecules; TAAs; inhibitory ligands such as FasL, TRAIL, PD-L1, TGF-β/LAP; adhesion molecules, notably ICAM, EPCAM, CD44, integrins; proteases such as MMPS and CD26; ectonucleotidases engaged in adenosine production, CD39/CD73; transmembrane receptors such as CXCR4 and c-Met; heat shock proteins (HSPs); and numerous tetraspanins frequently used as “exosome markers.” In the TEX lumen are nucleic acids, including DNA, mRNA, and miRNA; cytosolic proteins including various enzymes; soluble factors, such as PGE2; cytokines; histones; transport proteins such as ALIX, Rabs, dynamin, LAMPs; cytoskeletal proteins, including actin, tubulin, vimentin, and others; oncoproteins; and a variety of signaling molecules, including MAPK, ERK1/2, Rho, catenin, Wnt, and many others. The TEX molecular and genetic content recapitulates that of the parent cell. However, it is unclear how much of the parent cell content is passed on to exosomes, and the estimates vary widely from 5 to 50%. Nevertheless, It has been convincingly shown that TEX are enriched in some of the key molecules characteristic of the parent cell and thus can serve, at least in part, as surrogates of the parent tumor cells [16].
One intriguing aspect of the cargo TEX carry is that, in addition to a plethora of immunoinhibitory molecules, they also carry tumor-associated antigens (TAA), costimulatory molecules, MHC class I and class II molecules, and intraluminal growth-promoting cytokines [10, 17]. This suggests that TEX are capable of stimulating immune cell responses and that TEX have the dual functional potential. This has led to a controversy regarding TEX and their biological role in cancer, with many investigators viewing TEX as vaccination-promoting vehicles capable of inducing effective anti-tumor immunity [18, 19]. It appears, however, that in the TME, where tumor cells are actively engaged in suppression of anti-tumor immunity and activities of immune cells are blocked, TEX are primarily utilized as an effective mechanism designed to promote tumor progression. It is reasonable to expect that the vesicle-based communication system driven by the tumor is operating to benefit tumor progression and to impair anti-tumor immune responses.
6.4 Communication of TEX with Their Cell Targets
TEX produced by parent cells and released in the extracellular space can interact with local and distant cellular targets. It is unknown whether TEX “carry an address.” But their ubiquitous presence in all body fluids suggests that TEX are freely distributed throughout the body and can interact with any recipient cell ready to commit itself and accept the vesicles. In fact, exosomes are admirably equipped to serve as communication vehicles. Their surface is decorated by the parent cell-derived signaling molecules. Their intra-vesicular content of genetic materials, enzymes, and soluble factors, all biologically active and capable of executing functional responses in target cells, is protected by a membrane from potential degradation by extracellular enzymes during transport. Thus, exosome content can be safely delivered to recipient cells and upon exosome up-take can lead to the cell re-programing [14]. Exosomes can interact with target cells utilizing one or more of the following mechanisms: (a) direct signaling via surface molecules to activate intracellular signaling pathways; (b) fusion with the target cell membrane followed by transfer of proteins or genes the cell lumen; (c) phagocytosis of opsonized exosomes and their internalization; (d) receptor-mediated endocytosis [11]. The cargo delivered by exosomes to recipient cells and taken up by phagocytosis or endocytosis may be either directed to the lysosomes for degradation and clearance or directly incorporated into the cellular machinery to initiate functional re-programming of the recipient cells.
The mechanisms through which TEX alter functions of recipient cells are only partly understood and are being intensively investigated. It appears that some of these mechanisms involve the receptor/ligand type signaling and others require up-take and internalization of TEX [11, 20]. In some cases, TEX fusion with the membrane of a recipient cell may be sufficient to generate signals that induce cellular re-programming [11, 20]. It may be that the recipient cell determines the mode of TEX up-take, which in turn activates downstream molecular/genetic events, culminating in the change of functions. Immune cells differ in their ability to internalize and process TEX. T cells interact with TEX via the receptor/ligand signaling, while other lymphocytes (B cells, NK cells) and monocytes internalize TEX [21]. TEX deliver receptor-mediated signals to T cells that initiate sustained Ca2+ flux [20] resulting in subsequent activation of the relevant downstream pathways, alterations in the recipient cell transcriptome and ultimately translate into modified functional responses [21]. Interestingly, TEX deliver negative signals to effector T cells and activating signals to regulatory T cell (Treg) and MDSC, as discussed below.
6.5 Mechanisms Used by TEX to Alter Function of Recipient Cells
All types of immune cells are sensitive to TEX-mediated interference. However, T lymphocytes seem to be especially vulnerable to negative messages delivered by TEX. The two key receptors on T cells are the T-cell receptor (TcR) and interleukin 2 receptor (IL-2R). We and others have reported that TEX negatively regulate functions of these receptors [22, 23]. Specifically, TEX-mediated down-regulation of the TcR zeta chain is consistently seen in T cells co-incubated with TEX [24]. TEX also reduced JAK expression and phosphorylation in activated T cells [22], and since the integrity of the JAK pathway is essential for functions of IL-2, IL-7 and IL-15, the cytokines sharing the y chain of the IL-2R, down-regulation of JAK activity by TEX is detrimental to T-cell proliferation [25]. TEX were shown to inhibit proliferation of CD8+ T cells but promote expansion of CD4+ T cells, specifically of Treg, while exosomes released by normal cells promoted proliferation of all T cells [22]. Consistent with these data, TEX were found to increase STAT5 phosphorylation in activated CD4+ T cells and to inhibit STAT5 phosphorylation in activated CD8+ T cells [25]. These data suggest that TEX modulate functions of transcription factors such as STATs in recipient T cells. In addition, TEX preferentially inhibited proliferation of human melanoma-specific CD8+ T cells generated in cultures of T cells with melanoma peptide-pulsed DC [22] suggesting that TEX can inhibit antigen-specific T-cell responses. There is solid evidence in support of the ability of TEX carrying a membrane form of FasL or PD-L1 to alter functions of immune cells [22, 26]. TEX-mediated signals leading to apoptosis of activated CD8+ T cells were associated with early membrane changes (i.e., Annexin V binding) in recipient cells, caspase3 cleavage, cytochrome C release from mitochondria, loss of mitochondrial membrane potential (MMP) and DNA fragmentation [27]. These data suggest that TEX induce apoptosis in activated CD8+ T cells by engaging extrinsic as well as intrinsic apoptotic cascades. Further, the PI3K/AKT pathway is the key target for TEX in activated CD8+ T cells: dramatic, time-dependent AKT dephosphorylation and concomitant decreases in expression levels of BCL-2, BCL-xL and MCL-1 accompanied by an increase in levels of pro-apoptotic BAX were observed in these cells during co-incubation with TEX [27].
In a recent study, we co-incubated TEX with subsets of human CD4+, CD8+ and CD4+ CD39+ Treg cells isolated from peripheral blood of normal donors [21]. The objective was to study mechanisms used by recipient T cells to translate TEX-delivered signals into transcriptional activity and functional changes. The qRTPCR was used to monitor expression levels of 24 immunoregulatory genes [21]. Interestingly, massive changes in expression levels of multiple immunoinhibitory and immunostimulatory genes in T cells were observed following co-incubation with TEX. We found that the only factors that significantly regulated TEX-induced transcriptional activity in T cells, including changes in expression levels of genes mediating immune suppression or immune activation, were: (a) the presence or absence of exosomes; (b) recipient cell type (CD4+, CD8+ or Treg); and (c) the activation status of the recipient cells. The observed massive changes in mRNA expression levels were equally induced by co-incubation with TEX or DEX (exosomes produced by human monocyte-derived cultured DC and used as control for TEX). However, TEX and DEX modulated different immunoregulatory genes, and some of the genes were modulated differently in Treg than in CD4+ or CD8+ cells. To show that TEX-mediated signals translated into relevant functions, we concomitantly measured CD69 (an activation marker) expression levels in CD4+ T effector cells by flow cytometry. TEX significantly decreased expression levels of CD69 on the surface of CD4+ T cells, which was consistent with TEX immunosuppressive functions [21]. Also, Treg co-incubated with TEX, which carry both CD39 and CD73 ectonucleotidases [28], significantly up-regulated production of immunosuppressive adenosine in a concentration- and time-dependent manner [21]. This set of data, together with the demonstration that T cells do not readily internalize TEX [20], provided evidence for the hypothesis that TEX signal by engaging surface receptors on recipient T cells and that this signaling negatively modulates T-cell responses.
Our studies of TEX-immune cell interactions have indicated that TEX may exert direct or indirect effects on human immune cells. Directly, TEX induce apoptosis of activated anti-tumor effector T cells [22, 29]; TEX inhibit functions necessary for sustaining anti-tumor responses such as activation, proliferation, and cytotoxicity [22]; TEX interfere with normal differentiation of immune cells [30, 31]; TEX polarize immune cells to tumor-promoting phenotypes and regulate mobilization of immune cells to the tumor [23, 32]. Indirectly, TEX expand proliferation of Treg and myeloid-derived suppressor cells (MDSC) and up-regulate suppressor activity of these cells thus contributing to tumor-induced immune suppression and the tumor immune escape [33, 34]. In addition, TEX can interfere with immune therapies. Antibody-based cancer therapies could be made less effective by TEX carrying TAAs which are targeted by therapeutic antibodies: TEX, ubiquitous in all body fluids, can “soak” therapeutic antibodies diminishing their anti-tumor effects [35]. Adoptively transferred activated T or NK cells may be especially vulnerable to TEX carrying multiple inhibitory ligands [30]. Further, following the delivery of anti-tumor vaccines, newly minted, activated T cells may be highly sensitive to apoptosis by TEX carrying, e.g., FasL among other inhibitory ligands [29]. Emerging evidence clearly points to TEX as a major barrier to successful immunotherapy with antibodies, vaccines or adoptively transferred immune cells in patients with cancer.
6.6 TEX Interactions with Other Immune Cells
T lymphocytes are not the only immune cells targeted by TEX. Activities of human NK cells, B cells, and monocytes are impaired by co-incubation in the presence of TEX. In NK cells, down-regulation in expression of the activating receptors, especially NKG2D, is induced by TEX carrying MICA and MICB ligands [36]. NK-cell activation and cytotoxicity is inhibited by TGF-β, which is prominently displayed on TEX as transforming growth factor-latency associated protein (TGF-LAP), the form necessary for TGF-β activation upon binding to integrins, e.g., α6βV, on the surface of recipient cells [36, 37]. TEX, which are able to make adenosine from ATP by virtue of carrying CD39 and CD73 [28] are implicated in inducing suppressive activity in activated B cells, because adenosine can convert activated B cells into regulatory B cells [38]. TEX have been reported to inhibit normal differentiation of monocytes and to convert monocytes into TGF-β-expressing DCs, which secreted prostaglandin E2 (PGE2) and interfered with the generation of cytolytic T cells [34, 39]. In addition, TEX skewed differentiation of myeloid precursor cells toward developing into highly suppressive MDSCs. This function of TEX was dependent on MyD88 signaling in monocytes and the presence of TGF-β and PGE2 in the TEX cargo [40]. In aggregate, TEX emerge as biologically active vesicles capable of negatively influencing functions of different types of immune cells by mechanisms engaging one or more than one molecular pathway responsible for functional changes in recipient cells.
6.7 Genetic Information Transfer by TEX
Nucleic acids present in the TEX lumen, including DNA, mRNA, and miRNA, play a major role in TEX-mediated delivery of genetic information to recipient cells. To date, relatively little information is available about DNA transfer by TEX [41]. On the other hand, exosomes are known to contain more than 10,000 distinct mRNA species, many of which are known to modulate immune regulation [42]. By far the greatest attention has been directed at miRNA carried by exosomes. MicroRNAs (miRNAs) are small (19–25 nucleotides) non-coding RNAs that suppress the translation of target mRNAs by binding to their 3′ untranslated region. MicroRNAs act as critical regulators of cellular processes such as proliferation, differentiation, apoptosis, and development [43]. MicroRNAs are a prominent component of the TEX cargo [44]. Upon TEX internalization by recipient cells, tumor-derived miRNAs alter gene expression by either repressing protein translation or degradation of multiple targeted mRNA species [45]. TEX are often called “oncomirs,” and miRNAs derived from the tumor and transported to recipient cells have been extensively studied because of their potential role as cancer biomarkers and as a mechanism responsible for transcriptional regulation [46]. Numerous studies have shown that expression of individual miRNAs or specific miRNA signatures can be linked to the diagnosis and prognosis of many cancer types [47]. Many tumor-associated miRNAs, such as miR-21, miR-155, miR-146a, or miR-568, which are frequently recognized as components of the TEX cargos, are known to negatively regulate functions of immune cells or induce apoptosis [45, 48]. Current literature is replete with reports of exosomal transfer of miRNA from tumor to recipient immune cells leading to altered expression levels of complementary mRNA and subsequently to alterations in the transcriptional profile of recipient cells.
6.8 Plasma-Derived Exosomes Vs. TEX
While supernatants of cultured tumor cell lines have been widely used as a source of pure TEX, plasma of patients with cancer contains mixtures of exosomes derived from tumor and normal cells. Thus, plasma-derived exosomes are a heterogeneous mix of vesicles. Immune cells are also a rich source of exosomes and, therefore, miRNA or protein signatures of exosomes isolated from plasma of cancer patients probably reflect those of immune cells as well as the tumor and other tissue cells. It follows that to be able to truly understand how TEX modulate functions of immune cells and to define miRNA or protein signatures of TEX, it will be essential to develop methodologies for separation of TEX from immune cell- and other cell-derived exosomes present in patients’ plasma. To this end, we and others are experimenting with methods for capture of TEX from patients’ plasma and their separation from total plasma exosomes [49]. Meanwhile, total plasma exosome fractions are being used to link the total protein content and molecular as well as genetic exosome profiles to immune dysregulation in patents’ with cancer. Remarkably, these studies appear to confirm the enrichment of exosomes bearing the immunosuppressive cargo in plasma of patients with cancer relative to normal donors [26]. Further, these studies confirm the correlations between the exosome immunosuppressive cargo and disease stage, activity and outcome [50].
6.9 Conclusions
Tumor-derived exosomes (TEX) carrying and delivering various inhibitory ligands to recipient immune cells in the TME are emerging as yet another category of CPIs. The available data support the critical role of exosomes in mediating tumor escape. Further, TEX appear to be implicated in down-regulation of effects of immune therapies in cancer. Rapid progress is being made in finding strategies for silencing of their suppressive cargo to protect immune cells from inhibitory signals TEX deliver and to restore anti-tumor responses. The potential role of TEX as non-invasive biomarkers of cancer diagnosis, progression and outcome is being explored. The future development of TEX as “liquid biopsies” together with measures of TEX impact on functions of immune cells in patients with cancer promises to significantly improve diagnosis and prognosis of human malignancies.
References
Whiteside TL, Demaria S, Rodriguez-Ruiz ME, Zarour HM, Melero I. Emerging opportunities and challenges in cancer immunotherapy. Clin Cancer Res. 2016;22(8):1845–55. https://doi.org/10.1158/1078-0432.CCR-16-0049.
Smyth MJ, Ngiow SF, Ribas A, Teng MW. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat Rev Clin Oncol. 2016;13(3):143–58. https://doi.org/10.1038/nrclinonc.2015.209.
Beatty GL, Gladney WL. Immune escape mechanisms as a guide for cancer immunotherapy. Clin Cancer Res. 2015;21(4):687–92. https://doi.org/10.1158/1078-0432.CCR-14-1860.
Abels ER, Breakefield XO. Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cell Mol Neurobiol. 2016;36(3):301–12. https://doi.org/10.1007/s10571-016-0366-z.
Gould SJ, Raposo G. As we wait: coping with an imperfect nomenclature for extracellular vesicles. J Extracell Vesicles. 2013;2. https://doi.org/10.3402/jev.v2i0.20389.
Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–83. https://doi.org/10.1083/jcb.201211138.
Keller S, Ridinger J, Rupp AK, Janssen JW, Altevogt P. Body fluid derived exosomes as a novel template for clinical diagnostics. J Transl Med. 2011;9:86. https://doi.org/10.1186/1479-5876-9-86.
van der Pol E, Boing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64(3):676–705. https://doi.org/10.1124/pr.112.005983.
Boyiadzis M, Whiteside TL. Information transfer by exosomes: a new frontier in hematologic malignancies. Blood Rev. 2015;29(5):281–90. https://doi.org/10.1016/j.blre.2015.01.004.
Whiteside TL. Exosomes and tumor-mediated immune suppression. J Clin Invest. 2016;126(4):1216–23. https://doi.org/10.1172/JCI81136.
Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3:24641. https://doi.org/10.3402/jev.v3.24641.
Whiteside TL. Tumor-derived exosomes and their role in tumor-induced immune suppression. Vaccine. 2016;4(4):35.
Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126(Pt 24):5553–65. https://doi.org/10.1242/jcs.128868.
Lo Cicero A, Stahl PD, Raposo G. Extracellular vesicles shuffling intercellular messages: for good or for bad. Curr Opin Cell Biol. 2015;35:69–77. https://doi.org/10.1016/j.ceb.2015.04.013.
Cocucci E, Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015;25(6):364–72. https://doi.org/10.1016/j.tcb.2015.01.004.
Atay S, Godwin AK. Tumor-derived exosomes: A message delivery system for tumor progression. Commun Integr Biol. 2014;7(1):e28231. https://doi.org/10.4161/cib.28231.
Whiteside TL. Tumor-derived exosomes and their role in cancer progression. Adv Clin Chem. 2016;74:103–41. https://doi.org/10.1016/bs.acc.2015.12.005.
Kunigelis KE, Graner MW. The dichotomy of tumor exosomes (TEX) in cancer immunity: is it all in the ConTEXt? Vaccines (Basel). 2015;3(4):1019–51. https://doi.org/10.3390/vaccines3041019.
Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14(3):195–208. https://doi.org/10.1038/nri3622.
Muller L, Simms P, Hong CS, Nishimura MI, Jackson EK, Watkins SC, et al. Human tumor-derived exosomes (TEX) regulate Treg functions via cell surface signaling rather than uptake mechanisms. OncoImmunology. 2016. https://doi.org/10.1080/2162402X.2016.1261243.
Muller L, Mitsuhashi M, Simms P, Gooding WE, Whiteside TL. Tumor-derived exosomes regulate expression of immune function-related genes in human T cell subsets. Sci Rep. 2016;6:20254. https://doi.org/10.1038/srep20254.
Wieckowski EU, Visus C, Szajnik M, Szczepanski MJ, Storkus WJ, Whiteside TL. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. J Immunol. 2009;183(6):3720–30. https://doi.org/10.4049/jimmunol.0900970.
Clayton A, Mitchell JP, Court J, Mason MD, Tabi Z. Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Cancer Res. 2007;67(15):7458–66. https://doi.org/10.1158/0008-5472.CAN-06-3456.
Taylor DD, Gercel-Taylor C, Lyons KS, Stanson J, Whiteside TL. T-cell apoptosis and suppression of T-cell receptor/CD3-zeta by Fas ligand-containing membrane vesicles shed from ovarian tumors. Clin Cancer Res. 2003;9(14):5113–9.
Whiteside TL. Immune modulation of T-cell and NK (natural killer) cell activities by TEXs (tumour-derived exosomes). Biochem Soc Trans. 2013;41(1):245–51. https://doi.org/10.1042/BST20120265.
Hong CS, Funk S, Muller L, Boyiadzis M, Whiteside TL. Isolation of biologically active and morphologically intact exosomes from plasma of patients with cancer. J Extracell Vesicles. 2016;5:29289. https://doi.org/10.3402/jev.v5.29289.
Czystowska M, Han J, Szczepanski MJ, Szajnik M, Quadrini K, Brandwein H, et al. IRX-2, a novel immunotherapeutic, protects human T cells from tumor-induced cell death. Cell Death Differ. 2009;16(5):708–18. https://doi.org/10.1038/cdd.2008.197.
Schuler PJ, Saze Z, Hong CS, Muller L, Gillespie DG, Cheng D, et al. Human CD4(+) CD39(+) regulatory T cells produce adenosine upon co-expression of surface CD73 or contact with CD73(+) exosomes or CD73(+) cells. Clin Exp Immunol. 2014;177(2):531–43. https://doi.org/10.1111/cei.12354.
Kim JW, Wieckowski E, Taylor DD, Reichert TE, Watkins S, Whiteside TL. Fas ligand-positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes. Clin Cancer Res. 2005;11(3):1010–20.
Valenti R, Huber V, Iero M, Filipazzi P, Parmiani G, Rivoltini L. Tumor-released microvesicles as vehicles of immunosuppression. Cancer Res. 2007;67(7):2912–5. https://doi.org/10.1158/0008-5472.CAN-07-0520.
Yu S, Liu C, Su K, Wang J, Liu Y, Zhang L, et al. Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J Immunol. 2007;178(11):6867–75.
Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, et al. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151(7):1542–56. https://doi.org/10.1016/j.cell.2012.11.024.
Szajnik M, Czystowska M, Szczepanski MJ, Mandapathil M, Whiteside TL. Tumor-derived microvesicles induce, expand and up-regulate biological activities of human regulatory T cells (Treg). PLoS One. 2010;5(7):e11469. https://doi.org/10.1371/journal.pone.0011469.
Xiang X, Poliakov A, Liu C, Liu Y, Deng ZB, Wang J, et al. Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer. 2009;124(11):2621–33. https://doi.org/10.1002/ijc.24249.
Battke C, Ruiss R, Welsch U, Wimberger P, Lang S, Jochum S, et al. Tumour exosomes inhibit binding of tumour-reactive antibodies to tumour cells and reduce ADCC. Cancer Immunol Immunother. 2011;60(5):639–48. https://doi.org/10.1007/s00262-011-0979-5.
Szczepanski MJ, Szajnik M, Welsh A, Whiteside TL, Boyiadzis M. Blast-derived microvesicles in sera from patients with acute myeloid leukemia suppress natural killer cell function via membrane-associated transforming growth factor-beta1. Haematologica. 2011;96(9):1302–9. https://doi.org/10.3324/haematol.2010.039743.
Hong CS, Muller L, Whiteside TL, Boyiadzis M. Plasma exosomes as markers of therapeutic response in patients with acute myeloid leukemia. Front Immunol. 2014;5:160. https://doi.org/10.3389/fimmu.2014.00160.
Figueiro F, Muller L, Funk S, Jackson EK, Battastini AM, Whiteside TL. Phenotypic and functional characteristics of CD39high human regulatory B cells (Breg). Oncoimmunology. 2016;5(2):e1082703. https://doi.org/10.1080/2162402X.2015.1082703.
Bretz NP, Ridinger J, Rupp AK, Rimbach K, Keller S, Rupp C, et al. Body fluid exosomes promote secretion of inflammatory cytokines in monocytic cells via toll-like receptor signaling. J Biol Chem. 2013;288(51):36691–702. https://doi.org/10.1074/jbc.M113.512806.
Liu Y, Xiang X, Zhuang X, Zhang S, Liu C, Cheng Z, et al. Contribution of MyD88 to the tumor exosome-mediated induction of myeloid derived suppressor cells. Am J Pathol. 2010;176(5):2490–9. https://doi.org/10.2353/ajpath.2010.090777.
Cai J, Wu G, Jose PA, Zeng C. Functional transferred DNA within extracellular vesicles. Exp Cell Res. 2016;349(1):179–83. https://doi.org/10.1016/j.yexcr.2016.10.012.
Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–6. https://doi.org/10.1038/ncb1800.
Ruan K, Fang X, Ouyang G. MicroRNAs: novel regulators in the hallmarks of human cancer. Cancer Lett. 2009;285(2):116–26. https://doi.org/10.1016/j.canlet.2009.04.031.
Esquela-Kerscher A, Slack FJ. Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer. 2006;6(4):259–69. https://doi.org/10.1038/nrc1840.
Ye SB, Li ZL, Luo DH, Huang BJ, Chen YS, Zhang XS, et al. Tumor-derived exosomes promote tumor progression and T-cell dysfunction through the regulation of enriched exosomal microRNAs in human nasopharyngeal carcinoma. Oncotarget. 2014;5(14):5439–52.
Whiteside TL. The potential of tumor-derived exosomes for noninvasive cancer monitoring. Expert Rev Mol Diagn. 2015;15(10):1293–310.
Sato-Kuwabara Y, Melo SA, Soares FA, Calin GA. The fusion of two worlds: non-coding RNAs and extracellular vesicles—diagnostic and therapeutic implications (review). Int J Oncol. 2015;46(1):17–27. https://doi.org/10.3892/ijo.2014.2712.
Carissimi C, Carucci N, Colombo T, Piconese S, Azzalin G, Cipolletta E, et al. miR-21 is a negative modulator of T-cell activation. Biochimie. 2014;107 Pt B:319–26. https://doi.org/10.1016/j.biochi.2014.09.021.
Hong CS, Muller L, Boyiadzis M, Whiteside TL. Isolation and characterization of CD34+ blast-derived exosomes in acute myeloid leukemia. PLoS One. 2014;9(8):e103310. https://doi.org/10.1371/journal.pone.0103310.
Funk S, Floros T, Hong CS, Jackson EK, Lang S, Whiteside TL. Suppression of lymphocyte functions by plasma exosomes correlates with disease activity in patients with head and neck cancer. Clin Cancer Res. 2017;23(16):4843–54.
Acknowledgments
Supported in part by NIH grants RO-1 CA 168628 and R-21 CA205644 to TLW.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Whiteside, T.L. (2017). Exosomes in Cancer: Another Mechanism of Tumor-Induced Immune Suppression. In: Kalinski, P. (eds) Tumor Immune Microenvironment in Cancer Progression and Cancer Therapy. Advances in Experimental Medicine and Biology, vol 1036. Springer, Cham. https://doi.org/10.1007/978-3-319-67577-0_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-67577-0_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-67575-6
Online ISBN: 978-3-319-67577-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)