Abstract
Stress causes symptom exacerbation in functional disorders of the urinary bladder. However, the potential mediators and underlying mechanisms of stress effects on micturition reflex function are unknown. We have characterized PACAP (Adcyap1) and PAC1 receptor (Adcyap1r1) signaling in stress-induced urinary bladder dysfunction in mice. We determined PACAP and PAC1 transcripts and protein expressions in the urinary bladder and lumbosacral dorsal root ganglia (DRG) and spinal cord in repeated variate stress (RVS) or control mouse (handling only) groups. RVS in mice significantly (p ≤ 0.01) increased serum corticosterone and urinary bladder NGF content and decreased weight gain. PACAP and PAC1 mRNA and protein were differentially regulated in lower urinary tract tissues with changes observed in lumbosacral DRG and spinal cord but not in urinary bladder. RVS exposure in mice significantly (p ≤ 0.01) increased (2.5-fold) voiding frequency as determined using conscious cystometry. Intrabladder administration of the PAC1 receptor antagonist, PACAP(6-38) (300 nM), significantly (p ≤ 0.01) increased infused volume (1.5–2.7-fold) to elicit a micturition event and increased the intercontraction interval (i.e., decreased voiding frequency) in mice exposed to RVS and in control mice, but changes were smaller in magnitude in control mice. We also evaluated the effect of PAC1 blockade at the level of the urinary bladder on pelvic sensitivity in RVS or control mouse groups using von Frey filament testing. Intrabladder administration of PACAP(6-38) (300 nM) significantly (p ≤ 0.01) reduced pelvic sensitivity following RVS. PACAP/receptor signaling in the CNS and PNS contributes to increased voiding frequency and pelvic sensitivity following RVS and may represent a potential target for therapeutic intervention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Normal micturition involves the filling and storage of urine in the bladder and the periodic voiding of urine at socially appropriate times. Storage and elimination functions involve the reciprocal functions of the bladder, urethra, and external urethral sphincter, which are controlled by the coordination of the structural features of the bladder and complex neural pathways organized in the central nervous system (CNS) and peripheral nervous system (PNS) (Andersson 2004; Elbadawi 1996). Bladder pain syndrome (BPS)/interstitial cystitis (IC) is an urologic, chronic pelvic pain syndrome characterized by pelvic pain, pressure, or discomfort with urinary symptoms (Hanno and Sant 2001). Stress-induced symptom exacerbation (flares) among patients with BPS/IC is well recognized and attributed to multiple triggers (Nickel et al. 2016; Sutcliffe et al. 2014, 2018). Flares are bothersome, disruptive, and associated with increased pelvic pain and urologic symptoms (Nickel et al. 2016; Sutcliffe et al. 2014, 2018). Effective treatments and greater understanding of the stress contribution to BPS/IC are needed.
PACAP peptides are expressed in and exhibit diverse functions in multiple organ systems (e.g., endocrine, nervous, urinary, gastrointestinal, cardiovascular systems) and in the CNS and PNS, including sensory and autonomic ganglia (Arimura 1998; Arimura et al. 1991; Beaudet et al. 1998, 2000; Braas and May 1996, 1999; Braas et al. 1998; Brandenburg et al. 1997; May et al. 1998; May and Braas 1995; Cheppudira et al. 2009; Ghatei et al. 1993; Holgert et al. 1996; Klimaschewski et al. 1996; Koves et al. 1990, 1991; Masuo et al. 1993; Moller et al. 1997a, b; Nogi et al. 1997; Portbury et al. 1995; Shiotani et al. 1995; Sundler et al. 1996; Tatsuno et al. 1994). Neuropeptides are expressed in neural and non-neural components of the lower urinary tract (LUT) including afferent neurons and pathways, plasma, areas of tissue inflammation or injury, bladder fibroblasts, detrusor, and the urothelium. The balance of neuropeptides in LUT pathways can be affected by disease, neural injury, and target organ inflammation and contributes to a hyper- or a hypo-active reflex state of the LUT (Arms and Vizzard 2011). PACAP-IR is expressed in C-fiber bladder afferents in the dorsal root ganglia (DRG) and in nerve fibers within the urinary bladder smooth muscle, suburothelial nerve plexus, and surrounding blood vessels (Fahrenkrug and Hannibal 1998). The urothelium expresses the PACAP receptor, PAC1, and upon PACAP stimulation, the urothelial cells release ATP to stimulate receptors on underlying sensory nerve fibers in the suburothelial plexus (Girard et al. 2008). Mice with a genetic disruption or deletion of PACAP or vasoactive intestinal polypeptide (VIP) exhibit altered bladder and somatic function (May and Vizzard 2010; Studeny et al. 2008; Jensen et al. 2008). PACAP and PAC1 receptor expression exhibits neuroplastic changes in both the neural and non-neural components of the LUT following nerve injury or inflammation (Larsen et al. 1997; Moller et al. 1997a; Vizzard 2000c; Zhang et al. 1995, 1996).
Symptom exacerbation due to stress is prevalent in many disease states, including functional disorders of the urinary bladder (e.g., overactive bladder (OAB), BPS/IC) (Nickel et al. 2016; Sutcliffe et al. 2014; Sutcliffe et al. 2018); however, the mechanisms underlying the effects of stress on the micturition reflex function are unclear. The repeated variate stress (RVS) model has been used previously to examine stress-induced changes in bladder function and pelvic sensation in rats (Girard et al. 2017; Gonzalez et al. 2016; Merrill et al. 2013; Merrill and Vizzard 2014). In this study, we extend the use of the RVS paradigm to mice and examine the role of PACAP/PAC1 signaling to RVS-induced changes in voiding frequency and pelvic sensation. We have characterized PACAP/PAC1 expression and modulation with RVS exposure as well as the effect of PAC1 receptor blockade at the level of the urinary bladder, in RVS-induced urinary bladder dysfunction using biochemical, molecular, and functional approaches. Portions of this study have been presented in abstract (May et al. 2017b, 2019).
Methods
Animals
For these studies, we used a PACAP promoter-dependent EGFP BAC transgenic mice strain (PACAP-EGFP) in which PACAP-expressing cells and neurons are tagged with green fluorescent protein (GFP) for easy visualization and tracking (Condro et al. 2016; May et al. 2015, 2017a). PACAP-EGFP transgenic mice, Tg(Adcyap1-EGFP)FB22Gsat/Mmucd (RRID:IMSR_MMRRC:012011), were generated using the BAC (RP24-358O1) by the Gene Expression Nervous System Atlas (GENSAT) project and obtained from the Mutant Mouse Resource and Research Centers (Condro et al. 2016). The PACAP-EGFP construct is regulated by the endogenous promoter. Therefore, it is possible to examine whether altered physiology, including stress, can differentially regulate specific neuronal PACAP populations in the CNS and PNS. We have examined the effects of CYP-induced cystitis in PACAP promoter-dependent EGFP BAC transgenic mice (May et al. 2015, 2017a). Mice in the current study are also being used to define LUT neural pathways and neural populations in CNS and PNS following RVS with or without characterization of bladder function (May et al. 2017b). In pilot studies, we verified that control female PACAP-EGFP (handled but no stress exposure) mice had bladder capacity and somatic sensitivity similar to that of C57Bl/6 wild-type mice (Vizzard et al., unpublished observations) (Girard et al. 2016). Mice were bred locally at the Larner College of Medicine at the University of Vermont (UVM) animal facilities, and animal genotype was confirmed by PCR analyses. The UVM Institutional Animal Care and Use Committee approved all experimental protocols involving animal (IACUC #08-085, #13-030, #X9-020). The UVM Office of Animal Care Management oversaw all animal use in accordance with AAALAC and NIH guidelines. All efforts were made to minimize the potential for animal pain. Separate cohorts of female PACAP-EGFP mice were used in the following experiments. We used female mice because of the female predominance of IC/BPS (24, 67, 74). Estrous cycle status was not determined for mice in these studies.
RVS
Mice were randomly assigned to control or RVS groups and weighed daily. Mice assigned to the RVS group (hereafter RVS mice) were exposed to 7 days of stressors with a single stressor being presented on each day between 9 am and 12 pm as described previously (Hammack et al. 2009; Merrill et al. 2013; Merrill and Vizzard 2014) (Fig. 1A, B). Control mice were handled daily and remained in home cages in the animal facility following weighing. All mice within the RVS group were exposed to the same order of stressors for the same duration (Fig. 1B):
A Experimental paradigm. Overview of studies using control mice (handled only) and mice exposed to repeated variate stress (RVS) and the two primary outcome measures measured in this study: urinary bladder function and referred somatic sensitivity of the pelvic region. B Repeated variate stress (RVS) protocol (7 days) in the order of stressor presentation. Five different stressors and the duration of each stressor for each day they are administered in the RVS protocol (Longden et al. 2014a, b; Merrill et al. 2013; Merrill and Vizzard 2014) are indicated. Swim and foot shock stressors are repeated on the last 2 days of RVS. hr hour, s seconds, min minutes
Oscillation stress: Mice were placed inside a plastic chamber 25 × 16 × 13 cm (L × W × H), which was secured to a clinical rotator (Fisher Scientific, Morris Plains, NJ) and oscillated at low to medium speed for 30 min (min). Forced swim: Mice were placed in a cylindrical container 29 × 37 cm (D × H) that was filled with room temperature water to a depth that prevented the tail from touching the bottom of the container. After 5 min of monitored swimming, mice were placed in a holding chamber for 30 min prior to being returned to their home cage. Electrical foot shock: Mice were placed inside a Plexiglass conditioning chamber (Med Associates, St. Albans, VT) 30 × 25 × 35 cm (L × W × H). After a 5-min acclimation period, two 0.2-mA, 5-s scrambled foot shocks were delivered through the grid floor with a 1 min intertrial interval. Restraint: Mice were placed in a cylindrical restraining device 30 × 115 mm (D × L) for 60 min. Pedestal: Mice were placed on an elevated (60 cm) platform 20 × 20 cm (L × W) for 30 min.
Euthanasia and Tissue Harvest
Female (control and RVS mice, n = 8 each) mice were deeply anesthetized with isoflurane (5%) and then euthanized via thoracotomy. The urinary bladder and lumbosacral (L6-S1) DRG and spinal cord were quickly dissected under RNase-free conditions as previously described (Girard et al. 2010; Girard et al. 2013). The bladder was cut open along the midline and pinned to a sylgard-coated dish, and the urothelium was removed with the aid of fine forceps and a dissecting microscope. All tissues were snap-frozen on dry ice prior to processing as previously described (Arms et al. 2010).
Real-Time Q-PCR
The urothelium + suburothelium was dissected (Schnegelsberg et al. 2010; Corrow et al. 2010) from the detrusor, and the specificity of the split bladder preparations was examined for the presence of α-smooth muscle actin (1:1000; Abcam, Cambridge, MA) and uroplakin II (1:25; American Research Products, Belmont, MA) by western blotting or Q-PCR (Corrow et al. 2010; Girard et al. 2011, 2013). We determined PACAP and PAC1 transcript expression in the urinary bladder (urothelium + suburothelium, detrusor), lumbosacral (L1, L2, L5-S1) spinal cord, and DRG of control and RVS mice (n = 8 each) using Q-PCR as described (Girard et al. 2010, 2011, 2013).
Measurement of Urinary Bladder, L6-S1 DRG, and Spinal Cord PACAP, PAC1, NGF, or Serum Corticosterone
We determined PACAP and PAC1 (PACAPR1) protein content in the urinary bladder of control (n = 8) or RVS mice (n = 8) using ELISA kits (MBS2503456, PACAP; MBS033808, PACAPR1; MyBioSource, Inc., San Diego, CA); tissue processing and ELISAs were performed as described previously (Vizzard 2000b; Guo et al. 2018). We determined serum corticosterone from control and RVS mice (n = 8 each) using ELISAs (ADI-900-097, Enzo Life Sciences, Farmingdale, NY). One hour after the last stressor of the RVS paradigm (day 7), blood from the abdominal aorta was collected in serum tubes between 9 am and 12 pm and allowed to coagulate at room temperature for 1 h. Samples were centrifuged in an Eppendorf 5415R centrifuge at 10,000 rpm for 10 min. Serum was aliquoted and frozen at – 20 °C until analysis. We determined NGF protein content in the urinary bladder of control (n = 8) and RVS mice (n = 8) using ELISAs (R&D Systems, Minneapolis, MN) as previously described (45, 75, 85). No samples were diluted, and all samples had absorbance values on the linear portion of the standard curve. Curve fitting of standards and evaluation of PACAP, PACAPR1, or corticosterone content of samples were performed using a least squares fit.
Conscious, Open Outlet, Continuous Fill Cystometry
Mice were anesthetized with isoflurane (3–4%), a lower midline abdominal incision was made, and polyethylene tubing (PE-10, Clay Adams, Parsippany, NJ) was inserted into the bladder dome and secured with a nylon purse-string suture (6-zero) (Schnegelsberg et al. 2010; Gonzalez et al. 2013; Girard et al. 2019). The end of the PE tubing was heat flared, but the catheter did not extend into the bladder body or neck, and it was not associated with inflammation or altered cystometric function (Schnegelsberg et al. 2010; Gonzalez et al. 2013; Girard et al. 2019). The distal end of the tubing was sealed, tunneled subcutaneously, and externalized at the back of the neck (Schnegelsberg et al. 2010; Gonzalez et al. 2013; Girard et al. 2019). Abdominal and neck incisions were closed with nylon sutures (6-0). Mice received postoperative analgesics (subcutaneous carprofen, 5.0 mg/kg, once a day for 2 days) and recovered from survival surgery for 72 h before performing cystometry.
For cystometry in conscious mice, an unrestrained animal was placed in a Plexiglas cage with a wire bottom. Before the start of the recording, the bladder was emptied and the catheter was connected via a T-tube to a pressure transducer (Grass Model PT300, West Warwick, RI) and microinjection pump (Harvard Apparatus 22, South Natick, MA). A Small Animal Cystometry Lab Station (MED Associates, Fairfax, VT) was used for urodynamic measurements (Schnegelsberg et al. 2010; Gonzalez et al. 2013; Girard et al. 2019). Saline solution was infused at room temperature into the bladder at a rate of 25 μl/min to elicit repetitive bladder contractions. At least six reproducible micturition cycles were recorded after the initial stabilization period of 25–30 min (Schnegelsberg et al. 2010; Gonzalez et al. 2013; Girard et al. 2019). The following cystometric parameters were recorded in each animal: baseline pressure (pressure at the beginning of the bladder filling), threshold pressure (bladder pressure immediately prior to micturition), peak micturition pressure, intercontraction interval (ICI; time between micturition events), infused volume (IV), and void volume (VV) (Schnegelsberg et al. 2010; Gonzalez et al. 2013; Girard et al. 2019). Mice in these studies had residual volume of less than 10 μl. At the conclusion of the experiment, the mouse was euthanized (5% isoflurane plus thoracotomy).
Conscious Cystometry and Effects of a PAC1 Selective Receptor Antagonist, PACAP(6-38), on Bladder Function in Control and RVS Mice
The effects of PACAP(6-38), a PAC1 selective receptor antagonist, on urinary bladder function in control mice and RVS mice were assessed using conscious, open outlet, cystometry with continuous instillation of intrabladder saline (Girard et al. 2019; Gonzalez et al. 2013; Schnegelsberg et al. 2010; Girard et al. 2016) (Fig. 1A, B). Two groups of mice were evaluated: control mice receiving intrabladder administration of vehicle (0.9% saline) and PACAP(6-38) (n = 8) and RVS mice receiving intrabladder administration of vehicle (0.9% saline) and PACAP(6-38) (n = 8). For intrabladder administration of PACAP(6-38) or vehicle, mice were anesthetized with 2% isoflurane and PACAP(6-38) (< 200 μl) was injected through the bladder catheter; the animals were maintained under anesthesia to prevent expulsion of PACAP(6-38) or vehicle via a voiding reflex. In this procedure, PACAP(6-38) or vehicle remained in the bladder for 30 min at which time, the bladder was drained and washed with saline and animals recovered from anesthesia for 20 min before experimentation. These experiments were performed in the same mice before and after treatment with PACAP(6-38). The concentration (300 nM) of PACAP(6-38) (Bachem, Torrance, CA) used was based upon previous studies (Braas et al. 2006; Girard et al. 2016). To summarize, the experimental design involves administration of a one time, intrabladder infusion of PACAP(6-38) (300 nM) with cystometric data collection occurring ~ 75 min after infusion. At the conclusion of the experiment, the animal was euthanized (5% isoflurane plus thoracotomy). Experiments were conducted at similar times of the day (9 am–12 pm) to avoid the possibility that circadian variations were responsible for changes in bladder capacity measurements (Girard et al. 2019). An individual blinded to treatment or group analyzed the cystometric data; groups were decoded after data analysis.
Exclusion Criteria
Mice were removed from the study when adverse events occurred that included a significant postoperative event, lethargy, pain, or distress not relieved by our IACUC-approved regimen of postoperative analgesics (Cheppudira et al. 2008; Schnegelsberg et al. 2010). In the present study, no mice were excluded from the study. In addition, behavioral movements such as grooming, standing, walking, and defecation rendered bladder pressure recordings during these events unusable.
Mechanical Sensitivity Testing in Control and RVS Mice
Referred (secondary) hyperalgesia was measured by testing the frequency of withdrawal responses to the application of calibrated von Frey monofilaments to the abdominal (Cheppudira et al. 2008; Schnegelsberg et al. 2010; Girard et al. 2019; Girard et al. 2016) region overlying the urinary bladder with PACAP antagonist, PACAP(6-38) (300 nM), or vehicle (0.9% saline) delivered into the bladder (intrabladder) via a transurethral catheter in control and RVS mice (n = 10 each) (Fig. 1A, B) (Girard et al. 2019). Four separate groups (n = 10 each) of mice were evaluated: control mice with vehicle, RVS mice with vehicle, control mice with PACAP(6-38), and RVS mice with PACAP(6-38). For these studies, a transurethral bladder catheterization method was used to avoid the need for an abdominal incision (Girard et al. 2019). In this procedure, PACAP(6-38) (< 200 μl) or vehicle remained in the bladder for 30 min at which time, the bladder was drained and washed with saline, the catheter removed, and animals recovered from anesthesia for 20 min before testing. Mechanical sensitivity assessment was performed using von Frey monofilaments (Stoelting, Wood Dale, IL) with forces of 0.1–4 g applied to the pelvic region (Cheppudira et al. 2008; Schnegelsberg et al. 2010; Girard et al. 2019). All mice were first habituated in a clear acrylic testing chamber 20 min/day for 4 days with a fan to generate ambient noise. On the day of testing, the mice were placed in the acrylic testing chamber on top of a metal mesh floor (IITC Life Science Inc., Woodland Hills, CA) and habituated again for 10 min before the application of von Frey filaments in an up-down method for 1–3 s with a minimum interstimulus interval of 2 min (Cheppudira et al. 2008; Schnegelsberg et al. 2010). The following behaviors were considered positive responses to pelvic region stimulation: sharp retraction of the abdomen, jumping, or immediate licking or scratching of the pelvic area (Cheppudira et al. 2008; Schnegelsberg et al. 2010). Separate cohorts of mice were used for cystometry and somatic sensitivity testing. All mechanical sensitivity testing was performed in a blinded manner. The groups were decoded after data analysis.
Statistical Analyses
All values represent mean ± SEM. Comparisons among experimental groups were made using analysis of variance (ANOVA), repeated measures ANOVA, and paired or unpaired t test where appropriate. When F test statistic exceeded the critical value at α = 0.05, the Sidak’s multiple comparisons test was used to compare group means.
Results
Serum Corticosterone Is Increased, and Body Weight Is Decreased in Mice Exposed to RVS
RVS (7 days) significantly (p ≤ 0.01) increased (4.4-fold) serum corticosterone measured 1 h after the final stressor presented on day 7 compared with control mice (handling only) (Fig. 2A). RVS significantly (p ≤ 0.01) decreased body weight in mice exposed to RVS by day 4 of the RVS paradigm. Significant (p ≤ 0.01) weight reductions were maintained through day 7 of the RVS paradigm (Fig. 2B). RVS significantly (p ≤ 0.01) increased (2.1-fold) whole urinary bladder NGF in RVS mice measured on day 7 of the RVS paradigm (Fig. 2C).
RVS effects on serum corticosterone, body weight, and whole urinary bladder NGF content in mice. A Serum corticosterone significantly increased with RVS exposure measured 1 h after the final day RVS (7 days). B Changes in body weight of mice during 7-day RVS. Beginning on day 4 and lasting for the full duration of 7-day RVS, mice exhibited significant weight gain attenuation compared with control mice. Body weights were significantly (p ≤ 0.01) decreased in the RVS group on days 4–7 of RVS. C Whole urinary bladder nerve growth factor (NGF) content was significantly (p ≤ 0.01) increased following 7-day RVS in mice. Samples size are n = 8. Values are means ± SEM. *p ≤ 0.01
PACAP and PAC1 Transcript and Protein Expression Is Increased in LUT Tissues in Mice Exposed to RVS
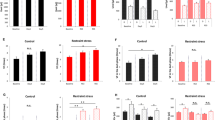
No changes in PACAP transcript expression were observed in urothelium or detrusor from RVS mice (Fig. 3A); however, mice exposed to RVS exhibited significantly (p ≤ 0.01) increased PACAP transcript expression in L1, L2, L6, and S1 dorsal root ganglia (DRG) (Fig. 3B) and S1 spinal cord (SC) (Fig. 3C). No changes in PAC1 transcript expression were observed in urothelium or detrusor smooth muscle or in any DRG level examined (Fig. 3D, E). PAC1 transcript expression was significantly increased in L5 and S1 SC segments with RVS exposure (Fig. 3F). RVS exposure did not affect PACAP or PAC1 protein expression in the whole urinary bladder (Fig. 4A); however, PACAP expression was significantly (p ≤ 0.01) increased in L6 and S1 DRG and spinal cord segments in RVS mice (Fig. 4A). RVS mice also exhibited significantly (p ≤ 0.01) increased PAC1 expression in L6 and S1 DRG (Fig. 4B); lumbosacral spinal cord segments were not examined.
Regulation of PACAP and PAC1 receptor transcript expression in lower urinary tract tissues from control and RVS mice. PACAP (A–C) and PAC1 (D–F) receptor expressions in control (handled only) and RVS-exposed mice in urothelium and detrusor smooth muscle (A, D), lumbosacral (L1, L2, L5-S1) dorsal root ganglia (DRG; B, E), and lumbosacral (L1, L2, L5-S1) spinal cord (SC; C, F). Relative expression of transcripts is expressed as a percentage of control urothelium (A, D), control L1 DRG (B, E), or control L1 SC (C, F) and normalized to the relative expression of the housekeeping gene, L32. Values are means ± SEM. Samples size are n = 8; *p ≤ 0.01 versus control
Regulation of PACAP and PAC1 protein expression in LUT tissues from control and RVS mice. PACAP (A) and PAC1 (B) receptor protein expressions in control (handled only) and RVS-exposed mice in whole urinary bladder, lumbosacral (L6, S1) dorsal root ganglia (DRG), and lumbosacral (L6, S1) spinal cord. No changes in PACAP or PAC1 receptor protein expression were observed in urinary bladder following 7-day RVS; however, PACAP (A) and PAC1 (B) receptor expressions were increased with RVS exposure in lumbosacral DRG and spinal cord. Values are means ± SEM. Samples size are n = 8; *p ≤ 0.01 versus control
PAC1 Receptor Blockade with Intrabladder Infusion of PACAP(6-38) Reduces Voiding Frequency and Increases Functional Bladder Capacity in Mice Exposed to RVS
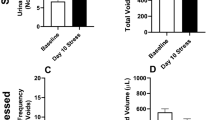
Conscious cystometry with continuous intrabladder infusion of vehicle (0.9% saline) was performed in control (handling only) and RVS-exposed female mice before intrabladder infusion of PACAP(6-38) to establish baseline voiding frequency, intercontraction interval, and infused volume (functional bladder capacity). Baseline voiding characteristics of control female PACAP-EGFP mice were similar to wild-type mice that we have previously characterized (Girard et al. 2019) (Figs. 5A, B and 6A–C). RVS-exposed mice exhibited significant (p ≤ 0.01) reductions (2.5-fold) in the infused volume (IV) of saline required to induce a micturition event (functional bladder capacity) (Figs. 5E, F and 6A) and significant (p ≤ 0.01) reductions in intercontraction interval (ICI;3.1-fold) that resulted in increased voiding frequency (Figs. 5E, F and 6B). This increase in voiding frequency with RVS exposure is consistent with previous studies in rats (Merrill et al. 2013; Merrill and Vizzard 2014). Following intrabladder infusion of the PAC1 receptor antagonist, PACAP(6-38) (300 nM), the same control PACAP-EGFP mice exhibited significantly (p ≤ 0.01) increased IV (1.5-fold) (Figs. 5C, D and 6A) and ICI (1.5-fold) (Figs. 5C, D and 6B). No changes in bladder pressure were observed in control PACAP-EGFP mice treated with PACAP(6-38) (300 nM) (Fig. 6C). The same PACAP-EGFP mice exposed to RVS also exhibited significantly (p ≤ 0.01) increased IV (2.7-fold) (Figs. 5G, H and 6A) and ICI (2.9-fold) (Figs. 5G, H and 6B) and significant (p ≤ 0.01) reduction in baseline (filling) bladder pressure (Fig. 6C) following intrabladder infusion of the PAC1 receptor antagonist, PACAP(6-38) (300 nM). All changes in urinary bladder function with intrabladder instillation of PACAP(6-38) persisted for the duration of the data collection period (~ 75 min).
Representative bladder function recordings from the same control (A–D) PACAP-EGFP or PACAP-EGFP RVS (E–H) mouse before (A, B; E, F) and after intrabladder instillation of PACAP(6-38) (300 nM) (C, D; G, H). A, B Control PACAP-EGFP mice (handling only) infused with vehicle (V) exhibit an infused volume (IV) necessary to elicit a micturition event (i.e., functional bladder capacity) that is similar to wild-type mice previously characterized. C, D After PACAP(6-38) instillation, control mice exhibited a reduction in voiding frequency (i.e., increased IV). E, F Prior to PACAP(6-38) instillation, RVS mice infused with V exhibit reduced infused volume (IV; E, F) required to elicit a micturition that results in increased voiding frequency compared with control mice. G, H After PACAP(6-38) instillation, RVS mice exhibit a reduction in voiding frequency (i.e., increased IV). BP bladder pressure
Summary histograms of infused volume (IV), intercontraction interval (ICI), and bladder pressure (BP) measured from bladder function testing in control (n = 8) and RVS mice (n = 8) before and after intrabladder instillation of PACAP(6-38) (300 nM). RVS mice exhibited significantly (*p ≤ 0.01) reduced IV (A) and ICI (B) compared with control (handled) mice. Intrabladder PACAP(6-38) significantly (*p ≤ 0.01) increased IV (A) and ICI (B) in RVS and control mice. Brackets indicate comparisons made between groups (e.g., control vs. RVS). Asterisks indicate comparisons of treatment with a group (e.g., control vs. control + PACAP(6-38)). C In RVS mice, intrabladder instillation of PACAP(6-38) significantly (*p ≤ 0.01) reduced baseline (i.e., filling) bladder pressure with no effects on threshold pressure or maximum pressure. PACAP(6-38) was without effect on bladder pressure in WT mice. Values are means ± SEM. Samples size are n = 8; *p ≤ 0.01 versus control
PAC1 Receptor Blockade with Intrabladder Infusion of PACAP(6-38) Reduces Pelvic Somatic Sensitivity in Mice Exposed to RVS
Pelvic somatic sensitivity was similar in control female PACAP-EGFP mice treated with intrabladder saline (vehicle) to wild-type mice previously studied (Girard et al. 2019; Schnegelsberg et al. 2010). Female PACAP-EGFP mice exposed to RVS and given intrabladder saline exhibited significantly (p ≤ 0.01) decreased somatic sensitivity in the pelvic region with all monofilament forces evaluated (0.1 to 4 g) (Fig. 7). Intrabladder infusion of PACAP(6-38) (300 nM) significantly (p ≤ 0.01) decreased the somatic sensitivity in the pelvic region in female PACAP-EGFP mice exposed to RVS (Fig. 7) with all monofilament forces evaluated (0.1 to 4 g) but was without effect in control mice (handling only) treated with intrabladder infusion of PACAP(6-38) (300 nM) (Fig. 7).
Effects of RVS on pelvic region sensitivity. Pelvic region testing with calibrated von Frey filaments was determined in control and RVS mice treated with intrabladder vehicle (V) or PAC1 receptor antagonist, PACAP(6-38). Stimulation was confined to the lower abdominal area overlying the urinary bladder. RVS mice (n = 10) had a significantly (*p ≤ 0.01) increased pelvic response frequency with all von Frey hairs (0.1–4 g) tested compared with control mice (n = 10). In RVS mice, intrabladder instillation of PACAP(6-38) significantly (*p ≤ 0.01) reduced pelvic response frequency with all von Frey filaments evaluated. No changes in pelvic sensitivity were observed in control mice (handling) following intrabladder PACAP(6-38) instillation. All somatic testing was performed in a blinded manner. Values are means ± SEM. Samples size are n = 10; *p ≤ 0.01 PACAP-EGFP + RVS + V compared with all other groups. Bracket and asterisk indicate comparison between groups PACAP-EGFP + RVS + V and PACAP-EGFP + RVS + PACAP(6-38)
Discussion
Stress-induced symptom exacerbation or “flare” is common in functional bladder disorders (e.g., OAB, BPS/IC) (Sutcliffe et al. 2014, 2018) and may be partly due to hypothalamic-pituitary-adrenal (HPA) axis dysregulation (Nazif et al. 2007; Westropp and Buffington 2002). The prevalence of micturition disorders is high among individuals with anxiety disorders; however, the mechanisms underlying stress effects on micturition reflex function are unclear. Growing evidence indicates that PACAP (Adcyap1) and the PAC1 receptor (Adcyap1r1) are novel stress mediators (Dore et al. 2013) with PACAP/PAC1 receptor transcripts increased in the limbic system following RVS exposure, CNS PACAP signaling is anxiogenic and anorexic, and PAC1 receptor antagonists block chronic stress-induced anxiety-related behaviors (Hammack et al. 2009; Lezak et al. 2014a, b; Missig et al. 2014; Roman et al. 2014). Furthermore, PACAP/receptor expression and signaling contributes to LUT function and dysfunction (Zvara and Vizzard 2007; Vizzard 2000c; Braas et al. 2006; Girard et al. 2008, 2010, 2012; Herrera et al. 2006): (1) PAC1 receptor is expressed in bladder nerve fibers (Braas et al. 2006) and tissues (Braas et al. 2006; Girard et al. 2010), (2) PACAP facilitates detrusor contractility (Braas et al. 2006) and ATP release from the urothelium (Girard et al. 2008), (3) PACAP and PAC1 mRNA and protein expression are regulated in LUT tissues in mice with chronic urothelial overexpression of NGF (NGF-OE) (Girard et al. 2010) or with CYP-induced cystitis (Girard et al. 2012; Gonzalez et al. 2016), and (4) the PAC1 receptor antagonist, PACAP(6-38), decreases voiding frequency and pelvic sensitivity in NGF-OE mice (Schnegelsberg et al. 2010) and in rats with CYP-induced cystitis (Braas et al. 2006). Thus, we examined whether PACAP/PAC1 signaling at the level of the urinary bladder could affect bladder function and pelvic sensitivity in mice exposed to 7-day repeated variate stress (RVS) that results in increased voiding frequency and somatic sensitivity. Intrabladder administration of PACAP(6-38) (300 nM) significantly (p ≤ 0.01) increased bladder capacity and the intercontraction interval and decreased filling pressure in RVS mice with similar effects (i.e., bladder capacity and ICI) on control mice being significant (p ≤ 0.01) but lesser in magnitude. Intrabladder administration of PACAP(6-38) (300 nM) also significantly (p ≤ 0.01) reduced pelvic sensitivity in RVS mice but was without effect in control mice. These studies add to growing evidence that blockade of urinary bladder PACAP/PAC1 signaling can reduce voiding frequency and pelvic sensitivity regardless of cause: CYP-induced cystitis (May et al. 2017a, b), urothelial overexpression of NGF (NGF-OE) (May et al. 2017a, b), or RVS.
A RVS paradigm, previously used to examine PACAP mRNA expression in the bed nucleus of the stria terminalis (BNST) (Hammack et al. 2009; King et al. 2017), was found to be anxiogenic, most likely mediated by BNST PACAP (Hammack et al. 2009; Lezak et al. 2014a, b; Missig et al. 2014; Roman et al. 2014). The RVS paradigm (Hammack et al. 2009; King et al. 2017) has multiple advantages including the following: (1) lack of habituation with novel stressor exposure, (2) reproducible and robust changes in urinary bladder function (Merrill et al. 2013; Merrill and Vizzard 2014), and (3) reproducible decrease (~ 10–25%) in weight gain during RVS as demonstrated in rats (Merrill et al. 2013; Merrill and Vizzard 2014) and confirmed in mice in the current study. In the current study, we demonstrated that serum corticosterone is increased in RVS mice, compared with control mice (handling only), when measured 1 h after the final stressor. Future studies should determine serum corticosterone levels in the RVS group compared with a control group that receives only the single, last stressor. This approach will determine if increased serum corticosterone reflects 7-day RVS or the single, last stressor. We have previously used the RVS protocol to characterize effects on bladder function and somatic sensitivity (Merrill et al. 2013; Merrill and Vizzard 2014) in rats. RVS in rats (Merrill et al. 2013; Merrill and Vizzard 2014) produced similar changes in bladder function and somatic sensation observed in the present study, including increased urinary frequency and somatic sensitivity of the hindpaw and pelvic region as well as increased NGF content in the urinary bladder. In addition, RVS in rats also caused additional changes in the inflammatory milieu of the urinary bladder including, changes in histamine, myeloperoxidase, and the chemokine, CXCL12 (Merrill et al. 2013; Merrill and Vizzard 2014). Current studies are examining more prolonged periods of RVS (2 or 4 weeks) and potential effects on bladder function and expression of various neuromodulators in LUT pathways. In addition, ongoing studies are using natural voiding assays (Urovoid assays) (Girard et al. 2019), without the need for survival surgery or bladder catheter implant, following daily stressor exposure in the RVS paradigm to assess bladder function.
We have previously examined the expression of PACAP, VIP, and associated receptors in the urinary bladder and lumbosacral DRG in NGF-OE mice (Girard et al. 2010) and in rodents with CYP-induced cystitis (May et al. 2015, 2017a; both animal models exhibit increased voiding frequency. PACAP mRNA expression in the urothelium may reflect expression in urothelial cells, axon terminals, as well as cells in the lamina propria (Ojala et al. 2018; Girard et al. 2017). PACAP mRNA expression in the detrusor may reflect expression in detrusor smooth muscle cells, axon terminals, as well as intramural ganglia (Ojala et al. 2018; Girard et al. 2017). Changes in PACAP, VIP, and associated receptor transcripts and protein expression in micturition pathways of NGF-OE mice resemble some, but not all, changes observed after CYP-induced cystitis (Girard et al. 2008) known to involve NGF production (Vizzard 2000c; Guerios et al. 2008; Klinger and Vizzard 2008; Bjorling et al. 2001). We demonstrated upregulation of PAC1 receptor transcript and PAC1-immunoreactivity (IR) in urothelium of NGF-OE mice, whereas PACAP transcript and PACAP-IR were decreased in urothelium (Girard et al. 2010); no changes in PACAP or associated receptors transcript expression were observed in lumbosacral DRG (Girard et al. 2010). In contrast, enhanced target-derived NGF availability has been shown to increase PACAP expression in small nociceptive neurons in DRG (Jongsma Wallin et al. 2001; Jongsma Wallin et al. 2003). With CYP-induced cystitis (48 h and chronic), PACAP and PAC1 transcript expressions were significantly increased in urinary bladder and L6 DRG (Girard et al. 2008). Consistent with CYP-induced cystitis (Vizzard 2000b), RVS exposure significantly increased urinary bladder NGF content measured at the conclusion of the RVS paradigm. In the present study, a major difference in the regulation of PACAP/PAC1 transcript or protein expression in LUT tissues with RVS exposure is the absence of change in the urinary bladder (urothelium or detrusor), whereas regulation was still observed in lumbosacral DRG and spinal cord. The absence of changes in PACAP/PAC1 regulation in the urinary bladder despite changes in urinary bladder function following RVS exposure may reflect the absence of a direct insult to the urinary bladder as achieved with CYP-induced cystitis (Vizzard 2000a, b, c) and the absence of urothelial transgene expression achieved in NGF-OE mice (Cheppudira et al. 2008; Schnegelsberg et al. 2010). Thus, changes in urinary bladder function as well as increased bladder NGF content may reflect secondary effects mediated by peripheral (lumbosacral DRG) and/or central (lumbosacral spinal cord) inputs. In addition, changes in PAC1 receptor transcript expression in L5 DRG in RVS mice were surprising given the lack of bladder innervation originating from L5. However, upregulation in L5 DRG may reflect the more systemic nature of the RVS. It would be of interest in future studies to determine hindpaw sensitivity in RVS mice. It would also be of interest in future studies to determine combined effects of CYP-induced cystitis or NGF-OE combined with RVS to address stress exacerbation on existing urinary bladder inflammation (Nazif et al. 2007; Westropp and Buffington 2002; Rothrock et al. 2001a, b; Lutgendorf et al. 2000).
In our previous studies using a CYP-induced cystitis model (Braas et al. 2006), the intrathecal or intrabladder administration of PAC1 receptor antagonist, PACAP(6-38), reduced cystitis-induced urinary frequency in rats and the intrabladder administration of PACAP(6-38) reduced voiding frequency in mice (May et al. 2015, 2017a). Intrabladder instillation of PACAP(6-38) similarly reduced voiding frequency in transgenic mice with chronic urothelial overexpression of NGF (NGF-OE). Our current results demonstrating intrabladder PACAP(6-38) reductions in urinary frequency in RVS mice are consistent with these previous studies. Although the site(s) of action of PACAP(6-38) in the current study is not known, PACAP(6-38) may affect PACAP/PAC1 signaling at the urothelium, in peripheral nerve terminals of DRG cells located in close proximity to the urothelium with demonstrated PAC1 expression (Braas et al. 2006). However, reductions in baseline (i.e., filling) pressure with intrabladder instillation of PACAP(6-38) suggest that detrusor smooth muscle cells may also be potential targets. The urothelium exhibits high transepithelial resistance (TER), and the effects of RVS on TER have not been studied; thus, the potential of PACAP(6-38) to reach the detrusor is unknown making the urothelium and adjacent nerve terminals more likely sites of action. Cold immobilization stress in rats (Ercan et al. 2001) produces morphological changes in the urinary bladder including urothelial barrier disruption; thus, future studies should consider changes in urothelial structure and function as an underlying mechanism contributing to increased voiding frequency with RVS.
Patients with disorders of the LUT and associated disease states report a worsening of symptoms during stress (Nazif et al. 2007; Westropp and Buffington 2002). A majority of these patients report exacerbation of symptoms by clinical stress (Rothrock et al. 2001a, b), and experimental stress increases bladder pain and urgency in these individuals (Lutgendorf et al. 2000). In addition, animal models of stress demonstrate symptoms of bladder dysfunction (e.g., increased micturition frequency, urgency, pain) as well as anxiety-like behavior (Birder and Andersson 2013) that may be due, in part, to disruption of the HPA axis (Pierce and Christianson 2015). Cortisol, by feeding back on the HPA axis, normally acts to attenuate inflammation; however, abnormalities in the feedback loop may cause dysregulation of the inflammatory response. Children exposed to chronic early life stress (ELS) (Taylor 2010) are at significant risk of developing HPA abnormalities (Pierce and Christianson 2015) (Fuentes and Christianson 2018), and individuals with functional bladder disorders have a higher incidence of ELS or trauma (Pierce and Christianson 2015; Fuentes and Christianson 2018). Therefore, patients with bladder dysfunction disorders may have abnormalities in the HPA axis, and stress could be attributed to the worsening of bladder symptoms (Nazif et al. 2007; Westropp and Buffington 2002).
Neural mechanisms and pathways linking psychosocial stress to altered behaviors and physiological disorders are still unclear. We are using PACAP-EGFP transgenic mice (Condro et al. 2016) in this study to map specific neuronal PACAP populations in the CNS and PNS and to determine if this transgenic mouse model can be used to examine and compare changes in PACAP populations with RVS and/or RVS and bladder function testing as suggested for other manipulations (e.g., facial nerve axotomy) (Condro et al. 2016). With 7-day RVS followed by bladder function testing, numbers of PACAP-EGFP+ cells increased dramatically, compared with RVS alone, in lumbosacral spinal cord segments and DRG involved in micturition reflexes, urothelial cells, and supraspinal locations including the following: locus coeruleus, Barrington’s nucleus, rostral ventrolateral medulla, PAG, raphe, and amygdala (May et al. 2019). These supraspinal regions, in turn, can project to and receive input from the HPA axis which may be a component of the anatomical substrates connecting stress, bladder function, and HPA regulation/dysregulation (Fuentes and Christianson 2018; Grundy et al. 2018; Pierce and Christianson 2015). Additional studies are needed to understand how stress or the inability to attenuate stress signaling can result in maladaptations and cumulative long-term damages (i.e., increased HPA and sympathetic reactivity) that can manifest a variety of disorders including those involving the urinary bladder (Grundy et al. 2018; Pierce and Christianson 2015; FitzGerald et al. 2009; Lovallo 2013; Mahon et al. 2013; Marshall and Garakani 2002; Mihaljevic et al. 2016; Turner-Cobb 2005).
References
Andersson KE (2004) Mechanisms of disease: central nervous system involvement in overactive bladder syndrome. Nat Clin Pract Urol 1(2):103–108. https://doi.org/10.1038/ncpuro0021
Arimura A (1998) Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. Jpn J Physiol 48(5):301–331
Arimura A, Somogyvari-Vigh A, Miyata A, Mizuno K, Coy DH, Kitada C (1991) Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes. Endocrinology 129(5):2787–2789. https://doi.org/10.1210/endo-129-5-2787
Arms L, Vizzard MA (2011) Neuropeptides in lower urinary tract function. Handb Exp Pharmacol 202:395–423. https://doi.org/10.1007/978-3-642-16499-6_19
Arms L, Girard BM, Vizzard MA (2010) Expression and function of CXCL12/CXCR4 in rat urinary bladder with cyclophosphamide-induced cystitis. Am J Physiol Ren Physiol 298(3):F589–F600. https://doi.org/10.1152/ajprenal.00628.2009
Beaudet MM, Braas KM, May V (1998) Pituitary adenylate cyclase activating polypeptide (PACAP) expression in sympathetic preganglionic projection neurons to the superior cervical ganglion. J Neurobiol 36(3):325–336
Beaudet MM, Parsons RL, Braas KM, May V (2000) Mechanisms mediating pituitary adenylate cyclase-activating polypeptide depolarization of rat sympathetic neurons. J Neurosci 20(19):7353–7361
Birder L, Andersson KE (2013) Urothelial signaling. Physiol Rev 93(2):653–680. https://doi.org/10.1152/physrev.00030.2012
Bjorling DE, Jacobsen HE, Blum JR, Shih A, Beckman M, Wang ZY, Uehling DT (2001) Intravesical Escherichia coli lipopolysaccharide stimulates an increase in bladder nerve growth factor. BJU Int 87(7):697–702
Braas KM, May V (1996) Pituitary adenylate cyclase-activating polypeptides, PACAP-38 and PACAP-27, regulation of sympathetic neuron catecholamine, and neuropeptide Y expression through activation of type I PACAP/VIP receptor isoforms. Ann N Y Acad Sci 805:204–216 discussion 217–208
Braas KM, May V (1999) Pituitary adenylate cyclase-activating polypeptides directly stimulate sympathetic neuron neuropeptide Y release through PAC(1) receptor isoform activation of specific intracellular signaling pathways. J Biol Chem 274(39):27702–27710
Braas KM, May V, Harakall SA, Hardwick JC, Parsons RL (1998) Pituitary adenylate cyclase-activating polypeptide expression and modulation of neuronal excitability in guinea pig cardiac ganglia. J Neurosci 18(23):9766–9779
Braas KM, May V, Zvara P, Nausch B, Kliment J, Dunleavy JD, Nelson MT, Vizzard MA (2006) Role for pituitary adenylate cyclase activating polypeptide in cystitis-induced plasticity of micturition reflexes. Am J Physiol Regul Integr Comp Physiol 290(4):R951–R962. https://doi.org/10.1152/ajpregu.00734.2005
Brandenburg CA, May V, Braas KM (1997) Identification of endogenous sympathetic neuron pituitary adenylate cyclase-activating polypeptide (PACAP): depolarization regulates production and secretion through induction of multiple propeptide transcripts. J Neurosci 17(11):4045–4055
Cheppudira BP, Girard BM, Malley SE, Schutz KC, May V, Vizzard MA (2008) Upregulation of vascular endothelial growth factor isoform VEGF-164 and receptors (VEGFR-2, Npn-1, and Npn-2) in rats with cyclophosphamide-induced cystitis. Am J Physiol Ren Physiol 295(3):F826–F836. https://doi.org/10.1152/ajprenal.90305.2008
Cheppudira BP, Girard BM, Malley SE, Dattilio A, Schutz KC, May V, Vizzard MA (2009) Involvement of JAK-STAT signaling/function after cyclophosphamide-induced bladder inflammation in female rats. Am J Physiol Ren Physiol 297(4):F1038–F1044. https://doi.org/10.1152/ajprenal.00110.2009
Condro MC, Matynia A, Foster NN, Ago Y, Rajbhandari AK, Van C, Jayaram B, Parikh S, Diep AL, Nguyen E, May V, Dong HW, Waschek JA (2016) High-resolution characterization of a PACAP-EGFP transgenic mouse model for mapping PACAP-expressing neurons. J Comp Neurol 524(18):3827–3848. https://doi.org/10.1002/cne.24035
Corrow K, Girard BM, Vizzard MA (2010) Expression and response of acid-sensing ion channels in urinary bladder to cyclophosphamide-induced cystitis. Am J Physiol Ren Physiol 298(5):F1130–F1139. https://doi.org/10.1152/ajprenal.00618.2009
Dore R, Iemolo A, Smith KL, Wang X, Cottone P, Sabino V (2013) CRF mediates the anxiogenic and anti-rewarding, but not the anorectic effects of PACAP. Neuropsychopharmacology 38(11):2160–2169. https://doi.org/10.1038/npp.2013.113
Elbadawi A (1996) Functional anatomy of the organs of micturition. Urol Clin N Am 23(2):177–210
Ercan F, Oktay S, Erin N (2001) Role of afferent neurons in stress induced degenerative changes of the bladder. J Urol 165(1):235–239. https://doi.org/10.1097/00005392-200101000-00070
Fahrenkrug J, Hannibal J (1998) Pituitary adenylate cyclase activating polypeptide immunoreactivity in capsaicin-sensitive nerve fibres supplying the rat urinary tract. Neuroscience 83(4):1261–1272
FitzGerald LZ, Kehoe P, Sinha K (2009) Hypothalamic-pituitary-adrenal axis dysregulation in women with irritable bowel syndrome in response to acute physical stress. West J Nurs Res 31(7):818–836. https://doi.org/10.1177/0193945909339320
Fuentes IM, Christianson JA (2018) The influence of early life experience on visceral pain. Front Syst Neurosci 12:2. https://doi.org/10.3389/fnsys.2018.00002
Ghatei MA, Takahashi K, Suzuki Y, Gardiner J, Jones PM, Bloom SR (1993) Distribution, molecular characterization of pituitary adenylate cyclase-activating polypeptide and its precursor encoding messenger RNA in human and rat tissues. J Endocrinol 136(1):159–166
Girard BM, Wolf-Johnston A, Braas KM, Birder LA, May V, Vizzard MA (2008) PACAP-mediated ATP release from rat urothelium and regulation of PACAP/VIP and receptor mRNA in micturition pathways after cyclophosphamide (CYP)-induced cystitis. J Mol Neurosci 36(1–3):310–320. https://doi.org/10.1007/s12031-008-9104-4
Girard BM, Malley SE, Braas KM, May V, Vizzard MA (2010) PACAP/VIP and receptor characterization in micturition pathways in mice with overexpression of NGF in urothelium. J Mol Neurosci 42(3):378–389. https://doi.org/10.1007/s12031-010-9384-3
Girard BM, Malley SE, Vizzard MA (2011) Neurotrophin/receptor expression in urinary bladder of mice with overexpression of NGF in urothelium. Am J Physiol Ren Physiol 300(2):F345–F355. https://doi.org/10.1152/ajprenal.00515.2010
Girard BM, Tompkins JD, Parsons RL, May V, Vizzard MA (2012) Effects of CYP-induced cystitis on PACAP/VIP and receptor expression in micturition pathways and bladder function in mice with overexpression of NGF in urothelium. J Mol Neurosci 48(3):730–743. https://doi.org/10.1007/s12031-012-9834-1
Girard BM, Merrill L, Malley S, Vizzard MA (2013) Increased TRPV4 expression in urinary bladder and lumbosacral dorsal root ganglia in mice with chronic overexpression of NGF in urothelium. J Mol Neurosci 51(2):602–614. https://doi.org/10.1007/s12031-013-0033-5
Girard BM, Malley SE, Mathews MM, May V, Vizzard MA (2016) Intravesical PAC1 receptor antagonist, PACAP(6-38), reduces urinary bladder frequency and pelvic sensitivity in NGF-OE mice. J Mol Neurosci 59(2):290–299. https://doi.org/10.1007/s12031-016-0764-1
Girard BM, Tooke K, Vizzard MA (2017) PACAP/receptor system in urinary bladder dysfunction and pelvic pain following urinary bladder inflammation or stress. Front Syst Neurosci 11:90. https://doi.org/10.3389/fnsys.2017.00090
Girard BM, Campbell SE, Perkins M, Hsiang H, Tooke K, Drescher C, Hennig GW, Heppner TJ, Nelson MT, Vizzard MA (2019) TRPV4 blockade reduces voiding frequency, ATP release, and pelvic sensitivity in mice with chronic urothelial overexpression of NGF. Am J Physiol Ren Physiol 317(6):F1695–F1706. https://doi.org/10.1152/ajprenal.00147.2019
Gonzalez EJ, Girard BM, Vizzard MA (2013) Expression and function of transforming growth factor-beta isoforms and cognate receptors in the rat urinary bladder following cyclophosphamide-induced cystitis. Am J Physiol Ren Physiol 305(9):F1265–F1276. https://doi.org/10.1152/ajprenal.00042.2013
Gonzalez EJ, Girard B, Braas KM, May V, Vizzard MA (2016) Neuroplasticity of PACAP expression and function in micturition reflex pathways. In: Reglodi D, Tamas A (eds) Pituitary adenylate cyclase activating polypeptide — PACAP. Springer International Publishing, Cham, pp 313–334. https://doi.org/10.1007/978-3-319-35135-3_19
Grundy L, Caldwell A, Brierley SM (2018) Mechanisms underlying overactive bladder and interstitial cystitis/painful bladder syndrome. Front Neurosci 12:931. https://doi.org/10.3389/fnins.2018.00931
Guerios SD, Wang ZY, Boldon K, Bushman W, Bjorling DE (2008) Blockade of NGF and trk receptors inhibits increased peripheral mechanical sensitivity accompanying cystitis in rats. Am J Physiol Regul Integr Comp Physiol 295(1):R111–R122. https://doi.org/10.1152/ajpregu.00728.2007
Guo M, Chang P, Hauke E, Girard BM, Tooke K, Ojala J, Malley SM, Hsiang H, Vizzard MA (2018) Expression and function of chemokines CXCL9-11 in micturition pathways in cyclophosphamide (CYP)-induced cystitis and somatic sensitivity in mice. Front Syst Neurosci 12:9. https://doi.org/10.3389/fnsys.2018.00009
Hammack SE, Cheung J, Rhodes KM, Schutz KC, Falls WA, Braas KM, May V (2009) Chronic stress increases pituitary adenylate cyclase-activating peptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST): roles for PACAP in anxiety-like behavior. Psychoneuroendocrinology 34(6):833–843. https://doi.org/10.1016/j.psyneuen.2008.12.013
Hanno PM, Sant GR (2001) Clinical highlights of the National Institute of Diabetes and Digestive and Kidney Diseases/Interstitial Cystitis Association Scientific Conference on Interstitial Cystitis. Urology 57(6 Suppl 1):2–6
Herrera GM, Braas KM, May V, Vizzard MA (2006) PACAP enhances mouse urinary bladder contractility and is upregulated in micturition reflex pathways after cystitis. Ann N Y Acad Sci 1070:330–336. https://doi.org/10.1196/annals.1317.040
Holgert H, Holmberg K, Hannibal J, Fahrenkrug J, Brimijoin S, Hartman BK, Hokfelt T (1996) PACAP in the adrenal gland--relationship with choline acetyltransferase, enkephalin and chromaffin cells and effects of immunological sympathectomy. Neuroreport 8(1):297–301
Jensen DG, Studeny S, May V, Waschek J, Vizzard MA (2008) Expression of phosphorylated cAMP response element binding protein (p-CREB) in bladder afferent pathways in VIP−/− mice with cyclophosphamide (CYP)-induced cystitis. J Mol Neurosci 36(1–3):299–309. https://doi.org/10.1007/s12031-008-9045-y
Jongsma Wallin H, Danielsen N, Johnston JM, Gratto KA, Karchewski LA, Verge VM (2001) Exogenous NT-3 and NGF differentially modulate PACAP expression in adult sensory neurons, suggesting distinct roles in injury and inflammation. Eur J Neurosci 14(2):267–282
Jongsma Wallin H, Pettersson LM, Verge VM, Danielsen N (2003) Effect of anti-nerve growth factor treatment on pituitary adenylate cyclase activating polypeptide expression in adult sensory neurons exposed to adjuvant induced inflammation. Neuroscience 120(2):325–331
King SB, Toufexis DJ, Hammack SE (2017) Pituitary adenylate cyclase activating polypeptide (PACAP), stress, and sex hormones. Stress:1–11. https://doi.org/10.1080/10253890.2017.1336535
Klimaschewski L, Hauser C, Heym C (1996) PACAP immunoreactivity in the rat superior cervical ganglion in comparison to VIP. Neuroreport 7(15–17):2797–2801
Klinger MB, Vizzard MA (2008) Role of p75NTR in female rat urinary bladder with cyclophosphamide-induced cystitis. Am J Physiol Ren Physiol 295(6):F1778–F1789. https://doi.org/10.1152/ajprenal.90501.2008
Koves K, Arimura A, Somogyvari-Vigh A, Vigh S, Miller J (1990) Immunohistochemical demonstration of a novel hypothalamic peptide, pituitary adenylate cyclase-activating polypeptide, in the ovine hypothalamus. Endocrinology 127(1):264–271. https://doi.org/10.1210/endo-127-1-264
Koves K, Arimura A, Gorcs TG, Somogyvari-Vigh A (1991) Comparative distribution of immunoreactive pituitary adenylate cyclase activating polypeptide and vasoactive intestinal polypeptide in rat forebrain. Neuroendocrinology 54(2):159–169
Larsen JO, Hannibal J, Knudsen SM, Fahrenkrug J (1997) Expression of pituitary adenylate cyclase-activating polypeptide (PACAP) in the mesencephalic trigeminal nucleus of the rat after transsection of the masseteric nerve. Brain Res Mol Brain Res 46(1–2):109–117
Lezak KR, Roelke E, Harris OM, Choi I, Edwards S, Gick N, Cocchiaro G, Missig G, Roman CW, Braas KM, Toufexis DJ, May V, Hammack SE (2014a) Pituitary adenylate cyclase-activating polypeptide (PACAP) in the bed nucleus of the stria terminalis (BNST) increases corticosterone in male and female rats. Psychoneuroendocrinology 45:11–20. https://doi.org/10.1016/j.psyneuen.2014.03.007
Lezak KR, Roman CW, Braas KM, Schutz KC, Falls WA, Schulkin J, May V, Hammack SE (2014b) Regulation of bed nucleus of the stria terminalis PACAP expression by stress and corticosterone. J Mol Neurosci 54(3):477–484. https://doi.org/10.1007/s12031-014-0269-8
Longden TA, Dabertrand F, Hill-Eubanks DC, Hammack SE, Nelson MT (2014a) Stress-induced glucocorticoid signaling remodels neurovascular coupling through impairment of cerebrovascular inwardly rectifying K+ channel function. Proc Natl Acad Sci U S A 111(20):7462–7467. https://doi.org/10.1073/pnas.1401811111
Longden TA, Hammack SE, Nelson MT (2014b) Channeling stress: inwardly-rectifying K+ channels in stress and disease. Channels (Austin) 8(4):296–297
Lovallo WR (2013) Early life adversity reduces stress reactivity and enhances impulsive behavior: implications for health behaviors. Int J Psychophysiol 90(1):8–16. https://doi.org/10.1016/j.ijpsycho.2012.10.006
Lutgendorf SK, Kreder KJ, Rothrock NE, Ratliff TL, Zimmerman B (2000) Stress and symptomatology in patients with interstitial cystitis: a laboratory stress model. J Urol 164(4):1265–1269
Mahon PB, Zandi PP, Potash JB, Nestadt G, Wand GS (2013) Genetic association of FKBP5 and CRHR1 with cortisol response to acute psychosocial stress in healthy adults. Psychopharmacology 227(2):231–241. https://doi.org/10.1007/s00213-012-2956-x
Marshall RD, Garakani A (2002) Psychobiology of the acute stress response and its relationship to the psychobiology of post-traumatic stress disorder. Psychiatr Clin North Am 25(2):385–395
Masuo Y, Suzuki N, Matsumoto H, Tokito F, Matsumoto Y, Tsuda M, Fujino M (1993) Regional distribution of pituitary adenylate cyclase activating polypeptide (PACAP) in the rat central nervous system as determined by sandwich-enzyme immunoassay. Brain Res 602(1):57–63
May V, Braas KM (1995) Pituitary adenylate cyclase-activating polypeptide (PACAP) regulation of sympathetic neuron neuropeptide Y and catecholamine expression. J Neurochem 65(3):978–987
May V, Vizzard MA (2010) Bladder dysfunction and altered somatic sensitivity in PACAP−/− mice. J Urol 183(2):772–779. https://doi.org/10.1016/j.juro.2009.09.077
May V, Beaudet MM, Parsons RL, Hardwick JC, Gauthier EA, Durda JP, Braas KM (1998) Mechanisms of pituitary adenylate cyclase activating polypeptide (PACAP)-induced depolarization of sympathetic superior cervical ganglion (SCG) neurons. Ann N Y Acad Sci 865:164–175
May V, Mathews ME, Malley S., Girard BM, Braas KM, Waschek JA, Vizzard MA (2015) Pituitary adenylate cyclase-activating polypeptide (PACAP) expression in lower urinary tract pathways (LUT) with cyclophosphamide (CYP)-induced cystitis in PACAP promoter-dependent EGFP BAC transgenic mice. 12th International Symposium on VIP, PACAP and Related Peptdies
May V, Mathews ME, Torres NS, McQuesten J, Chang PL, Hauke E, Ojala J, Malley S, Girard BM, Braas KM, Waschek JA, Vizzard MA (2017a) Pituitary adenylate cyclase-activating polypeptide (PACAP) expression in lower urinary tract pathways (LUT) Following cyclophosphamide (CYP)-induced cystitis in PACAP promoter-dependent EGFP BAC transgenic mice. 13th International Symposium on VIP, PACAP and Related Peptdies
May V, Mathews ME, Torres NS, McQuesten J, Chang PL, Hauke E, Ojala J, Malley S, Girard BM, Braas KM, Waschek JA, Vizzard MA (2017b) Role of PACAP/PAC1 signaling in micturition reflexes and somatic sensitivity following repeated variate stress (RVS) in PACAP promoter-dependent EGFP BAC transgenic mice. 12th International Symposium on VIP, PACAP and Related Peptdies
May V, Ojala J, Tooke K, Perkins M, Hsiang H, Campbell SE, Girard BM, Braas KM, Waschek JA, Vizzard MA (2019) PACAP/Receptor Mechanisms Contribute To Micturition Dysfunction With Repeated Variate Stress (RVS) In Mice PACAP/receptor mechanisms contribute to micturition dysfunction with repeated variate stress (RVS) in mice. Akira Arimura Memorial VIP/PACAP and Related Peptides Symposium: 30 years after PACAP Discovery
Merrill L, Vizzard MA (2014) Intravesical TRPV4 blockade reduces repeated variate stress-induced bladder dysfunction by increasing bladder capacity and decreasing voiding frequency in male rats. Am J Physiol Regul Integr Comp Physiol 307(4):R471–R480. https://doi.org/10.1152/ajpregu.00008.2014
Merrill L, Malley S, Vizzard MA (2013) Repeated variate stress in male rats induces increased voiding frequency, somatic sensitivity, and urinary bladder nerve growth factor expression. Am J Physiol Regul Integr Comp Physiol 305(2):R147–R156. https://doi.org/10.1152/ajpregu.00089.2013
Mihaljevic M, Zeljic K, Soldatovic I, Andric S, Mirjanic T, Richards A, Mantripragada K, Pekmezovic T, Novakovic I, Maric NP (2016) The emerging role of the FKBP5 gene polymorphisms in vulnerability-stress model of schizophrenia: further evidence from a Serbian population. Eur Arch Psychiatry Clin Neurosci. https://doi.org/10.1007/s00406-016-0720-7
Missig G, Roman CW, Vizzard MA, Braas KM, Hammack SE, May V (2014) Parabrachial nucleus (PBn) pituitary adenylate cyclase activating polypeptide (PACAP) signaling in the amygdala: implication for the sensory and behavioral effects of pain. Neuropharmacology 86:38–48. https://doi.org/10.1016/j.neuropharm.2014.06.022
Moller K, Reimer M, Ekblad E, Hannibal J, Fahrenkrug J, Kanje M, Sundler F (1997a) The effects of axotomy and preganglionic denervation on the expression of pituitary adenylate cyclase activating peptide (PACAP), galanin and PACAP type 1 receptors in the rat superior cervical ganglion. Brain Res 775(1–2):166–182
Moller K, Reimer M, Hannibal J, Fahrenkrug J, Sundler F, Kanje M (1997b) Pituitary adenylate cyclase-activating peptide (PACAP) and PACAP type 1 receptor expression in regenerating adult mouse and rat superior cervical ganglia in vitro. Brain Res 775(1–2):156–165
Nazif O, Teichman JM, Gebhart GF (2007) Neural upregulation in interstitial cystitis. Urology 69(4 Suppl):24–33. https://doi.org/10.1016/j.urology.2006.08.1108
Nickel JC, Stephens A, Landis JR, Mullins C, van Bokhoven A, Lucia MS, Ehrlich GD, Network MR (2016) Assessment of the lower urinary tract microbiota during symptom flare in women with urologic chronic pelvic pain syndrome: a MAPP Network Study. J Urol 195(2):356–362. https://doi.org/10.1016/j.juro.2015.09.075
Nogi H, Hashimoto H, Hagihara N, Shimada S, Yamamoto K, Matsuda T, Tohyama M, Baba A (1997) Distribution of mRNAs for pituitary adenylate cyclase-activating polypeptide (PACAP), PACAP receptor, vasoactive intestinal polypeptide (VIP), and VIP receptors in the rat superior cervical ganglion. Neurosci Lett 227(1):37–40
Ojala J, Tooke K, Hsiang H, Girard BM, May V, Vizzard MA (2018) PACAP/PAC1 expression and function in micturition pathways. J Mol Neurosci. https://doi.org/10.1007/s12031-018-1170-7
Pierce AN, Christianson JA (2015) Stress and chronic pelvic pain. Prog Mol Biol Transl Sci 131:509–535. https://doi.org/10.1016/bs.pmbts.2014.11.009
Portbury AL, McConalogue K, Furness JB, Young HM (1995) Distribution of pituitary adenylyl cyclase activating peptide (PACAP) immunoreactivity in neurons of the guinea-pig digestive tract and their projections in the ileum and colon. Cell Tissue Res 279(2):385–392
Roman CW, Lezak KR, Hartsock MJ, Falls WA, Braas KM, Howard AB, Hammack SE, May V (2014) PAC1 receptor antagonism in the bed nucleus of the stria terminalis (BNST) attenuates the endocrine and behavioral consequences of chronic stress. Psychoneuroendocrinology 47:151–165. https://doi.org/10.1016/j.psyneuen.2014.05.014
Rothrock NE, Lutgendorf SK, Kreder KJ, Ratliff T, Zimmerman B (2001a) Stress and symptoms in patients with interstitial cystitis: a life stress model. Urology 57(3):422–427
Rothrock NE, Lutgendorf SK, Kreder KJ, Ratliff TL, Zimmerman B (2001b) Daily stress and symptom exacerbation in interstitial cystitis patients. Urology 57(6 Suppl 1):122
Schnegelsberg B, Sun TT, Cain G, Bhattacharya A, Nunn PA, Ford AP, Vizzard MA, Cockayne DA (2010) Overexpression of NGF in mouse urothelium leads to neuronal hyperinnervation, pelvic sensitivity, and changes in urinary bladder function. Am J Physiol Regul Integr Comp Physiol 298(3):R534–R547. https://doi.org/10.1152/ajpregu.00367.2009
Shiotani Y, Kimura S, Ohshige Y, Yanaihara C, Yanaihara N (1995) Immunohistochemical localization of pituitary adenylate cyclase-activating polypeptide (PACAP) in the adrenal medulla of the rat. Peptides 16(6):1045–1050
Studeny S, Cheppudira BP, Meyers S, Balestreire EM, Apodaca G, Birder LA, Braas KM, Waschek JA, May V, Vizzard MA (2008) Urinary bladder function and somatic sensitivity in vasoactive intestinal polypeptide (VIP)−/− mice. J Mol Neurosci 36(1–3):175–187. https://doi.org/10.1007/s12031-008-9100-8
Sundler F, Ekblad E, Hannibal J, Moller K, Zhang YZ, Mulder H, Elsas T, Grunditz T, Danielsen N, Fahrenkrug J, Uddman R (1996) Pituitary adenylate cyclase-activating peptide in sensory and autonomic ganglia: localization and regulation. Ann N Y Acad Sci 805:410–426 discussion 427–418
Sutcliffe S, Colditz GA, Goodman MS, Pakpahan R, Vetter J, Ness TJ, Andriole GL, Lai HH (2014) Urological chronic pelvic pain syndrome symptom flares: characterisation of the full range of flares at two sites in the Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network. BJU Int 114(6):916–925. https://doi.org/10.1111/bju.12778
Sutcliffe S, Jemielita T, Lai HH, Andriole GL, Bradley CS, Clemens JQ, Gallop R, Hooton TM, Kreder KJ, Krieger JN, Kusek JW, Labus J, Lucia MS, Mackey S, Naliboff BD, Robinson NA, Rodriguez LV, Stephens-Shields A, van Bokhoven A, Wolin KY, Yan Y, Yang CC, Landis JR, Colditz GA, Network MR (2018) A case-crossover study of urological chronic pelvic pain syndrome flare triggers in the MAPP Research Network. J Urol 199(5):1245–1251. https://doi.org/10.1016/j.juro.2017.12.050
Tatsuno I, Somogyvari-Vigh A, Arimura A (1994) Developmental changes of pituitary adenylate cyclase activating polypeptide (PACAP) and its receptor in the rat brain. Peptides 15(1):55–60
Taylor SE (2010) Mechanisms linking early life stress to adult health outcomes. Proc Natl Acad Sci U S A 107(19):8507–8512. https://doi.org/10.1073/pnas.1003890107
Turner-Cobb JM (2005) Psychological and stress hormone correlates in early life: a key to HPA-axis dysregulation and normalisation. Stress 8(1):47–57. https://doi.org/10.1080/10253890500095200
Vizzard MA (2000a) Alterations in spinal cord Fos protein expression induced by bladder stimulation following cystitis. Am J Physiol Regul Integr Comp Physiol 278(4):R1027–R1039
Vizzard MA (2000b) Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp Neurol 161(1):273–284. https://doi.org/10.1006/exnr.1999.7254
Vizzard MA (2000c) Up-regulation of pituitary adenylate cyclase-activating polypeptide in urinary bladder pathways after chronic cystitis. J Comp Neurol 420(3):335–348
Westropp JL, Buffington CA (2002) In vivo models of interstitial cystitis. J Urol 167(2 Pt 1):694–702
Zhang Q, Shi TJ, Ji RR, Zhang YZ, Sundler F, Hannibal J, Fahrenkrug J, Hokfelt T (1995) Expression of pituitary adenylate cyclase-activating polypeptide in dorsal root ganglia following axotomy: time course and coexistence. Brain Res 705(1–2):149–158
Zhang YZ, Hannibal J, Zhao Q, Moller K, Danielsen N, Fahrenkrug J, Sundler F (1996) Pituitary adenylate cyclase activating peptide expression in the rat dorsal root ganglia: up-regulation after peripheral nerve injury. Neuroscience 74(4):1099–1110
Zvara P, Vizzard MA (2007) Exogenous overexpression of nerve growth factor in the urinary bladder produces bladder overactivity and altered micturition circuitry in the lumbosacral spinal cord. BMC Physiol 7:9. https://doi.org/10.1186/1472-6793-7-9
Acknowledgments
The authors thank Dr. James A. Waschek for his generous gift of transgenic PACAP-EGFP mice breeding pairs. The authors also gratefully acknowledge the research efforts of summer undergraduate research students, some of whom were supported by a NINDS Summer Research Experience in Neuroscience for Undergraduates (R25 NS090623) through the Summer Undergraduate Neuroscience Fellowship Program at UVM. Student researchers included William (Trey) Walker, Michelle Hernandez, Hina Rattu, Neysharie Sánchez-Torres, Jenna McQuesten, Morgan Mathews, Diana Grinberg, Phat (Tony) Chan, Eric Hauke, and Gabrielle Krumgalz.
Funding
This work was funded by National Institutes of Health (NIH) grants DK051369 (MAV), DK060481 (MAV), DK065989 (MAV), and DK120108 (MAV). This publication was also supported by grants from the National Center for Research Resources (5 P30 RR 032135) and the National Institute of General Medical Sciences (8 P30 GM 103498) from the NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The UVM Institutional Animal Care and Use Committee approved all experimental protocols involving animal (IACUC #08-085, #13-030, #X9-020). The UVM Office of Animal Care Management oversaw all animal use in accordance with AAALAC and NIH guidelines.
Conflict of Interest
The authors declare that they have no conflict of interest.
Disclaimer
The funding entity, NIH, had no role in the studies described including the following: design, data collection and analysis of studies performed in the Vizzard laboratory, decision to publish, or the preparation of the manuscript. The contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Girard, B.M., Campbell, S.E., Beca, K.I. et al. Intrabladder PAC1 Receptor Antagonist, PACAP(6-38), Reduces Urinary Bladder Frequency and Pelvic Sensitivity in Mice Exposed to Repeated Variate Stress (RVS). J Mol Neurosci 71, 1575–1588 (2021). https://doi.org/10.1007/s12031-020-01649-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-020-01649-x