Abstract
Pituitary adenylate cyclase-activating peptide (PACAP) peptides are expressed in micturition pathways, and PACAP expression is regulated by urinary bladder inflammation. Previous physiological studies have demonstrated roles for PACAP27 and PACAP38 in detrusor smooth muscle (DSM) contraction and a PAC1 receptor antagonist reduced cyclophosphamide (CYP)-induced bladder hyperreflexia. To gain insight into PACAP signaling in micturition and regulation with cystitis, receptor characterization by real-time quantitative polymerase chain reaction and physiological assays were performed. PACAP receptors were identified in tissues of rat micturition pathway, including DSM, urothelium (U), and dorsal root ganglia (DRG) after acute (4 h), intermediate (48 h) or chronic (8 days) CYP-induced cystitis. PAC1 messenger RNA expression significantly (p ≤ 0.05) increased in U and DSM after 48 h and chronic CYP-induced cystitis after an initial decrease at 4 h. VPAC1 and VPAC2 transcripts increased in U and DSM after acute and intermediate CYP-induced cystitis followed by a decrease in VPAC2 expression with chronic cystitis. Application of PACAP27 (100 nM) to cultured urothelial cells evoked adenosine triphosphate (ATP) release that was blocked by the PAC1 specific antagonist, M65 (1 μM). PACAP38 (100 nM) also evoked ATP release from cultured urothelial cells, but ATP release was less than that observed with PACAP27. PACAP transcripts were increased in the U with intermediate and chronic cystitis, whereas vasoactive intestinal polypeptide (VIP) expression in both tissues was very low and showed no regulation with cystitis. Regulation of PACAP, galanin, and substance P transcripts expression was observed in lumbosacral DRG, but no regulation for VIP was observed. The current data demonstrate PACAP and PAC1 regulation in micturition pathways with inflammation and PACAP-mediated ATP release from urothelium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pain and micturition dysfunction in interstitial cystitis (IC) or painful bladder syndrome (PBS) may involve an alteration in bladder sensory neuron and urinary bladder function. Many studies have suggested the involvement of the neuropeptide, pituitary adenylate cyclase-activating polypeptide (PACAP) in lower urinary tract (LUT) function after bladder inflammation (Vizzard 2000c; Braas et al. 2006; Herrera et al. 2006) or spinal cord injury (Zvarova et al. 2005; Zvara et al. 2006;). PACAP is a member of the vasoactive intestinal polypeptide (VIP), secretin, glucagon family of hormones and exhibits ~68% homology to VIP (Arimura 1992). PACAP can act on three types of G-protein-coupled receptors including VPAC1 and VPAC2 that respond to PACAP and VIP, whereas PAC1 is a specific receptor for PACAP (Arimura 1998; Sherwood et al. 2000; Vaudry et al. 2000).
Dense PACAP-immunoreactive sensory fibers are found within the bladder wall and suburothelial plexus (Fahrenkrug and Hannibal 1998; Mohammed et al. 2002; Braas et al. 2006), and PACAP expression in dorsal root ganglia (DRG) and afferent projections in the spinal cord are upregulated after bladder inflammation induced by cyclophosphamide (CYP; Vizzard 2000c) or after spinal cord injury (Zvarova et al. 2005; Zvara et al. 2006). Consistent with the direct effects of PACAP and VIP in other smooth muscle systems (Schworer et al. 1993; Steenstrup et al. 1994; Linden et al. 1999; Fizanne et al. 2004), we have previously demonstrated specific PACAP receptor expression in bladder smooth muscle, urothelium, lumbosacral DRG, and spinal cord suggesting that PACAP and PAC1 receptor signaling participate at multiple levels along the LUT (Braas et al. 2006). One goal of the present study was to characterize the regulation of PACAP and VIP and associated receptor expression in urothelium, detrusor smooth muscle, and lumbosacral DRG as a function of increasing duration of bladder inflammation induced by CYP using real-time quantitative reverse transcription-polymerase chain reaction (Q-PCR). We also examined expression of two other neuropeptides, substance P (Vizzard 2001) and galanin (Zvarova and Vizzard 2006), not members of the PACAP/VIP family but expressed in LUT pathways and regulated with bladder inflammation (Vizzard 2001; Zvarova and Vizzard 2006).
Purinergic signaling pathways may play an important role in regulating normal urinary bladder function including detrusor smooth muscle contraction (Brown et al. 1979; King et al. 1997) and bladder filling sensation (Burnstock 2000; Rong et al. 2002), and changes in purinergic signaling have been implicated in bladder dysfunction (Bodin and Burnstock 1998; Sun et al. 2001a; Sun et al. 2001b; Birder et al. 2003, 2004). Earlier studies have indicated that the urothelium releases adenosine triphosphate (ATP) in response to various stimuli (Birder 2006). In addition, it has been suggested that ATP released from the serosal surface of the urothelium during bladder filling stimulates P2X3 receptors on suburothelial sensory nerve fibers and contributes to bladder filling sensation (Cockayne et al. 2000). ATP in the cell cytoplasm can be released extracellularly by several mechanisms including exocytosis of ATP-containing vesicles (Bodin and Burnstock 2001; Novak 2003). An additional goal of this study was to determine if PACAP and VIP receptors in normal bladder urothelium are functional by measurement of PACAP and/or VIP-evoked ATP release.
Materials and Methods
CYP-Induced Cystitis
Acute, intermediate, and chronic CYP-induced cystitis rat models were examined in these studies (Corrow and Vizzard 2007; Klinger et al. 2008). For chronic CYP-induced cystitis, rats received drug injection (75 mg/kg; intraperitoneal, i.p.) every third day for 8 days. For intermediate CYP-induced cystitis, rats received a single injection (150 mg/kg, i.p.), and tissues were examined 48 h later. For acute CYP-induced cystitis, rats received a single injection (150 mg/kg, i.p.) and survived for 4 h. Control rats received volume-matched injections of saline (0.9%; i.p.) or no treatment, and no difference among the control groups was observed. All injections were performed under isoflurane (2%) anesthesia. The CYP model of bladder inflammation produces an increase in voiding frequency with small micturition volumes and is associated with inflammatory cell infiltrates in the urinary bladder including mast cells, macrophages, and neutrophils (Vizzard 2000a; Hu et al. 2003; LaBerge et al. 2006). All experimental protocols involving animal use were approved by the University of Vermont Institutional Animal Care and Use Committee (IACUC number 06–014). Animal care was under the supervision of the University of Vermont’s Office of Animal Care Management in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and National Institutes of Health guidelines. All efforts were made to minimize the potential for animal pain, stress, or distress.
Tissue Harvest
After CYP treatment or in control situations (control, 4 hr, or chronic, 48 h; n = 5–7 for each), animals were deeply anesthetized with isoflurane (3–4%) and then euthanized via thoracotomy. The urinary bladder and DRG were quickly removed. Whole bladder was dissected under RNase-free conditions. The bladder was cut open along the midline and pinned to a sylgard-coated dish, and the urothelium was removed as previously described (Zvarova and Vizzard 2005). The urothelium has suburothelial structures associated with it; the term urothelium in this paper refers to both urothelial and suburothelial structures. DRG were identified and isolated as previously described (Vizzard 1997; Vizzard 2000a,c) under RNase-free conditions. Lumbosacral DRG (L6, S1) were specifically chosen for analysis based upon the previously determined segmental representation of urinary bladder circuitry (Donovan et al. 1983; Keast and de Groat 1992; Nadelhaft and Vera 1995). Bladder afferents are not distributed within the L4–L5 DRG (Donovan et al. 1983; Keast and de Groat 1992) that contain only somatic afferents nor are neurons that are involved in urinary bladder function observed in the L4–L5 spinal segments (Nadelhaft and Vera 1995). Harvest of DRG was restricted to L6–S1 because previous studies have demonstrated the presence of PAC1 and VPAC2 receptors in these tissues using non-quantitative reverse-transcription PCR (Braas et al. 2006).
Q-PCR Analysis
Total RNA was extracted using the STAT-60 total RNA/mRNA isolation reagent (Tel-Test ‘B’, Friendswood, TX, USA) as previously described (Girard et al. 2002; Klinger et al. 2008). One to 2 mg of RNA per sample was used to synthesize complementary DNA using SuperScript II reverse transcriptase and random hexamer primers with the SuperScript II Preamplification System (Invitrogen, Carlsbad, CA, USA) in a 20-μl final reaction volume.
The quantitative PCR standards for all transcripts were prepared with the amplified PAC1, VPAC1, VPAC2, PACAP, VIP, Gal, Sub P, and L32 complementary DNA products ligated directly into pCR2.1 TOPO vector using the TOPO TA cloning kit (Invitrogen). The nucleotide sequences of the inserts were verified by automated fluorescent dideoxy dye terminator sequencing (Vermont Cancer Center DNA Analysis Facility). To estimate the relative expression of the receptor transcripts, tenfold serial dilutions of stock plasmids were prepared as quantitative standards. The range of standard concentrations was determined empirically.
Q-PCR was performed using SYBR Green I detection (Girard et al. 2002; Klinger et al. 2008). Complementary DNA templates, diluted fivefold to minimize the inhibitory effects of the reverse transcription reaction components, were assayed using SYBR Green I JumpStart™. Taq ReadyMix™ (Sigma, St. Louis, MO, USA) containing 5 mM MgCl2, 200 mM 2′-deoxyadenosine 5′-triphosphate, 2′-deoxyguanosine 5′-triphosphate, 2′-deoxycytidine 5′-triphosphate, and 2′-deoxythymidine 5′-triphosphate, 0.64 U Taq DNA polymerase, and 300 nM of each primer in a final 25-μl reaction volume. The Q-PCR was performed on an Applied Biosystems 7500 Fast real-time PCR system (Applied Biosystems, Foster City, CA, USA; Girard et al. 2002; Klinger et al. 2008) using the following standard conditions: (1) 94°C for 2 min; (2) amplification over 40 cycles at 94°C for 15 s; and 60 to 64°C depending on primers set for 40 s.
The amplified product from these amplification parameters was subjected to SYBR Green I melting analysis by ramping the temperature of the reaction samples from 60°C to 95°C. A single DNA melting profile was observed under these dissociation assay conditions demonstrating amplification of a single unique product free of primer dimers or other anomalous products. Oligonucleotide primer sequences for PACAP (Girard et al. 2002), VIP (Girard et al. 2002), PAC1 (Braas and May 1999), VPAC1 (Girard et al. 2006), VPAC2 (Girard et al. 2006), substance P (Girard et al. 2002), galanin (Girard et al. 2002), and L32 (Klinger et al. 2008) used in these studies have been previously described.
For data analyses, a standard curve was constructed by amplification of serially diluted plasmids containing the target sequence. Data were analyzed at the termination of each assay using the Sequence Detection Software version 1.3.1 (Applied Biosystems, Norwalk, CT, USA). In standard assays, default baseline settings were selected. The increase in SYBR Green I fluorescence intensity (ΔRn) was plotted as a function of cycle number, and the threshold cycle was determined by the software as the amplification cycle at which the ΔRn first intersects the established baseline. All data are expressed as the relative quantity of the gene of interest normalized to the relative quantity of the housekeeping gene L32. Control samples are set equal to 100%.
Urothelial Cell Preparation and ATP Release Measurements
Urothelial cells were cultured 18–72 h before testing as previously described (Chopra et al. 2005; Wang et al. 2005). For each urothelial culture, at least three rat bladders were required to achieve sufficient numbers of dissociated cells for the ATP release assay. For ATP release, a perfusate of 100 μl of Hank’s buffered salt solution (5 mM KCl, 0.3 mM KH2PO4, 138 mM NaCl, 4 mM NaHCO3, 0.3 mM Na2HPO4, 5.6 mM glucose, 2 mM CaCl2, 1 mM MgCl2, and 10 mM 4-2-hydroxyethyl-1-piperazineethanesulfonic acid, pH 7.4) was collected every 30 s. After measuring background release of ATP from the cultures for 5–10 min, the test compounds were applied through the perfusion system (Rainin Peristaltic Pump RP-1, Oakland, CA, USA) for another 5–10 min. ATP measurements were calculated based on the luciferin–luciferase reaction using a standard curve (Adenosine Triphosphate Assay Kit, Sigma; Turner Systems luminometer TD-20/20, Sunnyvale, CA, USA). Data were obtained from at least three independent cultures and at least n = 3 for each culture. Data were analyzed by calculating the area under the curve during each portion of the experiment and by calculating the fold increase in ATP release using the initial perfusates as onefold.
Materials
All standard chemicals were obtained from Sigma-Aldrich or Fisher and were either analytical or laboratory grade. PACAP27 and PACAP38 were purchased from American Peptide Company, Sunnyvale, CA, USA. The PAC1 selective antagonist, M65, was a gift from Dr. Ethan Lerner, Harvard Medical School.
Statistical Analysis
All data are presented as mean ± SEM. One-way analysis of variance (ANOVA) was used to evaluate differences among groups for Q-PCR. Data obtained from the ATP release assays violated the assumptions of the ANOVA. Thus, these data were analyzed using a non-parametric analysis, the Mann–Whitney rank sum test. Differences were considered statistically significant if p ≤ 0.05. When F ratios exceeded the critical value (p ≤ 0.05), the Newman–Keul’s post-hoc test was used to compare the experimental means.
Results
Time Course of CYP-Induced Cystitis and Bladder Hyperreflexia
Previous studies (Vizzard 2000a; Hu et al. 2003) have detailed the histological examinations and myeloperoxidase (MPO) assays that were used to establish inflammation of the urinary bladder after CYP. Gross microscopic analysis of urinary bladders from animals treated chronically with CYP resulted in extensive regions of mucosal erosion, ulcerations, edema, and, in some instances, petechial hemorrhages. Histological changes evident after chronic CYP treatment included edema of the lamina propria and plasma cell infiltrates in the lamina propria, submucosa, and perivascular tissue. Inflammatory changes are evident in urinary bladder as early as 2 h after CYP treatment and increase in severity with time and repeated dosing with CYP. Gross microscopic analyses of bladders from animals treated with CYP 4 or 48 h before examination showed a few, scattered regions of mucosal erosion on the luminal surface (Hu et al. 2003). The number and distribution of mast cells and macrophages as well as MPO activity increased with the duration of CYP treatment (chronic > 48 h > 4 h; Hu et al. 2003). Bladder hyperreflexia is evident as early as 4 h after CYP treatment (Hu et al. 2003).
PAC1, VPAC1, and VPAC2 Receptor Transcript Expression and Regulation in Urothelium and Detrusor with CYP-Induced Cystitis
PAC1, VPAC1 and VPAC2 receptor transcripts were expressed in the urothelium and detrusor smooth muscle of control (non-inflamed) urinary bladder (Fig. 1A–C) as previously described (Braas et al. 2006). With acute (4 h) CYP-induced cystitis, PAC1 receptor transcript exhibited a significant (p ≤ 0.05) decrease in expression in both urothelium and detrusor with a significant (p ≤ 0.05) increase in PAC1 receptor transcript expression in urothelium and detrusor smooth muscle induced by intermediate (48 h) and chronic (8 days) CYP-induced cystitis (Fig. 1A). CYP-induced changes in VPAC1 and VPAC2 expression were similar in both urothelium and detrusor (Fig. 1B,C). Acute (4 h) and intermediate (48 h) CYP-induced cystitis increased VPAC1 and VPAC2 receptor transcript expression in both urothelium and detrusor. In contrast, chronic (8 days) CYP-induced cystitis decreased VPAC2 receptor transcript expression in both urothelium and detrusor (Fig. 1C).
Regulation of urinary bladder PAC1, VPAC1, and VPAC2 receptor transcript levels in control and after acute (4 h), intermediate (48 h), and chronic (8 days, d) cyclophosphamide (CYP-induced bladder inflammation. Relative expression of the urothelium (gray bars) and detrusor receptor transcripts (black bars) are expressed as a percentage of control and normalized to the relative expression of the housekeeping gene, L32. A PAC1 mRNA expression. B VPAC1 mRNA expression. C VPAC2 mRNA expression. Samples sizes are n of 5–7; *p ≤ 0.05 versus control
PACAP, VIP, Galanin, and Substance P Transcript Expression and Regulation in Urothelium and Detrusor with CYP-Induced Cystitis
Transcripts for each of these neuropeptides were present in urothelium and detrusor of control (non-inflamed) urinary bladder, but the effects of CYP-induced cystitis on each neuropeptide expression differed (Fig. 2A,B; Table 1). PACAP transcript expression significantly (p ≤ 0.05) increased in the urothelium with intermediate (48 h) and chronic (8 days) CYP-induced cystitis, whereas no changes were observed with acute (4 h) CYP-induced cystitis in either urothelium or detrusor smooth muscle (Fig. 2A). No regulation of VIP transcript expression was detected at any duration of CYP treatment in urothelium or detrusor (Fig. 2B). With respect to bladder inflammation-induced changes in galanin or substance P transcript expression, very different regulation was demonstrated (Table 1). Acute (4 h) CYP-induced cystitis significantly (p ≤ 0.05) increased galanin transcript expression in the urothelium whereas chronic (8 day) CYP treatment significantly (p ≤ 0.05) increased substance P transcript expression in the detrusor smooth muscle (Table 1).
Regulation of urinary bladder PACAP and VIP transcript levels in control and after cyclophosphamide (CYP)-induced cystitis of varying duration [acute (4 h), intermediate (48 h) and chronic (8 day, d)]. Relative expression of the urothelium (gray bars) and detrusor transcripts (black bars) are expressed as a percentage of control and normalized to the relative expression of the housekeeping gene, L32. A PACAP mRNA expression. B VIP mRNA expression. Samples sizes are n of 5–7; *p ≤ 0.05 versus control
PAC1, VPAC1, and VPAC2 Receptor Transcript Expression and Regulation in L6-S1 DRG with CYP-Induced Cystitis
PAC1, VPAC1, and VPAC2 receptor transcripts are expressed in the lumbosacral (L6–S1) DRG (Fig. 3A–C) as previously described (Braas et al. 2006). CYP-induced inflammation of the urinary bladder only affected receptor transcript expression after acute (4 h) CYP-induced cystitis (Fig. 3A–C). PAC1 and VPAC2 receptor transcript expression significantly (p ≤ 0.05) decreased in both L6 and S1 DRG with acute (4 h) CYP-induced cystitis (Fig. 3A,C). For VPAC1 receptor transcript expression, a significant (p ≤ 0.05) increase in expression was demonstrated in the S1 DRG after acute CYP-induced cystitis (Fig. 3B). No changes in PAC1, VPAC1, or VPAC1 receptor expression in L6 or S1 DRG were demonstrated with intermediate (48 h) or chronic (8 days) CYP-induced cystitis (Fig. 3A–C).
Regulation of PACAP/VIP receptors mRNA expression in lumbosacral (L6–S1) dorsal root ganglia (DRG) with CYP-induced cystitis of varying duration (acute (4 h), intermediate (48 h) and chronic (8 day, d). Relative expression of the PACAP/VIP receptor transcripts in the L6 DRG (white bars) and S1 DRG (black bars) are expressed in a percentage of control and normalized to the relative expression of the housekeeping gene, L32. A PAC1 mRNA expression. B VPAC1 mRNA expression. C VPAC2 mRNA expression. Samples sizes are n of 5–7; *p ≤ 0.05 versus control
PACAP, VIP, Galanin, and Substance P Transcript Expression and Regulation in L6-S1 DRG with CYP-Induced Cystitis
In contrast to the few changes in neuropeptide transcript expression demonstrated in the urothelium and detrusor smooth muscle with CYP-induced cystitis, greater regulation was demonstrated in the L6 and S1 DRG (Fig. 4A,B; Table 1). Changes in PACAP transcript with CYP-induced cystitis mirrored those observed in the urinary bladder with acute CYP-induced cystitis decreasing and intermediate (48 h) and chronic (8 day) treatments significantly (p ≤ 0.05) increasing PACAP transcript expression in both the L6 and S1 DRG (Fig. 4A). Similar to the lack of regulation demonstrated for VIP transcript in the urinary bladder with CYP-induced cystitis, cystitis was also without effect on VIP regulation in the L6 and S1 DRG (Fig. 4B). Galanin transcript expression significantly (p ≤ 0.05) decreased in the L6 DRG after 4 h or 8 day CYP-induced cystitis (Table 1). In contrast, substance P transcript expression significantly (p ≤ 0.05) increased in the L6 DRG with intermediate (48 h) and chronic (8 day) cystitis (Table 1). Acute (4 h) CYP treatment significantly (p ≤ 0.05) decreased substance P transcript expression in the L6 DRG (Table 1).
Regulation of lumbosacral (L6–S1) PACAP and VIP transcript levels in control and after cyclophosphamide (CYP)-induced cystitis of varying duration [acute (4 h), intermediate (48 h), and chronic (8 day, d)]. Relative expression of the L6 DRG (white bars) and S1 DRG (black bars) neuropeptide transcripts are expressed in a percentage of control after CYP-induced cystitis. A PACAP mRNA expression. B VIP mRNA expression. Samples sizes are n of 5–7; *p ≤ 0.05 versus control
PACAP-Evoked ATP Release from Cultured Rat Urothelial Cells
ATP release from normal rat urothelial cell cultures was significantly (p ≤ 0.01) increased after stimulation with PACAP27 (100 nM; Fig. 5). PACAP27-evoked release of ATP was significantly (p ≤ 0.01) inhibited by the PAC1 specific receptor antagonist, M65 (1 μM). PACAP38 (100 nM) also increased ATP release from normal rat urothelial cell cultures, but release was less than that observed with PACAP38.
Discussion
PACAP peptides have diverse functions in the endocrine, nervous, gastrointestinal, and cardiovascular systems (Braas and May 1996; Arimura 1998) through PAC1, VPAC1, and VPAC2 G-protein-coupled receptors. High levels of PACAP and VIP expression have been identified in many central nervous system neurons and in sensory and autonomic ganglia (Arimura et al. 1991; Sundler et al. 1996; Moller et al. 1997a,b; Arimura 1998; Braas et al. 1998). Both PACAP and VIP immunoreactivity have been identified in urinary bladder (Fahrenkrug and Hannibal 1998). The majority of the PACAP nerve fibers are derived from sensory neurons (Zhang et al. 1995; Fahrenkrug and Hannibal 1998; Zvarova et al. 2005). By contrast, bladder VIP-immunoreactive fibers exhibited distribution patterns that were distinct from PACAP and were not sensitive to capsaicin, suggesting that they may be derived from pelvic autonomic ganglia (Fahrenkrug and Hannibal 1998).
PACAP and VIP peptides regulate smooth muscle function, either directly or by facilitating cholinergic and nitric oxide mechanisms in a tissue- and species-specific manner (Mizumoto et al. 1992; Fox-Threlkeld et al. 1999; Onaga et al. 1998; Seebeck et al. 2002; Zizzo et al. 2004). A number of studies have implicated PACAP in LUT function (Vizzard 2000c; Zvarova et al. 2005; Braas et al. 2006; Herrera et al. 2006; Zvara et al. 2006). Similar to previous studies for calcitonin gene-related peptide and substance P (Mohammed et al. 2002), CYP-induced cystitis altered the expression of PACAP in micturition pathways. Acute and chronic CYP treatment in rats increased PACAP staining in lumbosacral DRG neurons and central projections to corresponding spinal cord segments (Vizzard 2000c). Recent studies also demonstrated upregulation of PACAP in LUT tissues after chronic spinal cord injury (Zvarova et al. 2005). These observations were consistent with neurophenotypic changes in DRG and spinal cord after injury and inflammation (Zhang et al. 1996; Zhang et al. 1998; Jongsma Wallin et al. 2003) and suggested that among other sensory peptides, increased PACAP expression in the micturition pathways may participate in the physiological pathologies associated with bladder inflammation. Thus, by extrapolation, we have suggested that PACAP peptides may contribute to the clinical syndrome of IC/PBS.
IC/PBS is a chronic inflammatory bladder disease syndrome characterized by urinary frequency, urgency, suprapubic, and pelvic pain. Although the etiology and pathogenesis of IC are unknown, numerous theories including infection, autoimmune disorder, toxic urinary agents, deficiency in bladder wall lining, and neurogenic causes have been proposed (Erickson and Davies 1998; Erickson 1999; Sant and Theoharides 1999; Rosamilia and Dwyera 2000). CYP administration to rats results in bladder inflammation and reduced bladder capacity and intercontraction intervals (Maggi et al. 1992; Maggi et al. 1993; Hu et al. 2003). We have previously demonstrated that PACAP expression is upregulated in spinal cord and DRG (Vizzard 2000c) with bladder inflammation. In the present study, we extend those findings to demonstrate that PACAP transcript expression is also increased in the urinary bladder and lumbosacral DRG with bladder inflammation. In experimental inflammation paradigms, the expression of several sensory peptides including PACAP is increased while VIP levels, by contrast, are unchanged (Donaldson et al. 1992; Lu et al. 2005). Consistent with these previous studies, no changes in VIP transcript expression were observed in the urinary bladder or L6-S1 DRG after CYP-induced cystitis. The changes in PACAP transcript in the lumbosacral DRG were very similar to those observed for substance P transcript expression after CYP-induced cystitis (Vizzard 2001). This similar regulation may reflect colocalization of PACAP and substance P in some sensory neurons. Both PACAP and substance P are expressed in bladder afferent neurons and CYP-induced cystitis increases the percentage of bladder afferent cells that express PACAP or substance P (Vizzard 2000c; Vizzard 2001). It would be of interest in the future to determine whether bladder afferent neurons in the DRG express both PACAP and substance P. In contrast, a small percentage of bladder afferent cells express galanin immunoreactivity, and this expression is largely unaffected by CYP-induced intermediate or chronic cystitis (Zvarova and Vizzard 2006). This finding is consistent with the present demonstration that intermediate or chronic cystitis has no effect on galanin transcript expression in L6–S1 DRG despite a reduction in transcript expression with acute CYP-induced cystitis.
PACAP- and VIP-immunoreactive fibers have been identified in the bladder wall and suburothelial plexus, PACAP peptides have been measured in bladder tissues (Fahrenkrug and Hannibal 1998), and we have recently identified PACAP/VIP receptor subtypes and isoforms in LUT (Braas et al. 2006). As PACAP and VIP share receptor subtypes coupled to different intracellular effectors, the identification of specific PACAP/VIP receptors can be important in revealing the relevant peptides and their signaling mechanisms in LUT physiology. Only PACAP peptides exhibit high affinity for the PAC1 receptor, whereas VIP and PACAP have similar high affinities for the VPAC1 and VPAC2 receptors (Arimura 1998; Sherwood et al. 2000; Vaudry et al. 2000). While VPAC receptors appear to be coupled solely to adenylyl cyclase, PAC1 receptor isoforms display unique patterns of adenylyl cyclase and phospholipase C activation that differ for the alternatively processed PACAP27 and PACAP38 peptides (Spengler et al. 1993; Arimura 1998; Sherwood et al. 2000; Vaudry et al. 2000). Although PACAP/VIP receptor subtypes were identified in LUT (Braas et al. 2006), there were still a number of deficits in our knowledge regarding PACAP/VIP receptors in the LUT including receptor regulation in LUT with bladder inflammation induced by CYP.
As demonstrated in the present study, PACAP/VIP receptors transcript expression is regulated by CYP-induced cystitis in both lumbosacral (L6–S1) DRG and in the urothelium and detrusor smooth muscle. In the present studies, we focused our examination of PACAP/VIP receptor regulation to the urinary bladder (urothelium, detrusor smooth muscle) and the lumbosacral DRG. We have previously demonstrated that a large percentage of bladder afferent cells in the DRG express PACAP after CYP treatment and intravesical (i.e., intrabladder) infusion of the PACAP antagonist, PACAP6-38, reduced urinary bladder hyperreflexia after CYP treatment (Braas et al. 2006). In agreement with the demonstration that PACAP (Vizzard 2000c) is upregulated in spinal cord, DRG, and urinary bladder after CYP-induced cystitis, the present data demonstrate that CYP-induced cystitis upregulates PAC1 receptor transcript expression in the urinary bladder at all durations of CYP treatment. Similarly, VPAC1 and VPAC2 receptor transcripts were also increased with acute and intermediate CYP-induced cystitis; however, VPAC2 receptor transcript decreased in urinary bladder after chronic (8 days) CYP treatment.
We have previously demonstrated the direct effects of PACAP on bladder smooth muscle contractility (Braas et al. 2006). Both PACAP27 and PACAP38 increased bladder smooth muscle tone and potentiated electric field stimulation (EFS)-induced contractions (Braas et al. 2006). EFS-induced contractions were superimposed on spontaneous muscle contractions and were tetrodotoxin-insensitive, suggesting that the responses were direct detrusor smooth muscle effects (Braas et al. 2006). Surprisingly, VIP had no apparent effects on either bladder tone or EFS-stimulated contractions despite VPAC2 receptor transcript expression in detrusor (Braas et al. 2006). However, as VIP innervation to the urinary bladder is minimal compared to that for PACAP (Fahrenkrug and Hannibal 1998), these results may be in keeping with suggestions that PACAP and PAC1 signaling are more prominent regulators of rat bladder physiology (Braas et al. 2006). These VIP results are consistent with previous studies, which demonstrated that VIP application to detrusor smooth muscle had not effect on spontaneous or carbachol-induced bladder contractions despite facilitation of micturition when VIP was administered intrathecally or intra-arterially close to the rat bladder (Igawa et al. 1993). In the present studies, PACAP27, PACAP38, and VIP application evoked ATP release from rat urothelial cell cultures; however, ATP release was greatest with PACAP27 treatment and significantly blocked by the PAC1 receptor selective antagonist, M65. The current data further support the suggestion that PACAP and PAC1 signaling are regulators of bladder physiology at the level of the urinary bladder and specifically the urothelium.
CYP-induced cystitis alters the expression of tissue-derived growth factors and receptors including NGF, TrkA, and p75NTR (Murray et al. 2004; Vizzard 2000b; Hu et al. 2005). Enhanced target-derived NGF availability has been shown to increase PACAP expression in small nociceptive neurons in DRG (Vizzard 2000c; Jongsma Wallin et al. 2001, 2003), which may result in increased PACAP signaling at PAC1 and/or VPAC2 receptors in peripheral tissue sites to exacerbate bladder dysfunction and sensory disturbances in cystitis (Fig. 6). As a corollary, NGF does not increase VIP expression in DRG, which may be consistent with the target tissue neurotrophic model to selectively augment PACAP expression in DRG (Mulderry and Lindsay 1990; V. May and K. Braas, unpublished observations). Ongoing studies are designed to address LUT function in PACAP−/− mice under normal conditions and in the context of urinary bladder inflammation.
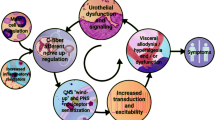
Potential mechanism underlying PACAP/PAC1 signaling in urinary bladder hyperreflexia with bladder inflammation. Previous studies have demonstrated an involvement of nerve growth factor (NGF) in the urinary bladder contributing to bladder hyperreflexia induced by CYP-induced cystitis (Hu et al. 2005; Vizzard 2000b,c; Zvara and Vizzard 2007). NGF is retrogradely transported from the urinary bladder to dorsal root ganglia (DRG). NGF can upregulate PACAP expression in DRG and spinal cord with CYP-induced cystitis (Vizzard 2000c). Local release of NGF in the urinary bladder may also upregulate PACAP expression in the urothelium and detrusor. The PAC1 receptor antagonist, PACAP6-38, was effective in reducing bladder hyperreflexia with either intrathecal or intravesical administration (Braas et al. 2006). The present studies demonstrated increased PAC1 expression in urinary bladder with CYP-induced cystitis. PACAP27 evokes ATP release from the urothelium that is inhibited by the PAC1 selective antagonist, M65. These studies suggest that PACAP acting through PAC1 receptors may function at multiple sites along the lower urinary tract (spinal cord, DRG, urothelium, and detrusor) to contribute to bladder hyperreflexia. DH Dorsal horn, EUS external urethral sphincter. Figure modified from (Braas et al. 2006)
References
Arimura, A. (1992). Pituitary adenylate cyclase activating polypeptide (PACAP): discovery and current status of research. Regulatory Peptide, 37(3), 287–303.
Arimura, A. (1998). Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. Japanese Journal of Physiology, 48(5), 301–331.
Arimura, A., Somogyvari-Vigh, A., Miyata, A., Mizuno, K., Coy, D. H., & Kitada, C. (1991). Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes. Endocrinology, 129(5), 2787–2789.
Birder, L. A. (2006). Urinary bladder urothelium: molecular sensors of chemical/thermal/mechanical stimuli. Vascular Pharmacology, 45(4), 221–226.
Birder, L. A., Barrick, S. R., Roppolo, J. R., et al. (2003). Feline interstitial cystitis results in mechanical hypersensitivity and altered ATP release from bladder urothelium. American Journal of Physiology– Renal Physiology, 285(3), F423–F429.
Birder, L. A., Ruan, H. Z., Chopra, B., et al. (2004). Alterations in P2X and P2Y purinergic receptor expression in urinary bladder from normal cats and cats with interstitial cystitis. American Journal of Physiology– Renal Physiology, 287(5), F1084–F1091.
Bodin, P., & Burnstock, G. (1998). Increased release of ATP from endothelial cells during acute inflammation. Inflammation Research, 47(8), 351–354.
Bodin, P., & Burnstock, G. (2001). Evidence that release of adenosine triphosphate from endothelial cells during increased shear stress is vesicular. Journal of Cardiovascular Pharmacology, 38(6), 900–908.
Braas, K. M., & May, V. (1996). Pituitary adenylate cyclase-activating polypeptides, PACAP-38 and PACAP-27, regulation of sympathetic neuron catecholamine, and neuropeptide Y expression through activation of type I PACAP/VIP receptor isoforms. Annals of the New York Academy of Sciences, 805, 204–216 (discussion 217–208).
Braas, K. M., & May, V. (1999). Pituitary adenylate cyclase-activating polypeptides directly stimulate sympathetic neuron neuropeptide Y release through PAC(1) receptor isoform activation of specific intracellular signaling pathways. Journal of Biological Chemistry, 274(39), 27702–27710.
Braas, K. M., May, V., Harakall, S. A., Hardwick, J. C., & Parsons, R. L. (1998). Pituitary adenylate cyclase-activating polypeptide expression and modulation of neuronal excitability in guinea pig cardiac ganglia. Journal of Neuroscience, 18(23), 9766–9779.
Braas, K. M., May, V., Zvara, P., et al. (2006). Role for pituitary adenylate cyclase activating polypeptide in cystitis-induced plasticity of micturition reflexes. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 290(4), R951–R962.
Brown, C., Burnstock, G., & Cocks, T. (1979). Effects of adenosine 5'-triphosphate (ATP) and beta-gamma-methylene ATP on the rat urinary bladder. British Journal of Pharmacology, 65(1), 97–102.
Burnstock, G. (2000). P2X receptors in sensory neurones. British Journal of Anaesthesia, 84(4), 476–488.
Chopra, B., Barrick, S. R., Meyers, S., et al. (2005). Expression and function of bradykinin B1 and B2 receptors in normal and inflamed rat urinary bladder urothelium. Journal of Physiology, 562(Pt 3), 859–871.
Cockayne, D. A., Hamilton, S. G., Zhu, Q. M., et al. (2000). Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X(3)-deficient mice. Nature, 407(6807), 1011–1015.
Corrow, K. A., & Vizzard, M. A. (2007). Phosphorylation of extracellular signal-regulated kinases in urinary bladder in rats with cyclophosphamide-induced cystitis. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 293(1), R125–134.
Donaldson, L. F., Harmar, A. J., McQueen, D. S., & Seckl, J. R. (1992). Increased expression of preprotachykinin, calcitonin gene-related peptide, but not vasoactive intestinal peptide messenger RNA in dorsal root ganglia during the development of adjuvant monoarthritis in the rat. Brain Res. Mol. Brain Res., 16(1–2), 143–149.
Donovan, M. K., Winternitz, S. R., & Wyss, J. M. (1983). An analysis of the sensory innervation of the urinary system of the rat. Brain Research Bulletin, 11, 321–324.
Erickson, D. R. (1999). Interstitial cystitis: Update on etiologies and therapeutic options. Journal of Women's Health & Gender-based Medicine, 8(6), 745–758.
Erickson, D. R., & Davies, M. F. (1998). Interstitial cystitis. International Urogynecology Journal, 9, 174–183.
Fahrenkrug, J., & Hannibal, J. (1998). Pituitary adenylate cyclase activating polypeptide immunoreactivity in capsaicin-sensitive nerve fibres supplying the rat urinary tract. Neuroscience, 83(4), 1261–1272.
Fizanne, L., Sigaudo-Roussel, D., Saumet, J. L., & Fromy, B. (2004). Evidence for the involvement of VPAC1 and VPAC2 receptors in pressure-induced vasodilatation in rodents. Journal of Physiology, 554(Pt 2), 519–528.
Fox-Threlkeld, J. A., McDonald, T. J., Woskowska, Z., Iesaki, K., & Daniel, E. E. (1999). Pituitary adenylate cyclase-activating peptide as a neurotransmitter in the canine ileal circular muscle. The Journal of Pharmacology and Experimental Therapeutics, 290(1), 66–75.
Girard, B. A., Lelievre, V., Braas, K. M., et al. (2006). Noncompensation in peptide/receptor gene expression and distinct behavioral phenotypes in VIP- and PACAP-deficient mice. Journal of Neurochemistry, 99(2), 499–513.
Girard, B. M., May, V., Bora, S. H., Fina, F., & Braas, K. M. (2002). Regulation of neurotrophic peptide expression in sympathetic neurons: quantitative analysis using radioimmunoassay and real-time quantitative polymerase chain reaction. Regulatory Peptide, 109(1–3), 89–101.
Herrera, G. M., Braas, K. M., May, V., & Vizzard, M. A. (2006). PACAP enhances mouse urinary bladder contractility and is upregulated in micturition reflex pathways after cystitis. Annals of the New York Academy of Sciences, 1070, 330–336.
Hu, V. Y., Malley, S., Dattilio, A., Folsom, J. B., Zvara, P., & Vizzard, M. A. (2003). COX-2 and prostanoid expression in micturition pathways after cyclophosphamide-induced cystitis in the rat. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 284(2), R574–R585.
Hu, V. Y., Zvara, P., Dattilio, A., et al. (2005). Decrease in bladder overactivity with REN1820 in rats with cyclophosphamide induced cystitis. Journal of Urology, 173(3), 1016–1021.
Igawa, Y., Persson, K., Andersson, K. E., Uvelius, B., & Mattiasson, A. (1993). Facilitatory effect of vasoactive intestinal polypeptide on spinal and peripheral micturition reflex pathways in conscious rats with and without detrusor instability. Journal of Urology, 149(4), 884–889.
Jongsma, W. H., Danielsen, N., Johnston, J. M., Gratto, K. A., Karchewski, L. A., & Verge, V. M. K. (2001). Exogenous NT-3 and NGF differentially modulate PACAP expression in adult sensory neurons, suggesting distinct roles in injury and inflammation. European Journal of Neuroscience, 14, 267–282.
Jongsma, W. H., Pettersson, L. M., Verge, V. M., & Danielsen, N. (2003). Effect of anti-nerve growth factor treatment on pituitary adenylate cyclase activating polypeptide expression in adult sensory neurons exposed to adjuvant induced inflammation. Neuroscience, 120(2), 325–331.
Keast, J. R., & de Groat, W. C. (1992). Segmental distribution and peptide content of primary afferent neurons innervating the urogenital organs and colon of male rats. Journal of Comparative Neurology, 319, 615–623.
King, J. A., Huddart, H., & Staff, W. G. (1997). Purinergic modulation of rat urinary bladder detrusor smooth muscle. General Pharmacology, 29(4), 597–604.
Klinger, M. B., Girard, B., & Vizzard, M. A. (2008). p75(NTR) expression in rat urinary bladder sensory neurons and spinal cord with cyclophosphamide-induced cystitis. Journal of Comparative Neurology, 507(3), 1379–1392.
LaBerge, J., Malley, S. E., Zvarova, K., & Vizzard, M. A. (2006). Expression of corticotropin-releasing factor and CRF receptors in micturition pathways after cyclophosphamide-induced cystitis. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 291(3), R692–R703s.
Linden, A., Cardell, L. O., Yoshihara, S., & Nadel, J. A. (1999). Bronchodilation by pituitary adenylate cyclase-activating peptide and related peptides. European Respiratory Journal, 14(2), 443–451.
Lu, C. L., Pasricha, P. J., Hsieh, J. C., et al. (2005). Changes of the neuropeptides content and gene expression in spinal cord and dorsal root ganglion after noxious colorectal distension. Regulatory Peptide, 131(1–3), 66–73.
Maggi, C. A., Lecci, A., Santicioli, P., Del Bianco, E., & Giuliani, S. (1992). Cyclophosphamide cystitis in rats: involvement of capsaicin-sensitive primary afferents. Journal of The Autonomic Nervous System, 38(3), 201–208.
Maggi, C. A., Lecci, A., Santicioli, P., Del Bianco, E., & Giuliani, S. (1993). Cyclophosphamide-induced cystitis in rats: involvement of capsaicin-sensitive primary afferents. Agents Actions, 38, C28–C30.
Mizumoto, A., Fujimura, M., Ohtawa, M., et al. (1992). Pituitary adenylate cyclase activating polypeptide stimulates gallbladder motility in conscious dogs. Regulatory Peptide, 42(1–2), 39–50.
Mohammed, H., Hannibal, J., Fahrenkrug, J., & Santer, R. (2002). Distribution and regional variation of pituitary adenylate cyclase activating polypeptide and other neuropeptides in the rat urinary bladder and ureter: effects of age. Urological Research, 30(4), 248–255.
Moller, K., Reimer, M., Ekblad, E., et al. (1997a). The effects of axotomy and preganglionic denervation on the expression of pituitary adenylate cyclase activating peptide (PACAP), galanin and PACAP type 1 receptors in the rat superior cervical ganglion. Brain Research, 775(1–2), 166–182.
Moller, K., Reimer, M., Hannibal, J., Fahrenkrug, J., Sundler, F., & Kanje, M. (1997b). Pituitary adenylate cyclase-activating peptide (PACAP) and PACAP type 1 receptor expression in regenerating adult mouse and rat superior cervical ganglia in vitro. Brain Research, 775(1–2), 156–165.
Mulderry, P. K., & Lindsay, R. M. (1990). Rat dorsal root ganglion neurons in culture express vasoactive intestinal polypeptide (VIP) independently of nerve growth factor. Neuroscience Letters, 108(3), 314–320.
Murray, E., Malley, S. E., Qiao, L. Y., Hu, V. Y., & Vizzard, M. A. (2004). Cyclophosphamide induced cystitis alters neurotrophin and receptor tyrosine kinase expression in pelvic Ganglia and bladder. Journal of Urology, 172(6 Pt 1), 2434–2439.
Nadelhaft, I., & Vera, P. L. (1995). Central nervous system neurons infected by pseudorabies virus injected into the rat urinary bladder following unilateral transection of the pelvic nerve. Journal of Comparative Neurology, 359, 443–456.
Novak, I. (2003). ATP as a signaling molecule: the exocrine focus. News in Physiological Sciences, 18, 12–17.
Onaga, T., Harada, Y., & Okamoto, K. (1998). Pituitary adenylate cyclase-activating polypeptide (PACAP) induces duodenal phasic contractions via the vagal cholinergic nerves in sheep. Regulatory Peptide, 77(1–3), 69–76.
Rong, W., Spyer, K. M., & Burnstock, G. (2002). Activation and sensitisation of low and high threshold afferent fibres mediated by P2X receptors in the mouse urinary bladder. Journal of Physiology, 541(Pt 2), 591–600.
Rosamilia, A., & Dwyera, P. L. (2000). Pathophysiology of interstitial cystitis. Current Opinion in Obstetrics and Gynecology, 12, 405–410.
Sant, G. R., & Theoharides, T. C. (1999). Interstitial cystitis. Current Opinion in Urology, 9, 297–302.
Schworer, H., Clemens, A., Katsoulis, S., Kohler, H., Creutzfeldt, W., & Schmidt, W. E. (1993). Pituitary adenylate cyclase-activating peptide is a potent modulator of human colonic motility. Scandinavian Journal of Gastroenterology, 28(7), 625–632.
Seebeck, J., Lowe, M., Kruse, M. L., et al. (2002). The vasorelaxant effect of pituitary adenylate cyclase activating polypeptide and vasoactive intestinal polypeptide in isolated rat basilar arteries is partially mediated by activation of nitrergic neurons. Regulatory Peptide, 107(1–3), 115–123.
Sherwood, N. M., Krueckl, S. L., & McRory, J. E. (2000). The origin and function of the pituitary adenylate cyclase-activating polypeptide (PACAP)/glucagon superfamily. Endocrine Reviews, 21(6), 619–670.
Spengler, D., Waeber, C., Pantaloni, C., et al. (1993). Differential signal transduction by five splice variants of the PACAP receptor. Nature, 365, 170–175.
Steenstrup, B. R., Ottesen, B., Jorgensen, M., & Jorgensen, J. C. (1994). Pituitary adenylate cyclase activating polypeptide induces vascular relaxation and inhibits non-vascular smooth muscle activity in the rabbit female genital tract. Acta Physiologica Scandinavica, 152(2), 129–136.
Sun, Y., Keay, S., De Deyne, P. G., & Chai, T. C. (2001a). Augmented stretch activated adenosine triphosphate release from bladder uroepithelial cells in patients with interstitial cystitis. Journal of Urology, 166(5), 1951–1956.
Sun, Y., Keay, S., DeDeyne, P., & Chai, T. (2001b). Stretch-activated release of adenosine triphosphate by bladder uroepithelia is augmented in interstitial cystitis. Urology, 57(6 Suppl 1), 131.
Sundler, F., Ekblad, E., Hannibal, J., et al. (1996). Pituitary adenylate cyclase-activating peptide in sensory and autonomic ganglia: localization and regulation. Annals of The New York Academy of Sciences, 805, 410–426.
Vaudry, D., Gonzalez, B. J., Basille, M., Yon, L., Fournier, A., & Vaudry, H. (2000). Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmaceutical Review, 52(2), 269–324.
Vizzard, M. A. (1997). Increased expression of neuronal nitric oxide synthase in bladder afferent and spinal neurons following spinal cord injury. Developmental Neuroscience, 19(3), 232–246.
Vizzard, M. A. (2000a). Alterations in spinal cord Fos protein expression induced by bladder stimulation following cystitis. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 278(4), R1027–R1039.
Vizzard, M. A. (2000b). Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Experimental Neurology, 161(1), 273–284.
Vizzard, M. A. (2000c). Up-regulation of pituitary adenylate cyclase-activating polypeptide in urinary bladder pathways after chronic cystitis. Journal of Comparative Neurology, 420(3), 335–348.
Vizzard, M. A. (2001). Alterations in neuropeptide expression in lumbosacral bladder pathways following chronic cystitis. Journal of Chemical Neuroanatomy, 21(2), 125–138.
Wang, E. C., Lee, J. M., Ruiz, W. G., et al. (2005). ATP and purinergic receptor-dependent membrane traffic in bladder umbrella cells. Journal of Clinical Investigation, 115(9), 2412–2422.
Zhang, Y. Z., Danielsen, N., Sundler, F., & Mulder, H. (1998). Pituitary adenylate cyclase-activating peptide is upregulated in sensory neurons by inflammation. Neuroreport, 9(12), 2833–2836.
Zhang, Y. Z., Hannibal, J., Zhao, Q., et al. (1996). Pituitary adenylate cyclase activating peptide expression in the rat dorsal root ganglia: up-regulation after peripheral nerve injury. Neuroscience, 74(4), 1099–1110.
Zhang, Q., Shi, T.-J., Ji, R.-R., et al. (1995). Expression of pituitary adenylate cyclase-activating polypeptide in dorsal root ganglia following axotomy: time course and coexistence. Brain Research, 705, 149–158.
Zizzo, M. G., Mule, F., & Serio, R. (2004). Interplay between PACAP and NO in mouse ileum. Neuropharmacology, 46(3), 449–455.
Zvara, P., Braas, K. M., May, V., & Vizzard, M. A. (2006). A role for pituitary adenylate cyclase activating polypeptide (PACAP) in detrusor hyperreflexia after spinal cord injury (SCI). Annals of the New York Academy of Sciences, 1070, 622–628.
Zvara, P., & Vizzard, M. A. (2007). Exogenous overexpression of nerve growth factor in the urinary bladder produces bladder overactivity and altered micturition circuitry in the lumbosacral spinal cord. BMC Physiology, 7(1), 9.
Zvarova, K., Dunleavy, J. D., & Vizzard, M. A. (2005). Changes in pituitary adenylate cyclase activating polypeptide expression in urinary bladder pathways after spinal cord injury. Experimental Neurology, 192(1), 46–59.
Zvarova, K., & Vizzard, M. A. (2005). Distribution and fate of cocaine- and amphetamine-regulated transcript peptide (CARTp)-expressing cells in rat urinary bladder: a developmental study. Journal of Comparative Neurology, 489(4), 501–517.
Zvarova, K., & Vizzard, M. A. (2006). Changes in galanin immunoreactivity in rat micturition reflex pathways after cyclophosphamide-induced cystitis. Cell & Tissue Research, 324(2), 213–224.
Acknowledgments
This work was funded by NIH grants DK051369, DK060481, and DK065989.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Girard, B.M., Wolf-Johnston, A., Braas, K.M. et al. PACAP-Mediated ATP Release from Rat Urothelium and Regulation of PACAP/VIP and Receptor mRNA in Micturition Pathways after Cyclophosphamide (CYP)-Induced Cystitis. J Mol Neurosci 36, 310–320 (2008). https://doi.org/10.1007/s12031-008-9104-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-008-9104-4