Abstract
Many homeostatic genes are thought to play a role in the susceptibility to migraine, making it a highly complex neurovascular disease. In this meta-analysis, our primary objective was to evaluate whether or not MTHFR variants (such as C677T and A1289C) and ACE I/D were associated with an increased risk of migraine. Using a PRISMA-based systematic literature-review guideline, internet sources such as PubMed and Google Scholar were searched to identify the genes of interest and migraine risk. To pool the data, odds ratios with 95% confidence intervals were calculated utilizing different genetic models. Cochran’s Q Test and I2 statistics were used to access heterogeneity, while Begg’s and Egger’s tests were used to identify publication bias. All tests were two-sided, and a p-value of < 0.05 was regarded as statistically significant. The present meta-analysis observed that the C677T variant is significantly associated with the increased risk of migraine (allele model: OR:1.19, CI [1.07–1.33], I2 = 78%) and its clinical subtype i.e., MA (allele model: OR: 1.26, CI [1.09–1.45], I2 = 80%) in the overall population. Concerning the ACE- I/D, it significantly increased the risk of overall migraine and both clinical subtypes after utilizing the dominant genetic models (OR: 1.14, CI [1.01–1.29], I2% = 32). Concerning the MTHFR A1289C, only the codominant model (HR vs HT) and recessive model significantly increased the risk of overall migraine. Therefore, the findings of the present meta-analysis showed that MTHFR-C677T is an important risk factor for migraine and its clinical subtype.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Migraine is generally recognized as the third most debilitating and prevalent condition and is classified as a neuro-vascular disorder (Steiner et al. 2020; Sudershan et al. 2022). Depending on whether or not an aura feature is present during an attack, the disease is classified as migraine with aura (MA) or migraine without aura (MWA) by the International Classification of Headache Disorder-III (ICHD-3.org/1-migraine/). Comprehending the susceptibility to diseases is an important factor, and in the case of migraine, it is defined by various risk variables that are roughly classified as environmental and genetic risk factors, with the former being responsible for hindering the sensitivity threshold of pain established by the latter, i.e., genetic risk factors (Sudershan et al. 2022). The most recent and updated meta-analysis of the genome-wide association study (GWAS) data has shown that many genes with modest effects are involved in the disease risk (Hautakangas et al. 2022). Other than the advanced GWAS, numerous independent candidate gene association studies (CGAS) have been carried out in various populations and have identified various genes that are responsible for disease-risk attribution (Sudershan et al. 2022). In migraineurs, changes in vascular endothelial function have been identified as a result, genes such asMTHFR and ACE are known to be associated with vascular or endothelial function and have emerged as prominent candidates for the involvement in migraine development (Gasparini et al. 2013).
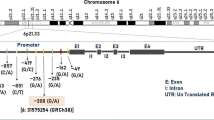
MTHFR (Methylenetetrahydrofolate-Reductase) (1p36.3) with two most-common missense mutations known as rs1801133 or C677T and A1298C/rs1801131 located in exon 4 and exon 7, respectively, (Fig. 1) is responsible for an increase in the level of methylene-THF leading to a subsequent reduction which further disturbs the DNA methylation level, increases the chance of DNA mutations, and increased the homocysteine levels (MTHFR Gene—GeneCards | MTHR Protein | MTHR Antibody). Another gene i.e., ACE (Angiotensin-Converting Enzyme) (17q23) is long (21 kilo-bases) comprised of 26 exons and 25 introns (Fig. 2) and encodes a vital enzyme in the renin–angiotensin–aldosterone system (RAS) and acts as a dipeptidyl carboxypeptidase (ACE Gene—GeneCards | ACE Protein | ACE Antibody). It is responsible for the conversion of an inactive decapeptide, i.e., angiotensin I into an active octapeptide, i.e., angiotensin II, which is a powerful vasoconstrictor (Guan et al. 2017). The most extensively studied polymorphism is the insertion and deletion variant (I/D)-rs1799752 in intron 16 responsible for increased serum levels of ACE and is also associated with the raised frequency of attacks of migraine in patients of MO (Paterna et al. 2000).
Many research studies have shown interest in genotyping MTHFR C677T and A1289C and ACE gene I/D polymorphism to determine its role in migraine but have found a contradictory conclusion (Tables 1, 2, and 3). This might be due to various factors such as diverse sample sizes, different populations (population stratification), and utilization of different genotyping methods. Therefore, to overcome such factors, in the present study, our primary aim was to pool the independent study to find out the precise risk of migraine due to the variants of interest. Secondly, we also aim to pool the results of different research studies to find out the frequency of risk genotypes within different countries.
Method
PROSPERO Registration
The study protocol was registered in the PROSPERO database which is an international prospective register of systematic reviews controlled by NIHR (National Institute for Health and Care Research). (Registration number: CRD42023457008).
Literature Survey
The presented meta-analysis aimed to find out the precise association between the selected variants such as MTHFR’s C677T and A1298C and ACE I/D and the risk of migraine and its clinical subtypes (MA and MWA). Therefore, a “systematic way of literature survey” was done from the online database and search engines such as PubMed-NCBI (National Center for Biotechnology Information) (Pubmed.NCBI.nlm.nih.gov), Google Scholar (Scholar.google.com.tw), and Semantic Scholars (Research Dashboard | Semantic Scholar), respectively. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standards were followed for both the search technique and the selection of relevant material for the review (www.prisma-statement.org). All the relavent information about the PRISMA is provided in PRISMA check list (Supplementary File-Tables 13 and 14). We did not perform a search in databases like the Web of Science, Elsevier, Springer, and others because PubMed already contains over 34 million citations for scientific publications drawn from a wide range of sources like MEDLINE (Medical Literature Analysis and Retrieval System Online), life science journals, and electronic books. Also, there was no language and time restriction for the data search.
Multiple key terms were used in our search strategy, including “C677T and risk of migraine OR MTHFR and risk of migraine”, “A1298C association with migraine OR A1298C and risk of migraine”, and “ACE I/D and risk of migraine”. To reduce the potential for bias, we have gone to great lengths to eliminate any unfavorable study data that were either unpublished/research square (www.researchsquare.com), incomplete, or only partially available book chapters, conference papers, etc. In doing so, we have tried our best to minimize the risk of using biased information.
Inclusion and Exclusion Features
Concerning the study inclusion criteria, the following inclusion criteria should be met and those include “a case–control or cohort study design must be the prime requirement”; second, “the authors must have investigated/ diagnosed the patients suffering from migraine according to the criteria of the International Headache Society (IHS) or ICHD-2/3 (International Classification of Headache Disorders-2 or 3)”; third, “the authors must have looked at the genetic polymorphisms/variants and must have provided the detailed description about the variant under study with proper reference ID (rsID) or change of nucleotide”; fourth, “the genotype frequencies for the polymorphisms studied among migraineurs and non-migraineurs must be reported in the paper in detail”; fifth, “all studies must be within the Hardy–Weinberg Equilibrium (HWE)”; sixth, “the studies should provide clear data to calculate the odds ratios (ORs) and the corresponding 95% confidence intervals (CI)”; and finally, “methodological quality of independent study”. Studies that were based on pedigrees, as well as genome scans, were not accepted for analysis because both of these types of studies investigate linkage. Patients suffering from tension-type headaches or any other type of primary or secondary headaches (according to ICHD-III) were excluded. Also, if an article does not contain the necessary data, the necessary data were either extracted from a previously published article or the author of the article in question was contacted.
Data Extraction
There were a variety of aspects from each study that were analyzed, such as the location, ethnicity, number of patients and healthy subjects, cohort data (if any), the source of the control group, the genotypic frequency of both cases and controls, the first authors along with the publication year, and, lastly, the genotyping method that was applied. These aspects were taken into consideration. If any statistical or numerical data were discovered to be absent, prior research and references were reviewed. First, our two authors, A.S. and A.C.P, were in charge of extracting all of the data and characteristics, and then our other two authors (P.K and H.K) were in charge of evaluating the data’s quality.
Quality Assessment-Newcastle–Ottawa Scale (NOS)
One of the most crucial factors in reaching a conclusive result in a meta-analysis is the quality of the papers included in the study. Given this, the current study evaluated the quality of all previously published studies using the Newcastle–Ottawa Scale (NOS) criteria, where a study will be invalidated if it obtains less than 5 stars (< 5) (or 5 points), and a study that receives more than 5 points is considered a good study and can only receive a maximum of nine stars (Ottawa Hospital Research Institute (ohri.ca)).If any differences in decision-making were noticed concerning article inclusion, data extraction, or quality assessment/NOS, the third investigator (H.K) investigated and concluded the matter.
Statistical Analysis
First, the genotypic and allelic frequencies were determined for each of the studies that were incorporated into the meta-analysis. Next, the chi-square test was performed to determine whether or not the population is in HWE (p > 0.05 for populations in HWE; p < 0.05 for populations not in HWE). The tests of Begg’s and Egger’s, as well as the χ2 based on Cochran’s Q Test with I-square (I2) tests, were used to evaluate the publication bias (p < 0.05 was considered as statistically significant) which included the reporting bias and the heterogeneity of the research papers, respectively. I2 is an estimate that defines the fraction of the variability found across studies that can be attributable to chance rather than to heterogeneity. For the heterogeneity p-value, if this value is lower than 0.10, it indicates the presence of heterogeneity.
The OR (odds ratio) model with 95% CI (confidence interval) and p-value < 0.05 was utilized in logistic regression to determine the strength of the relationship between the variant of interest and the risk of migraine. This was done to find out how strongly the two are associated with one another. Different genetic models such as allelic (rare allele vs. wild allele), dominant (dominant vs. heterozygote + pure recessive), recessive (recessive vs. dominant + heterozygote), and over-dominant (heterozygote vs. pure dominant + recessive) were used to observe the strength of association (OR) using random: DerSimonian and Laird method or fixed model (inverse variance method) based on I2 (I2 > 55%: random model).
In addition, we carried out the sensitivity analysis to examine the impact of individual studies on the pooled ORs and 95% confidence intervals using the criteria “exclusion of each study.” The Cochrane recommendations were followed throughout each step of the current meta-analysis process, from deciding which statistical tests to use to conduct the actual analysis of the findings (training.cochrane.org/handbook/current). The statistical analysis was carried out entirely using the online statistical analysis system software provided by Meta-Genyo (MetaGenyo: Meta-Analysis of Genetic Association Studies).
Genetic Model Selection
Choosing the proper genetic model for risk association is crucia; hence, in the present study, we employed the Thakkinstian algorithm to do so. It is well known that a single nucleotide polymorphism (SNP) can have either a dominant (DD) or recessive (RR) allele, and in the present meta-analysis, we estimated the pool OR values, i.e., OR1, OR2, and OR3 for DD versus RR, DR versus RR, and DD versus DR, along with 95% CIs, respectively.
Condition 1: If OR1 = OR3 ≠ 1 and OR2 = 1 then a recessive model is suggested, condition 2:if OR1 = OR2 ≠ 1 and OR3 = 1 then a dominant model is suggested, condition 3: if OR2 = 1/OR3 ≠ 1 and OR1 = 1 then a complete over-dominant model is suggested, condition 4: if OR1 > OR2 > 1 and OR1 > OR3 > 1 (or OR1 < OR2 < 1 and OR1 < OR3 < 1) then a codominant model is selected (Thakkinstian et al. 2005).
False-Positive Report Probability
To determine whether the association between the genetic variant and the migraine is noteworthy, we used false-positive report probability (FPRP); a classical statistical test which is defined as probability or PR (null hypothesis (H0) is true | association is deemed statistically significant) = Pr (H0 is true | T > zα), where T is a statistical test and zα is the α point of the standard normal distribution. FPRP can be simply defined as the probability of no true association between a genetic variant and disease given a statistically significant finding. Many factors are responsible for FPRP and these include the observed P value, the prior probability that the association between the genetic variant and the disease is real, and the statistical power of the test. Assuming a three-level prior chance (high: 0.1, moderate: 0.01, and low: 0.001), the FPRP threshold was set to 0.2. If the FPRP value is below a preset FPRP value (F), the association between the genetic variant and the disease is deemed noteworthy (Wacholder et al. 2004).
Trial Sequential Analysis
In the current meta-analysis, a novel approach known as trial sequential analysis (TSA) was used to limit random errors by identifying whether or not the studies included in the meta-analysis exceeded the needed sample size. The required information size was determined using the TSA tool (Copenhagen Trial Unit, Denmark) based on a 5% overall risk and a relative risk-reduction of 10–20% (with 80% power) (TSA – ctu.dk). However, there are two basic scenarios: either no additional research is needed if the cumulative Z value/curve meets the RIS (required information size) barrier, or more thorough research is needed if the Z curve remains below the RIS criterion.
Meta-Regression
Based on population age, ethnicity, diagnostic criteria, and genotyping technology, we conducted subgroup analysis and Bayesian meta-regression analysis to identify particular sources of heterogeneity across the included research. Using Microsoft Excel-2019 with Analysis ToolPak, parameter data for the Bayesian meta-regression, such as R-squared, intercept coefficient, standard error, t-value, confidence interval, and p-value, were retrieved as mentioned in our previous meta-analysis (Sudershan et al. 2023).
Result
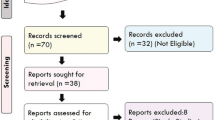
By conducting a systematic literature search following the PRISMA guidelines (Fig. 3), we were able to locate a large number of studies, such as forty-three studies that explored the risk-association between the MTHFR-C677T variant and the risk of migraine in different populations representing different ethnic groups (Table 1). Concerning other MTHFR polymorphism, i.e., A1289C, eight studies explored the association between the respective variant and risk of migraine and its two clinical subtypes (Kara et al. 2003; Bottini et al. 2006; Pizza et al. 2010; Lorenz et al. 2014; Saeedeh et al. 2015; Kaur et al. 2018; Salehi et al. 2018; Thomas et al. 2022). Twenty-four different research studies explored an additional significant variant of the ACE gene known as the I/D polymorphism (Table 3). This involves the presence (insertion, I) or absence (deletion, D) of a 287-base-pair (bp) ALU sequence of DNA in intron 16. Furthermore, the methodological quality of the studies reviewed for the current meta-analysis was depicted (Tables 4 and 5).
MTHFRC677T
After critical analysis of literature from online databases, it was found that a total of 43 studies explored the risk related to MTHFR C677T and the risk of migraine (Table 1) which showed high D allele frequency in Poland, South Korea, Japan, etc. (Fig. 4A). After excluding multiple studies (Magis et al. 2007; Tietjen et al. 2009; Pizza et al. 2010; Liu et al. 2010; Szczygioł et al. 2012; Essmeister et al. 2016; Osman et al. 2019; Thomas et al. 2022) using the criteria such as described in the”Literature Survey” section, only 33 studies were found to be eligible for final pooling of results.
After utilizing different genetic models, it was found that the C677T variant was significantly associated with the increased risk of migraine in the overall population with an associated value of OR:1.19, CI [1.07–1.33], I2 = 78% (random model) under allele model (Fig. 5); OR: 1.19, CI [1.04–1.36], I2 = 72% (random model) for a dominant model; OR: 1.30, CI [1.06–1.59], I2 = 67% (random model) under recessive model; OR: 1.34, CI [1.08–1.67], I2 = 69% (random model) for co-dominant model (HR vs HW); and OR: 1.24, CI [1.02–1.50], I2 = 60% (random model) (HR vs HT) in contrast to the over dominant model (OR: 1.04, CI [0.99–1.09], I2 = 52%, fixed model). In the subgrouping based on ethnicity concerning overall migraine cases, C677T showed a significant association with the risk of migraine in the Caucasian population (allele model-OR: 1.19, CI [1.04–1.37], I2 = 83%, random model) (Supplementary File-Table 2).
After the subgrouping of migraine into clinical subtypes such as MA and MWA, it was observed that allele (OR: 1.26, CI [1.09–1.45], I2 = 80%, random model), dominant (OR: 1.03, CI [1.03–1.42], I2 = 70%, random model), recessive (OR: 1.52, CI [1.18–1.96], I2 = 70%, random model), co-dominant (HR vs HW) (OR: 1.56, CI [1.18–2.06], I2 = 72%, random model), and HR vs HT (OR: 1.42, CI [1.13–1.80], I2 = 60%, random model) in contrast to MWA where in the non-significant association was observed (Supplementary File-Table 1). Significant risk association was observed between the C677T and the risk of MA within the Caucasian population (allele: OR: 1.26 [1.12–1.90]) (Supplementary File-Table 3) in contrast to the non-significant association in MWA (Supplementary File-Table 4).
A scatter plot showed the plotted studies with high precision studies on the top and with low precision below in the plot and created and resulted in a “funnel plot” (Fig. 6). All genetic models produced symmetrical funnel plots, indicating the absence of publication bias. By omitting each study one at a time, sensitive analysis for all genetic models was also examined. It was shown that no pooled OR were significantly impacted, showing a strong stability of the meta-analysis findings (Fig. 7).
MTHFRA1298C
Concerning the second polymorphism i.e., A1298C, a total of eight studies (Kara et al. 2003; Bottini et al. 2006; Pizza et al. 2010; Lorenz et al. 2014; Saeedeh et al. 2015; Kaur et al. 2018; Salehi et al. 2018; Thomas et al. 2022) were found that discussed the association between the risk of migraine and MTHFR A1298C. But after exclusion using criteria such as “not being in the HWE” two studies were excluded (Kara et al. 2003; Thomas et al. 2022) and also one study by Pizza and group which did not provide any control data in their article (Pizza et al. 2010).
After exclusion, a total of five studies (Bottini et al. 2006; Lorenz et al. 2014; Saeedeh et al. 2015; Kaur et al. 2018; Salehi et al. 2018) were used for pooling the result, a non-significant association was observed between the allele, dominant, overdominant, and codominant (HR vs HW and HT vs HW) in contrast to the codominant model (HR vs HT) {OR: 1.47, CI [1.04–2.08], I2 = 24% (fixed model)} and the recessive model {OR: 1.45, CI [1.05–2.01], I2 = 14%, (fixed model)} which significantly increased the risk of overall migraine (Supplementary File: Table 5). After subgrouping migraine into MA and MWA, it was observed that only two studies explored the variant of interest and risk of clinical subtypes which support the non-significant association between the risk of the variant and both clinical subtypes (Supplementary File: Table 5).
Concerning the subgrouping according to criteria of”Ethnicity”, no association was observed with overall migraine as well with both clinical subtypes (MA and MWA) except recessive {OR: 2.09, CI [1.06–4.10]} and codominant model (HR vs HT) {OR: 2.15, CI [1.04–4.44]} in the overall migraine within the Asian population (Supplementary File-Table 6).
ACEI/D
There were a total of 24 research studies that explored the relationship between ACE-I/D polymorphism and migraine risk. (Table 3). However, after a critical analysis, we found that the article published by author Aluclu and group in 2013 was repeatedly published in 2021 by Ekinci and group (Ekinci et al. 2021) and is therefore excluded. There was one research that was carried out by Pizza and colleagues that compared the migraine group with the stroke group. Since the controls in the investigation were people who had experienced an acute myocardial infarction, the study was disregarded as well (Pizza et al. 2010). Also, one study published by Tietjen and the group (Tietjen et al. 2009) was excluded due to the non-availability of the control group. After exclusion, a total of 21 studies were considered eligible for meta-analysisbut it was found that three studies were not found in HWE and thus were excluded (Schürks et al. 2009; Ozbey et al. 2010; Sipahi et al. 2013).
After exclusion, only 17 studies (Paterna et al.2000; Alicakmak et al. 2003; Kowa et al. 2005; Lea et al. 2005; Lin et al. 2005; Kara et al. 2007; Tronvik et al. 2008; Alaşehirliet al. 2009; Joshi et al. 2009; Aluclu et al. 2015; An et al. 2013; Palmirotta et al. 2014; Güllüoğlu et al. 2015; Wani et al. 2016; Essmeister et al. 2016; Jasrotia et al. 2018; Hamdan et al. 2020) were used for pooling the result. It was found that ACE I/D significantly increased the risk of overall migraine after utilizing the dominant and codominant model (HR vs HW) with a risk value of OR: 1.14, CI [1.01–1.29], I2 = 32% (fixed model) and OR: 1.19, CI [1.03–1.39], I2: 47% (fixed model), respectively, in contrast to allele {OR: 1.07, CI [0.99–1.14], I2 = 54% (fixed model)}; recessive {OR: 1.06, CI [0.88–1.28], I2 = 61% (random model)}; over-dominant {OR: 1.07, CI [0.97–1.18], I2 = 47% (fixed model); codominant model {HT vs HW with a risk value of OR: 1.13, CI [0.99–1.28], I2 = 26% (fixed model); and HR vs HT with an associated value of OR: 1.02, CI [0.84–1.23], I2 = 58% (random model)} (Supplementary File-Table 9).
After subgroup migraine cases into its two clinical subtype such as MA and MWA, it was found that only the dominant {OR: 1.21, CI [1.00–1.46], I2 = 32% (Fixed model)} and codominant model (HR vs HW) {OR: 1.34, CI [1.06–1.71], I2 = 35% (Fixed model)} significantly increased the risk of MA. Concerning the case of MWA, only the dominant model was found to be associated with the risk of MWA {OR: 1.17, CI [1.00–1.37], I2 = 10% (fixed model)} (Supplementary File-Table 9).
Concerning the subgrouping using criteria of “Ethnicity” it was observed that the recessive {OR: 2.01, CI [1.32–3.05], I2 = 26% (Fixed model)} codominant (HR vs HW) {OR: 2.11, CI [1.33–3.36], I2 = 42% (fixed model)} and (HR vs HT) {OR: 1.88, CI [1.20–2.95], I2 = 9% (fixed model)} are significantly associated with the risk of MA within the Asian population (Supplementary File-Table 11). Concerning MWA, only the over-dominant {OR: 1.2, CI [1.02–1.41], I2 = 0% (fixed model)} and the codominant (HT vs HW) {OR: 1.25, CI [1.00–1.56], I2 = 16% (fixed model)} were found to be associated with the risk of MWA in the Caucasian population (Supplementary File-Table 12). Linear regression model-based Egger’s test showed the plotted studies created a symmetrical funnel plot for all genetic models which directed that there was no publication bias. Sensitive analysis was also checked for all the genetic models by excluding each study one by one and observed that no pooled ORs were substantially affected, indicating high stability of themeta-analysis results.
Determination of Appropriate Model
As we found the significant risk of migraine under the different models of MTHFR C677T (see “Result: MTHFR C677T” section), therefore, to find out the best genetic model for the risk association we utilized the Thakkinstian model/algorithm which showed codominant as a perfect model (OR1 (1.34) > OR2 (1.24) > 1 and OR1 (1.34) > OR3 (1.12) > 1).
False-Positive Report Probability
FRPR was calculated for the MTHFR C677T due to its significant association with the condition of interest (Table 6).
Trial Sequential Analysis
The necessary sample size and the reliability of the meta-analysis were calculated using TSA statistics and it was noted that the cumulative Z score cross the required information size for MTHFR-C677T (Fig. 8) and ACE-I/D (Fig. 9). Thus, TSA supports that it is an acceptable indication of our conclusions and no further trials/studies are now required. Concerning the MTHFR-A1289C, the required sample size estimation was done for the allele model which showed that the last point of the Z-curve reached or positioned within the conventional boundary which is considered a statistically non-significant zone (Supplementary File- Fig. 1). As a result, we are unable to draw any firm conclusions on the possible causal relationship between the investigated variant and the disease. Therefore, to achieve power, further studies are required.
Bayesian Meta-Regression Analysis
To further understand the sources of variation in results for MTHFR-C677T across studies that satisfied our inclusion criteria, we conducted a meta-regression analysis, taking into account potential impacts such as ethnicity, year of publication, diagnostic criteria, control source, and genotyping technique. The meta-regression analysis showed that most of the predicted heterogeneity parameters did not cause more heterogeneity (Fig. 10B). This was in contrast to the recessive (Fig. 10A) and codominant genetic model (HR vs HW) of the “genotyping technique used,” where a strong linear relationship was seen (Table 7).
Discussion
Migraine is known as a complex neurovascular disorder featured with unilateral headache and is generally recognized as the prevalent and third most debilitating condition (Steiner et al. 2020; Sudershan et al. 2023b). Other than the pathogenic mechanisms, such as CSD, neurogenic inflammation, and the activation and desensitization of the trigeminovascular system, disease susceptibility is one of the most important factors to consider (Sudershan et al. 2022, 2023c). CGASs have shown that genes like the MTHFR and ACE (Tables 1, 2, and 3), which are known to be related to vascular or endothelial function, have emerged as major possibilities for the participation in migraine progression. Many research studies have already shown a significant association of selected candidate genes in different populations but in contradictory to others (Tables 1, 2, and 3). Therefore, to overcome such factors, in the present study, our primary aim was to pool the independent study to find out the precise risk of migraine due to the variants of interest.
The MTHFR gene (gene ID: 4524) was first isolated by Goyette and colleagues (Goyette et al. 1994), which is located on chromosome 1 (1p36.3) (Fig. 1), featured 2.2 kb in length with 12 exons, and encodes methylenetetrahydrofolate reductase (574 amino acids long chain (70 kDa)(MTHFR Gene—GeneCards | MTHR Protein | MTHR Antibody). This enzyme catalyzes the conversion of 5,10-methylenetetrahydrofolate (a primary circulatory form of folate) to 5-methyltetrahydrofolate in NAD(P)-dependent manner which is utilized in homocysteine re-methylation to methionine (Fig. 1). GnomAD (GnomAD (Broadinstitute.Org) data have enlightened that the MTHFR has 182, 342, and 20 synonymous, missense, and pLOF mutations, respectively (Fig. 1B). But the most common missense mutation known as rs1801133 or C677T which is located in exon 4 results in the decreased activity of the MTHFR enzyme from 50 to 70% when exposed to heat (heat-labile variant) thus leading to lower 5-methyl THF levels and higher 5,10-methylene THF and plasma homocysteine levels (Lievers et al. 2001; Leclerc & Rozen, 2007; Škovierová et al. 2016).
Multiple genetic association studies have shown variable results (Table 1) but after pooling diverse independent studies we observed that the C677T variant, i.e., TT (genotype)/ T (variant allele) was significantly associated with the increased risk of migraine in the overall population (Supplementary File). In the subgrouping based on ethnicity concerning overall migraine cases, C677T showed a significant association with the risk of migraine in the Caucasian population (allele model-OR: 1.19, CI [1.04–1.37], I2 = 83%, random model) (Supplementary File-Table 2). After the subgrouping of migraine into clinical subtypes such as MA and MWA, it was observed that allele, dominant, recessive, and co-dominant such as HR vs HW, HR vs HT in contrast to MWA where in the non-significant association (Supplementary File-Table 1). It is very important to understand the relevance of the dominant and recessive models, we have simply defined the dominant model for the “T” allele (C677T), where we assume that having one or more copies of the “T” allele increases the risk compared to C (TT + CT = higher risk vs CC); whereas, for the recessive model for “T” allele, we assume that two copies of “T” allele are required to alter risk (TT = higher risk vs CT + CC).
Multiple meta-analyses that were published previously are featured with different numbers of studies included such as 13 studies (Schürks et al. 2010), 15 studies (Samaan et al. 2011), 16 studies (Liu et al. 2014), 26 studies (Liu et al. 2019), 20 studies (Aiswarya et al. 2020), 24 studies (Rai and Kumar 2022) in comparison to our analysis where 33 studies were found eligible after excluding 10 studies based on exclusion presented in the “Literature Survey” section. All published meta-studies were found to be consistent with our study, thus the present analysis confirmed the results of the previous meta-analysis. Also, it is important to note that the rs1801133 variant that was found to have a significant association with disease risk in this meta-analysis was not found or replicated in the most recent GWAS meta-analysis by Hautakangas and colleagues (Hautakangas et al. 2022). This could be because the sample used in the GWAS was all of European descent, or it could be due to methodological differences such as CGAS having small sample sizes that are underpowered to detect genetic variants with small effects and also have different approaches based on priori hypothesis which is completely different from the GWAS strategy (Jorgensen et al. 2009; Bron et al. 2021).
C667T is a crucial missense variant that results from a C (cytosine) to T (thymine) substitution at nucleotide 677 (C677T), leading to the replacement of an Alanine with a Valine in the final protein (MTHFR Gene—GeneCards | MTHR Protein). Changes in amino acids reduce protein function, resulting in elevated plasma homocysteine levels (Hyper-Homo-Cysteinemia/ HHcy). Homocysteine acid (Hcy) has a large excitatory effect on neurons by functioning as an endogenous NMDA receptor agonist, which is responsible for modifying cortical spreading depression—a known contributor to migraine pathogenesis—and hence has a considerable influence on migraine onset, development, and duration (Hoffmann and Charles 2018; Sudershan et al. 2022). Research has shown that vascular inflammation is brought on by HHcy’s increased oxidative stress and its associated signaling pathways (Fu et al. 2018). According to studies based on model organisms, the combination of clinically relevant migraine-related variables such as nitric oxide and homocysteine results in a complicated algesic phenotype with enhanced photophobia, anxiety, and mechanical hyperalgesia as well as increased neuronal activity in specific cerebral cortical layers (Gerasimova et al. 2022).Also, genotype TT homozygous generally has lower levels of DNA methylation relative to CC homozygotes and influences the susceptibility to various multifactorial diseases including migraine (Brustolin et al. 2010). With a classical example, Wan and group have found lower methylation levels at different CpG units such as + 89, + 94, and + 96 in the RAMP1 gene where they found that decreasing the methylation will significantly increase the migraine, thus showing an inverse relationship; they also found + 25, + 27, + 31 CpG island are associatied with migraine having a family history (Wan et al. 2016).
ACE gene (angiotensin-converting enzyme) (17q23) is another long gene (21 kilo-bases) comprising 26 exons and 25 intronsand encodesa key enzyme in the renin–angiotensin–aldosterone system (RAS) (ACE Gene—GeneCards | ACE Protein | ACE Antibody). It acts as a dipeptidyl carboxypeptidase responsible for converting the inactive decapeptide, i.e., angiotensin I to an active octapeptide and potent vasoconstrictor, i.e., angiotensin II (Riordan 2003). There are currently over 160 known variants in the ACE gene, with the presence or absence of a 287-bp ALU DNA sequence in intron 16 being the polymorphism that has received the most attention. Many research studies have shown interest in ACE gene I/D polymorphism to determine its role in health and disease states including migraine (Horasanli et al. 2012).
In the present meta-analysis, we observed that ACE-I/D significantly increased the risk of overall migraine after utilizing the dominant and codominant model (HR vs HW) with a risk value of OR: 1.19, CI [1.03–1.39], I2: 47% (fixed model). After subgrouping the migraine, it was shown that both the dominant and codominant models (HR vs HW) considerably enhanced the risk of MA. This was in contrast to the findings regarding MWA, where it was discovered that only the dominant model was related to the risk (Supplementary File-Table 9). Concerning the subgrouping using the criterion of “Ethnicity,” it was observed that recessive, codominant (HR vs HW), and (HR vs HT) were significantly associated with the risk of MA within the Asian population (Supplementary File-Table 11). This was in contrast to the findings regarding MWA, where only dominant and codominant were found to be associated with the risk of MWA in the Caucasian population (Supplementary File-Table 12). Different meta-analyses have been previously published which were found to be consistent with our study (Guan et al. 2017) in contrast to another study (Wan et al. 2015).
Research has shown that the mean ACE activity levels are different depending on the type of I/D genotype such as higher serum level corresponding withthe DD genotype. Higher ACE in the circulation provides a transformation of angiotensin I to angiotensin II (vasoconstrictor) and degrades bradykinin (vasodilator) to inactive fragments (Brewster and Perazella 2004; Fleming 2006). Ang-II has been shown in several studies to have significant proinflammatory effects on the vascular wall, producing ROS, inflammatory cytokines, and adhesion molecule synthesis (Benigni et al. 2010). By interacting with AT1 receptors, Ang-II stimulates the NADPH-oxidase system and encourages ROS production in the vascular cells and macrophages. These actions increase oxidative stress in the vessel wall and activate redox-sensitive genes, such as those that code for proinflammatory cytokines (IL-6) (Schieffer 2003).
The DD genotype of the ACE gene is found usually to be associated with increased vWF activity, a sign of endothelial dysfunction (Tietjen et al. 2009), and raised frequency of attacks in MA patients (Paterna et al. 2000) which was found to be inconsistent with what was observed by Lin and group (Lin et al. 2005). On the other hand, the ACE I/I genotype is associated with multiple phenotypes such as lower use of preventative migraine medication in patients with chronic migraine, negative migraine family history in MA patients, and reduced attack frequency (Palmirotta et al. 2014). One possible explanation is that I/I carriers have a negative effect on neuronal excitability via a decrease in glutamatergic excitatory postsynaptic potential coupled with an increase in synaptic GABA release, thereby decreasing the chances of CSD (Dalkara et al. 2010; Kowa et al. 2005). It is interesting to note that Lea and colleagues’ research has demonstrated that using logistic regression analysis made it clear to see the potential combined effects of the ACE and MTHFR polymorphisms on migraine risk, specifically showing that the risk of MA is threefold higher in those with the MTHFR TT genotype and at least one copy of the ACE D gene (Lea et al. 2005).
Enclosing the section, the present study showed a significant association between the C677T and I/D with the overall risk of migraine and subtype MA.
Strengths and Limitations of the Present Meta-Analysis
The greater number of included studies, which closely correlates with the larger sample size, is the primary strength of the present meta-analysis which is followed by the strategy utilized for the literature survey, then the inclusion of searched studies, precise statistical analysis, utilization of of Newcastle–Ottawa Scale quality instrument (Tables 4 and 5), finding risk between variants and subtypes of migraine (MA and MWA) among general and different ethnic groups, determination of “required sample size”, and source of heterogeneity. We also utilized the Thakkinstian algorithm and FPRP to find the best genetic model and to find out whether our association valuesare noteworthy or not, respectively.
The first limitation of the present study is that even though every study employed the ICHD-3/IHS criteria to identify the suspected individual, there is still a potential for misclassification because migraine is such a diverse condition. This is because migraine sufferers sometimes have symptoms that overlap with those of other headache disorders. Second, the present research was only able to perform clinical subgrouping, and there was no attempt made to perform gender-based subgrouping of the data. Third, the risk posed by variants that are not statistically related can be altered by a variety of modifier genes that were not investigated in this particular study. Furthermore, the interaction between markers of the same gene, such as C677T and A1289C of MTHFR or otherSNPs/variants can be in linkage disequilibrium, thus interfering with the results. Unfortunately, this potential connection was not investigated in the current study.
Future Perspective
In the present study, we aimed to find out the association between the candidate variants of vascular homeostasis using a high statistical meta-analytical research approach. Variants such as C677T and I/D of MTHFR and ACE respectively showed a significant association with overall migraine and specifically with the MA subgroup. On the other hand, the A1289C mutation of MTHFR was shown to be associated with a risk of migraines; however, this link was not statistically significant. Possible explanations for this include a smaller number of studies that investigated the link, or more particularly, the fact that the group was not even present in HWE (Kara et al. 2003; Pizza et al. 2010; Thomas et al. 2022). As a consequence of this, it is of the utmost importance to keep in mind the requirement for more research to be carried out that provides evidence for the risk attribution hypothesis which is also shown by TSA (Supplementary File- Fig. 1). More intriguingly, the ratio between case and control should be identical (that is, 1:1), and this is because the chi-square test for independence is at its most convincing when the number of cases and controls is proportional to one another (Jewell 2003).
Conclusion
Migraine is a complex disease, and several potential genes can raise an individual’s risk of developing the migraine disease, and few of which are MTHFR and ACE with their respective polymorphism i.e., C677T, A1298C, and I/D, respectively. The current analysis observed a statistically significant association between the C677T and the risk of migraine and MA both overall as well as in the Caucasian population. Only the recessive and codominant forms of A1298C were found to significantly increase migraine risk in contrast to a non-significant association with MA and MWA. Concerning ACE-I/D, genetic models such as dominant and co-dominant showed a significant risk of association with overall migraine in the overall population and MA in both overall as well as in the Asian population.
Data Availability
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Abbreviations
- ICHD-3:
-
International classification of headache disorder 3rd edition
- MA:
-
Migraine aura
- MWA:
-
Migraine without aura
- CSD:
-
Cortical spreading depression
- GWAS:
-
Genome-wide association study
- CGAS:
-
Candidate gene association study
- ACE :
-
Angiotensin-converting enzyme
- MTHFR :
-
Methylenetetrahydrofolate reductase
- ICHD-3:
-
International classification of headache disorders
- NCBI:
-
National Center for Biotechnology Information
- MEDLINE:
-
Medical literature analysis and retrieval system online
- PRISMA:
-
Preferred Reporting Items for Systematics Reviews and Meta-Analysis
- HIS:
-
International Headache Society
- HWE:
-
Hardy-Weinberg Equilibrium
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- I 2 :
-
I Square
- SNP:
-
Single-nucleotide polymorphism
- FPRP:
-
False-positive report probability
References
Aiswarya PS, Husain RA, Kesavan P, Subramaniyan K, Ahmed SS, Ramakrishnan V (2020) Association of rs1801133 polymorphism with migraine susceptibility: a case-control study followed by updated meta-analysis and trial sequential analysis. Gene Reports 21:100881
Alaşehirli B, Gür M, Akçalı A, Geyik S, Bülbül B, Sayar D, Neyal M (2009) Angiotensin converting enzyme gene insertion/deletion polymorphism in migraine patients. Turkish J Neurol 15(4)
Alicakmak E, Cataloluk O, Yoldas T, Arslan A, Herken H, Barlas O, Yigiter R (2003) Migraine and angiotensin-converting enzyme association in Turkish patients. Pain Clin 15(4):473–477
Aluclu M, Unan F, Tekes S (2015) ACE gene polymorphism and of MTHFR parameters effects on migraine. J Neurol Sci 357:e446
An XK, Lu CX, Ma QL, Zhang XR, Burgunder JM, Lin Q, Qu HL (2013) Association of MTHFR C677T polymorphism with susceptibility to migraine in the Chinese population. Neurosci Lett 549:78–81. https://doi.org/10.1016/j.neulet.2013.06.028
Azimova JE, Sergeev AV, Korobeynikova LA, Kondratieva NS, Kokaeva ZG, Shaikhaev GO, Skorobogatykh KV, Fokina NM, Tabeeva GR, Klimov EA (2013) Effects of MTHFR gene polymorphism on the clinical and electrophysiological characteristics of migraine. BMC Neurol 13:103. https://doi.org/10.1186/1471-2377-13-103
Bahadir A, Eroz R, Dikici S (2013) Investigation of MTHFR C677T gene polymorphism, biochemical and clinical parameters in Turkish migraine patients: association with allodynia and fatigue. Cell Mol Neurobiol 33(8):1055–1063. https://doi.org/10.1007/s10571-013-9972-1
Benigni A, Cassis P, Remuzzi G (2010) Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med 2(7):247–257. https://doi.org/10.1002/emmm.201000080
Bokhari FA, Shakoori TA, Hassan SA, Qureshi HJ, Qureshi GA (2010) Plasma homocysteine in patients of migraine without aura. J Ayub Med College, Abbottabad : JAMC 22(2):52–55
Bottini F, Celle ME, Calevo MG, Amato S, Minniti G, Montaldi L, Di Pasquale D, Cerone R, Veneselli E, Molinari AC (2006) Metabolic and genetic risk factors for migraine in children. Cephalalgia : Int J Headache 26(6):731–737. https://doi.org/10.1111/j.1468-2982.2006.01107.x
Brewster UC, Perazella MA (2004) The renin-angiotensin-aldosterone system and the kidney: effects on kidney disease. Am J Med 116(4):263–272. https://doi.org/10.1016/j.amjmed.2003.09.034
Bron C, Sutherland HG, Griffiths LR (2021) Exploring the hereditary nature of migraine. Neuropsychiatr Dis Treat 17:1183–1194. https://doi.org/10.2147/NDT.S282562
Brustolin S, Giugliani R, Félix TM (2010) Genetics of homocysteine metabolism and associated disorders. Brazilian journal of medical and biological research = Revistabrasileira de pesquisasmedicas e biologicas, 43(1):1–7. https://doi.org/10.1590/s0100-879x2009007500021
Dalkara T, Nozari A, Moskowitz MA (2010) Migraine aura pathophysiology: the role of blood vessels and microembolisation. Lancet Neurol 9(3):309–317
de Tommaso M, Difruscolo O, Sardaro M, Losito L, Serpino C, Pietrapertosa A, Santeramo MT, Dicuonzo F, Carella A, Lamberti P, Livrea P (2007) Influence of MTHFR genotype on contingent negative variation and MRI abnormalities in migraine. Headache 47(2):253–265. https://doi.org/10.1111/j.1526-4610.2006.00690.x
Ekinci FU, Aluclu MU, Ekinci MH (2021) Investigation of the effects of ACE gene polymorphism and MTHFR parameter on migraine. Analytical and Quantitative Cytopathology and Histopathology 43(5):327–336
Essmeister R, Kress HG, Zierz S, Griffith L, Lea R, Wieser T (2016) MTHFR and ACE polymorphisms do not increase susceptibility to migraine neither alone nor in combination. Headache 56(8):1267–1273. https://doi.org/10.1111/head.12893
Ferro A, Castro MJ, Lemos C, Santos M, Sousa A, Pereira-Monteiro J, Sequeiros J, Maciel P (2008) The C677T polymorphism in MTHFR is not associated with migraine in Portugal. Dis Markers 25(2):107–113. https://doi.org/10.1155/2008/178679
Fleming I (2006) Signaling by the angiotensin-converting enzyme. Circ Res 98(7):887–896. https://doi.org/10.1161/01.RES.0000217340.40936.53
Fu Y, Wang X, Kong W (2018) Hyperhomocysteinaemia and vascular injury: advances in mechanisms and drug targets. Br J Pharmacol 175(8):1173–1189. https://doi.org/10.1111/bph.13988
Gasparini CF, Sutherland HG, Griffiths LR (2013) Studies on the pathophysiology and genetic basis of migraine. Curr Genomics 14(5):300–315. https://doi.org/10.2174/13892029113149990007
Gerasimova E, Yakovleva O, Enikeev D, Bogatova K, Hermann A, Giniatullin R, Sitdikova G (2022) Hyperhomocysteinemia increases cortical excitability and aggravates mechanical hyperalgesia and anxiety in a nitroglycerine-induced migraine model in rats. Biomolecules 12(5):735. https://doi.org/10.3390/biom12050735
Goyette P, Sumner JS, Milos R, Duncan AM, Rosenblatt DS, Matthews RG, Rozen R (1994) Human methylenetetrahydrofolate reductase: isolation of cDNA, mapping and mutation identification. Nat Genet 7(2):195–200. https://doi.org/10.1038/ng0694-195
Guan X, Dong X, Yan Y, Zhang C, Liu D, Wan Q (2017) ACE I/D and 5-HTT STin2 VNTR polymorphisms in migraine: a meta-analysis. Int J Clin Exp Med 10(1)
Güllüoğlu H, Gökçay F, Durmaz A, Akin H, Calli C, Köse t (2015) ACE I/D and MTHFR C677T Gene Polymorphisms and matrix metalloproteinase-9 gene expression in migraine patients with and without aura and correlation with cranial magnetic resonance imaging findings: a case-control study. J Neurol Sci 32(2)
Hamdan F, Jabir APDAK (2020) Study the biochemical and molecular role of angiotensin converting enzyme in migraine and non-migraine headache. Int J Pharm Res 12(2)
Hautakangas H, Winsvold BS, Ruotsalainen SE, Bjornsdottir G, Harder AVE, Kogelman LJA, Thomas LF, Noordam R, Benner C, Gormley P, Artto V, Banasik K, Bjornsdottir A, Boomsma DI, Brumpton BM, Burgdorf KS, Buring JE, Chalmer MA, de Boer I, Dichgans M, Pirinen M (2022) Genome-wide analysis of 102,084 migraine cases identifies 123 risk loci and subtype-specific risk alleles. Nature Gen 54(2):152–160. https://doi.org/10.1038/s41588-021-00990-0
Hemat K, Haghjooy Javanmard S, Saadatnia M, Rafiee L, Zandifar A, Tajaddini M (2012) Genetic polymorphisms of C677T MTHFR gene in migraine patients compared with controls. Journal of Isfahan Medical School 30(214):1935–1941
Hesami O, Asadollahi M, Lima BS (2017) Correlation between MTHFR Genotype polymorphisms and different types of migraine headache in iranian patients: a case control study. ARCHIVOS DE MEDICINA 8(S4):228
Hoffmann J, Charles A (2018) Glutamate and its receptors as therapeutic targets for migraine. Neurother: J Am Soc Exp Neurother 15(2):361–370. https://doi.org/10.1007/s13311-018-0616-5
Horasanlı B, Ataç FB, Çöven İ, Karakurum Goksel B, Benli S (2012) Angiotensin I-converting enzyme gene (I/D) polymorphism in patients with migraine. Headache: J Head Face Pain 53(1):161–164. https://doi.org/10.1111/head.12008
Ibrahim SMH, Magzoub MSE, Abdalla SF, Mohamed HS, Ibrahim ME (2018) The Methylene tetrahydrofolate reductase gene variant C677T is not associated with migraine in twelve sudanese pedigrees with migraine. J Neurol Stroke 8(1):00279. https://doi.org/10.15406/jnsk.2018.08.00279
Ishii M, Shimizu S, Sakairi Y, Nagamine A, Naito Y, Hosaka Y, Naito Y, Kurihara T, Onaya T, Oyamada H, Imagawa A, Shida K, Takahashi J, Oguchi K, Masuda Y, Hara H, Usami S, Kiuchi Y (2012) MAOA, MTHFR, and TNF-β genes polymorphisms and personality traits in the pathogenesis of migraine. Mol Cell Biochem 363(1–2):357–366. https://doi.org/10.1007/s11010-011-1188-4
Jasrotia R, Raina JK, Sharma M, Panjaliya RK, Kundal BR, Kumar P (2018) Relationship of MTHFR and ACE gene variations with migraine susceptibility: a case-control study in the population of North India (Jammu). Biosci, Biotechnol Res Asia 15(4):851–860
Jewell NP (2003) Statistics for epidemiology. CRC Press
Jorgensen TJ, Ruczinski I, Kessing B, Smith MW, Shugart YY, Alberg AJ (2009) Hypothesis-driven candidate gene association studies: practical design and analytical considerations. Am J Epidemiol 170(8):986–993. https://doi.org/10.1093/aje/kwp242
Joshi G, Pradhan S, Mittal B (2009) Role of the ACE ID and MTHFR C677T polymorphisms in genetic susceptibility of migraine in a north Indian population. J Neurol Sci 277(1–2):133–137. https://doi.org/10.1016/j.jns.2008.11.002
Kara I, Ozkok E, Aydin M, Orhan N, Cetinkaya Y, Gencer M, Tireli H (2007) Combined effects of ACE and MMP-3 polymorphisms on migraine development. Cephalalgia 27(3):235–243
Kara I, Sazci A, Ergul E, Kaya G, Kilic G (2003) Association of the C677T and A1298C polymorphisms in the 5,10 methylenetetrahydrofolate reductase gene in patients with migraine risk. Brain Res Mol Brain Res 111(1–2):84–90. https://doi.org/10.1016/s0169-328x(02)00672-1
Kaunisto MA, Kallela M, Hämäläinen E, Kilpikari R, Havanka H, Harno H, Nissilä M, Säkö E, Ilmavirta M, Liukkonen J, Teirmaa H, Törnwall O, Jussila M, Terwilliger J, Färkkilä M, Kaprio J, Palotie A, Wessman M (2006) Testing of variants of the MTHFR and ESR1 genes in 1798 Finnish individuals fails to confirm the association with migraine with aura. Cephalalgia: Int J Headache 26(12):1462–1472. https://doi.org/10.1111/j.1468-2982.2006.01228.x
Kaur S, Ali A, Pandey AK, Singh B (2018) Association of MTHFR gene polymorphisms with migraine in North Indian population. Neurological Sciences: Official Journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology 39(4):691–698. https://doi.org/10.1007/s10072-018-3276-7
Kim BK, Kim M, Kang L, Bae HJ, Koo JS, Kwon O, Lee SS (2005) Methylenetetrahydrofolate reductase gene polymorphism in Korean patients with migraine or ischemic stroke. J Korean Neurol Assoc 176–180
Kowa H, Fusayasu E, Ijiri T, Ishizaki K, Yasui K, Nakaso K, Nakashima K (2005) Association of the insertion/deletion polymorphism of the angiotensin I-converting enzyme gene in patients of migraine with aura. Neurosci lett 374(2):129–131
Kowa H, Yasui K, Takeshima T, Urakami K, Sakai F, Nakashima K (2000) The homozygous C677T mutation in the methylenetetrahydrofolate reductase gene is a genetic risk factor for migraine. Am J Med Genet 96(6):762–764. https://doi.org/10.1002/1096-8628(20001204)96:6%3c762::aid-ajmg12%3e3.0.co;2-x
Lea RA, Ovcaric M, Sundholm J, MacMillan J, Griffiths LR (2004) The methylenetetrahydrofolate reductase gene variant C677T influences susceptibility to migraine with aura. BMC Med 2:3. https://doi.org/10.1186/1741-7015-2-3
Lea RA, Ovcaric M, Sundholm J, Solyom L, MacMillan J, Griffiths LR (2005) Genetic variants of angiotensin converting enzyme and methylenetetrahydrofolate reductase may act in combination to increase migraine susceptibility. Mol Brain Res 136(1–2):112–117
Leclerc D, Rozen R (2007) Génétiquemoléculaire de MTHFR: les polymorphismes ne sont pas tousbénins [Molecular genetics of MTHFR: polymorphisms are not all benign]. Medecinesciences : M/s 23(3):297–302. https://doi.org/10.1051/medsci/2007233297
Lievers KJ, Boers GH, Verhoef P, den Heijer M, Kluijtmans LA, van der Put NM, Trijbels FJ, Blom HJ (2001) A second common variant in the methylenetetrahydrofolate reductase (MTHFR) gene and its relationship to MTHFR enzyme activity, homocysteine, and cardiovascular disease risk. J Mol Med (berl) 79(9):522–528. https://doi.org/10.1007/s001090100253
Lin J, Wang P, Chen C, Yueh K, Lin S, Harn H (2005) Homozygous deletion genotype of angiotensin converting enzyme confers protection against migraine in man. Acta NeurologicaTaiwanica 14(3):120
Liu A, Menon S, Colson NJ, Quinlan S, Cox H, Peterson M, Tiang T, Haupt LM, Lea RA, Griffiths LR (2010) Analysis of the MTHFR C677T variant with migraine phenotypes. BMC Res Notes 3:213. https://doi.org/10.1186/1756-0500-3-213
Liu L, Yu Y, He J, Guo L, Li H, Teng J (2019) Effects of MTHFR C677T and A1298C polymorphisms on migraine susceptibility: a meta-analysis of 26 studies. Headache 59(6):891–905. https://doi.org/10.1111/head.13540
Liu R, Geng P, Ma M, Yu S, Yang M, He M, Dong Z, Zhang W (2014) MTHFR C677T polymorphism and migraine risk: a meta-analysis. J Neurol Sci 336(1–2):68–73. https://doi.org/10.1016/j.jns.2013.10.008
Lorenz AL, Kahre T, Mihailov E, Nikopensius T, Lotman EM, Metspalu A, Kolk A (2014) Are methylenetetrahydrofolate reductase (MTHFR) gene polymorphisms C677T and A1298C associated with higher risk of pediatric migraine in boys and girls?. J Biomed Sci Eng 2014
Magis D, Allena M, Coppola G, Di Clemente L, Gérard P, Schoenen J (2007) Search for correlations between genotypes and electrophysiological patterns in migraine: the MTHFR C677T polymorphism and visual evoked potentials. Cephalalgia: Int J Headache 27(10):1142–1149. https://doi.org/10.1111/j.1468-2982.2007.01412.x
Osman MM, Elzoghby DM, Heba H, Randa M, Dalia ME, Nehal EM, Ahmad Sweed MD (2019) Methylenetetrahydrofolate reductase (MTHFR) gene polymorphism (677CT) and increased susceptibility to migraine in egyptian population: a case-control study. Med J Cairo Univ 87(December):4165–4173
Oterino A, Toriello M, Valle N, Castillo J, Alonso-Arranz A, Bravo Y, Ruiz-Alegria C, Quintela E, Pascual J (2010) The relationship between homocysteine and genes of folate-related enzymes in migraine patients. Headache 50(1):99–168. https://doi.org/10.1111/j.1526-4610.2009.01484.x
Oterino A, Valle N, Bravo Y, Muñoz P, Sánchez-Velasco P, Ruiz-Alegría C, Castillo J, Leyva-Cobián F, Vadillo A, Pascual J (2004) MTHFR T677 homozygosis influences the presence of aura in migraineurs. Cephalalgia : an International Journal of Headache 24(6):491–494. https://doi.org/10.1111/j.1468-2982.2004.00692.x
Oterino A, Valle N, Pascual J, Bravo Y, Muñoz P, Castillo J, Ruiz-Alegría C, Sánchez-Velasco P, Leyva-Cobián F, Cid C (2005) Thymidylate synthase promoter tandem repeat and MTHFD1 R653Q polymorphisms modulate the risk for migraine conferred by the MTHFR T677 allele. Brain Res Mol Brain Res 139(1):163–168. https://doi.org/10.1016/j.molbrainres.2005.05.015
Ozbey U, Etem E, Ozel S, Berilgen MS (2010) Is there a relation between insertion/deletion polymorphism of the angiotensin converting enzyme gene in patients of migraine with aura and migraine without aura in region of eastern Turkey. TurkiyeKlinikleri J Med Sci 30(2):502–506
Palmirotta R, Barbanti P, Ludovici G, De Marchis ML, Ialongo C, Egeo G, Guadagni F (2014) Association between migraine and ACE gene (insertion/deletion) polymorphism: the BioBIM study. Pharmacogenomics 15(2):147–155
Pandith AA, Wani IY, Qasim I, Shah ZA, Sheikh S (2017) Evaluation of risk related to MTHFR 677C> T gene polymorphism in migraine patients in Kashmiri population. Open J Prev Med 7(8):151–161
Paterna S, Di Pasquale P, D’Angelo A, Seidita G, Tuttolomondo A, Cardinale A, Licata G (2000) Angiotensin-converting enzyme gene deletion polymorphism determines an increase in frequency of migraine attacks in patients suffering from migraine without aura. Eur Neurol 43(3):133–136
Pezzini A, Grassi M, Del Zotto E, Giossi A, Monastero R, Dalla Volta G, Archetti S, Zavarise P, Camarda C, Gasparotti R, Magoni M, Camarda R, Padovani A (2007) Migraine mediates the influence of C677T MTHFR genotypes on ischemic stroke risk with a stroke-subtype effect. Stroke 38(12):3145–3151. https://doi.org/10.1161/STROKEAHA.107.491506
Pizza V, Bisogno A, Lamaida E, Agresta A, Bandieramonte G, Volpe A, Capasso A (2010) Migraine and coronary artery disease: an open study on the genetic polymorphism of the 5, 10 methylenetetrahydrofolate (MTHFR) and angiotensin I-converting enzyme (ACE) genes. Cent Nerv Syst Agents Med Chem (formerly current medicinal chemistry-central nervous system agents) 10(2):91–96
Rai V, Kumar P (2022) Relation between methylenetetrahydrofolate reductase polymorphisms (C677T and A1298C) and migraine susceptibility. Indian Journal of Clinical Biochemistry : IJCB 37(1):3–17. https://doi.org/10.1007/s12291-021-01000-0
Riordan JF (2003) Angiotensin-I-converting enzyme and its relatives. Genome Biol 4(8):225. https://doi.org/10.1186/gb-2003-4-8-225
Saeedeh S, Ali Akbar O, Mohammad A, Majid G, Ahmad E, Mohammad Sadegh F (2015) MTHFR Gene polymorphisms and susceptibility to migraine attacks
Salehi M, Amin-Beidokhti M, Safarpour Lima B, Gholami M, Javadi GR, Mirfakhraie R (2018) The rs4846049 polymorphism in the 3’UTR region of the MTHFR gene increases the migraine susceptibility in an Iranian population. J Pain Res 11:145–149. https://doi.org/10.2147/JPR.S152930
Samaan Z, Gaysina D, Cohen-Woods S, Craddock N, Jones L, Korszun A, Owen M, Mente A, McGuffin P, Farmer A (2011) Methylenetetrahydrofolate reductase gene variant (MTHFR C677T) and migraine: a case control study and meta-analysis. BMC Neurol 11:66. https://doi.org/10.1186/1471-2377-11-66
Scher AI, Eiriksdottir G, Garcia M, Feit P, Smith AV, Harris TB, Roecklein KA, Gudmundsson LS, Gudnason V, Launer LJ (2013) Lack of association between the MTHFR C677T variant and migraine with aura in an older population: could selective survival play a role?. Cephalalgia: Int J Headache 33(5):308–315. https://doi.org/10.1177/0333102412469739
Scher AI, Terwindt GM, Verschuren WM, Kruit MC, Blom HJ, Kowa H, Frants RR, van den Maagdenberg AM, van Buchem M, Ferrari MD, Launer LJ (2006) Migraine and MTHFR C677T genotype in a population-based sample. Ann Neurol 59(2):372–375. https://doi.org/10.1002/ana.20755
Schieffer B (2003) Interaction of interleukin-6 and angiotensin II in atherosclerosis: culprit for inflammation?. Eur Heart J Suppl 5(suppl_A):A25-A30
Schürks M, Rist PM, Kurth T (2010) MTHFR 677C>T and ACE D/I polymorphisms in migraine: a systematic review and meta-analysis. Headache 50(4):588–599. https://doi.org/10.1111/j.1526-4610.2009.01570.x
Schürks M, Zee RY, Buring JE, Kurth T (2008) Interrelationships among the MTHFR 677C>T polymorphism, migraine, and cardiovascular disease. Neurology 71(7):505–513. https://doi.org/10.1212/01.wnl.0000316198.34558.e5
Schürks M, Zee RY, Buring JE, Kurth T (2009) ACE D/I polymorphism, migraine, and cardiovascular disease in women. Neurology 72(7):650–656
Seo JH, Kim HJ, Lee IH, Kim OJ, Choi BO (2004) Association between migraine with aura and both homocysteine and MTHFR C677T polymorphism. J Korean Neurol Assoc 200–205
Sipahi T, Güldiken B, Kabayel L, Palabiyik O, Özkan H, Kiliç TO, Süt N, Turgut N (2013) Endothelial nitric oxide synthase and angiotensin converting enzyme gene polymorphisms in migraine patients. Noropsikiyatriarsivi 50(3):274–278. https://doi.org/10.4274/npa.y6665
Škovierová H, Vidomanová E, Mahmood S, Sopková J, Drgová A, Červeňová T, Halašová E, Lehotský J (2016) The molecular and cellular effect of homocysteine metabolism imbalance on human health. Int J Mol Sci 17(10):1733. https://doi.org/10.3390/ijms17101733
Steiner TJ, Stovner LJ, Jensen R, Uluduz D, Katsarava Z, The L, Burden: the Global Campaign against Headache (2020) Migraine remains second among the world’s causes of disability, and first among young women: findings from GBD2019. J Headache Pain 21(1):137. https://doi.org/10.1186/s10194-020-01208-0
Sudershan A, Mahajan K, Singh K, Dhar MK, Kumar P (2022) The complexities of migraine: a debate among migraine researchers: a review. Clin Neurol Neurosurg 214:107136. https://doi.org/10.1016/j.clineuro.2022.107136
Sudershan A, Pushap AC, Younis M, Sudershan S, Bhagat S, Kumar H, Panjalyia RK, Kumar P (2023c) Neuroepidemiology study of headache in the region of Jammu of north Indian population: a cross-sectional study. Front Neurol 13:1030940. https://doi.org/10.3389/fneur.2022.1030940
Sudershan A, Sudershan S, Younis M, Bhagat M, Pushap AC, Kumar H, Kumar P (2023a) Enlightening the association between TNF-α -308 G > A and migraine: a meta-analysis with meta-regression and trial sequential analysis. BMC Neurol 23(1):159. https://doi.org/10.1186/s12883-023-03174-x
Sudershan A, Younis M, Sudershan S, Kumar P (2023b) Migraine as an inflammatory disorder with microglial activation as a prime candidate. Neurol Res 45(3):200–215. https://doi.org/10.1080/01616412.2022.2129774
Szczygioł D, Motta E, Gołba A, Stęposz A, Witecka J, Dębski M, Sieroń, A (2012) Frequency of the C677T variant of the methylenetetrahydrofolate reductase (MTHFR) gene in patients with migraine with or without aura–a preliminary report. NeurologiaiNeurochirurgia Polska 46(5):443–449
Thakkinstian A, McElduff P, D’Este C, Duffy D, Attia J (2005) A method for meta-analysis of molecular association studies. Stat Med 24(9):1291–1306. https://doi.org/10.1002/sim.2010
Thomas ASS, Saraswathy R, Thayanithy M (2022) Association of MTHFR (C677T and A1298C) gene variants polymorphisms with migraineurs: a case-control study. Appl Sci Eng Prog 15(3):5531–5531
Tietjen GE, Herial NA, Utley C, White L, Yerga-Woolwine S, Joe B (2009) Association of von Willebrand factor activity with ACE I/D and MTHFR C677T polymorphisms in migrainecha. Cephalalgia 29(9):960–968
Todt U, Freudenberg J, Goebel I, Netzer C, Heinze A, Heinze-Kuhn K, Göbel H, Kubisch C (2006) MTHFR C677T polymorphism and migraine with aura. Ann Neurol 60(5):621–622. https://doi.org/10.1002/ana.20911
Tronvik E, Stovner LJ, Bovim G, White LR, Gladwin AJ, Owen K, Schrader H (2008) Angiotensin-converting enzyme gene insertion/deletion polymorphism in migraine patients. BMC Neurol 8(1):1–5
Wacholder S, Chanock S, Garcia-Closas M, El Ghormli L, Rothman N (2004) Assessing the probability that a positive report is false: an approach for molecular epidemiology studies. J Natl Cancer Inst 96(6):434–442. https://doi.org/10.1093/jnci/djh07
Wan D, Hou L, Zhang X, Han X, Chen M, Tang W, Liu R, Dong Z, Yu S (2015) DNA methylation of RAMP1 gene in migraine: an exploratory analysis. J Headache Pain 16(1). https://doi.org/10.1186/s10194-015-0576-7
Wan D, Wang C, Zhang X, Tang W, Chen M, Dong Z, Yu S (2016) Association between angiotensin-converting enzyme insertion/deletion polymorphism and migraine: a meta-analysis. Int J Neurosci 126(5):393–399
Wani IY, Sheikh S, Shah ZA, Pandith AA, Wani M, Asimi R, Wani M, Sheikh S, Mehraj I (2016) Association of ACE Gene I/D polymorphism with migraine in Kashmiri population. Ann Indian Acad Neurol 19(1):89–93. https://doi.org/10.4103/0972-2327.167698
Acknowledgements
The authors are highly thankful to the Institute of Human Genetics, the University of Jammu, and the Department of Human Genetics (Sri Pratap College, Srinagar, Cluster University Srinagar) for the support in the present study.
Author information
Authors and Affiliations
Contributions
Detail of the authors’ contribution according to the CRediT (Contributor Roles Taxonomy) System: PK and AS conceptualized the study and provided supervision; AS and ACP downloaded and filtered the data; AS and PK conducted all the statistical analysis and interpretation, drafted the manuscript, and edited the pictures and tables; HK and PK edited the manuscript; and PK finalize the manuscript. All authors provided critical feedback on the drafts and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sudershan, A., Pushap, A.C., Kumar, H. et al. A Comprehensive Investigation into the Association Between Mthfr C677t, A1298c, and Ace I/D Variants and Risk of Migraine: an Updated Meta-Analysis of Genetic Association Studies with Trial Sequential Analysis and Meta-Regression. J Mol Neurosci 73, 884–911 (2023). https://doi.org/10.1007/s12031-023-02164-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-023-02164-5