Abstract
Polymorphisms in MTHFR gene are mostly associated with increased levels of homocysteine in the absence of dietary folate and are a risk factor for complex neurovascular diseases like migraine. The aim of present case-control study was to determine the association between MTHFR gene polymorphisms (C667T; rs 1801133, A1298C; rs 1801131) with migraine susceptibility. In total, 100 patients with migraine (23with MA and 77 with MO) and age-sex matched 100 healthy controls were included in this study from OPD of ESIC Medical College & Hospital, Faridabad. Genotyping was done by PCR-RFLP method. Genotypic and allelic frequencies were compared by SPSS 24 version. Genotypic results indicated a non-significant increase in frequencies of CT and TT in C667T SNP in migraine patients with control (52 and 10% vs. 42 and 7%: p > 0.05), but CC genotype in A1298C was found to be a risk factor in migraine patients than controls (30 vs. 17% respectively: p < 0.05). On comparing migraine subclasses, migraine with aura (MA) and without aura (MO) with control groups, the present study suggests that in MTHFR polymorphisms, the prevalence of 677CT genotype and T allele in C667T SNP influences susceptibility to MA (p < 0.05) but not to MO. Meanwhile, CC genotype in A1298C SNP could be a risk factor for migraine patients without aura (p < 0.05).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Migraine is a chronic disorder characterized by moderate to severe headaches and nausea. Although some believe it to be a neurological disorder, there is no evidence to confirm this theory. Migraines are about three times more common in women than in men. A typical migraine headache is unilateral (affecting one half of the head) and pulsating in nature and lasting from 4 to 72 h; symptoms include nausea, vomiting, photophobia (increased sensitivity to light), and phonophobia (increased sensitivity to sound); the symptoms are generally aggravated by routine activity. [1] Two main types of migraine are distinguished based on the presence of an aura that can precede the headache: migraine with aura (MA) or without aura (MO) [2, 3]. Although MA and MO have been considered distinct disease entities, it is now more and more accepted that they represent different manifestations of the same disease [4,5,6]. Approximately one-third of people who suffer from migraine headaches perceive an aura—transient visual, sensory, language, or motor disturbances signaling the migraine will soon occur [3]. It is thought that people who experience migraines may have an inherited abnormality in the nerves and blood vessels in the brain. The headache associated with migraines may be caused by blood vessels dilating abnormally, and the “aura”—blind spots, flashes of light, and abnormal sensations experienced before the headache—may be caused by slow electrical waves spreading out across the brain from where the migraine originates. Migraines are extremely painful headaches and are caused by a neurological disorder in the brain. About 300 million people worldwide suffer from recurring migraines, with 8% of the male population and a whopping 17% of the female population being inflicted with this painful disorder [6, 7]. Both genetic susceptibility and environmental triggers could play a role in the pathogenesis of migraine. Many genes have been considered as predisposing factors in initiating migraine attacks mainly in MA than in MO [3]. Many studies have indicated various susceptibility genes for common migraine including MTHFR and some other genes [4, 5]. Methylenetetrahydrofolate reductase catalyzes the conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, a cosubstrate for homocysteine remethylation to methionine. Mutations in the MTHFR gene could be one of the factors leading to increased risk of developing migraine [8]. Among polymorphisms in MTHFR gene, the C677T (rs1801133) and A1298C (rs1801131) substitutions have been investigated so far. The substitution of alanine for valine in the MTHFR protein resulted from C677T (rs1801133) polymorphism which is located within the catalytic domain of the enzyme. In this regard, the presence of T allele was associated with decrease in enzyme activity; thereby alteration in circulating levels of homocysteine and folate metabolites [9].
Similarly, polymorphism A1298C (rs1801131) affects the enzyme activity of MTHFR as well as folate concentrations, although less than those in C677T [10]. Additionally, this polymorphism is located within the regulatory domain. High levels of homocysteine are associated with vascular and endothelial damage and with cerebrovascular and cardiovascular diseases [11]. In particular, it has been assumed that hyperhomocysteinemia is involved in reducing brain blood flow and producing the depolarization wave defined as CSD. These facts associated with reduced oxygen transfer could act as trigger of migraine attack. The endothelial damage induced by high levels of homocysteine could decrease nitric oxide release and leads to the initiation and maintenance of migraine attacks [12]. Hence, the investigation of MTHFR polymorphisms and its effect on metabolic pathway of folate has been considered as an interesting issue in recent years.
Here, we undertook the case control study to see whether an association exists between MTHFR gene polymorphisms (C667T; rs 1801133 and A1298C; rs 1801131) and migraine patients in North Indian population.
Materials and methods
This study was conducted in Jamia Millia Islamia, New Delhi in the Department of Biosciences. The project was approved by the Ethics Committees of Jamia Millia Islamia and ESIC Medical College & Hospital, Faridabad. One hundred migraine patients were selected from those who came to OPD in ESIC Medical College & Hospital, Faridabad. Migraine was diagnosed according to the criteria from the International Headache Society [13]. A detailed medical history was obtained from each patient, and all cases thoroughly taken for a physical examination. The migraine patients who had any cardiovascular or cerebrovascular disease or hypertension, and stroke history were excluded from this study. All patients with migraine were unrelated. One hundred healthy controls, which had never experienced a migraine headache, and had no family history of migraine, were selected after interviewing and examining by a physician.
Genomic DNA extraction
Venous blood samples were collected from both the groups, and a written informed consent was obtained from all the cases and controls. Three milliliters of blood was obtained from each subject in ethylene tetra acetic acid (EDTA) containing vials and stored at − 20 °C till further use. Genomic DNA was extracted from EDTA-treated whole-blood samples from white blood cells using whole genomic DNA extraction kit (Banglore Genei) as well as by salting out method [14]. DNA content was quantified by Nanodrop spectrophotometer (BioLab).
Polymerase chain reaction for amplification of MTHFR gene
C677T and A1298C polymorphisms were detected by PCR-RFLP (restriction fragment length polymorphism) methods using primers (Table 1) obtained from previous studies [7, 8]. PCR amplification was performed in a final volume of 25 μl containing 3 μl 10× PCR buffer containing 50 mM of MgCl2, 0.7 μl of each 10 pmol forward and reverse primer, 1 μl of 10 mM dNTPs, 1 μl of DNA template, and 0.5 μl of 5u/μl Tag DNA polymerase. Thermal cycling was performed in Eppendorf master cycler (Germany) with an initial denaturation of 5 min at 94 °C, then 40 cycles of denaturation at 94 °C for 30 s, annealing at 65 and 56 °C for 30 s for C667T and A1298C respectively, and extension at 70 °C for 1 min 30 s followed by a final extension at 70 °C for 10 min. Amplified DNA were visualized by using gel electrophoresis on 1% agarose and staining.
Restriction fragment length polymorphism
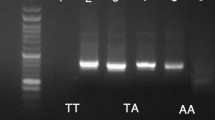
Further restriction digestion of PCR products was done by HinfI and MboII restriction enzymes (Fermentas, USA) to identify specific alleles in C677T and A1298C polymorphisms, respectively. The digestion reaction for each PCR product was carried out in a final volume of 20 μL containing 2 μL buffer, 0.1 μL restriction enzyme and 10 μL PCR product. Then, it was incubated at 37 °C overnight. Digested products were visualized on 3% agarose gel stained with ethidium bromide. C677T PCR product (198 bp) was digested into 175 and 23 bp fragments by Hinf I for T allele. A1298C PCR product (256 bp) was digested into 212, 183, 30, 28, and 14 bp fragments by Mbo II for C allele. The possible results of PCR products and restriction digestion of these SNPs are presented in (Figs. 1, 2, 3 and 4).
Statistical analysis
Statistical analysis was done by SPSS 24.0, and p < 0.05 was considered significant. χ2 test was done to evaluate genotype and allele frequencies and to determine whether they were in Hardy–Weinberg equilibrium. The risk of migraine for mutant allele carriers was reported as odds ratios (OR) and 95% confidence intervals between groups. Continuous variables were expressed as means ± SD and evaluated between groups by t test. Categorical variables were presented as numbers and percentages. For C667T SNP with minor allele T, dominant model is CC vs. CT + TT and recessive model is TT vs CC + CT, and for A1298C SNP with minor allele C, dominant model is AA vs. AC + CC and recessive model is CC vs. AC + AA in this study.
Results
Study subjects
A case-control study, comprising of 100 migraine patients who were matched to gender with 100 healthy controls from the same geographic region, was done. Out of 100 migraineurs, there were 72 women and 28 men, and healthy cohort comprised of 38 males and 62 females. No specific gender-related differences were seen between the groups as shown in Table 2 (p = 0.77 and p = 0.13). Out of 100 migraine patients, 23% were having migraine with aura, and 77% had migraine without aura. Mean age at entry was 35.13 ± 6.0, 36.40 ± 5.2, and 34.45 ± 7.6 years old in MA, MO, and control group, respectively.
Genotypic association analysis
CT and TT genotypes were more frequent in C667T SNP polymorphism in migraine patients (52 and 10%) as compared to controls (42 and 7%) as shown in Table 3, and genotypic frequencies were in Hardy–Weinberg equilibrium and did not show any significant association (p = 0.79). The distribution of MTHFR C667T variant genotypes (CT/TT) in migraine patients was higher (62.0%) compared with control group (49%), but no statistical difference was found (p > 0.05). Distribution of TT allele was higher in patients (10%) as against controls (7%), but results were not significant as p > 0.05 and did not relate with an increased risk of migraine. C allele was more frequent in C667T SNP as was shown in Table 3, but this frequency was in Hardy–Weinberg equilibrium among migraineurs and controls (p = 0.09). A significantly higher frequency of CC in A1298C was observed in patients as compared to controls (30 and 17% respectively, p < 0.05) that was associated with increased risk of migraine in patients (OR = 2.15; 95%CI: 1.04–4.44). However, AA/AC genotype in A1298C SNP also showed significant results with p = 0.03 on comparing with CC genotype. Also non-significantly higher frequency of C allele was observed in migraineurs as compared to controls (50.5 vs. 42%).
On comparing migraine subclasses, MA and MO with control groups in Tables 4 and 5, 667CT genotype showed more frequency in MA (60.9%) than MO (49.4%) and control (42%) and show significant association (p = 0.049) in C667T SNP. A significantly higher frequency of T allele in MA had been found as compared with control (p < 0.05). Even non-significant higher frequency of T allele was observed in MO against controls (33.7 vs. 28%) which reveal that T allele could be a risk factor for migraine patients in both subgroups. In MA subgroup of migraine in A1298C polymorphism, a non-significant increase in CC genotype had been found against control (26.1 vs. 17% respectively, p > 0.05). But a significant result was revealed in MO when compared with control (p = 0.03; OR = 2.35; 95%CI 1.09–5.07). This suggests that CC genotype may be a potential marker for migraine in particularly MO. Almost similar frequencies were observed with C allele in both groups of migraine in A1298C SNP when compared with control (50, 50.7, and 42% respectively).
Moreover, by comparing subgroups of migraine against each other in Table 6, a non-significant increase in frequencies of CT genotype and T allele in C667T SNP was observed in MA against MO (60.9 vs. 49.4% and 43.5 vs. 33.7% respectively) (p > 0.05). In A1298C SNP the frequency of CC genotype was non-significantly higher in MO (31.2%) vs. MA (26.1%), but the C allele was almost equal in MO (50.7%) and MA (50%) groups of migraine.
Discussion
MTHFR gene polymorphisms could be related to a wide range of vascular and neurological disorders. Therefore, study of polymorphisms in MTHFR with the migraine is an interesting topic. As migraine is a common cause of headache disorder mostly in middle-age women. Migraine affects the productivity and health-related quality of life mostly in women. Because of multifactorial and multigenic property of migraine, many candidate genes like MTHFR, ACE, and PGR were involved as role players in migraine attacks in MA [15,16,17]. Heterogeneity may be the reason for failure to replicate various genetic association studies in common migraine. Conflicting results have been reported across the world by various studies on the association between the MTHFR polymorphisms and migraine [18,19,20]. Linkage analysis and candidate gene association study have been popular tools for common forms of migraine genetic studies [21].
As candidate genes using case control study is a powerful method, we compared the association of C667T (rs 1801133) and A1298C (1801131) polymorphisms in MTHFR gene in migraine patients with healthy controls. Results showed that CT and TT genotype in C667T were more prevalent in migraine as compared with control group, whereas 1298CC genotype was more common in migraine patients and these results were in compliance with findings shown by Loenz A.L. et al. [22] and Bahadir A et al. [23] that showed higher frequencies of minor alleles of A1298C and C667T in migraine patients. Another study by Kara I et al. [24] nicely supported that these alleles have higher genotype frequencies in migraineurs in comparison with the control group. Bottini et al. stated that a slight trend toward an increased risk for migraine was present in people who carried the homozygous variant on mutant A1298C or C677T in the Italian population [25]. In the Croatian pediatric migraine population, no statistically significant association with migraine was found, but a trend toward an increased risk for migraine in patients with 677TT genotype was revealed [26]. A study by A. A. Pandith et al. [27] showed that higher distribution of TT mutant genotype was found in controls as against the cases in C667T SNP, but this association was not significant (p > 0.05).
On the other hand, various other previous studies found a significant association between the MTHFR C667T variant and migraine and therefore coincide with our report [8, 9]. During subgroup analysis in this study, it was found that the frequency of T allele was more in MA as compared with MO in C667T, whereas C allele in A1298C was more in MO group. These results were similar to Lea et al. [18] who found that the homozygous TT genotype was significantly associated with MA (p = 0.017). Our study also coincides with Oterino et al. [28] who found twofold risk of migraine with aura to be associated with the T allele among the patients. More recently, a meta-analysis combining all studies assessing the association of MTHFR C677T polymorphism with migraine further suggests that the TT-genotype is a genetic risk factor for migraine with aura, but not to migraine overall [29]. Another study by Saeedi et al. [30] indicated that the prevalence of the MTHFR genotypes 677TT/AC1298 were significantly associated with migraine than control (p < 0.01).
We also compared MA with MO and independently against controls where genotype and allele frequencies were found to be almost in equal proportion, and the difference between the two groups was in were significant (p > 0.05). Similar results were shown by Joshi et al. that revealed that no significant differences were found on comparing migraine with either disease controls or healthy controls [17]. Ferro et al. observed that there was no significant difference in the frequencies of MTHFR C677T genotypes or of the T allele among the Portuguese migraineurs when compared to controls. There was also no association of migraine with aura with MTHFR genotypes or with the T allele, in contrast with our study [31]. Some studies showed positive relevance between migraineurs and both A1298C and C667T genotypes. As homozygote genotypes might cause more enzyme disability that could have major effects in patients. Similar results were found by Kara et al. [23] that individuals with 677CC/1298CC and 677TT/1298AA were more susceptible to migraine than controls having normal genotype of 667CC/1298AA.
Conclusion
We conclude that MTHFR polymorphisms are not significantly associated with a risk for the development of migraine in our study. Different environmental conditions may affect the degree to which genes are activated and thereby modulating the extent of the association. However, we might infer that the TT genotype in C677T SNP may slightly increase the risk for MA but not in MO in our patients and CC genotype in A1298C SNP genotype is a marker for susceptibility to MO. But these need to be validated in a large series of samples. It is assumed that polymorphisms in this gene alone cannot serve as a predictive factor for the risk of migraine. To understand pathophysiology of migraine condition properly, more studies including genetic, clinical, and biochemical parameters should be done.
References
De Vries B, Haan J, Frants RR, Van den Maagdenberg AM, Ferrari MD (2006) Genetic biomarkers for migraine. Headache 46(7):1059–1068. https://doi.org/10.1111/j.1526-4610.2006.00499.x
Wessman M, Terwindt GM, Kaunisto MA, Palotie A, Ophoff RA (2007) Migraine: a complex genetic disorder. Lancet Neurol 6(6):521–532. https://doi.org/10.1016/S1474-4422(07)70126-6
Mulder EJ, Van Baal C, Gaist D, Kallela M, Kaprio J, Svensson DA et al (2003) Genetic and environmental influences on migraine, a twin study across six countries. Twin Res 6(5):422–431. https://doi.org/10.1375/136905203770326420
Estevezm M, Gardner KL (2004) Update on the genetics of migraine. Hum Genet 114(3):225–235. https://doi.org/10.1007/s00439-003-1055-9
Lee JY, Kim M (2005) Current issues in migraine genetics. J Clin Neurol 1(1):8–13. https://doi.org/10.3988/jcn.2005.1.1.8
Stovner LJ, Hagen K (2006) Prevalence, burden, and cost of headache disorders. Curr Opin Neurol 19(3):281–285. https://doi.org/10.1097/01.wco.0000227039.16071.92
Bigal ME, Lipton RB (2009) The epidemiology, burden, and comorbidities of migraine. Neurol Clin 27(2):321–334. https://doi.org/10.1016/j.ncl.2008.11.011
Kowa H, Yasui K, Takeshima T, Urakami K, Sakai F, Nakashima K (2000) The homozygous C677T mutation in the methylenetetrahydrofolate reductase gene is a genetic risk factor for migraine. Am J Med Genet 96(6):762–764. https://doi.org/10.1002/1096-8628(20001204)96:6<762::AID-AJMG12>3.0.CO;2-X
Friedman G, Goldschmidt N, Friedlander Y, Ben-Yehuda A, Selhub J, Babaey S, Mendel M, Kidron M, Bar-On H (1999) A common mutation A1298C in human methylenetetrahydrofolate reductase gene: association with plasma total homocysteine and folate concentrations. J Nutr 129(9):1656–1661
Scher AI, Terwindt GM, Verschuren WM, Kruit MC, Blom HJ, Kowa H et al (2006) Migraine and MTHFR C677T genotype in a population-based sample. Ann Neurol 59(2):372–375. https://doi.org/10.1002/ana.20755
Bhargava S, Ali A, Parakh RR, Saxena R, Srivastava LM (2012) Higher incidence of C677T polymorphism of the MTHFR gene in North Indian patients with vascular disease. Vascular 20(2):88–95. https://doi.org/10.1258/vasc.2011.oa0320
Menon S, Lea RA, Ingle (2015) Effects of dietary folate intake on migraine disability and frequency. Headache 55(2):301–319. https://doi.org/10.1111/head.12490
Headache Classification Subcommittee of the International Headache Society (2013) The international classification of headache disorders, 3rd Edition (beta version). Cephalalgia 33(9):629–808. https://doi.org/10.1177/0333102413485658
Miller SA, Dykes DD, Polesky HF (1998) A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 16(3):1215
Stuart S, Cox HC, Lea RA, Griffiths LR (2012) The role of the MTHFR gene in migraine. Headache 52(3):515–520. https://doi.org/10.1111/j.1526-4610.2012.02106.x
Schürks M, Zee RYL, Buring JE, Kurth T (2009) ACE D/I polymorphism, migraine, and cardiovascular disease in women. Neurology 72(7):650–656. https://doi.org/10.1212/01.wnl.0000342517.97178.f6
Joshi G, Pradhan S, Mittal B (2009) Role of the ACE ID and MTHFR C677T polymorphisms in genetic susceptibility of migraine in a north Indian population. J Neurol Sci 277(1-2):133–137. https://doi.org/10.1016/j.jns.2008.11.002
Lea RA, Ovcaric M, Sundholm J, MacMillan J, Griffiths LR (2004) The methylenetetrahydrofolate reductase gene variant C677T influences susceptibility to migraine with Aura. BMC Med 2(1):3. https://doi.org/10.1186/1741-7015-2-3
Lea RA, Ovcaric M, Sundholm J, Solyom L, Macmillan J, Griffiths LR (2005) Genetic variants of angiotensin converting enzyme and methylenetetrahy-drofolate reductase may act in combination to increase migraine susceptibility. Brain Res Mol Brain Res 136(1-2):112–117. https://doi.org/10.1016/j.molbrainres.2005.01.006
Lin JJ, Wang PJ, Chen CH, Yueh KC, Lin SZ, Harn HJ (2005) Homo-zygous deletion genotype of angiotensin converting enzyme confers protection against migraine in man. Acta Neurol Taiwanica 14(3):120–125
Schurks M (2012) Genetics of migraine in the age of genome-wide association studies. J Headache Pain 13(1):1–9. https://doi.org/10.1007/s10194-011-0399-0
Lorenz AL, Kahre T, Mihailov E, Nikopensius T, Lotman EM, Metspalu A, Kolk A (2014) Are methylenetetrahydrofolate reductase (MTHFR) gene polymorphisms C677T and A1298C associated with higher risk of pediatric migraine in boys and girls? J Biomed Sci Eng 7(08):464–472. https://doi.org/10.4236/jbise.2014.78049
Bahadir A, Eroz R, Dikici S (2013) Investigation of MTHFR C677T gene polymorphism. Biochem Clin Parameters Turk Migraine Patients 33(8):1055–1063. https://doi.org/10.1007/s10571-013-9972-1
Kara I, Sazei A, Ergul E, Kaya G, Kilie G (2003) Association of the C677T and A1298C polymorphisms in the 5, 10 methylenetetrahy-drofolate reductase gene in patients with migraine risk. Brain Res Mol Brain Res 111:84–90
Bottini F, Celle ME, Calevo MG, Amato S, Minniti G, Montaldi L, di Pasquale D, Cerone R, Veneselli E, Molinari AC (2006) Metabolic and genetic risk factors for migraine in children. Cephalalgia 26(6):731–737. https://doi.org/10.1111/j.1468-2982.2006.01107.x
Liu A, Menon S, Colson NJ, Quinlan S, Cox H, Peterson M, Tiang T, Haupt LM, Lea RA, Griffiths LR (2010) Analysis of the MTHFR C677T variant with migraine phenotypes. BMC Res Notes 3(1):213. https://doi.org/10.1186/1756-0500-3-213
Pandith AA, Wani IY, Qasim I, Shah ZA, Sheikh S (2017) Evaluation of risk related to MTHFR 677C>T gene polymorphism in migraine patients in Kashmiri population. Open J Prev Med 7(08):151–161. https://doi.org/10.4236/ojpm.2017.78012
Oterino A, Valle N, Bravo Y, Muñoz P, Sánchez-Velasco P, Ruiz-Alegría C et al (2004) MTHFR T677 homozygosis influences the presence of aura in migraineurs. Cephalalgia Int J Headache 24:491–494
Rubino E, Ferrero M, Rainero I, Binello E, Vaula G, Pinessi L (2007) Association of the C677T polymorphism in the MTHFR gene with migraine: a meta-analysis. Cephalalgia 29:818–825
Saeedi S, Owji AA, Ansari M, Ghafarpour M, Ebrahimi A, Fallah MS (2015) MTHFR gene polymorphisms and susceptibility to migraine attacks. Arch Med Lab Sci 1(2):61–66
Ferro A, Castro MJ, Lemos C, Santos M, Sousa A, Pereira-Monteiro J, Sequeiros J, Maciel P (2008) The C677T polymorphism in MTHFR is not associated with migraine in Portugal. Dis Markers 25(2):107–113. https://doi.org/10.1155/2008/178679
Acknowledgements
The authors sincerely thank Dr. Praveen Malik of ESIC Medical College & Hospital, Faridabad, India, for providing samples. Financial support from UGC, New Delhi, India, to Sukhvinder kaur as a UGC-PDF is also highly acknowledged.
Funding
This work was supported by PDF given to Sukhvinder Kaur by Union Grant Commission (UGC), New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kaur, S., Ali, A., Pandey, A.K. et al. Association of MTHFR gene polymorphisms with migraine in North Indian population. Neurol Sci 39, 691–698 (2018). https://doi.org/10.1007/s10072-018-3276-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-018-3276-7