Abstract
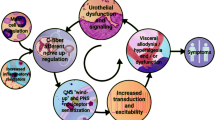
Micturition, the storage and periodic elimination of urine, requires a complex neural control system that coordinates the activities of the smooth muscle of the urinary bladder and urethra and the smooth and striated muscle of the urethral sphincters. The lower urinary tract (LUT) reflex mechanisms, organized at the level of the lumbosacral spinal cord, are modulated predominantly by supraspinal controls. Complex neural organization is necessary for the coordination of the reciprocal functions of the urinary bladder, urethra, and urethral sphincters to result in normal micturition function. Injury or diseases of the nervous system, as well as disorders of the peripheral organs, can produce LUT dysfunction. Numerous neuropeptide/receptor systems are expressed in central and peripheral nervous system pathways that regulate the LUT and expression can also be found in both neural and non-neural (e.g., urothelium) components. Pituitary adenylate cyclase-activating polypeptide (PACAP; Adcyap1) and its cognate receptor, PAC1 (Adcyap1r1), have tissue-specific distributions in diverse systems including the LUT. PACAP and associated receptors exhibit neurophenotypic changes with neural injury, inflammation, stress, and disease of the LUT. Changes in the balance of the PACAP/receptor system in central and peripheral bladder reflex pathways may underlie and/or contribute to LUT dysfunction including urinary urgency, increased voiding frequency, nocturia, urinary incontinence, detrusor dyssynergia, and/or pain. The PACAP/receptor system in LUT pathways may thus represent a potential target for therapeutic intervention.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Lower urinary tract
- Spinal cord
- Dorsal root ganglia
- Urinary bladder
- Neurochemistry
- Urothelium
- Detrusor smooth muscle
- Nerve growth factor
- Knockout mice
- Cystitis
- Spinal cord injury
Introduction
Micturition Reflex Pathways to the Urogenital Tract
Micturition is organized between two modes of operation: storage and elimination. During storage, somatosympathetic excitatory inputs to the urethral sphincters and sympathetic inputs to the bladder wall are tonically active [1, 2]. In contrast, during reflexive or voluntary elimination, parasympathetic inputs to the urinary bladder wall are active whereas somatosympathetic inputs to the bladder wall and urethral sphincters are inhibited [3]. Although spinal reflexes underlie most of the storage phase, reflexive or voluntary micturition reflex mechanisms are modulated by supraspinal regulation in the pontine micturition center [4].
Slowly adapting mechanoreceptors in the urinary bladder wall underlie the switch from storage to elimination [5]. The thinly myelinated Aδ afferent fibers of the hypogastric and pelvic nerves increase their activity as hydrostatic pressure rises [6]. Bladder afferent nerves that terminate peripherally in the urinary bladder may also signal through unmyelinated C-fibers that respond to nociceptive stimulation by chemicals, inflammation, and elevated intravesical pressures [7–9]. Although C-fibers are quiescent during normal bladder filling, their activation may contribute to the development of lower urinary tract (LUT) symptoms and functional disorders of the urinary bladder [10, 11].
Bladder afferent fibers from the pelvic nerve project into Lissauer’s tract where collateral branches extend along the superficial laminae of the dorsal horn (DH) [2, 8, 12] (Fig. 19.1). The ventromedial collateral branch follows the medial edge of the DH (i.e., medial collateral pathways) into the dorsal commissure (DCM) and receives inputs from the pudendal nerve and urogenital structures [2, 8, 12]. The ventrolateral collateral branch (i.e., lateral collateral pathway, LCP) projects on the lateral edge (lamina I) of the DH into the sacral parasympathetic nucleus (SPN) that contains preganglionic parasympathetic neurons projecting to the periphery [2, 8, 12] (Fig. 19.1). In addition to synapsing directly on preganglionic parasympathetic neurons in some species, primary bladder afferent fibers also synapse on interneurons in the lumbosacral DCM, superficial DH, and SPN [2, 6]. These interneurons project locally in the spinal cord or to supraspinal cortical modulatory centers and are important in the normal micturition reflex [2, 6].
Wiring diagram of micturition reflexes emphasizing the reflex elements that express PACAP-IR. The PACAP/receptor system has been identified in micturition reflex pathways with contributions to normal LUT function as well as that after neural injury, disease, or inflammation. PACAP-IR is expressed in normal LUT pathways but expression is dramatically increased following injury, disease, and inflammation of the urinary bladder (see text for details). Robust PACAP-IR is expressed in lumbosacral DRG (2), spinal cord (1), including the superficial laminae of the DH (1) and the LCP of Lissauer’s tract (white arrows, 1). Bladder afferent cells in the DRG, retrogradely labeled with the conventional tracer, Fast Blue (FB), express PACAP-IR (white arrows, 2). Not all PACAP-IR cells in the lumbosacral DRG are bladder afferent cells (blue arrows, 2). Not all presumptive bladder afferent cells expressing FB, also exhibit PACAP-IR (yellow arrow, 2). PACAP-IR is also present in the urinary bladder including expression in urothelial cells that line the urinary bladder and in nerve fibers of the suburothelial nerve plexus (3). Postganglionic neurons in the major pelvic ganglia (MPG) also express PACAP-IR (not shown). INT interneurons, PGN preganglionic neurons, SPN sacral parasympathetic nucleus, CC central canal, PMC pontine micturition center, EUS external urethral sphincter
Urothelial Signaling
The urothelium lines the bladder mucosa and responds to mechanical, chemical, and thermal stimuli [13]. In response to these stimuli, urothelial cells secrete factors like urinary proteins and signaling molecules suggesting a role in urinary bladder sensory transduction [13, 14]. Urothelial cells also express receptors and mechanosensitive channels to respond to the extracellular environment [15–18]. Given that the urothelium may have a sensory influence on micturition reflex function, any disruption to urothelial signaling mechanisms and/or the underlying neural network may contribute to pathological conditions of the urinary bladder [13].
Neurochemistry of Micturition Pathways
Bladder afferent fibers contain a variety of neuropeptides, including calcitonin-gene related peptide (CGRP), substance P (SP), neurokinin A, neurokinin B, vasoactive intestinal polypeptide (VIP), pituitary adenylate cyclase-activating polypeptide (PACAP), cholecystokinin, and enkephalins [12, 19–24] (Fig. 19.1). With the exception of CGRP, all of these substances are predominantly expressed in small diameter (presumably C-fiber) afferents [12, 19, 20, 25–32]. The administration of capsaicin, which acts selectively on small-diameter afferents to deplete neurotransmitter stores, reduces the levels of SP, neurokinin A, and CGRP but not VIP or enkephalin within the pelvic viscera [33]. These findings are consistent with SP, CGRP, and related tachykinins expression in afferent pathways to the pelvic viscera [33]. The following sections focus on the expression, distribution, and functional plasticity of members of the VIP–secretin–glucagon family of hormones, PACAP and VIP in micturition reflex pathways (Fig. 19.1). The contributions of other peptides to micturition reflex pathways have recently been described [24].
Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) and VPAC/PAC1 Receptor Signaling
PACAP belongs to the VIP/secretin/glucagon family of bioactive peptides and was isolated from hypothalami based on its stimulation of anterior pituitary adenylyl cyclase (AC) activity [34, 35]. The rat PACAP precursor protein consists of 175 amino acid residues with posttranslational processing resulting in two ∝-amidated forms, PACAP38 and PACAP27 [34, 36–39]. PACAP38 has 38 amino acid residues, whereas PACAP27 has the carboxyl terminus truncated and exhibits 68 % homology to VIP [35, 37, 40]. The distribution of these two forms is tissue-specific with PACAP38 typically predominating expression in most tissues [34, 41]. PACAP38 remains identical among mammalian species suggesting similar physiologically important roles such as signaling modulation and trophic functions in the nervous and endocrine systems [34, 42].
There are three distinct G-protein-coupled receptors for PACAP and VIP: PAC1, VPAC1, and VPAC2 [43–48] (Fig. 19.2). PAC1 receptors exhibit high affinity for PACAP and display unique patterns of AC and phospholipase C (PLC) activation for PACAP27 or PACAP38 [48–52]. The potency of PACAP27 and PACAP38 to PAC1 receptors is affected by alternative splicing to receptor transcripts resulting in the presence (short) or absence (very-short) of a 21-residue insert into the amino-terminal extracellular domain [53] (Fig. 19.2). Other variants from the alternative splicing of two 84 base pair HIP and HOP cassettes result in the unique patterns of AC and PLC activation [48] (Fig. 19.2). VPAC1 and VPAC2 receptors, on the other hand, exhibit high affinity for both PACAP and VIP and are solely coupled to AC [48]. The expression of PAC1 and VPAC receptors is tissue- and cell type-specific. It was previously shown that rat superior cervical ganglia sympathetic neurons express PAC1(short)HOP1 while VPAC receptors were sparsely expressed in ganglion non-neuronal cells [49–51, 54–60].
LUT tissues express PAC1 receptor variants. Complementary DNA templates were prepared from rat S1 spinal cord, S1 DRG, and bladder detrusor and urothelium total RNA. The region spanning the alternative splice site for the HIP and HOP exons within the third cytoplasmic loop was amplified using PACAPR1/2 oligonucleotide primers. Six third cytoplasmic loop isoform fragments containing neither, one or both HIP and HOP cassettes can potentially be amplified with these primers. LUT tissues express PAC1 receptor isoforms in a tissue-specific manner. S1 DRG express predominantly the one cassette isoform; other tissues possess both the null and the one cassette variant. Schematic shading: Dark grey, short region containing exons 4 and 5; light grey, HIP exon cassette; black, HOP cassette. Thick line, region amplified using PACAPR1/2 primers. LUT tissue expression of PAC1 receptor isoforms also results from alternative splicing in amino-terminal extracellular domain. Complementary DNA templates from LUT samples described above were amplified using primers PACAPR3/4, which flank the amino-terminal extracellular domain splice site. The amplified fragments of indicated sizes represent isoforms with both (short) or neither (very short) exons 4 and 5. All LUT tissues express the short variant; urinary detrusor smooth muscle also demonstrates very short PAC1 receptor expression. Shading in schematic denotes alternatively spliced exons. Thick line, region amplified using primers PACAR3/4. Figure modified from ref. [98]
PACAP and PAC1 Receptor Neuronal Functions in the LUT
PACAP peptides have diverse functions in endocrine, nervous, gastrointestinal, and cardiovascular systems and are expressed in many central nervous system neurons and sensory and autonomic ganglia [34, 36, 41, 42, 61–76]. PACAP facilitates neuronal calcium influx, induces depolarization of the membrane, activates AC and PLC, and stimulates neurotransmitter secretion [49, 50, 54, 55, 58–60, 77–79]. Widespread PACAP-immunoreactivity (IR) has been demonstrated in nerve fibers within the urinary bladder smooth muscle, suburothelial plexus and surrounding blood vessels [80] (Fig. 19.1). Neonatal capsaicin treatment significantly reduced PACAP suggesting these fibers are derived from sensory neurons [80]. These results are consistent with the expression of PACAP in DRG and its neurochemical plasticity following nerve injury or inflammation [42, 81–83] (Fig. 19.1).
PACAP- and VIP-Mediated Effects on Urothelium and Detrusor Smooth Muscle
The urothelium acts as a selective barrier to prevent urinary constituents from penetrating the underlying tissue [84, 85]. A disruption to the properties of barrier function may occur through trauma, infection or disorders affecting the bladder like bladder pain syndrome/interstitial cystitis (BPS/IC) or spinal cord injury (SCI) [86, 87]. It has been suggested that this loss of barrier integrity contributes to the altered sensory processing observed in cystitis. Recent studies have demonstrated that the urothelium expresses PAC1 receptors that upon stimulation release ATP to stimulate receptors on underlying sensory nerve fibers [88] (Fig. 19.2). ATP release was evoked by PACAP27, PACAP38, and VIP application to cultured urothelial cells with PACAP27 and PAC1 receptor antagonism blocking ATP release [88]. These results suggest PACAP and PAC1 signaling may regulate micturition reflex function at the level of the urothelium [88] (Figs. 19.1 and 19.2).
PACAP and VIP have direct effects on smooth muscle cells. PACAP or VIP elicit relaxation of guinea pig stomach, rat ileum, rabbit iris sphincter and dilator muscles, cat and human esophageal sphincter, and human and guinea pig airways but elicit contraction in guinea pig ileum and gall bladder [89]. The effects of PACAP on urinary bladder smooth muscle have not been well described despite PACAP, PAC1, and VPAC expression in nerve fibers within the detrusor [80] (Figs. 19.1 and 19.2). PACAP27 has a small effect on isolated bladder smooth muscle strips even though it facilitates micturition in conscious, open outlet rats [90]. These studies, however, did not take into account PAC1 and VPAC receptor cross talk and the peptide selectivity of various PAC1 isoforms.
Unlike PACAP, VIP is expressed in postganglionic efferent neurons of the major pelvic ganglia (MPG) and minimally innervates the urinary bladder [80, 91–93]. VIP administration to the detrusor smooth muscle had no effect on spontaneous or carbachol-induced bladder contractions but intrathecal or intra-arterial VIP administration facilitated micturition [94]. These conflicting roles may result from VIP receptor distribution varying across species and target tissue [94–97]. Taken together, it appears that PACAP/receptor signaling has more influence on micturition reflex function than VIP/receptor signaling [94–98].
PACAP and VIP Expression and Effects on MPG Neurons
PACAP/receptor expression has been demonstrated in the MPG, ganglia that supply autonomic (sympathetic and parasympathetic) innervation to the urinary bladder, following neuronal injury. PAC1, VPAC1, and VPAC2 transcripts were reported in the MPG in cell culture for 4 h [99], with only VPAC2 transcript significantly increased by day 3 [100]. PACAP transcript and PACAP-IR similarly increased in the MPG by day 3 in culture [100]. Unlike PACAP, VIP transcript expression remained unchanged in a 3-day culture [100]. Furthermore, the application of VIP, PACAP, and maxadilan, a PAC1-selective agonist, increased neuronal excitability and decreased after-hyperpolarization in the MPG [99]. Taken together, these studies suggest PACAP/receptor signaling in the MPG may have a role in micturition reflex function following injury (Fig. 19.1).
PACAP or VIP Knockout Mice Exhibit Altered Micturition Reflexes
Bladder dysfunction and altered somatic sensation have previously been demonstrated in mice with a genetic disruption or deletion to PACAP or VIP. PACAP (+/−) and PACAP (−/−) mice display less somatic sensitivity to mechanical stimuli in the pelvic and hindpaw regions relative to controls [101]. On the other hand, VIP (−/−) mice display increased somatic sensitivity in the pelvis and decreased paw pressure threshold following inflammation [102]. These dissimilarities may reflect distinct roles for VIP and PACAP in bladder sensory function. In contrast to the observed mechanosensitivity differences, both knockout mice exhibited an increase in bladder mass with hypertrophy specific to the lamina propria and detrusor smooth muscle in PACAP (−/−) mice and only the detrusor smooth muscle in VIP (−/−) mice [101, 103].
Functionally, PACAP (−/−) mice have increased bladder capacity, void volume, and longer intercontraction intervals relative to controls [101]. It has been argued that these mice exhibit partial outlet obstruction because of extended and incomplete emptying of the bladder [101]. VIP (−/−) mice, however, do not functionally present with changes in the basal tone of the bladder but do have increased void volume, longer intercontraction intervals, and complete emptying of the bladder [102]. Bladder dysfunction in VIP (−/−) mice may result from functional changes within the bladder itself or neuroplasticity among bladder afferent cells. Along with the increase in tissue mass, the urinary bladders of VIP (−/−) mice have increased urea permeability, increased basal expression of NGF and an exaggerated proinflammatory response to inflammation [102–104]. Additionally, there are elevated basal levels of phosphorylated cAMP response-element binding protein (pCREB) in lumbosacral (L1, L2, L5–S1) DRG and in L6 and S1 afferent neurons projecting directly from the bladder of VIP (−/−) mice suggesting elevated afferent activity of the urinary bladder [103].
Neuroplasticity of PACAP/Receptor Expression and Function with Cystitis
The regulation of transcript and peptide expression of PACAP and its receptors previously demonstrated with cyclophosphamide (CYP)-induced cystitis has been suggested to underlie the development of urinary bladder dysfunction. Following a downregulation in transcript expression after acute (4 h) CYP-induced cystitis, PACAP transcript expression is dramatically upregulated in the urothelium and L6 and S1 DRG after intermediate (48 h) or chronic (10 day) CYP-induced cystitis [88]. Similarly, transcript expression of the PAC1 receptor is down-regulated in the urothelium, detrusor smooth muscle and L6 and S1 DRG after acute (4 h) CYP-induced cystitis, but upregulated in the urothelium and detrusor smooth muscle after intermediate (48 h) or chronic (10 day) CYP-induced cystitis [88]. VPAC1 and VPAC2 transcript expression, however, remains upregulated in the urothelium and detrusor smooth muscle from acute (4 h) CYP-induced cystitis to intermediate (48 h) CYP-induced cystitis with the down-regulation of VPAC2 transcript expression occurring after chronic (10 day) CYP-induced cystitis [88]. PACAP-IR in the spinal cord is restricted to nerve fibers and is increased in micturition reflex associated regions after intermediate (48 h) or chronic (10 day) CYP-induced cystitis [22, 105]. Following intermediate or chronic CYP-induced cystitis, PACAP-IR is increased in the superficial laminae (I-II) of the DH, medial to lateral extent of the dorsal horn, LCP of Lissauer, SPN, and S1 spinal segments [22, 105] (Fig. 19.1). Additionally, the percentage of Fast Blue labeled bladder afferent cells positive for PACAP increased in L1-L2, L6, and S1 DRG following chronic (10 day) CYP-induced cystitis [22] (Fig. 19.1).
The aforementioned regulation of PACAP and its receptors in areas associated with the micturition reflex suggests this neuropeptide may have a role in bladder dysfunction with inflammation (Fig. 19.2). In support of this, the intrathecal (L6-S1) or intravesical administration of a PAC1 receptor antagonist, PACAP6-38, was able to increase bladder capacity but not intravesical pressure with intermediate (48 h) CYP-induced cystitis [98]. The different routes of administration with similar functional effects suggest PACAP6-38 may have multiple sites of action. Administration of PACAP6-38 at the level of the spinal cord may be acting on superficial DH neurons to block PACAP release from C-fiber afferents, whereas, PACAP at the level of the urinary bladder may be acting on urothelial, suburothelial or detrusor smooth muscle cells [98] (Fig. 19.1). Despite not yet knowing its specific site of action, the inhibition of aberrant PACAP signaling seems to be a promising target to reduce voiding frequency with cystitis.
PACAP Expression in LUT with CYP-Induced Cystitis in PACAP Promoter-Dependent EGFP BAC Transgenic Mice
We previously demonstrated an upregulation of PACAP expression in rodent micturition pathways following CYP-induced cystitis [98]. We subsequently examined the effects of CYP-induced cystitis (4 h, 48 h, chronic) in PACAP promoter-dependent EGFP BAC transgenic mice [106]. We induced bladder inflammation in adult mice by injecting CYP intraperitoneally to produce acute (150 mg/kg; 4 h), intermediate (150 mg/kg; 48 h), and chronic (75 mg/kg; every third day for 10 days) cystitis. In control (no inflammation) animals, low basal expression of PACAP-EGFP+ fibers was present in the superficial DH at all segmental levels examined (L1, L2, L4–S1). Dorsal root ganglia (DRG; L1, L2, L6, S1) from control animals also exhibited PACAP-EGFP+ cells. After CYP-induced cystitis, PACAP-EGFP+ cells increased dramatically in spinal segments and DRG (L1, L2, L6, and S1) involved in micturition reflexes. Small diameter, PACAP-EGFP+ DRG cells co-localized with TRPV1- and TRPV4-IR [106]. The density of PACAP-EGFP+ nerve fibers was increased in the superficial laminae (I–II) of the L1, L2, L6, and S1 DH. No changes in PACAP-EGFP+ nerve fibers were observed in the L4–L5 segments. PACAP-EGFP+ nerve fibers also increased in the lateral collateral pathway in L6–S1 spinal cord. Following CYP-induced cystitis, PACAP-EGFP+ urothelial cells were observed and the number of PACAP-EGFP+ urothelial cells increased with duration of cystitis. PACAP-EGFP+ urothelial cells were co-localized with TRPV4-IR [106]. Changes in PACAP expression in LUT pathways after cystitis may play a role in altered visceral sensation (allodynia) and/or increased voiding frequency in the chronic inflammatory pain syndrome, interstitial cystitis/bladder pain syndrome.
Neuroplasticity of PACAP/Receptor Expression and Function with Spinal Cord Injury (SCI)
SCI has been demonstrated to regulate the transcript and peptide expression of PACAP and its receptors within the spinal cord and urinary bladder. An increase in PACAP and PAC1 receptor transcript expression is observed in the spinal cord following a moderate compression model of SCI [107]. PACAP- and PAC1-immunoreactive cells are also increased around the site of injury and co-localized with NeuN-positive cells (i.e., neuronal marker) [107]. In other studies utilizing spinal cord (Thoracic (T)7–T9) transection, PACAP-IR is increased 6 weeks after SCI in micturition reflex associated regions [108]. Within the upper lumbar (L1–L2) spinal cord, PACAP-IR increased in the superficial laminae (I–II) of the DH, medial to lateral extent of the DH, and a fiber bundle extending laterally from Lissauer’s tract [108]. Similarly within the lumbosacral (L6–S1) spinal cord, PACAP-IR increased in the DH, DCM, SPN, and LCP of Lissauer [108]. Increased PACAP-IR is not limited to the spinal cord, but the percentage of PACAP-positive bladder afferent cells labeled with Fast Blue are also increased in L1–L2, L6, and S1 DRG 48 h to 6 weeks after SCI [108]. Unlike the increased PACAP-IR observed at the level of the spinal cord and DRG, the urinary bladder has decreased PACAP-IR in the urothelium and detrusor smooth muscle from 5 days to 3 weeks after SCI [108].
The regulation of PACAP and its receptors around the site of SCI and micturition reflex regions suggests this neuropeptide may have a protective role to help facilitate bladder function. In support of this, the intrathecal administration of PACAP-38 following transection of the T8–T9 spinal cord resulted in large amplitude and long duration bladder contractions under isovolumetric conditions [109]. Additionally, intrathecal administration of a PAC1 receptor antagonist, PACAP6-38, following transection of the T8–T10 spinal cord reduced filling, threshold and peak micturition pressures, number and amplitude of non-voiding contractions, and had shorter intercontraction intervals [110]. Taken together, these studies suggest PACAP may act on parasympathetic efferent pathways at the level of the spinal cord and/or DRG [109]. Aside from its possible role in bladder function, PACAP may also be protecting from a loss of motor function. PACAP (+/−) mice showed a greater injury volume surrounding SCI and also exhibited lower Basso Mouse Scale motor scores on days 3, 7, and 14 suggesting impaired motor function [107]. These studies demonstrate the significance of PACAP regulation following SCI and argue for a role of PACAP in both bladder and somatomotor function with injury.
Role of Nerve Growth Factor (NGF) and Associated Receptors in LUT Plasticity
Cytokines and growth factors, including NGF, are upregulated at the site of tissue injury, inflammation, and/or target organ hypertrophy [111–115]. Following noxious peripheral stimulation, for example, levels of neuroactive compounds (e.g., enkephalin [112], dynorphin [116], CGRP [115, 117, 118], SP [23, 112, 116, 118], neuropeptide Y [112]; neuronal nitric oxide synthase (nNOS) [28, 119, 120] and PACAP [22, 121]) have been demonstrated to increase in DRG and spinal cord neurons. NGF, in particular, is also released from the target organ for tyrosine kinase receptor (Trk) type 1 (TrkA) binding and retrograde transport in DRG afferent neurons [122]. The subsequent increase in NGF expression within the DRG neurons may induce increased production of neuropeptides (i.e., SP, CGRP, and PACAP) and alter sensory transduction [115, 117, 118]. In addition, a large percentage of pelvic visceral afferent neurons express neurotrophic factor receptors, including Trk for NGF and related substances [123–126]. Following cystitis or SCI, neurotrophic factor receptors exhibit neuroplastic increases in TrkA- and TrkB-IR and Trk phosphorylation in bladder afferent neurons [125, 126].
CYP-Induced Cystitis
Increases in the number of TrkA-immunoreactive cell profiles were detected in the L1 and L6 DRG (fourfold) and the S1 DRG (1.5-fold) but not in the L2, L4, and L5 DRG with CYP-induced cystitis of acute and chronic duration compared with control rats [125]. The number of TrkB-IR cell profiles increased in the L1 and L2 DRG (L1: 2.6-fold; L2: 1.4-fold) and in the L6 and S1 DRG (L6: 2.2-fold; S1: 1.3-fold) only after acute CYP treatment (8 h) [125]. After CYP treatment, the percentage of bladder afferent cell profiles expressing TrkA-IR (~50 %) increased in L1 and L6 DRG. The percentage of bladder afferent cell profiles expressing TrkB-IR (~45 %) in L1, L2, L6, and S1 DRG also increased compared with control cell profiles [125]. The increase in TrkA-IR in bladder afferent cells occurred 8 h after CYP treatment and was maintained in L1 DRG with chronic (10 days) CYP-induced cystitis. However, the increase in bladder afferent cells expressing TrkB-IR only occurred at the most acute time point examined (8 h). TrkA-IR and TrkB-IR cell profiles also demonstrated phosphorylated Trk-IR with acute and/or chronic CYP-induced cystitis [125].
Spinal Cord Injury (SCI)
After SCI, a significant increase in the number of TrkB-immunoreactive cells was also detected in the L6–S1 DRG and in the L1–L2 DRG but not in the L4–L5 DRG compared with control rats [127]. After SCI, the percentage of FB-labeled cells expressing TrkA- or TrkB-IR in L1 and L6 DRG significantly increased compared with control DRG. After SCI, the percentage of TrkA-immunoreactive cells expressing phosphorylated (p)-Trk-IR significantly increased (1.5- to 2.3-fold increase) in the L1, L6, and S1 DRG. The percentage of TrkB-immunoreactive cells expressing p-Trk-IR after SCI also increased (1.3-fold increase) in the L1 and L6 DRG [127]. These results demonstrate that (1) TrkA- and TrkB-IR is increased in bladder afferent cells after SCI and (2) TrkA and TrkB receptors are phosphorylated in DRG after SCI. Neuroplasticity of LUT reflexes after SCI may be mediated by both NGF and brain-derived neurotrophic factor in target tissues [127].
NGF and PACAP Interactions
Recent reports have demonstrated reciprocal regulatory interactions between NGF and PACAP in rat pheochromocytoma (PC)12 cells and in DRG cells. Recent studies [128] have implicated NGF as a positive regulator of PACAP expression in nociceptive DRG cells. In rat PC12 cells, both NGF and PACAP can induce PC differentiation into a neuronal phenotype [129]. Upon PC12 transfection of a PACAP promoter-luciferase construct, exogenously applied PACAP and NGF, added either alone or in combination, upregulated PACAP gene expression [130, 131]. In addition, the neurotrophins can also facilitate expression of the PACAP-selective PAC1 receptor. NGF upregulated the PAC1 receptor promoter in PC12 cells; both NGF and BDNF induced PAC1 receptor promoter activity and mRNA expression in cerebellar granule cells [132].
Conversely, PACAP has also been shown to upregulate TrkA and TrkB receptor expression and/or phosphorylation in PC12 cells and hippocampal neurons, respectively in a Src-dependent manner [133]. Studies with sympathetic neuroblasts also demonstrated that PACAP can augment TrkA and TrkC expression in the neuronal differentiation process [134]. The ability for the PACAP and NGF signaling pathways to demonstrate reciprocal regulatory processes may be a primary example of an important feed-forward mechanism to amplify a trophic survival or differentiation response during neuronal development or regeneration. In bladder inflammation or other pathophysiological events, the same feed-forward mechanism may present complications and exacerbate dysfunction.
Transgenic Mouse Model with Chronic Urothelial Overexpression of NGF (NGF-OE)
Our laboratory has characterized a transgenic mouse model of urothelium-specific, NGF-OE that represents a novel approach to exploring the role of NGF in urinary bladder inflammation and sensory function [135]. Functionally, NGF-OE mice exhibit urinary bladder hyperreflexia with frequent urination and the presence of non-voiding bladder contractions as well as referred somatic pelvic hypersensitivity [135]. No changes in the electrical properties of the MPG neurons of NGF-OE mice were detected using intracellular recording, suggesting that the urinary bladder phenotype in NGF-OE mice is not influenced by changes in the efferent limb of the micturition reflex. NGF-OE mice may represent a useful animal model of BPS/IC because the changes observed in the urinary bladders of these mice are consistent with certain changes observed in this syndrome. Pleiotropic changes, subsequent to NGF-OE, including changes in the expression of growth factors, neuroactive compounds, and ion channels (e.g., transient receptor potential (TRP) channels) [136, 137] can also directly modulate pain and bladder/visceral sensory function and could contribute to altered urinary bladder function in NGF-OE mice [11, 137–139].
Recent studies also demonstrate changes in PACAP/VIP and receptor expression in micturition pathways in NGF-OE mice [140]. Results demonstrate upregulation of PAC1 receptor transcript and PAC1-IR in urothelium of NGF-OE mice whereas PACAP transcript and PACAP-IR were decreased in urothelium of NGF-OE mice [140]. In contrast, VPAC1 receptor transcript was decreased in both urothelium and detrusor smooth muscle of NGF-OE mice [140]. VIP transcript expression and VIP-IR was not altered in urinary bladder of NGF-OE mice [140]. Changes in PACAP, VIP and associated receptors transcripts and peptide expression in micturition pathways resemble some, but not all, changes observed after induction of urinary bladder inflammation known to involve NGF production.
Contributions of PACAP/Receptor Signaling to Increased Voiding Frequency and Somatic Sensitivity in NGF-OE
Given the presence of PAC1-IR fibers, the expression of PAC1 receptor expression in bladder tissues, and the abilities of PACAP to facilitate detrusor contractility, whether PACAP/receptor signaling contributes to bladder hyperreflexia and somatic sensitivity was recently evaluated [141]. Intravesical administration of PACAP6-38 (300 nM) significantly increased bladder capacity (2.0-fold), intercontraction interval and void volume in NGF-OE mice. Intravesical instillation of PACAP6-38 also decreased filling and peak micturition pressure in NGF-OE mice [141]. PACAP6-38 had no effects on WT mice. Intravesical administration of PACAP6-38 (300 nM) significantly reduced pelvic sensitivity in NGF-OE mice but was without effect in WT mice. PACAP/receptor signaling contributes to the increased voiding frequency and pelvic sensitivity observed in NGF-OE mice [141].
We have extended these studies to address the contribution of target-derived NGF in combination with CYP-induced cystitis to determine whether additional changes in neuropeptides/receptors are observed in micturition reflex pathways due to the presence of additional inflammatory mediators in the urinary bladder [142]. Quantitative polymerase chain reaction (PCR) was used to determine PACAP/ VIP, SP, galanin, and receptor transcript expression in the urinary bladder (urothelium, detrusor) in NGF-OE mice and wild type (WT) mice with CYP-induced cystitis (4 h, 48 h, and chronic) [142]. With CYP-induced cystitis (4 h), WT and NGF-OE mice exhibited similar changes in galanin transcript expression in the urothelium (30-fold increase) and detrusor (threefold increase). In contrast, PACAP, VIP, and SP transcripts exhibited differential changes in WT and NGF-OE with CYP-induced cystitis. PAC1, VPAC1, and VPAC2 transcript expression also exhibited differential responses in NGF-OE mice that were tissue (urothelium vs. detrusor) and CYP-induced cystitis duration-dependent [142]. Using conscious cystometry, NGF-OE mice treated with CYP exhibited significant increases in voiding frequency above that observed in control NGF-OE mice [142]. These studies are consistent with target-derived NGF and other inflammatory mediators affecting neurochemical plasticity and the reflex function of micturition pathways.
We now have determined whether additional changes in neuropeptides/receptors and growth factor/receptors are observed in the urinary bladder (urothelium, detrusor) and lumbosacral dorsal root ganglia (DRG) involved in micturition reflexes in NGF-OE mice with CYP-induced cystitis (Girard and Vizzard, unpublished observations). Quantitative PCR was used to determine NGF, BDNF, VEGF, and receptors (TrkA, TrkB, p75NTR) and PACAP/VIP and receptors (PAC1, VPAC1, VPAC2) transcripts expression in tissues from NGF-OE and wild type (WT) mice with CYP-induced cystitis (4 h, 48 h, and chronic). As expected in urothelium of control NGF-OE mice, NGF mRNA was significantly increased. Urothelial expression of NGF mRNA in NGF-OE mice treated with CYP (4 h 48 h, and chronic) was not further increased but maintained with all durations of CYP treatment evaluated. In contrast, CYP-induced cystitis (4 h 48 h, and chronic) in NGF-OE mice demonstrated significant regulation in BDNF, VEGF mRNA, TrkA, TrkB, and P75NTR in urothelium and detrusor smooth muscle. Similarly, CYP-induced cystitis (4 h 48 h, and chronic) in NGF-OE mice resulted in significant differential changes in WT and NGF-OE in transcript expression for NGF, BDNF, and receptors (TrkA, TrkB, p75NTR) and PACAP/VIP and receptors (PAC1, VPAC1, VPAC2) in lumbosacral DRG that was also CYP-induced cystitis duration-dependent. These studies are consistent with target-derived NGF and other inflammatory mediators affecting neurochemical plasticity and contributing to reflex function of micturition pathways.
Perspectives and Significance
PACAP (Adcyap1) and its cognate receptor, PAC1 (Adcyap1r1), have tissue-specific distributions in diverse systems including micturition reflex pathways including expression in both neural and non-neural (e.g., urothelium) components. PACAP and associated receptors exhibit neuroplastic changes in expression and function with neural injury, inflammation, and diseases of the LUT. Changes in the PACAP/receptor system in micturition pathways may underlie and/or contribute to LUT dysfunction including the symptoms of urinary urgency, increased voiding frequency, nocturia, urinary incontinence, detrusor dyssynergia, and/or pain. The PACAP/receptor system in micturition reflexes may represent a potential target for therapeutic intervention.
Abbreviations
- ATP:
-
Adenosine triphosphate
- BPS/IC:
-
Bladder pain syndrome/interstitial cystitis
- CGRP:
-
Calcitonin gene-related peptide
- CYP:
-
Cyclophosphamide
- DCM:
-
Dorsal commissure
- DH:
-
Dorsal horn
- DRG:
-
Dorsal root ganglia
- h:
-
Hours
- IR:
-
Immunoreactivity
- L:
-
Lumbar
- LCP:
-
Lateral collateral pathway
- LUT:
-
Lower urinary tract
- MPG:
-
Major pelvic ganglia
- NGF-OE:
-
Nerve growth factor-overexpression
- nNOS:
-
Neuronal nitric oxide synthase
- p:
-
Phosphorylated
- PACAP:
-
Pituitary adenylate cyclase-activating polypeptide
- PC:
-
Pheochromocytoma
- PCR:
-
Polymerase chain reaction
- pCREB:
-
Phosphorylated cAMP-response element binding protein
- PLC:
-
Phospholipase C
- SCI:
-
Spinal cord injury
- SP:
-
Substance P
- SPN:
-
Sacral parasympathetic nucleus
- T:
-
Thoracic
- Trk:
-
Receptor tyrosine kinase
- VIP:
-
Vasoactive intestinal polypeptide
- WT:
-
Wild type
References
Kuru M. Nervous control of micturition. Physiol Rev. 1965;45:425–94.
de Groat WC, Kruse MN. Central processing and morphological plasticity in lumbosacral afferent pathways from the lower urinary tract. In: Mayer EA, Raybould HE, editors. Basic and clinical aspects of chronic abdominal pain research and clinical management, vol. 9. Amsterdam: Elsevier Science Publishers; 1993. p. 219–35.
Middleton JW, Keast JR. Artificial autonomic reflexes: using functional electrical stimulation to mimic bladder reflexes after injury or disease. Auton Neurosci. 2004;113:3–15.
Griffiths DJ. The pontine micturition centres. Scand J Urol Nephrol Suppl. 2002;210:21–6.
Kingsley RE, Gable SR, Kingsley TR, Saint Joseph Medical Center (South Bend Ind.). Magnetic Resonance Imaging Center. Concise text of neuroscience. Baltimore: Williams & Wilkins; 1996. viii, 564 p. 335–434.
Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9:453–66.
Habler HJ, Janig W, Koltzenburg M. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. J Physiol. 1990;425:545–62.
Andersson KE. Bladder activation: afferent mechanisms. Urology. 2002;59(5 Suppl 1):43–50.
Mazieres L, Jiang C, Lindstrom S. The C fibre reflex of the cat urinary bladder. J Physiol. 1998;513(Pt 2):531–41.
de Groat WC, Booth AM, Yoshimura N. Neurophysiology of micturition and its modification in animal models of human disease. In: Maggi CA, editor. The autonomic nervous system. London: Harwood Academic Publishers; 1993. p. 227–90.
Yoshimura N, Seki S, Chancellor MB, de Groat WC, Ueda T. Targeting afferent hyperexcitability for therapy of the painful bladder syndrome. Urology. 2002;59(5 Suppl 1):61–7.
Donovan MK, Winternitz SR, Wyss JM. An analysis of the sensory innervation of the urinary system of the rat. Brain Res Bull. 1983;11:321–4.
Birder L, Andersson KE. Urothelial signaling. Physiol Rev. 2013;93:653–80.
Birder L, Wyndaele JJ. From urothelial signalling to experiencing a sensation related to the urinary bladder. Acta Physiol (Oxf). 2013;207:34–9.
Kullmann FA, Shah MA, Birder LA, de Groat WC. Functional TRP and ASIC-like channels in cultured urothelial cells from the rat. Am J Physiol Renal Physiol. 2009;296:F892–901.
Moro C, Tajouri L, Chess-Williams R. Adrenoceptor function and expression in bladder urothelium and lamina propria. Urology. 2013;81:211e1–7.
Wang EC, Lee JM, Ruiz WG, Balestreire EM, von Bodungen M, Barrick S, et al. ATP and purinergic receptor-dependent membrane traffic in bladder umbrella cells. J Clin Invest. 2005;115:2412–22.
Bschleipfer T, Schukowski K, Weidner W, Grando SA, Schwantes U, Kummer W, et al. Expression and distribution of cholinergic receptors in the human urothelium. Life Sci. 2007;80:2303–7.
de Groat WC, Vizzard MA, Araki I, Roppolo J. Spinal interneurons and preganglionic neurons in sacral autonomic reflex pathways. Prog Brain Res. 1996;107:97–111.
de Groat WC, Kawatani M, Hisamitsu T, Booth AM, Roppolo JR, Thor K, et al. Neural control of micturition: the role of neuropeptides. J Auton Nerv Syst. 1986;Suppl:369–87.
Keast JR. Patterns of co-existence of peptides and differences of nerve fibre types associated with noradrenergic and non-noradrenergic (putative cholinergic) neurons in the major pelvic ganglion of the male rat. Cell Tissue Res. 1991;266:405–15.
Vizzard MA. Up-regulation of pituitary adenylate cyclase-activating polypeptide in urinary bladder pathways after chronic cystitis. J Comp Neurol. 2000;420:335–48.
Vizzard MA. Alterations in neuropeptide expression in lumbosacral bladder pathways following chronic cystitis. J Chem Neuroanat. 2001;21:125–38.
Arms L, Vizzard MA. Neuropeptides in lower urinary tract function. Handbook Exp Pharmacol. 2011;202:395–423.
Ek A, Alm P, Andersson KE, Persson K. Adrenergic and cholinergic nerves of the human urethra and urinary bladder. Acta Physiol Scand. 1977;99:345–52.
Keast JR, de Groat WC. Segmental distribution and peptide content of primary afferent neurons innervating the urogenital organs and colon of male rats. J Comp Neurol. 1992;319:615–23.
Su HC, Polak JM, Mulderry PK, Ghatei MA, Gibson SJ, Terenghi G, et al. Calcitonin gene-related peptide immunoreactivity in afferent neurons supplying the urinary tract: combined retrograde tracing and immunohistochemistry. Neuroscience. 1986;18:727–47.
Vizzard MA, de Groat WC. Increased expression of neuronal nitric oxide synthase (NOS) in bladder afferent pathways following chronic bladder irritation. J Comp Neurol. 1996;370:191–202.
Vizzard MA, Erdman SL, de Groat WC. Localization of NADPH-diaphorase in bladder afferent and postganglionic efferent neurons of the rat. J Auton Nerv Syst. 1993;44:85–90.
Vizzard MA, Erdman SL, de Groat WC. Localization of NADPH-diaphorase in pelvic afferent and efferent pathways of the rat. Neurosci Lett. 1993;152:72–6.
Vizzard MA, Erdman SL, de Groat WC. Increased expression of neuronal nitric oxide synthase in dorsal root ganglion neurons after systemic capsaicin administration. Neuroscience. 1995;67:1–5.
Vizzard MA, Erdman SL, Förstermann U, de Groat WC. Differential distribution of nitric oxide synthase in neural pathways to the urogenital organs (urethra, penis, urinary bladder) of the rat. Brain Res. 1994;646:279–91.
de Groat WC. Neuropeptides in pelvic afferent pathways. Experientia. 1987;43:801–13.
Arimura A. Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. Jpn J Physiol. 1998;48:301–31.
Ogi K, Kimura C, Onda H, Arimura A, Fujino M. Molecular cloning and characterization of cDNA for the precursor of rat pituitary adenylate cyclase activating polypeptide (PACAP). Biochem Biophys Res Commun. 1990;173:1271–9.
Braas KM, May V, Harakall SA, Hardwick JC, Parsons RL. Pituitary adenylate cyclase-activating polypeptide expression and modulation of neuronal excitability in guinea pig cardiac ganglia. J Neurosci. 1998;18:9766–79.
Kimura C, Ohkubo S, Ogi K, Hosoya M, Itoh Y, Onda H, et al. A novel peptide which stimulates adenylate cyclase: molecular cloning and characterization of the ovine and human cDNAs. Biochem Biophys Res Commun. 1990;166:81–9.
Ohkubo S, Kimura C, Ogi K, Okazaki K, Hosoya M, Onda H, et al. Primary structure and characterization of the precursor to human pituitary adenylate cyclase activating polypeptide. DNA Cell Biol. 1992;11:21–30.
Okazaki K, Itoh Y, Ogi K, Ohkubo S, Onda H. Characterization of murine PACAP mRNA. Peptides. 1995;16(7):1295–9.
Miyata A, Jiang L, Dahl RD, Kitada C, Kubo K, Fujino M, et al. Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP38). Biochem Biophys Res Commun. 1990;170(2):643–8.
Arimura A, Somogyvari-Vigh A, Miyata A, Mizuno K, Coy DH, Kitada C. Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes. Endocrinology. 1991;129:2787–9.
Moller K, Reimer M, Ekblad E, Hannibal J, Fahrenkrug J, Kanje M, et al. The effects of axotomy and preganglionic denervation on the expression of pituitary adenylate cyclase activating peptide (PACAP), galanin and PACAP type 1 receptors in the rat superior cervical ganglion. Brain Res. 1997;775:166–82.
Hashimoto H, Ishihara T, Shigemoto R, Mori K, Nagata S. Molecular cloning and tissue distribution of a receptor for pituitary adenylate cyclase-activating polypeptide. Neuron. 1993;11:333–42.
Hosoya M, Onda H, Ogi K, Masuda Y, Miyamoto Y, Ohtaki T, et al. Molecular cloning and functional expression of rat cDNAs encoding the receptor for pituitary adenylate cyclase activating polypeptide (PACAP). Biochem Biophys Res Commun. 1993;194:133–43.
Inagaki N, Yoshida H, Mizuta M, Mizuno N, Fujii Y, Gonoi T, et al. Cloning and functional characterization of a third pituitary adenylate cyclase-activating polypeptide receptor subtype expressed in insulin-secreting cells. Proc Natl Acad Sci U S A. 1994;91:2679–83.
Ishihara T, Shigemoto R, Mori K, Takahashi K, Nagata S. Functional expression and tissue distribution of a novel receptor for vasoactive intestinal polypeptide. Neuron. 1992;8:811–9.
Lutz EM, Sheward WJ, West KM, Morrow JA, Fink G, Harmar AJ. The VIP2 receptor: molecular characterisation of a cDNA encoding a novel receptor for vasoactive intestinal peptide. FEBS Lett. 1993;334:3–8.
Spengler D, Waeber C, Pantaloni C, Holsboer F, Bockaert J, Seeburg PH, et al. Differential signal transduction by five splice variants of the PACAP receptor. Nature. 1993;365:170–5.
Braas KM, May V. Pituitary adenylate cyclase-activating polypeptides directly stimulate sympathetic neuron neuropeptide Y release through PAC(1) receptor isoform activation of specific intracellular signaling pathways. J Biol Chem. 1999;274:27702–10.
Braas KM, May V. Pituitary adenylate cyclase-activating polypeptides, PACAP-38 and PACAP-27, regulation of sympathetic neuron catecholamine, and neuropeptide Y expression through activation of type I PACAP/VIP receptor isoforms. Ann N Y Acad Sci. 1996;805:204–16.
Braas KM, May V. Novel activation and interaction of sympathetic neuron signal transduction pathways by pituitary adenylate cyclase activating polypeptides. Soc Neurosci Abstr. 1995;21:1845.
Deutsch PJ, Sun Y. The 38-amino acid form of pituitary adenylate cyclase-activating polypeptide stimulates dual signaling cascades in PC12 cells and promotes neurite outgrowth. J Biol Chem. 1992;267:5108–13.
Pantaloni C, Brabet P, Bilanges B, Dumuis A, Houssami S, Spengler D, et al. Alternative splicing in the N-terminal extracellular domain of the pituitary adenylate cyclase-activating polypeptide (PACAP) receptor modulates receptor selectivity and relative potencies of PACAP-27 and PACAP-38 in phospholipase C activation. J Biol Chem. 1996;271:22146–51.
Beaudet MM, Parsons RL, May V. Electrophysiological actions and signal transduction mechanisms of pituitary adenylate cyclase activating polypeptide (PACAP) in the rat superior cervical ganglion. Soc Neurosci Abstr. 1998;24:2050.
Beaudet MM, Parsons RL, Braas KM, May V. Mechanisms mediating pituitary adenylate cyclase-activating polypeptide depolarization of rat sympathetic neurons. J Neurosci. 2000;20:7353–61.
DiCicco-Bloom E, Deutsch PJ, Maltzman J, Zhang J, Pintar JE, Zheng J, et al. Autocrine expression and ontogenetic functions of the PACAP ligand/receptor system during sympathetic development. Dev Biol. 2000;219:197–213.
Lu N, Zhou R, DiCicco-Bloom E. Opposing mitogenic regulation by PACAP in sympathetic and cerebral cortical precursors correlates with differential expression of PACAP receptor (PAC1-R) isoforms. J Neurosci Res. 1998;53:651–62.
May V, Braas KM. Pituitary adenylate cyclase-activating polypeptide (PACAP) regulation of sympathetic neuron neuropeptide Y and catecholamine expression. J Neurochem. 1995;65:978–87.
May V, Beaudet MM, Parsons RL, Hardwick JC, Gauthier EA, Durda JP, et al. Mechanisms of pituitary adenylate cyclase activating polypeptide (PACAP)-induced depolarization of sympathetic superior cervical ganglion (SCG) neurons. Ann N Y Acad Sci. 1998;865:164–75.
May V, Braas KM. Pituitary adenylate cyclase activating polypeptide (PACAP) stimulation of the sympathetic neuron mitogen-activated protein kinase (MAPK) pathway. Soc Neurosci Abstr. 1998;24:116.
Brandenburg CA, May V, Braas KM. Expression, secretion and plasticity of endogenous pituitary adenylate cyclase activating polypeptides (PACAP) in rat superior cervical ganglion neurons. Soc Neurosci Abstr. 1995;21:1598.
Brandenburg CA, May V, Braas KM. Induction of novel pituitary adenylate cyclase activating polypeptide (PACAP) precursor messenger RNA in rat superior cervical ganglion neurons. Soc Neurosci Abstr. 1996;22:1764.
Brandenburg CA, May V, Braas KM. Identification of endogenous sympathetic neuron pituitary adenylate cyclase-activating polypeptide (PACAP): depolarization regulates production and secretion through induction of multiple propeptide transcripts. J Neurosci. 1997;17:4045–55.
Ghatei MA, Takahashi K, Suzuki Y, Gardiner J, Jones PM, Bloom SR. Distribution, molecular characterization of pituitary adenylate cyclase-activating polypeptide and its precursor encoding messenger RNA in human and rat tissues. J Endocrinol. 1993;136:159–66.
Holgert H, Holmberg K, Hannibal J, Fahrenkrug J, Brimijoin S, Hartman BK, et al. PACAP in the adrenal gland—relationship with choline acetyltransferase, enkephalin and chromaffin cells and effects of immunological sympathectomy. Neuroreport. 1996;8:297–301.
Klimaschewski L, Hauser C, Heym C. PACAP immunoreactivity in the rat superior cervical ganglion in comparison to VIP. Neuroreport. 1996;7(15–17):2797–801.
Koves K, Arimura A, Somogyvari-Vigh A, Vigh S, Miller J. Immunohistochemical demonstration of a novel hypothalamic peptide, pituitary adenylate cyclase-activating polypeptide, in the ovine hypothalamus. Endocrinology. 1990;127:264–71.
Koves K, Arimura A, Gorcs TG, Somogyvari-Vigh A. Comparative distribution of immunoreactive pituitary adenylate cyclase activating polypeptide and vasoactive intestinal polypeptide in rat forebrain. Neuroendocrinology. 1991;54:159–69.
Masuo Y, Suzuki N, Matsumoto H, Tokito F, Matsumoto Y, Tsuda M, et al. Regional distribution of pituitary adenylate cyclase activating polypeptide (PACAP) in the rat central nervous system as determined by sandwich-enzyme immunoassay. Brain Res. 1993;602:57–63.
Moller K, Zhang YZ, Hakanson R, Luts A, Sjolund B, Uddman R, et al. Pituitary adenylate cyclase activating peptide is a sensory neuropeptide: immunocytochemical and immunochemical evidence. Neuroscience. 1993;57:725–32.
Moller K, Reimer M, Hannibal J, Fahrenkrug J, Sundler F, Kanje M. Pituitary adenylate cyclase-activating peptide (PACAP) and PACAP type 1 receptor expression in regenerating adult mouse and rat superior cervical ganglia in vitro. Brain Res. 1997;775:156–65.
Nogi H, Hashimoto H, Hagihara N, Shimada S, Yamamoto K, Matsuda T, et al. Distribution of mRNAs for pituitary adenylate cyclase-activating polypeptide (PACAP), PACAP receptor, vasoactive intestinal polypeptide (VIP), and VIP receptors in the rat superior cervical ganglion. Neurosci Lett. 1997;227:37–40.
Portbury AL, McConalogue K, Furness JB, Young HM. Distribution of pituitary adenylyl cyclase activating peptide (PACAP) immunoreactivity in neurons of the guinea-pig digestive tract and their projections in the ileum and colon. Cell Tissue Res. 1995;279:385–92.
Shiotani Y, Kimura S, Ohshige Y, Yanaihara C, Yanaihara N. Immunohistochemical localization of pituitary adenylate cyclase-activating polypeptide (PACAP) in the adrenal medulla of the rat. Peptides. 1995;16:1045–50.
Sundler F, Ekblad E, Hannibal J, Moller K, Zhang YZ, Mulder H, et al. Pituitary adenylate cyclase-activating peptide in sensory and autonomic ganglia: localization and regulation. Ann N Y Acad Sci. 1996;805:410–26.
Tatsuno I, Somogyvari-Vigh A, Arimura A. Developmental changes of pituitary adenylate cyclase activating polypeptide (PACAP) and its receptor in the rat brain. Peptides. 1994;15:55–60.
May V, Beaudet MM, Parsons RL, Braas KM. PACAP modulates rat sympathetic neuron depolarization through IP3. Ann N Y Acad Sci. 2000;921:186–94.
Murase T, Kondo K, Otake K, Oiso Y. Pituitary adenylate cyclase-activating polypeptide stimulates arginine vasopressin release in conscious rats. Neuroendocrinology. 1993;57:1092–6.
Tatsuno I, Yada T, Vigh S, Hidaka H, Arimura A. Pituitary adenylate cyclase activating polypeptide and vasoactive intestinal peptide increase cytosolic free calcium concentration in cultured rat hippocampal neurons. Endocrinology. 1992;131:73–81.
Fahrenkrug J, Hannibal J. Pituitary adenylate cyclase activating polypeptide immunoreactivity in capsaicin-sensitive nerve fibres supplying the rat urinary tract. Neuroscience. 1998;83:1261–72.
Larsen JO, Hannibal J, Knudsen SM, Fahrenkrug J. Expression of pituitary adenylate cyclase-activating polypeptide (PACAP) in the mesencephalic trigeminal nucleus of the rat after transection of the masseteric nerve. Mol Brain Res. 1997;46:109–17.
Zhang Q, Shi TJ, Ji RR, Zhang YZ, Sundler F, Hannibal J, et al. Expression of pituitary adenylate cyclase-activating polypeptide in dorsal root ganglia following axotomy: time course and coexistence. Brain Res. 1995;705:149–58.
Zhang Y-Z, Hannibal J, Zhao Q, Moller K, Danielsen N, Fahrenkrug J, et al. Pituitary adenylate cyclase activating peptide expression in the rat dorsal root ganglia: up-regulation after peripheral nerve injury. Neuroscience. 1996;74:1099–110.
Negrete HO, Lavelle JP, Berg J, Lewis SA, Zeidel ML. Permeability properties of the intact mammalian bladder epithelium. Am J Physiol. 1996;271(4 Pt 2):F886–94.
Zeidel ML. Low permeabilities of apical membranes of barrier epithelia: what makes watertight membranes watertight? Am J Physiol. 1996;271(2 Pt 2):F243–5.
Lavelle JP, Meyers SA, Ruiz WG, Buffington CAT, Zeidel ML, Apodaca G. Urothelial pathophysiological changes in feline interstitial cystitis: a human model. Am J Physiol Renal Physiol. 2000;278:F540–53.
Apodaca G, Kiss S, Ruiz WG, Meyers S, Zeidel M, Birder L. Disruption of bladder epithelial function after spinal cord injury. Am J Physiol. 2003;284:F966–76.
Girard BM, Wolf-Johnston A, Braas KM, Birder LA, May V, Vizzard MA. PACAP-mediated ATP release from rat urothelium and regulation of PACAP/VIP and receptor mRNA in micturition pathways after cyclophosphamide (CYP)-induced cystitis. J Mol Neurosci. 2008;36:310–20.
Fahrenkrug J. VIP and autonomic neurotransmission. Pharmacol Therap. 1989;41:515–34.
Ishizuka O, Alm P, Larsson B, Mattiasson A, Andersson KE. Facilitatory effect of pituitary adenylate cyclase-activating polypeptide on micturition in normal, conscious rats. Neuroscience. 1995;66:1009–14.
Chapple CR, Milner P, Moss HE, Burnstock G. Loss of sensory neuropeptides in the obstructed human bladder. Br J Urol. 1992;70:373–81.
Smet PJ, Moore KH, Jonavicius J. Distribution and colocalization of calcitonin gene-related peptide, tachykinins, and vasoactive intestinal peptide in normal and idiopathic unstable human urinary bladder. Lab Invest. 1997;77:37–49.
Wanigasekara Y, Kepper ME, Keast JR. Immunohistochemical characterisation of pelvic autonomic ganglia in male mice. Cell Tissue Res. 2003;311:175–85.
Igawa Y, Persson K, Andersson KE, Uvelius B, Mattiasson A. Facilitatory effect of vasoactive intestinal polypeptide on spinal and peripheral micturition reflex pathways in conscious rats with and without detrusor instability. J Urol. 1993;149:884–9.
Erol K, Ulak G, Donmez T, Cingi MI, Alpan RS, Ozdemir M. Effects of vasoactive intestinal polypeptide on isolated rat urinary bladder smooth muscle. Urol Int. 1992;49:151–3.
Hernandez M, Barahona MV, Recio P, Benedito S, Martinez AC, Rivera L, et al. Neuronal and smooth muscle receptors involved in the PACAP- and VIP-induced relaxations of the pig urinary bladder neck. Br J Pharmacol. 2006;149:100–9.
Uckert S, Stief CG, Lietz B, Burmester M, Jonas U, Machtens SA. Possible role of bioactive peptides in the regulation of human detrusor smooth muscle—functional effects in vitro and immunohistochemical presence. World J Urol. 2002;20:244–9.
Braas KM, May V, Zvara P, Nausch B, Kliment J, Dunleavy JD, et al. Role for pituitary adenylate cyclase activating polypeptide in cystitis-induced plasticity of micturition reflexes. Am J Physiol Regul Integr Comp Physiol. 2006;290:R951–62.
Tompkins JD, Girard BM, Vizzard MA, Parsons RL. VIP and PACAP effects on mouse major pelvic ganglia neurons. J Mol Neurosci. 2010;42:390–6.
Girard BM, Galli JR, Young BA, Vizzard MA, Parsons RL. PACAP expression in explant cultured mouse major pelvic ganglia. J Mol Neurosci. 2010;42:370–7.
May V, Vizzard MA. Bladder dysfunction and altered somatic sensitivity in PACAP-/- mice. J Urol. 2010;183:772–9.
Studeny S, Cheppudira BP, Meyers S, Balestreire EM, Apodaca G, Birder LA, et al. Urinary bladder function and somatic sensitivity in vasoactive intestinal polypeptide (VIP)(-/-) mice. J Mol Neurosci. 2008;36:175–87.
Jensen DG, Studeny S, May V, Waschek J, Vizzard MA. Expression of phosphorylated cAMP response element binding protein (p-CREB) in bladder afferent pathways in VIP-/- mice with cyclophosphamide (CYP)-induced cystitis. J Mol Neurosci. 2008;36:299–309.
Girard BM, Malley SE, Braas KM, Waschek JA, May V, Vizzard MA. Exaggerated expression of inflammatory mediators in vasoactive intestinal polypeptide knockout (VIP-/-) mice with cyclophosphamide (CYP)-induced cystitis. J Mol Neurosci. 2008;36:188–99.
Herrera GM, Braas KM, May V, Vizzard MA. PACAP enhances mouse urinary bladder contractility and is upregulated in micturition reflex pathways after cystitis. Ann N Y Acad Sci. 2006;1070:330–6.
Mathews ME, Malley, S, Girard, BM, Braas, KM, Wascek, JA, May, V, Vizzard, MA. Pituitary adenylate cyclase-activating polypeptide (PACAP) expression in lower urinary tract pathways (LUT) with cyclophosphamide (CYP)-induced cystitis in PACAP promoter-dependent EGFP BAC transgenic mice. Soc Neurosci Abstr Planner. 2015. In press.
Tsuchikawa D, Nakamachi T, Tsuchida M, Wada Y, Hori M, Farkas J, et al. Neuroprotective effect of endogenous pituitary adenylate cyclase-activating polypeptide on spinal cord injury. J Mol Neurosci. 2012;48:508–17.
Zvarova K, Dunleavy JD, Vizzard MA. Changes in pituitary adenylate cyclase activating polypeptide expression in urinary bladder pathways after spinal cord injury. Exp Neurol. 2005;192:46–59.
Yoshiyama M, de Groat WC. Effects of intrathecal administration of pituitary adenylate cyclase activating polypeptide on lower urinary tract functions in rats with intact or transected spinal cords. Exp Neurol. 2008;211:449–55.
Zvara P, Braas KM, May V, Vizzard MA. A role for pituitary adenylate cyclase activating polypeptide (PACAP) in detrusor hyperreflexia after spinal cord injury (SCI). Ann N Y Acad Sci. 2006;1070:622–8.
Dray A. Inflammatory mediators of pain. Br J Anaesth. 1995;75:125–31.
Lewin GR, Mendell L. Nerve growth factor and nociception. Trends Neurosci. 1993;16:353–9.
Lindholm D, Heumann R, Meyer M, Thoenen H. Interleukin-1 regulates synthesis of nerve growth factor in non-neuronal cells of the rat sciatic nerve. Nature. 1987;330:658–9.
Meller ST, Cummings CP, Traub RJ, Gebbhart GF. The role of nitric oxide in the development and maintenance of the hyperalgesia produced by intraplantar injection of carrageenan in the rat. Neuroscience. 1994;60:367–74.
Woolf CJ, Allchorne A, Safieh-Garabedian B, Poole S. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor. Br J Pharmacol. 1997;121:417–24.
Ruda MA, Iadarola MJ, Cohen LV, Yound II WS. In situ hybridization, histochemistry and immunocytochemistry reveal an increase in spinal dynorphin biosynthesis in a rat model of peripheral inflammation and hyperalgesia. Proc Natl Acad Sci U S A. 1988;85:622–6.
Donnerer J, Schuligoi R, Stein C. Increased content and transport of substance P and calcitonin gene-related peptide in sensory nerves innervating inflamed tissue: evidence for a regulatory function of nerve growth factor in vivo. Neuroscience. 1992;49:693–8.
Gary MB, Hargreaves KM. Enhanced release of immunoreactive CGRP and substance P from spinal dorsal horn slices occurs during carrageenan inflammation. Brain Res. 1992;582:139–42.
Vizzard MA, Erdman SL, de Groat WC. Increased expression of neuronal nitric oxide synthase (NOS) in visceral neurons after nerve injury. J Neurosci. 1995;15:4033–45.
Vizzard MA. Increased expression of neuronal nitric oxide synthase in bladder afferent and spinal neurons following spinal cord injury. Dev Neurosci. 1997;19:232–46.
Jongsma H, Danielsen N, Sundler F, Kanje M. Alteration of PACAP distribution and PACAP receptor binding in the rat sensory nervous system following sciatic nerve transection. Brain Res. 2000;853:186–96.
Johnson Jr EM, Taniuchi M, Clark HB, Springer JE, Koh S, Tayrien MW, et al. Demonstration of the retrograde transport of nerve growth factor receptor in the peripheral and central nervous system. J Neurosci. 1987;7:923–9.
McMahon SB, Armanini MP, Ling LH, Phillips HS. Expression and coexpression of Trk receptors in subpopulations of adult primary sensory neurons projecting to identified peripheral targets. Neuron. 1994;12:1161–71.
Wright DE, Snyder WD. Neurotrophin receptor mRNA expression defines distinct populations of neurons in rat dorsal root ganglia. J Comp Neurol. 1995;351:329–38.
Qiao LY, Vizzard MA. Cystitis-induced upregulation of tyrosine kinase (TrkA, TrkB) receptor expression and phosphorylation in rat micturition pathways. J Comp Neurol. 2002;454:200–11.
Qiao LY, Vizzard MA. Up-regulation of tyrosine kinase (Trka, Trkb) receptor expression and phosphorylation in lumbosacral dorsal root ganglia after chronic spinal cord (T8-T10) injury. J Comp Neurol. 2002;449:217–30.
Qiao LY, Vizzard MA. Spinal cord injury-induced expression of TrkA, TrkB, phosphorylated CREB, and c-Jun in rat lumbosacral dorsal root ganglia. J Comp Neurol. 2005;482:142–54.
Jongsma Wallin H, Danielsen N, Johnston JM, Gratto KA, Karchewski LA, Verge VMK. Exogenous NT-3 and NGF differentially modulate PACAP expression in adult sensory neurons, suggesting distinct roles in injury and inflammation. Eur J Neurosci. 2001;14:267–82.
Grumolato L, Louiset E, Alexandre D, AitAli D, Turquier V, Fournier A, et al. PACAP and NGF regulate common and distinct traits of the sympathoadrenal lineage: effects on electrical properties, gene markers and transcription factors in differentiating PC12 cells. Eur J Neurosci. 2003;17:71–82.
Hashimoto H, Hagihara N, Koga K, Yamamoto K, Shintani N, Tomimoto S, et al. Synergistic induction of pituitary adenylate cyclase-activating polypeptide (PACAP) gene expression by nerve growth factor and PACAP in PC12 cells. J Neurochem. 2000;74:501–7.
Yamamoto K, Hashimoto H, Hagihara N, Nishino A, Fujita T, Matsuda T, et al. Cloning and characterization of the mouse pituitary adenylate cyclase-activating polypeptide (PACAP) gene. Gene. 1998;211:63–9.
Jamen F, Bouschet T, Laden JC, Bockaert J, Brabet P. Up-regulation of the PACAP type-1 receptor (PAC1) promoter by neurotrophins in rat PC12 cells and mouse cerebellar granule cells via the Ras/mitogen-activated protein kinase cascade. J Neurochem. 2002;82:1199–207.
Lee FS, Rajagopal R, Kim AH, Chang PC, Chao MV. Activation of Trk neurotrophin receptor signaling by pituitary adenylate cyclase-activating polypeptides. J Biol Chem. 2002;277:9096–102.
DiCiccoBloom E, Deutsch PJ, Maltzman J, Zhang JW, Pintar JE, Zheng J, et al. Autocrine expression and ontogenetic functions of the PACAP ligand/receptor system during sympathetic development. Develop Biol. 2000;219:197–213.
Schnegelsberg B, Sun TT, Cain G, Bhattacharya A, Nunn PA, Ford AP, et al. Overexpression of NGF in mouse urothelium leads to neuronal hyperinnervation, pelvic sensitivity, and changes in urinary bladder function. Am J Physiol Reg Integr Comp Physiol. 2010;298:R534–47.
Allen SJ, Dawbarn D. Clinical relevance of the neurotrophins and their receptors. Clin Sci (Lond). 2006;110:175–91.
Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Ann Rev Neurosci. 2006;29:507–38.
Szallasi A, Cortright DN, Blum CA, Eid SR. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov. 2007;6:357–72.
Ford AP, Gever JR, Nunn PA, Zhong Y, Cefalu JS, Dillon MP, et al. Purinoceptors as therapeutic targets for lower urinary tract dysfunction. Br J Pharmacol. 2006;147 Suppl 2:S132–43.
Girard BM, Malley SE, Braas KM, May V, Vizzard MA. PACAP/VIP and receptor characterization in micturition pathways in mice with overexpression of NGF in urothelium. J Mol Neurosci. 2010;42:378–89.
Girard BM, Malley, S, Mathews, ME, Vizzard, MA. Contributions of pituitary adenylate cyclase-activating polypeptide (PACAP)/receptor signaling to increased voiding frequency and somatic sensitivity in mice with urothelium-specific overexpression (OE) of nerve growth factor (NGF) in the urinary bladder. Soc Neurosci Abstr Planner. 2015. In press.
Girard BM, Malley SE, Vizzard MA. Neurotrophin/receptor expression in urinary bladder of mice with overexpression of NGF in urothelium. Am J Physiol Renal Physiol. 2011;300:F345–55.
Acknowledgements
The authors thank current and former members of the Vizzard laboratory who have contributed to the studies described within including: Mary Beth Klinger, Susan Malley, Abbey Peterson, Kimberly Corrow, Katarina Zvarova, Peter Zvara, Li-ya Qiao, and Bopaiah P. Cheppudira. Gratitude is expressed to Dr. James A. Waschek, Dept. of Psychiatry and Behavioral Sciences, David Geffen School of Medicine, University of California Los Angeles for providing the PACAP-EGFP mice. The authors thank Dr. Jan Fahrenkrug, University of Copenhagen, Copenhagen, Denmark, for his generosity in supplying the monoclonal PACAP antisera used in the studies described.
Grants
Research from the Vizzard laboratory described herein was funded by the National Institutes of Health (NIH) grants DK051369 (MAV), DK060481 (MAV). Additional support was also provided by grants from the National Center for Research Resources (5 P30 RR 032135) and the National Institute of General Medical Sciences (8 P30 GM 103498) from the NIH.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Gonzalez, E.J., Girard, B., Braas, K.M., May, V., Vizzard, M.A. (2016). Neuroplasticity of PACAP Expression and Function in Micturition Reflex Pathways. In: Reglodi, D., Tamas, A. (eds) Pituitary Adenylate Cyclase Activating Polypeptide — PACAP. Current Topics in Neurotoxicity, vol 11. Springer, Cham. https://doi.org/10.1007/978-3-319-35135-3_19
Download citation
DOI: https://doi.org/10.1007/978-3-319-35135-3_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-35133-9
Online ISBN: 978-3-319-35135-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)