Abstract
With a feature of complex pathogenic mechanisms, migraine is a well-known common neurovascular disorder. Multiple genes are responsible for hindering the susceptibility of pain threshold one of which is the eNOS gene and its variants. Multiple independent observational studies with case–control design produced conflicting findings, which can be attributed to a variety of factors including varying sample sizes, demographic stratification, technique application, etc. Therefore, in the present study we aimed to find out the precise risk between the selected variant of eNOS and the risk of migraine and its clinical subtypes using a meta-analysis approach. To find the association between the risk variants of the eNOS gene and migraine, a PRISMA-based systematic literature review strategy was utilized to search via online resources including PubMed and Google Scholar. Using several genetic models, odds ratios with 95% confidence intervals were computed to pool the data. To access heterogeneity, Cochran's Q Test and I2 statistics were utilized, while Begg's and Egger's tests were used to determine publication bias. A p-value of 0.05 or below was deemed statistically significant for all two-sided tests. The present meta-analysis was able to find out the significant protective association between rs743506 and migraine after using dominant (OR: 0.66, CI [0.49–0.86]), over-dominant (OR: 0.56, CI [0.42–0.75]), codominant model (OR: 0.58, CI[0.43–0.77]). Only significant risk association was found between rs1799983, rs3918226, and risk of migraine with aura after utilizing recessive and codominant models i.e., HR vs HW and HR vs HT. The present meta-analysis showed that rs743506 showed a protective association in comparison to rs1799983, rs3918226 which showed significant risk in the MA group. Also, TSA showed non-significant results and therefore, in conclusion, more studies are required to establish risk.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Featured with high prevalence and the third most debilitating disorder, migraine is considered a complex neurovascular disorder (Steiner et al. 2020; Sudershan et al. 2023a, b). The International Classification of Headache Disorder-III has classified migraine into two sub-clinical types i.e., Migraine with Aura (MA) or Migraine without Aura (MWA) based on the criteria of presence or absence of aura phenotype” (ICHD-3.org/1-Migraine/). Susceptibility to migraine is generally attributed to various risk variables which are roughly classified as environmental and genetic factors where the former is accountable for hindering the sensitivity threshold of pain established by the latter, i.e., genetic risk factors (Sudershan et al. 2022). The significance of genes for vascular endothelial function in migraine, including eNOS (Endothelial Nitric Oxide Synthase), has been demonstrated by a large number of independent candidate gene association studies carried out in a variety of populations (Toriello et al. 2008; Gruber et al. 2010; Güler et al. 2015; Eröz et al. 2014; Logan et al. 2005; Gonçalves et al. 2011, 2012; Zakerjafari et al. 2016; García-Martín et al. 2020).
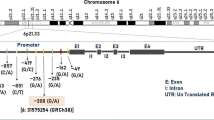
eNOSis an endothelial form of NOS (Nitric Oxide Synthase) which is long (26 exons spanning 21 kb DNA) and located on the 17th chromosome (7q36.1) (Fig. 1A) and encodes a long protein that acts as a key enzyme act in homodimer (Fig. 1C) for the synthesis of Nitric Oxide (NO) from L-arginine (Oliveira-Paula et al. 2016).Nitric oxide (NO) is an effective, reactive, endogenous vasodilator that easily oxidizes to form stable byproducts like nitrate and nitrite. There are multiple biological processes that it regulates which include platelet aggregation inhibition, cerebral blood flow regulation, activation of nociceptors in the trigeminovascular system, and release of vasoactive neuropeptides during the neurogenic inflammatory response (Chen et al. 2008) and has been implicated in the pathophysiology of migraine (Olesen 2008; Sudershan et al. 2023a, b). eNOS represent multiple variants such as -786 T > C, + 884 G > A, VNTR, rs743506, and rs3918226 which are responsible for altering the enzymatic activity and thus have a direct correlation with the level of NO (Oliveira-Paula et al. 2016). These variations (Table 1) have been investigated by several studies to determine the risk of diseases such as rs2070744 (Logan et al. 2005; Gonçalves et al. 2011, 2012; Eröz et al. 2014; Güler et al. 2015; Zakerjafari et al. 2016; García-Martín et al. 2020), rs3918226 Gonçalves et al. 2011, 2012; Güler et al. 2015), VNTR / Intronic-4 variant (Sipahi et al. 2013; Gonçalves et al. 2011, 2012), rs1799983 / + 894 G > A (Borroni et al. 2006; Toriello et al. 2008; Gruber et al. 2010; Gonçalves et al. 2011; Bahadir et al. 2012; Gonçalves et al. 2012; Tajehmiri et al. 2013; Eröz et al. 2014; Güler et al. 2015), rs743506 / Intronic-20 variant (Gonçalves et al. 2011, 2012; Güler et al. 2015) but have shown conflicting results.
A:eNOS gene with multiple common polymorphisms (MAF > 1%) infographics (Common (1000 Genomes Phase 3 MAF > = 1%) Short Genetic Variants from dbSNP Release 153 (ucsc.edu) B Alignment of different regions of eNOS: Cofactors: FAD (flavin adenine dinucleotide) &BH4 tetrahydrobiopterin, Cav-1 (caveolin 1), CaM (calmodulin), NADPH (Nicotinamide adenine dinucleotide phosphate). C Alignment of a homodimer of eNOS protein
As a result, the current study intended to determine the precise risk of migraine and its two sub-clinical types, MA and MWA, related to the presence of risk variants such as -786 T > C, + 884 G > A, VNTR, rs743506, and rs3918226 of eNOS.
Method
Literature Survey
A systematic way of literature survey was utilized in the present meta-analysis which was aimed to find out the precise association between the selected variants such as -786 T > C, + 884 G > A, VNTR (Variable number of tandem repeats), rs743506, rs3918226and the risk of migraine and its clinical subtypes (MA and MWA). Online databases and search engines such as PubMed-NCBI (National Center for Biotechnology Information) (Pubmed.NCBI.nlm.nih.gov), and Google Scholar (Scholar.google.com.tw), Semantic Scholars (Research Dashboard | Semantic Scholar) respectively were utilized.
For the literature survey, we used multiple key terms such as “gene polymorphism and risk of migraine”, OR “eNOS variants and risk of migraine”, OR “rs743506 and risk of migraine”, OR “intronic-20 variant and risk of migraine”, AND “rs1799983 and risk of migraine”, OR “ + 894 G > A and risk of migraine”, AND “rs2070744, and risk of migraine” OR “-786 T > C and risk of migraine”, AND “rs3918226 and risk of migraine” OR “-665 C > T and risk of migraine”, AND “VNTR and risk of migraine” OR “intron-4 variant and risk of migraine”.
Both the search strategy and the decision-making process for choosing appropriate information for the review complied with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria (www.prisma-statement.org). We went to considerable measures to eliminate any unfavorable study data that was either unpublished / Research Square (www.researchsquare.com), incomplete, or only partially available, book chapters, conference papers, etc., to reduce the possibility of bias. Three of our authors (A.S, M.B,& K.S) were responsible for the literature survey.
Inclusion and Exclusion Features
Concerning the study inclusion criteria as mentioned in the previous study (Sudershan et al. 2023a, b), such as (1): variants of interest such as -786 T > C, + 884 G > A, VNTR (Variable number of tandem repeats), rs743506, rs3918226 must be investigated with a case–control or cohort study design must be the prime requirement, (2): the “authors must have diagnosed the patients according to the criteria of the International Headache Society (IHS) or ICHD-2/3” (International Classification of Headache Disorders-II/ III), (3): “the genotype frequencies for the polymorphisms studied among migraineurs and non-migraineurs must be reported in the paper in detail”, (4): “all studies must be within the Hardy–Weinberg Equilibrium (HWE)”, (5):“ studies should provide clear data to calculate the odds ratios (ORs) and the corresponding 95% confidence intervals (CI)”. Studies that were based on pedigrees, as well as genome scans, were not accepted for analysis because both of these types of studies investigate linkage. Also, if an article does not contain the necessary data, the necessary data were either extracted from a previously published article or the author of the article in question was contacted.
Data Extraction
Different features from each included study were extracted such as the number of subjects (case and control), source of control such as population-based (PB) or Hospital Based (HB), source of patients such as Clinic/Hospital Based or Population-Based, and what diagnostic criteria were used. Also, location, ethnicity, the genotypic and allelic frequency of both cases and controls, the first authors along with the publication year, and, lastly, the genotyping method that was applied were collected. Additionally, it was verified that each study had received ethical board clearance before conducting its observation study. Prior research and references were checked if any statistical or numerical data were identified to be missing. Our three authors, M.B, A.C.P, and K.S., were in charge of extracting all of the data and characteristics, and our other two authors (A.S. and P.K.) were responsible for analyzing the quality of the data.
Quality Assessment-Newcastle–Ottawa Scale (NOS)
The evaluation of the quality of the included studies is one of the most essential factors to consider when conducting a meta-analysis. Therefore, the current study evaluated the quality of all prior published studies using the criteria present in the Newcastle–Ottawa scale (NOS). (Ottawa Hospital Research Institute (ohri.ca). If any differences in decision-making were noticed concerning article inclusion, data extraction, or quality assessment/ NOS, the third investigator (H.K) investigated and concluded the matter.
Statistical Analysis
First, the genotypic and allelic frequencies were determined for each of the studies that were incorporated into the meta-analysis. Next, the Chi-square test was performed to determine whether or not the population is in HWE (p > 0.05 for populations in HWE; p < 0.05 for populations not in HWE). The tests of Begg's and Egger's, as well as the χ2 based on Cochran's Q Test with I-square (I2) tests, were used to evaluate the publication bias, which included the reporting bias, and the heterogeneity of the research papers, respectively.
The current study used logistic regression using the OR (Odds Ratio) model along with a 95% CI (Confidence Interval) and a p-value of ≤ 0.05 to determine the precise association between the variations of interest and migraine. Different genetic models such as allelic (Rare/R vs Wild/W), dominant (HR + HT vs HW), recessive (HR vs HW + HT), and over-dominant (HT vs HW + HR), Codominant model (HR vs HW, HR vs HT, HT vs HW) were used to observe the strength of association (OR) using random: Dersimonian and Laird method or fixed model (Inverse variance method) based on I2 (I2 > 50%: Random model).
Additionally, using the criteria "exclusion of each study," we performed a sensitivity analysis to determine how different studies affected the pooled ORs. Every test was conducted using a two-sided approach, and a result of less than 0.05 was regarded as statistically significant. Every step of the current meta-analysis procedure, from choosing the statistical tests to use to assess the findings, was carried out following the Cochrane principles (Training.cochrane.org/handbook/current). The meta-analysis was conducted by the online tool “MetaGenyo: Meta-Analysis of Genetic Association Studies (GAS), which has been exclusively designed by Marugan and group to carry out the meta-analysis of gene association study (Martorell-Marugan et al. 2017).
Trial Sequential Analysis
Trial Sequential Analysis (TSA), a modern technique, was employed in the current meta-analysis to reduce random errors by determining whether or not the studies included in the meta-analysis had insufficient sample sizes. TSA tool was used (Copenhagen Trial Unit, Denmark) based on a 5% overall risk and a relative risk reduction of 10–20% (with 80% power) (TSA – ctu.dk).
Result
Study Characteristics
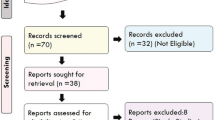
By conducting a systematic literature search following the PRISMA guidelines, we found 123 studies after an initial search in the database. After critical analysis as depicted in the PRISMA flow diagram (Fig. 2), we selected 15 final studies that describe the association of different variants of the eNOS gene with migraine. After selection, the features (depicted in Section "Data Extraction") were recorded, where it was found incomplete data of Papasavva and group (Papasavva et al. 2023) was therefore excluded from the study. After the inclusion of the studies, we checked the quality of each independent study using the New-Ottawa Scale (NOS) where a threshold of > 5 points was considered a good study (Table 2). After the quality assessment, two studies were excluded (Ishii et al. 2014; Tutunchi & Akhavan, 2016) due to its poor quality (quality score: <5). After a close look at the published studies by Güler et al. (2015), Gruber et al. (Gruber et al. 2010), and Toriello et al. (2008), we found that they gave the wrong rsID (for example, rs1800779) for the -786 variation. Also, the prior meta-analysis by Ding et al. (2018) made an error by misrepresenting the work/ genotypic data of Eroz et al. (2014).
Meta-Analysis
rs2070744 / -786 T > C
Another upstream variant ie., -786 T > C located in the promoter region and is found to be studied by 9 independent studies (Logan et al. 2005; Gonçalves et al. 2011, 2012; Gruber et al. 2010; Eröz et al. 2014; Güler et al. 2015; Toriello et al. 2008; Zakerjafari et al. 2016; García-Martín et al. 2020) in different regions (Table 3). The pooled sample size was 1437 for cases and 1265 for control and it was observed that the pooled risk variant genotypic frequency is decreased (HR: 20.66%) than control (HR: 22.21%) in the overall migraine group as well as in MA and MWA types (Table 4). The pooled frequency of risk variants was found slightly higher in overall migraine cases (q = 0.4606) than in controls (q = 0.4573).
After pooling, we observed a non-significant risk association using different genetic models such as allelic (OR: 1.08, CI [0.82–1.42], I2:81.16%-random model) (Fig. 3A), recessive (OR: 0.91, CI [0.62–1.32], I2: 65.66%- random model), etc. (Table 5). After subgrouping into clinical subtypes, a non-significant association was observed for all genetic models utilized in MWA and MA (Table 5). Also, subgroups using the criteria of “ethnicity” non-significant were observed for all genetic models in overall migraine as well in both types (SF: Tables 1–4).
Egger's test performed using a linear regression model revealed no evidence of publication bias (Fig. 3B). By removing each study one at a time, sensitive analysis was carried out for all genetic models (Fig. 4). It was shown that none of the pooled ORs were significantly impacted, demonstrating strong stability of the meta-analysis findings (SF: Tables 1–4).
rs3918226/ Intronic-1 Variant
The third variant i.e., intronic-1 variant (T > C)/ rs3918226, three studies explored the risk (Gonçalves et al. 2011, 2012; Güler et al. 2015) (Table 6). The pooled sample was n = 503 cases and n = 341 in controls which showed that the risk variant genotypic frequency was slightly increased in overall migraine as well as in both of its types (Table 4). However, a slight decrease in risk allele frequency was observed in case (q = 0.062) in contrast to control (q = 0.068).
Using the pooled OR, the present didn’t observe any significant association with overall migraine in contrast to recessive, codominant variant (HR vs HT and HT vs HW) which showed significant risk association with an association value of OR: 7.20, CI [1.12–46.1], OR: 6.95, CI [1.08–44.6] and 8.76, CI [1.27–60.3] respectively in MA group (Table 5). After subgroup analysis in different ethnic groups such as Caucasian and Brazilian, no significant association was observed in the overall migraine group as well as in either group (SF: Tables 3 and 5). All genetic models had a p-value larger than 0.05, indicating that there was no publication bias. A sensitive study performed on all genetic models by methodically deleting individual studies revealed that the pooled ORs were not significantly affected, validating the meta-analysis's high stability (SF: Tables 3 and 5).
VNTR / Intronic-4 Variant/rs61722009
Concerning the VNTR, 3 studies (Sipahi et al. 2013; Gonçalves et al. 2011, 2012) have explored the risk (Table 7). After pooling the result of independent studies which showed a pooled sample size of n = 433 and n = 313 in case and control respectively. The pooled risk genotypic frequency (HR: 20.78%) (q = 0.3475) was lower in overall migraine cases than in control (HR: 23.32%) (q = 0.3945). However, after subgrouping, it was observed that the risk variant genotypic frequency was quite higher in both MA as well in MWA than the control (Table 4). The present meta-analysis observed a non-significant association in both MA and MWA as well with overall migraine (Table 5). After subgrouping into different ethnic groups, no significant association was observed (SF: Tables 7–8).
Using Egger's test, it was observed that all genetic models had p-values larger than 0.05, indicating that there was no publication bias. The pooled ORs were not significantly changed, according to the results of a sensitive analysis that was conducted on all genetic models by methodically deleting individual studies, demonstrating the high stability of the meta-analysis(SF: Tables 7–8).
rs1799983 / + 894 G > A
Concerning rs1799983, a total of 9 studies (Table 9) with a sample size of n = 1345 in cases and n = 1157 in controls, were found to be eligible for pooling the result (Borroni et al. 2006; Toriello et al. 2008; Gruber et al. 2010; Gonçalves et al. 2011, 2012; Bahadir et al. 2012; Tajehmiri et al. 2013; Eröz et al. 2014; Güler et al. 2015). Concerning the genotypic and allelic (q) frequency, the risk genotype and allele (q)were found higher in the overall migraine (HR: 11.52%) (q = 0.3312 vs q = 0.3150) group as well as in MA (HR: 15.15%) than in control (HR: 9.85% & 10.69%) respectively in contrast to MWA cases (Table 4).
After pooling, we observed a non-significant association between the risk variant and overall migraine (Table 5). But after subgrouping into clinical sub-type, a significant risk association was observed after utilizing recessive (OR: 1.57, CI [1.14–2.16]) codominant (HR vs HW and HR vs HT) with an association value of OR: 1.69, CI [1.18–2.41] and OR: 1.58, CI [1.12–2.22] respectively in MA group in contrast to MWA where the non-significant association was observed (Table 5).
Subgrouping into different ethnic groups, we observed a significantly increased risk in Iranian (dominant, over-dominant, and codominant model) and Brazilian populations (SF: Table 5). In the case of MA and MWA sub-types, an overall decreased risk was observed in the Brazilian population using different models in both groups (SF: Tables 10 and 11).
All genetic models exhibited p-values greater than 0.05, according to Egger's test, proving that there was no publication bias. A sensitive analysis was performed on all genetic models by eliminating individual trials, and the findings showed that the pooled ORs were not significantly changed, demonstrating the great stability of the meta-analysis(SF: Tables 5–11).
rs743506 / Intronic-20 Variant
Concerning the intronic-20 variant which was studied by 3 independent groups (Gonçalves et al. 2011, 2012; Güler et al. 2015) (Table 8) and after pooling, we observed a significant increase in risk genotypic frequency in overall migraine as well as in both subtype (Table 4). However, a decreased frequency of risk variant in case (q = 0.2554) in contrast to control (q = 0.2917) was observed.
Concerning the risk attribution, the present meta-analysis was able to observe a decreased risk of overall migraine after utilizing different genetic models such as dominant (OR: 066, CI [0.49–0.86]) (Fig. 5A), over-dominant (OR: 0.56, CI [0.42–0.75], and codominant model (HT vs HW) with an association value of OR: 0.58, CI [0.43–0.77] (Table 5). After subgrouping into MA and MWA, the decreased risk was observed for both sub-types (Table 5) in contrast to significantly increased risk in MA using recessive and codominant model (HR vs HT) with an association value of OR: 2.10, CI [1.08–4.06] and OR: 2.79, CI [1.37–5.65] respectively.
Egger's test, which is based on the connection between standard error and strength of association (log of OR), was used to examine publication bias across all studies included in the meta-analysis. By placing the most accurate research on top and the least precise studies at the bottom of a scatter plot, we were able to create a "funnel plot" that displays the distribution of accuracy across all investigations. All genetic models resulted in symmetrical funnel plots, indicating that no publication bias existed (Fig. 5B). The findings of a sensitive analysis performed on all genetic models by systematically removing individual studies showed that the pooled ORs were not significantly altered, confirming the excellent stability of the meta-analysis (Fig. 5C).
After subgrouping into different ethnic group, we observed decreased risk in Brazilian population in overall migraine using dominant (OR: 0.63, CI [0.44–0.88], over-dominant (OR: 0.46, CI [0.31–0.64], and codominant model (OR: 0.50, CI [0.34–0.71]) in contrast to recessive model (OR: 2.70, CI [1.31–5.54]) (SF: Table 12). Also, decreased risk was observed in both MA and MWA in the Brazilian population (SF: Tables 12–14).
Using Egger's test, it was observed that all genetic models had p-values larger than 0.05, indicating that there was no publication bias. The pooled ORs were not significantly changed, according to the results of a sensitive analysis that was conducted on all genetic models by methodically deleting individual studies, demonstrating the high stability of the meta-analysis(SF: Tables 12–14).
Enclosing the section, the pooled analysis has shown that only rs743506 was found to be associated with migraine and its clinical subtype where it decreases the risk of decrease. Another important variant i.e., rs1799983 showed increased risk only in MA patients.
Trial Sequential Analysis
After combining the independent studies to find whether the pooled sample is adequate for confirming the risk of diseases or not? TSA graphs have shown that the last point of the Z-curve reached or is positioned within the conventional boundary which is considered a statistically non-significant zone therefore, we cannot conclude that there is any risk association between the variant under study (rs1799983) and diseases (Fig. 6). Therefore, to achieve power further studies are required (SF: Figs. 1–4).
Discussion
Polygenic inheritance is believed to be responsible for determining the pain threshold in migraine, which contributes to the disorder's complexity (Sudershan et al. 2022). The eNOS gene is recognized as one of the candidate genes that is crucial for the normal functioning of endothelial cells / vascular systems (Tran et al. 2022). The gene is long (26 exons spanning 21 kb DNA) (Fig. 1A) and encodes a long protein (1203 aa) (Fig. 1B) (NOS3 Gene—GeneCards | NOS3 Protein | NOS3 Antibody) which consists of multiple domains (Fig. 1B) such as FAD (flavin adenine dinucleotide) & BH4 tetrahydrobiopterin, Cav-1 (caveolin 1), CaM (calmodulin), NADPH (Nicotinamide adenine dinucleotide phosphate) important for the binding of the respective cofactors (See review) (Alderton et al. 2001). The active form of protein occurs in homodimer form (Fig. 1C) and is responsible for the synthesis of Nitric Oxide (NO) (Nathan and Xie 1994) which is an effective endogenous vasodilator that regulates cerebral blood flow regulation, nociceptors in the trigeminovascular system, and regulate the release of vasoactive neuropeptides during the neurogenic inflammatory response (Chen et al. 2008; Olesen 2008; Sudershan et al. 2023a, b). Also, NO enhances the CGRP release which in turn results in enhanced vasodilation and mast cell degranulation (Messlinger et al. 2012).
For enzymes to be activated, a high concentration of calcium must be present in the cytoplasm, which is secreted from the endoplasmic reticulum after being activated by numerous signaling molecules such as estrogen, membrane depolarization, neurotransmitters autocoid (serotonin), etc. (Fig. 7) (KEGG: Kyoto Encyclopedia of Genes and Genomes). Upon production of NO in endothelial cells, the NO is transported into smooth muscle cells where it acts as a ligand for soluble Guanine Cyclase which is responsible for the production of cGMP (Cyclic Guanosine Monophosphate) (Fig. 8). NO is immediately converted into nitrate and nitrite where migraine patients it’s both types (MA and MWA) have shown a significantly increased nitrate (Gruber et al. 2010).
KEGG pathways showed that multiple primary signaling molecules such as Estrogen (E2), membrane depolarization, and Neurotransmitters autacoids such as serotonin activates multiple pathway which increase the calcium/ calmodulin complex required for the activation of eNOS. (KEGG: Kyoto Encyclopedia of Genes and Genomes)
The eNOS protein is necessary for nitric oxide production, however, any change in the regulation of the enzyme's activity will affect nitric oxide production (Forstermann and Sessa 2012). Numerous variations of eNOS, including -786 T > C, + 884 G > A, VNTR, rs743506, and rs3918226 alter the regulation of enzyme's function and thus have a direct impact on nitric oxide levels (Oliveira-Paula et al. 2016). Many independent candidate gene association studies have been carried out in various populations (Toriello et al. 2008; Gruber et al. 2010; Güler et al. 2015; Eröz et al. 2014; Logan et al. 2005; Gonçalves et al. 2011, 2012; Zakerjafari et al. 2016; García-Martín et al. 2020) but have shown conflicting results. Determining the precise risk of migraine and its two subclinical kinds, MA and MWA, with the presence of risk variants such -786 T > C, + 884 G > A, VNTR, rs743506, and rs3918226 of eNOS was the goal of the current study.
In the present study, we have observed a non-significant association between different risk variants such as rs2070744/ -786 T > C and VNTR with the risk of migraine or both types i.e., MA and MWA. However, a considerable risk has been identified between other variations such as rs3918226 which significantly increases the risk of MA in the general population (SF: Table 8), and after using dominant, over-dominant, and co-dominant models, rs179983 was found to be a critical risk factor which considerably increases the susceptibility of total migraine in the Iranian population. (SF: Table 12). However, a decreased risk of MA and MWA was found in the Brazilian population after using the dominant, over-dominant, and codominant models (SF: Tables 13 and 14).Another variant i.e., rs743506 significantly decreases the risk of migraine and MWA in the Brazilian population (SF: Tables 15 and 17) in contrast to what was observed in the MA group (SF: Table 16).
Also, we utilize LDmatrix Tool (Genome Assembly: GRCh37 for All Population setting) to create an interactive heatmap matrix of pairwise linkage disequilibrium statistics of multiple eNOS gene variants which have been studied in association with migraine such as rs1800779, rs2070744, rs3918226, rs61722009, rs1799983, rs148554851, rs743506, rs7830. Multiple query variant such as rs207468799 rs61722009 rs148554851 is missing from 1000G (GRCh37) data but rs2070744 and rs1800779 are found in high disequilibrium (r2 = 0.977) (Fig. 9) (SF: Table 15) (LDlink | An Interactive Web Tool for Exploring Linkage Disequilibrium in Population Groups (nih.gov).
An interactive heatmap matrix of pairwise linkage disequilibrium statisticsof multiple variants of the eNOS gene (LDlink | An Interactive Web Tool for Exploring Linkage Disequilibrium in Population Groups (nih.gov)
Concerning the previously published meta-analysis studied + 894 G > T/ rs1799983 (Chen et al. 2015), rs179983 and rs1800779 (Qin et al. 2016), and -786 T > C (Dong et al. 2018). + 894 G > T analyzed by Chen et al. (2015) and Qin et al. (2016) where Qin and group showed rs1799983 was associated with susceptibility to MA in the genetic recessive (OR = 1.41 [1.02- 1.96]) and co-dominant (TT vs GG) (OR = 1.50 [1.04–2.15]) models. But no sub-grouping based on ethnicity was done by Qin and group. Also, a non-significant association was observed between rs1800779 and the risk of migraine of MA and MWA by Qin et al. (2016). Whereas Chen and group observed an increased risk of migraine among the non-caucasian population and with MA in both the overall and non-caucasian populations. Dong and group have observed that risk variant (-786 T > C) increased the risk of overall migraine in the Caucasian population after pooling the results of six studies (Logan et al. 2005; Gonçalves et al. 2011, 2012; Eröz et al. 2014; Güler et al. 2015; Zakerjafari et al. 2016) in contrast to the present study. Such contradiction might be due to the inclusion of more studies (García-Martín et al. 2020; Toriello et al. 2008).
Enclosing the section, the present meta-study might not support the fact that eNOS is a good candidate gene for increasing the risk of migraine or its two clinical subtypes. This is possible due to the lack of power in the present meta-study as supported by trial sequential analysis (SF: Figs. 1–4). However, this is not the actual scenario because studies conducted on a model organism have shown that there is an enhanced expression of eNOS in the dura mater and pia mater, an expression that was not suppressed by sumatriptan or other similar drugs (Zinck et al. 2006). Other evidence has shown that the formation of NO from organic nitrates like nitroglycerin (NTG), which is extremely permeable, lipophilic, and causes headaches comparable to migraines, is controlled by the enzyme eNOS (See review) (Sureda-Gibert et al. 2022). Further evidence for the importance of the NO-sGC pathway in migraine has been provided by studies showing that a PDE5 inhibitor (sildenafil) can also cause headaches in healthy controls and delayed migraine headaches in patients (Kruuse et al. 2002, 2003). Also, the present study hasn’t looked into the combined risk potential of variants on risk of disease which was supported by independent studies (described in Section "Strengths and Limitations with Future Perspective") (Gonçalves et al. 2012). Cumulative all the pieces of evidence mentioned above, have shown a possible relation of the eNOS gene with the pathophysiology of migraine. Therefore, we cannot rule out the possibility that the NOS3 gene may have a minor impact/ no association with migraine given that it is already known that it is a complicated and heterogeneous condition.
Strengths and Limitations with Future Perspective
The primary strength of the present meta-analysis is that it is the first of the kind in which multiple variants of the gene of interest have been analyzed. The second strength is the method used to conduct the literature review, followed by a selection of studies that met the inclusion criteria outlined in subsection "Inclusion and Exclusion Features". Third, precise statistical analysis is used to determine the risk association between the various risk variations and the diseases under review. Fourth, we found out the risk attribution that exists between the selected polymorphisms and the two subtypes of migraines, which are MA and MWA. Fifth, a precise risk attribution has been established for the migraine and its clinical subtypes risk among various ethnicities. In addition, we determine the necessary sample size for the meta-analysis using a novel approach known as TSA.
The first limitation is the small sample size of the present meta-analysis which was evaluated by trial sequential analysis. Also, we tried to contact the author (Papasavva et al. 2023) but no reply was received but if we had the data we could be able to find the significant result. Secondly, despite all studies having used the ICHD-3 / HIS criteria to diagnose the suspected individual, there is still a chance of misclassification because migraine is such a heterogeneous disorder. Third, the present study was only able to do clinical sub-grouping, and there was no attempt to subgroup the data according to gender. Fourth, the risk posed by variations that are not statistically related can be altered by a variety of modifier genes that were not investigated in this particular study. Furthermore, the interaction between markers of the same gene can be linked to the risk of disease. Unfortunately, this potential connection was not investigated in the current study.
Concerning future perspective as shown by TSA graphs, there are more studies needed to confirm the risk due to the presence of a variant of interest of the eNOS gene. Also, it is very important to find out the combined impact of variants of the same gene and other genes (gene–gene interaction) on the risk of diseases of interest.
Conclusion
Migraine has been known as a complex neuro-vascular disorder with a polygenic inheritance and eNOS is one of the genes which is responsible for the disease’s susceptibility. In the present study, we have observed that the rs743506 showed a protective association in comparison to rs1799983, rs3918226 which showed significant risk in the MA group.
Data Availability
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Abbreviations
- ICHD-3 :
-
International Classification of Headache Disorder 3rd edition
- MA :
-
Migraine Aura
- MWA :
-
Migraine without Aura
- eNOS :
-
Endothelial Nitric Oxide Synthase
- ICHD-3 :
-
International Classification of Headache Disorders
- NCBI :
-
National Centre for Biotechnology Information
- MEDLINE :
-
Medical Literature Analysis and Retrieval System Online
- PRISMA :
-
Preferred Reporting Items for Systematics Reviews and Meta-Analysis
- IHS :
-
International Headache Society
- HWE :
-
Hardy-Weinberg Equilibrium
- OR :
-
Odds Ratio
- CI :
-
Confidence Interval
- I 2 :
-
I Square
- GAS :
-
Gene Association Study
References
Alderton WK, Cooper CE, Knowles RG (2001) Nitric oxide synthases: structure, function and inhibition. Biochem J 357(Pt 3):593–615. https://doi.org/10.1042/0264-6021:3570593
Bahadir A, Recep ERÖZ, Dikici S (2012) Düzce İli Migren Tanısı Almış Hastalarda ENOS Ekzon (G894T) Polimorfizminin Araştırılması. Duzce Med J 14(3):22–27
Borroni B, Rao R, Liberini P, Venturelli E, Cossandi M, Archetti S, Caimi L, Padovani A (2006) Endothelial nitric oxide synthase (Glu298Asp) polymorphism is an independent risk factor for migraine with aura. Headache 46(10):1575–1579. https://doi.org/10.1111/j.1526-4610.2006.00614.x
Chen K, Pittman RN, Popel AS (2008) Nitric oxide in the vasculature: where does it come from and where does it go? A quantitative perspective. Antioxid Redox Signal 10(7):1185–1198. https://doi.org/10.1089/ars.2007.1959
Chen M, Tang W, Hou L, Liu R, Dong Z, Han X, Zhang X, Wan D, Yu S (2015) Tumor necrosis factor (TNF) -308G>A, nitric oxide synthase 3 (NOS3) +894G>T polymorphisms and migraine risk: a meta-analysis. PLoS ONE 10(6):e0129372. https://doi.org/10.1371/journal.pone.0129372
Dong H, Wang ZH, Dong B, Hu YN, Zhao HY (2018) Endothelial nitric oxide synthase (-786T>C) polymorphism and migraine susceptibility: a meta-analysis. Medicine 97(36):e12241. https://doi.org/10.1097/MD.0000000000012241
Eröz R, Bahadir A, Dikici S, Tasdemir S (2014) Association of endothelial nitric oxide synthase gene polymorphisms (894G/T, -786T/C, G10T) and clinical findings in patients with migraine. Neuromol Med 16(3):587–593. https://doi.org/10.1007/s12017-014-8311-0
Förstermann U, Sessa WC (2012) Nitric oxide synthases: regulation and function. Eur Heart J 33(7):829–837d. https://doi.org/10.1093/eurheartj/ehr304
García-Martín E, Navarro-Muñoz S, Rodriguez C, Serrador M, Alonso-Navarro H, Calleja M, Turpín-Fenoll L, Recio-Bermejo M, García-Ruiz R, Millán-Pascual J, Navacerrada F, Plaza-Nieto JF, García-Albea E, Agúndez JAG, Jiménez-Jiménez FJ (2020) Association between endothelial nitric oxide synthase (NOS3) rs2070744 and the risk for migraine. Pharmacogenomics J 20(3):426–432. https://doi.org/10.1038/s41397-019-0133-x
Gonçalves FM, Luizon MR, Speciali JG, Martins-Oliveira A, Dach F, Tanus-Santos JE (2012) Interaction among nitric oxide (NO)-related genes in migraine susceptibility. Mol Cell Biochem 370(1–2):183–189. https://doi.org/10.1007/s11010-012-1409-5
Gonçalves FM, Martins-Oliveira A, Speciali JG, Luizon MR, Izidoro-Toledo TC, Silva PS, Dach F, Tanus-Santos JE (2011) Endothelial nitric oxide synthase haplotypes associated with aura in patients with migraine. DNA Cell Biol 30(6):363–369. https://doi.org/10.1089/dna.2010.1152
Gruber HJ, Bernecker C, Lechner A, Weiss S, Wallner-Blazek M, Meinitzer A, Höbarth G, Renner W, Fauler G, Horejsi R, Fazekas F, Truschnig-Wilders M (2010) Increased nitric oxide stress is associated with migraine. Cephalalgia 30(4):486–492. https://doi.org/10.1111/j.1468-2982.2009.01964.x
Güler S, Gürkan H, Tozkir H, Turan N, Çelik Y (2015) An Investigation of the relationship between the eNOS Gene polymorphism and diagnosed migraine. Balkan J Med Genet 17(2):49–59. https://doi.org/10.2478/bjmg-2014-0074
Ishii M, Yahara M, Katoh H, Kawamura M, Shimizu S (2014) Polymorphisms of nitric oxide synthase and GTP cyclohydrolase I genes in Japanese patients with medication overuse headaches. Neurol Asia 19(3)
Kruuse C, Thomsen LL, Birk S, Olesen J (2003) Migraine can be induced by sildenafil without changes in middle cerebral artery diameter. Brain 126(Pt 1):241–247. https://doi.org/10.1093/brain/awg009
Kruuse C, Thomsen LL, Jacobsen TB, Olesen J (2002) The phosphodiesterase 5 inhibitor sildenafil has no effect on cerebral blood flow or blood velocity, but nevertheless induces headache in healthy subjects. J Cereb Blood Flow Metab 22(9):1124–1131. https://doi.org/10.1097/00004647-200209000-00010
Logan JF, Chakravarthy U, Hughes AE, Patterson CC, Jackson JA, Rankin SJ (2005) Evidence for association of endothelial nitric oxide synthase gene in subjects with glaucoma and a history of migraine. Invest Ophthalmol Vis Sci 46(9):3221–3226. https://doi.org/10.1167/iovs.05-0368
Martorell-Marugan J, Toro-Dominguez D, Alarcon-Riquelme ME, Carmona-Saez P (2017) MetaGenyo: a web tool for meta-analysis of genetic association studies. BMC Bioinformatics 18(1):563. https://doi.org/10.1186/s12859-017-1990-4
Messlinger K, Lennerz JK, Eberhardt M, Fischer MJ (2012) CGRP and NO in the trigeminal system: mechanisms and role in headache generation. Headache 52(9):1411–1427. https://doi.org/10.1111/j.1526-4610.2012.02212.x
Nathan C, Xie QW (1994) Regulation of biosynthesis of nitric oxide. J Biol Chem 269(19):13725–13728
Olesen J (2008) The role of nitric oxide (NO) in migraine, tension-type headache and cluster headache. Pharmacol Ther 120(2):157–171. https://doi.org/10.1016/j.pharmthera.2008.08.003
Oliveira-Paula GH, Lacchini R, Tanus-Santos JE (2016) Endothelial nitric oxide synthase: From biochemistry and gene structure to clinical implications of NOS3 polymorphisms. Gene 575(2 Pt 3):584–599. https://doi.org/10.1016/j.gene.2015.09.061
Papasavva M, Vikelis M, Siokas V, Katsarou MS, Dermitzakis EV, Raptis A, Kalliantasi A, Dardiotis E, Drakoulis N (2023) Variability in oxidative stress-related genes (SOD2, CAT, GPX1, GSTP1, NOS3, NFE2L2, and UCP2) and susceptibility to migraine clinical phenotypes and features. Front Neurol 13:1054333. https://doi.org/10.3389/fneur.2022.1054333
Qin B, Cai L, Zhang W, Tang Z, Zheng Y (2016) Effects of rs1799983 and rs1800779 polymorphisms in the endothelial nitric oxide synthase gene on migraine susceptibility with and without aura: a meta-analysis. Int J Clin Exp Med 9(8):15467–15476
Sipahi T, Güldiken B, Kabayel L, Palabiyik O, Özkan H, Kiliç TO, Süt N, Turgut N (2013) Endothelial nitric oxide synthase and angiotensin converting enzyme gene polymorphisms in migraine patients. Noro Psikiyatri Arsivi 50(3):274–278. https://doi.org/10.4274/npa.y6665
Steiner TJ, Stovner LJ, Jensen R, Uluduz D, Katsarava Z, Lifting The Burden: the Global Campaign against Headache (2020) Migraine remains second among the world’s causes of disability, and first among young women: findings from GBD2019. J Headache Pain 21(1):137. https://doi.org/10.1186/s10194-020-01208-0
Sudershan A, Mahajan K, Singh K, Dhar MK, Kumar P (2022) The complexities of migraine: a debate among migraine researchers: a review. Clin Neurol Neurosurg 214:107136. https://doi.org/10.1016/j.clineuro.2022.107136
Sudershan A, Pushap AC, Younis M, Sudershan S, Bhagat S, Kumar H, Panjalyia RK, Kumar P (2023a) Neuroepidemiology study of headache in the region of Jammu of north Indian population: A cross-sectional study. Front Neurol 13:1030940. https://doi.org/10.3389/fneur.2022.1030940
Sudershan A, Younis M, Sudershan S, Kumar P (2023b) Migraine as an inflammatory disorder with microglial activation as a prime candidate. Neurol Res 45(3):200–215. https://doi.org/10.1080/01616412.2022.2129774
Sureda-Gibert P, Romero-Reyes M, Akerman S (2022) Nitroglycerin as a model of migraine: clinical and preclinical review. Neurobiol Pain (cambridge, Mass) 12:100105. https://doi.org/10.1016/j.ynpai.2022.100105
Tajehmiri A, Sadeghi H, Mehmandousti S, Kaveh N, Mohammadi F, Mazdapour M (2013) Association of the G894T polymorphism of the endothelial nitric oxide synthase gene with migraine: an Iranian case-control study. J Biol Today’s World 2:417–424
Toriello M, Oterino A, Pascual J, Castillo J, Colás R, Alonso-Arranz A, Ruiz-Alegría C, Quintela E, Montón F, Ruiz-Lavilla N (2008) Lack of association of endothelial nitric oxide synthase polymorphisms and migraine. Headache 48(7):1115–1119. https://doi.org/10.1111/j.1526-4610.2008.01181.x
Tran N, Garcia T, Aniqa M, Ali S, Ally A, Nauli SM (2022) Endothelial nitric oxide synthase (eNOS) and the cardiovascular system: in physiology and in disease states. Am J Biomed Sci Res 15(2):153–177
Tutunchi S, Akhavan S (2016) Evaluation of NOS3 gene rs1800779 polymorphism in Iranian patients affected by migraine and normal individuals. Adv Biores 7(5)
Zakerjafari M, Dehkordi FF, Yaghoubi H (2016) Evaluation of NOS3 T-C 786 gene polymorphism in Iranian patients affected by migraine and normal individuals. Adv Biores 7:16–19
Zinck T, Illum R, Jansen-Olesen I (2006) Increased expression of endothelial and neuronal nitric oxide synthase in dura and pia mater after air stress. Cephalalgia 26(1):14–25. https://doi.org/10.1111/j.1468-2982.2005.00978.x
Acknowledgements
The authors are highly thankful to the Institute of Human Genetics, University of Jammu, and Department of Human Genetics (Sri Pratap College, Srinagar, Cluster University Srinagar) for support in the present study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Detail of the author’s contribution, according to the CRediT (Contributor Roles Taxonomy) System: PK & AS conceptualized the study and provided supervision, M.B, A.C.P &KSdownloaded and filtered the data, AS, KSconducted all the statistical analysis, and interpretation. A.Sdrafted the manuscript, pictures, and tables editing, HK & PK edited the manuscript and PK finalized the manuscript. All authors provided critical feedback on drafts and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sudershan, A., Bhagat, M., Singh, K. et al. A Comprehensive Investigation of Risk Association Between the -786 T > C, + 884 G > A, VNTR, rs743506, rs3918226 of eNOS and Susceptibility of Migraine: A Updated Meta-Analysis Utilizing Trial Sequential Analysis. J Mol Neurosci 73, 956–975 (2023). https://doi.org/10.1007/s12031-023-02167-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-023-02167-2