Abstract
Ischemic preconditioning (IPC) has been demonstrated to provide a neuroprotection against brain damage produced by focal cerebral ischemia. However, it is elusive whether ischemic preconditioning attenuates ischemic brain damage through modulating phosphatidylinositol 3-kinase/Akt (PI3K/Akt) and extracellular signal-regulated kinase 1/2 (ERK1/2) signaling pathway. In the present study, we first explored the best scheme of repetitive ischemic preconditioning (RIPC) to protect rat brain against ischemic damage and then further investigated the underlying mechanisms in RIPC’s neuroprotection. Adult male Sprague-Dawley rats underwent ischemic preconditioning or (and) middle cerebral artery occlusion (MCAO). LY294002 or (and) PD98059 were injected intracerebroventricularly to selectively inhibit the activation of PI3K/Akt or ERK1/2. Neurological deficit scores, cerebral infarct volume, and morphological characteristic were detected at corresponding time after cerebral ischemia. The enzymatic activity of myeloperoxidase (MPO) was measured 24 h after cerebral ischemia. Expressions of p-Akt, t-Akt, p-ERK1/2, t-ERK1/2, nuclear factor-kappa B (NF-κB) p65, and cyclooxygenase-2 (COX-2) in ischemic brain were determined by Western blot. The release of tumor necrosis factor-α (TNF-α) in blood was examined by ELISA. In the various schemes of RIPC, IPC2 × 5 min causes less neuronal damage in the cortex and subcortex of ischemic brain and provides an obvious alleviation of cerebral infarction and neurological deficit after lethal ischemia. IPC2 × 5 min significantly reduces cerebral infarct volume, neurological deficit scores, and MPO activity; all of which were diminished by LY294002 or (and) PD98059. IPC2 × 5 min significantly upregulates the expressions of p-Akt and p-ERK1/2, which were inhibited by LY294002 or (and) PD98059. IPC2 × 5 min significantly downregulates the expressions of NF-κB p65 and COX-2 and attenuates the release of TNF-α; all of which were abolished by LY294002 or (and) PD98059. IPC2 × 5 min is the best scheme of RIPC to protect rat brain against cerebral ischemia. IPC2 × 5 min attenuates brain damage in rats subjected to lethal ischemia, and this neuroprotection is associated with inhibition of neuroinflammation through modulating PI3K/Akt and ERK1/2 signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebral ischemia produced by transient or permanent focal cerebral ischemia develops a series of pathological processes that results in mediating ischemic brain damage. Post-ischemic neuroinflammation has been demonstrated to be involved in the process of ischemic brain damage, eventually leading to the aggravation of neuronal damage and extension of cerebral infarction (Cuartero et al. 2013). Inhibition of inflammatory reaction and the expression of inflammatory mediators have been reported to play a neuroprotection against brain damage following an ischemic stroke (Liesz et al. 2013). Inflammation has been considered to be an important therapeutic target for acute ischemic stroke (del Zoppo 2010).
Several reports demonstrated that ischemic preconditioning (IPC) reduces ischemia-induced brain damage after permanent of transient focal cerebral ischemia (Sommer 2008; Thompson et al. 2013; Zhan et al. 2013). Various schemes of IPC have been reported to induce ischemic tolerance and protect brain against ischemic damage. To our best knowledge, however, whether repetitive ischemic preconditioning (RIPC) provides more neuroprotection than IPC remains unknown until now.
Moreover, the neuroprotective mechanisms of IPC against ischemic brain damage also needed further investigation in the future. Thompson et al. (2012) showed that IPC attenuates ischemia-induced brain damage via inhibiting the oxidative stress. Xia et al. (2013) demonstrated that hypoxic preconditioning exits a neuroprotective role in a rat cerebral ischemic injury model through autophagy activation and apoptosis inhibition. Zhang et al. (2006) showed that IPC reduces ischemic brain damage by increasing the expression of matrix metalloproteinase-9 (MMP-9) and improving the blood-brain barrier (BBB) permeability in rat brain. However, never was explored that whether RIPC attenuates ischemic brain damage by inhibiting inflammatory reaction.
Phosphatidylinositol 3-kinase/Akt (PI3K/Akt) pathway and extracellular signal-regulated kinase 1/2 (ERK1/2) pathway are the two most important signaling pathways involved in the neuroprotection against ischemic brain damage, likely playing a critical role in promoting neuronal survival after ischemia (Kilic et al. 2005; Zhu et al. 2013). Classically, the neuroprotective properties of PI3K/Akt and ERK1/2 have been primarily attributed to the anti-apoptotic actions (Sirén et al. 2001) or the anti-oxidative actions (Wang et al. 2012). Leptin was demonstrated to protect against ischemic neuronal death in rat hippocampal CA1 by activating the pro-survival states of PI3K/Akt and ERK1/2 signaling pathways (Zhang and Chen 2008).
In the present study, we demonstrated that RIPC exerts a neuroprotective role in a rat model of focal cerebral ischemia. Then, we explored the inhibitory effect of RIPC on neuroinflammation following an experimental stroke. To further confirm the molecular mechanisms underlying the RIPC’s neuroprotection and anti-neuroinflammation, we investigated whether RIPC attenuates the inflammatory reaction in rat brain by activating PI3K/Akt and ERK1/2 signaling pathways. The expressions of downstream inflammatory mediators were also determined.
Materials and Methods
Animals and Experimental Design Flow
All animal experiments were conducted according to the National Institute of Health Guide for the Care and Use of Laboratory Animals. Adult male Sprague-Dawley rats weighing 250–300 g were obtained from Shanghai Laboratory Animal Center, Chinese Academy of Sciences. Animals were housed in a colony room under controlled temperature (22 °C) and a 12:12 light-dark cycle, with food and water available. The flow of experimental designs is present in Figs. 1 and 2.
Ischemic preconditioning groups and route diagram for experimental design. In the first part, rats underwent different cerebral IPC schemes by occluding bilateral CCA involved repeatedly transient cerebral ischemia with a 15-min intermission, including sham IPC, IPC 1 × 5 min, IPC 2 × 5 min, IPC 3 × 5 min, and IPC 1 × 15 min groups. Then, cerebral infarction and neuronal injury were estimated 72 h after IPC. In the second part, rats that received various IPC schemes were subjected to lethal middle cerebral artery occlusion (MCAO) after 48-h reperfusion. Neurological deficit scores (NDS) and cerebral infarct volume were measured 24 h after lethal ischemia
Flowsheet for intracerebroventricular injection, ischemic preconditioning, establishing MCAO model, and sample collection. LY294002 or PD98059 was injected intracerebroventricularly 15 min before IPC to inhibit PI3K/Akt or ERK1/2 signaling pathway. Then, cerebral RIPC was produced by occluding bilateral common carotid artery twice with a 15-min intermission. After 48-h reperfusion, rats were subjected to middle cerebral artery occlusion. Twenty-four hours after lethal ischemia, the rats were sacrificed and rat brains were collected
IPC Schemes
To explore which RIPC was the best scheme of RIPC protecting rat brain against focal cerebral ischemia, the experimental design was separated into two parts (Fig. 1). In the first part, to observe the histopathological characteristic produced by various RIPC schemes, adult male SD rats underwent different cerebral IPC by occluding bilateral common carotid artery (CCA) involved repeatedly in transient cerebral ischemia with a 15 min intermission, which includes sham IPC, IPC 1 × 5 min, IPC 2 × 5 min, IPC 3 × 5 min, and IPC 1 × 15 min groups. Then, 2,3,5-triphenyltetrazolium chloride (TTC) staining and hematoxylin and eosin (HE) staining were performed 72 h after simple IPC. In the second part, to explore the best scheme of RIPC against ischemic brain damage, SD rats received various sublethal IPC schemes respectively and then underwent lethal middle cerebral artery occlusion (MCAO) after 48-h reperfusion. Neurological deficit scores and cerebral infarct volume were measured 24 h after lethal ischemia.
MCAO
After 48-h reperfusion, rats were anesthetized again with an intraperitoneal injection of chloral hydrate (300 mg/kg) and subjected to MCAO as described previously (Shi et al. 2013). In brief, the right CCA, external carotid artery (ECA), and internal carotid artery (ICA) were carefully exposed. A monofilament suture with a distal cylinder (0.32 mm in diameter) was inserted from the ECA into the ICA and advanced to occlude the origin of the middle cerebral artery (MCA). The sham-control rats underwent the same surgery, except that the filament was inserted only 10 mm and withdrawn a minute later. Rectal temperature was maintained at 37.0 °C with a heating pad and warm light during the surgical procedure.
ICV Injection
To investigate whether PI3K/Akt and ERK1/2 signaling mediates the IPC’s neuroprotection, LY294002 (selective Akt inhibitor) or PD98059 (selective ERK1/2 inhibitor) was injected intracerebroventricularly 15 min before IPC to inhibit PI3K/Akt or ERK1/2 signaling pathway (Fig. 2). In brief, LY294002 or PD98059 was dissolved in dimethyl sulfoxide (DMSO) and diluted with PBS to 10 μM. With use of a stereotaxic device (Reward, Shenzhen, China), 10 μL of LY294002 or (and) PD98059 solution was injected into the right ventricle of rats. The stereotactic intracerebroventricular (ICV) injection site was chosen at the following sites from bregma: anteroposterior, 0.8 mm; lateral, 1.5 mm; and depth, 3.5 mm.
Histological Examination
After 72 h of IPC, rats were anesthetized and perfused with 4 % paraformaldehyde in phosphate-buffered saline. Then, brains were removed, fixed, and embedded in paraffin. Coronal sections (4 μm thick) were obtained from embedded paraffin and deparaffinized with xylene and rehydrated with graded alcohol. To detect whether simple IPC produces neuronal damage, HE staining was performed to observe the morphological characteristic of neurons in the cortex and subcortex of rat brains.
TTC Staining
To detect whether simple IPC produces cerebral infarction in the cortex and subcortex of rat brains. After 72 h of IPC, rats were sacrificed and brains were rapidly removed and coronally sliced into 2.0-mm-thick sections. Brain slices were incubated in 2 % TTC for 20 min. The infarcted brain tissue will appear white, whereas the noninfarcted region will appear red.
Assessment of Neurological Deficit Scores
Neurological deficit scores were estimated at 24 h after lethal ischemia according to the previous method described by Bederson et al. (1986), as follows: 0, no observable deficit; 1, contralateral forelimb flexion; 2, decreased resistance to lateral push without circling; and 3, circling to the contralateral side.
Assessment of Cerebral Infarct Volume
Rats were sacrificed under deep anesthesia, and brains were rapidly removed and coronally sliced into 2.0-mm-thick sections. Brain slices were incubated in 2 % TTC for 20 min. The infarcted brain tissue appeared white, whereas the noninfarcted region appeared red. The sections were digitized, and the infarct areas were measured using Photoshop software by tracing around the white area in each brain section. Infarct volume was calculated according to the following formula: V = t × (A 1 + A 2 + . . . An). V is the infarct volume, t is the thickness of slice, and A is the infarct area. Correction for edema of infarct area was performed as described by Lin et al. (1993).
Biochemical Analysis
The neutrophil infiltration, an index of neuroinflammation in ischemic brain, was determined by measuring the enzymatic activity of myeloperoxidase (MPO). The activity of MPO in rat brain was measured according to the manufacturer’s instructions from the assay kit (Nanjing Jiancheng Bioengineering Institute, China). The results were expressed as units per gram tissue.
Western Blot
Samples from ischemic brain were used for experiments, and total protein was extracted using protein extraction kit (Beyotime Biotech. CO., China) according to the manufacturer’s instructions. Protein samples (50 μg) were separated on 10 % SDS polyacrylamide gels, then transferred to nitrocellulose (NC) membranes, and blocked in 5 % nonfat dry milk buffer. The membranes were incubated overnight at 4 °C with a rabbit polyclonal antibody against p-Akt (1:200, Santa Cruz, USA), or a rabbit polyclonal antibody against t-Akt (1:1,000, Santa Cruz, USA), or a mouse monoclonal antibody against p-ERK1/2 (1:1,000, Santa Cruz, USA), or a rabbit polyclonal antibody against t-ERK1/2 (1:2,000, Santa Cruz, USA), or a mouse monoclonal antibody against NF-κB p65 (1:200, Santa Cruz, USA), or a rabbit polyclonal antibody against COX-2 (1:200, Wuhan Boster Biological Technology, LTD, Wuhan, Chian), followed by incubation with horseradish-peroxidase conjugated secondary antibodies (1:2,000, KPL Inc.). Protein expression was detected with an ECL detection system and exposed on an X-ray film. GAPDH was used as a loading control. The optical densities of protein bands on the X-ray film were quantitatively analyzed with Quantity One software.
ELISA Assay
After 24 h of lethal ischemia, blood samples (1 ml) were drawn from femoral vein of rats. After centrifugation at 3,000 r/min for 15 min, the supernatant was collected and stored at −80 °C in the refrigerator. Serum level of TNF-α was measured using a rat TNF-α immunoassay enzyme-linked immunosorbent assay (ELISA) kits (R&D system).
Statistical Analysis
Experimental data were presented as mean ± SD. Statistical analysis was performed using ANOVA followed by LSD test for individual comparisons between group means. A value of P < 0.05 was considered statistically significant.
Results
Estimate the Destructive Extent of Simple RIPCs on Rat Brain
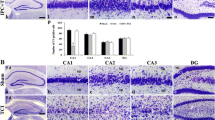
To estimate the destructive extent of various RIPC schemes on rat brains, TTC staining and HE staining were performed 72 h after simple RIPC. Experimental results showed that IPC 1 × 15 min and IPC3 × 5 min, but not IPC 2 × 5 min, schemes induce slight cerebral infarction in the subcortex and an obvious neuronal injury in the cortex and subcortex (Fig. 3).
Cerebral infarction and neuronal injury produced by various IPCs in rat brains were determined by the methods of TTC staining and HE staining. Experimental results showed that IPC 1 × 15 min or IPC3 × 5 min induce slight cerebral infarction in the subcortex (arrowhead) and an obvious neuronal injury in the cortex and subcortex (arrow), but not IPC 2 × 5 min scheme
Effect of Various RIPCs on Neurological Deficit Scores and Cerebral Infarct Volume
Experimental results showed that all of IPC2 × 5 min, IPC3 × 5 min, and IPC1 × 15 min significantly reduce neurological deficit scores after cerebral ischemia (P < 0.05 or P < 0.01), but not IPC1 × 5 min (P > 0.05, Fig. 4c). All of IPC1 × 5 min, IPC2 × 5 min, IPC3 × 5 min, and IPC1 × 15 min could significantly reduce cerebral infarct volume after cerebral ischemia (P < 0.05 or P < 0.01, Fig. 4b). Cortex infarction was significantly reduced by various IPC strategies (P < 0.05 or P < 0.01, Fig. 4d), and subcortex infarction was reduced by IPC2 × 5 min, IPC3 × 5 min, and IPC1 × 15 min (P < 0.05 or P < 0.01, Fig. 4d).
Effect of various IPCs on the neurological deficit scores (NDS) and cerebral infarct volume including cortex and subcortex after cerebral ischemia. Experimental results showed that IPC2 × 5 min, IPC3 × 5 min, and IPC1 × 15 min could reduce NDS (c). Representative TTC-stained brain sections were shown where rats were subjected to various IPC strategies and lethal MCAO (a), and the experimental results were summarized in (b, d). Cortex infarction was significantly reduced by various IPC strategies, and subcortex infarction was reduced by IPC2 × 5 min, IPC3 × 5 min, and IPC1 × 15 min. n = 6, *P < 0.05, and #P < 0.01 compared to control group and sham IPC group. NS means no significant difference
IPC2 × 5 min Reduces Ischemic Brain Damage via PI3K/Akt and ERK1/2 Signaling Pathway
To investigate the potential roles of PI3K/Akt and ERK1/2 signaling pathway in the IPC2 × 5 min neuroprotection against ischemic brain damage, LY294002 (selective Akt inhibitor) or (and) PD98059 (selective ERK1/2 inhibitor) was injected intracerebroventricularly to inhibit the activation of PI3K/Akt or ERK1/2 signaling. The experimental results showed that IPC2 × 5 min reduced cerebral infarct volume in rats after focal cerebral ischemia (P < 0.01, Fig. 5a), which was abolished by administration of LY294002 or (and) PD98059 (P < 0.05 or P < 0.01, Fig. 5b), demonstrating that IPC2 × 5 min provides a neuroprotective role in cerebral ischemia through modulating PI3K/Akt and ERK1/2 signaling pathway. IPC2 × 5 min reduced neurological deficit scores in rats after focal cerebral ischemia (P < 0.01, Fig. 5c), which was abolished by administration of LY294002 or (and) PD98059 (P < 0.05 or P < 0.01, Fig. 5c), indicating that IPC2 × 5 min exerts a neuroprotection against ischemic brain damage through modulating PI3K/Akt and ERK1/2 signaling pathway.
PI3K/Akt and ERK1/2 mediate IPC2 × 5 min’s neuroprotection in rats of focal cerebral ischemia. Representative TTC-stained brain sections were shown where rats were subjected to intracerebroventricular injection of LY294002 or (and) PD98059, IPC2 × 5 min, and lethal MCAO (a), and the experimental results were summarized in (b). IPC2 × 5 min reduced cerebral infarct volume in rats after focal cerebral ischemia, which was abolished by administration of LY294002 or (and) PD98059. IPC2 × 5 min reduced neurological deficit scores in rats after focal cerebral ischemia, which was abolished by administration of LY294002 or (and) PD98059 (c). n = 6, *P < 0.05, and **P < 0.01 compared to IPC2 × 5 min group, #P < 0.01 compared to LY294002 group or PD98059 group
Time-Course Expression of PI3K/Akt and ERK1/2 in Rats of Focal Cerebral Ischemia
Experimental results showed that the expression of p-Akt was upregulated 12 h after cerebral ischemia (P < 0.01) and then downregulated 24–72 h (P < 0.01, Fig. 6). The expression of p-ERK1/2 was upregulated 6 h in ischemic rat brains (P < 0.01) and decreased 12–72 h after cerebral ischemia (P < 0.01, Fig. 6). The expressions of t-Akt and t-ERK1/2 were sustained from 6 to 72 h after cerebral ischemia (P > 0.05).
Time-course expression of PI3K/Akt and ERK1/2 in rats of focal cerebral ischemia. Representative protein expression bands of p-Akt, t-Akt, p-ERK1/2, t-ERK1/2, and GAPDH in ischemic rat brains after focal cerebral ischemia were detected by Western blot (a), and the data are summarized in (b). The expression of p-Akt was upregulated 12 h after cerebral ischemia and then downregulated 24–72 h. The expression of p-ERK1/2 was upregulated 6 h after cerebral ischemia and then decreased 12–72 h. The expressions of t-Akt and t-ERK1/2 were sustained from 6 to 72 h after cerebral ischemia. n = 6, #P < 0.01 compared to sham control
Effect of LY294002 or PD98059 on the PI3K/Akt and ERK1/2 Signaling Pathway in Rats Subjected to IPC2 × 5 min and Lethal Ischemia
MCAO caused the downregulated expression of p-Akt and p-ERK1/2 24 h after focal cerebral ischemia in rats. IPC2 × 5 min upregulated the expression of p-Akt and p-ERK1/2 in rat brain (P < 0.01), both of which were abolished by administration of LY294002 or (and) PD98059 (P < 0.05 or P < 0.01, Fig. 7), demonstrating that LY294002 and PD98059 could inhibit the activation of PI3K/Akt and ERK1/2 in rats of cerebral ischemia, respectively. The expressions of t-Akt and t-ERK1/2 were not changed.
Effect of LY294002 or (and) PD98059 on the expression of PI3K/Akt and ERK1/2 in rats subjected to IPC2 × 5 min and focal cerebral ischemia. Representative protein expression bands of p-Akt, t-Akt, p-ERK1/2, t-ERK1/2, and GAPDH in ischemic rat brains were detected by Western blot (a), and the data are summarized in (b). MCAO caused the downregulated expression of p-Akt and p-ERK1/2 in rats 24 h after focal cerebral ischemia. IPC2 × 5 min upregulated the expression of p-Akt and p-ERK1/2 in ischemic rat brain, both of which were abolished by administration of LY294002 or (and) PD98059. There were no changes in the expression of t-Akt and t-ERK1/2. n = 6, #P < 0.01 compared to MCAO group. *P < 0.05 and **P < 0.01 compared to IPC2 × 5 min group
IPC2 × 5 min Reduces Neutrophil Infiltration via PI3K/Akt and ERK1/2 Signaling Pathway
The enzymatic activity of MPO was measured as an indicator of the accumulation of granulocytes and neuroinflammation in ischemic brain. MCAO caused the elevated enzymatic activities of MPO in rat brains 24 h after focal cerebral ischemia (P < 0.01). IPC2 × 5 min decreased the MPO activities (P < 0.01), which was inhibited by administration of LY294002 or (and) PD98059 (P < 0.05, Fig. 8).
PI3K/Akt and ERK1/2 mediate IPC2 × 5 min anti-neuroinflammation in rats of focal cerebral ischemia. MCAO caused the elevated activities of MPO in rat brains 24 h after focal cerebral ischemia. IPC2 × 5 min decreased MPO activities, which was abolished by administration of LY294002 or (and) PD98059. n = 6, *P < 0.05 compared to IPC2 × 5 min group
IPC2 × 5 min Downregulates the Over-Expression of NF-κB p65 and COX-2 via PI3K/Akt and ERK1/2 Signaling Pathway
IPC2 × 5 min downregulated the over-expression of nuclear factor-kappa B (NF-κB) p65 and cyclooxygenase-2 (COX-2) in rat brain produced by ischemic stroke (P < 0.01), both of which were inhibited by administration of LY294002 or (and) PD98059 (P < 0.05 or P < 0.01, Fig. 9).
IPC2 × 5 min inhibits the expression of NF-κB and COX-2 via PI3K/Akt and ERK1/2 signaling pathway. Representative protein expression bands of NF-κB p65, COX-2, and GAPDH in ischemic rat brains were detected by Western blot (a), and the data were summarized in b. MCAO caused the upregulated expression of NF-κB p65 and COX-2 in rats 24 h after focal cerebral ischemia. IPC2 × 5 min downregulated the expression of NF-κB p65 and COX-2 in ischemic rat brain, both of which were inhibited by administration of LY294002 or (and) PD98059. n = 6, #P < 0.01 compared to MCAO group. *P < 0.05 and **P < 0.01 compared to IPC2 × 5 min group
IPC2 × 5 min Decreases the Release of TNF-α in Blood via PI3K/Akt and ERK1/2 Signaling Pathway
MCAO caused the elevated release of TNF-α in blood 24 h after focal cerebral ischemia (P < 0.01). IPC2 × 5 min decreased the release of TNF-α in blood (P < 0.01), which was significantly inhibited by administration of LY294002, but not PD98059 (P < 0.05 or P < 0.01, Fig. 10).
IPC2 × 5 min decreases the release of TNF-α via PI3K/Akt and ERK1/2 signaling pathway. MCAO caused the elevated release of TNF-α in blood 24 h after focal cerebral ischemia. IPC2 × 5 min decreased the release of TNF-α in blood, which was inhibited by administration of LY294002, but not PD98059. n = 6, *P < 0.05, and **P < 0.01 compared to IPC2 × 5 min group
Discussion
Stroke is the second to third most common cause of mortality and the leading cause of adult neurological disability. Thrombolytic therapy (TT) and neuroprotective therapy (NT) are the two most important treatments for acute ischemic stroke (AIS) (Durukan and Tatlisumak 2007). In the ischemic penumbra surrounding the infarct core following an ischemic stroke, neuronal viability is time-dependent (Smith 2004). To date, tissue-plasminogen activator (t-PA) is the only FDA-approved thrombolytic therapeutic drug for AIS within a 3–4.5-h time window (Hacke et al. 2008; Chavez et al. 2009). However, only 1.8–2.1 % of patients receive thrombolytic therapy in USA, which might be attributed to the short time window (Kleindorfer et al. 2008). In animal models of AIS, neuroprotective therapy targeted at enhancing neuronal viability to widen the time window of thrombolytic therapy has been investigated (Fisher and Albers 2013).
Ischemic preconditioning (IPC) has been widely reported to reduce ischemia-induced brain damage after focal cerebral ischemia (Sommer 2008; Thompson et al. 2013; Zhan et al. 2013). To our best knowledge, however, it is unknown whether repetitive IPC (RIPC) also provides a neuroprotective effect against permanent focal cerebral ischemia. In the present study, we first demonstrated that various RIPCs exert neuroprotective effect in rats of permanent focal cerebral ischemia. IPC2 × 5 min is the best scheme of RIPCs to protect rat brain against cerebral ischemia. Moreover, IPC2 × 5 min reduces neuronal injury more obviously than other RIPC schemes.
Neuroinflammation, one of the most important pathological mechanisms in the process of ischemia-induced brain damage, has been widely considered to be the major therapeutic target for ischemic stroke. Previous studies from our laboratory demonstrated that inhibition of inflammatory reaction would reduce ischemic brain damage in rats of focal cerebral ischemia (Tu et al. 2009, 2010a, 2011). Ischemic preconditioning has been demonstrated to exert a protective effect via reducing inflammatory reaction in experimental animal model of heart ischemia-reperfusion injury (Zubakov et al. 2003), liver ischemia-reperfusion injury (Romanque et al. 2010), renal ischemia-reperfusion injury (Kinsey et al. 2010; Chen et al. 2009), intestine ischemia-reperfusion injury (Aksöyek et al. 2002), limb ischemia-reperfusion injury (Mansour et al. 2012), and acute pancreatitis (Warzecha et al. 2007). Bowen et al. (2006) showed that IPC induced neuroprotection against focal cerebral ischemia by preventing inflammatory reaction. The present study showed that a novel ischemic preconditioning scheme, repetitive IPC, attenuates the enzymatic activities of MPO in ischemic rat brain, suggesting that RIPC’s neuroprotection might be associated with the inhibition of neuroinflammation.
Then, we further investigated the potential roles of PI3K/Akt and ERK1/2 signaling pathways in mediating RIPC’s neuroprotection and anti-neuroinflammation against ischemic brain damage. Previous studies showed that IPC protects brain ischemia through inhibiting the oxidative stress (Thompson et al. 2012), inhibiting neuronal apoptosis (Xia et al. 2013), attenuating ubiquitin aggregation (Lee et al. 2014), and improving the blood-brain barrier permeability (Zhang et al. 2006). IPC was reported to attenuate ischemic injury of the neuron or brain by activating PI3K/Akt signaling pathway in vitro and in vivo (Bhuiyan et al. 2011; Prasad et al. 2011; Gao et al. 2010). Similarly, IPC was also reported to protect brain damage by activating ERK1/2 signaling pathway following an experimental stroke (Jones and Bergeron 2004; Autheman et al. 2012). The present study showed that IPC2 × 5 min attenuates neurological deficit scores, cerebral infarct volume, and the enzymatic activity of MPO in rats subjected to focal cerebral ischemia, which were abolished by administration of LY294002 or (and) PD98059, suggesting that PI3K/Akt and ERK1/2 mediates IPC2 × 5 min neuroprotection and anti-neuroinflammation.
To further explore the molecular mechanisms underlying RIPC’s anti-neuroinflammation, we detected the expression changes of inflammatory mediators that involved in the pathological process of ischemic brain damage under the inhibitory condition of PI3K/Akt and ERK1/2. NF-κB signaling pathway, which initiates the expression of inflammatory genes and leads to expansion of cerebral infarction, has been considered to be an important therapeutic target for ischemic stroke (Dong et al. 2013). Inflammatory mediators such as COX-2 and TNF-α were the downstream signaling of NF-κB pathway. NF-κB has been shown to regulate the expression of COX-2 and TNF-α that contributes to the evolution of ischemic brain damage (Tu et al. 2010b). Attenuation of COX-2 expression and TNF-α production has been demonstrated to protect brain against ischemic damage (Zhou et al. 2013). The present study showed that IPC2 × 5 min attenuates the NF-κB activation, COX-2 expression, and TNF-α release in rats subjected to focal cerebral ischemia, all of which were inhibited by administration of LY294002 or (and) PD98059, suggesting that IPC2 × 5 min inhibits the expression and activation of inflammatory mediators through activating PI3K/Akt and ERK1/2 signaling pathway.
Conclusions
The present studies were performed to evaluate the effect of repetitive ischemic preconditioning on focal cerebral ischemia in rats. In the first stage of research, we found that IPC2 × 5 min is the best scheme of RIPCs to protect rat brain against focal cerebral ischemia. Then, we found that IPC2 × 5 min reduces ischemic brain damage and neuroinflammation via modulating PI3K/Akt and ERK1/2 signaling pathway. Furthermore, IPC2 × 5 min attenuates the expression of NF-κB/COX-2/TNF-α inflammatory pathway through activating PI3K/Akt and ERK1/2 signaling pathway. These results suggest that PI3K/Akt and ERK1/2 signaling pathway might be the therapeutic targets for ischemic stroke in the future.
Abbreviations
- AIS:
-
Acute ischemic stroke
- BBB:
-
Blood-brain barrier
- COX-2:
-
Cyclooxygenase-2
- DMSO:
-
Dimethyl sulfoxide
- ELISA:
-
Enzyme-linked immunosorbent assay
- ERK1/2:
-
Extracellular signal-regulated kinase 1/2
- HE:
-
Hematoxylin and eosin
- ICV:
-
Intracerebroventricular
- IPC:
-
Ischemic preconditioning
- MCAO:
-
Middle cerebral artery occlusion
- MMP-9:
-
Matrix metalloproteinase-9
- MPO:
-
Myeloperoxidase
- NF-κB:
-
Nuclear factor-kappa B
- NT:
-
Neuroprotective therapy
- PI3K/Akt:
-
Phosphatidylinositol 3-kinase/Akt
- RIPC:
-
Repetitive ischemic preconditioning
- TNF-α:
-
Tumor necrosis factor-α
- t-PA:
-
Tissue-plasminogen activator
- TT:
-
Thrombolytic therapy
- TTC:
-
2,3,5-Triphenyltetrazolium chloride
References
Aksöyek S, Cinel I, Avlan D, Cinel L, Oztürk C, Gürbüz P, Nayci A, Oral U (2002) Intestinal ischemic preconditioning protects the intestine and reduces bacterial translocation. Shock 18(5):476–480
Autheman D, Sheldon RA, Chaudhuri N, von Arx S, Siegenthaler C, Ferriero DM, Christen S (2012) Glutathione peroxidase overexpression causes aberrant ERK activation in neonatal mouse cortex after hypoxic preconditioning. Pediatr Res 72(6):568–575
Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H (1986) Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke 17(3):472–476
Bhuiyan MI, Jung SY, Kim HJ, Lee YS, Jin C (2011) Major role of the PI3K/Akt pathway in ischemic tolerance induced by sublethal oxygen-glucose deprivation in cortical neurons in vitro. Arch Pharm Res 34(6):1023–1034
Bowen KK, Naylor M, Vemuganti R (2006) Prevention of inflammation is a mechanism of preconditioning-induced neuroprotection against focal cerebral ischemia. Neurochem Int 49(2):127–135
Chavez JC, Hurko O, Barone FC, Feuerstein GZ (2009) Pharmacologic interventions for stroke: looking beyond the thrombolysis time window into the penumbra with biomarkers, not a stopwatch. Stroke 40(10):e558–e563
Chen X, Liu X, Wan X, Wu Y, Chen Y, Cao C (2009) Ischemic preconditioning attenuates renal ischemia-reperfusion injury by inhibiting activation of IKKbeta and inflammatory response. Am J Nephrol 30(3):287–294
Cuartero MI, Ballesteros I, Moraga A, Nombela F, Vivancos J, Hamilton JA, Corbí ÁL, Lizasoain I, Moro MA (2013) N2 neutrophils, novel players in brain inflammation after stroke: modulation by the PPARγ agonist rosiglitazone. Stroke 44:3498–3508
del Zoppo GJ (2010) Acute anti-inflammatory approaches to ischemic stroke. Ann N Y Acad Sci 1207:143–148
Dong L, Qiao H, Zhang X, Zhang X, Wang C, Wang L, Cui L, Zhao J, Xing Y, Li Y, Liu Z, Zhu C (2013) Parthenolide is neuroprotective in rat experimental stroke model: downregulating NF-κB, phospho-p38MAPK, and caspase-1 and ameliorating BBB permeability. Mediat Inflamm 2013:370804
Durukan A, Tatlisumak T (2007) Acute ischemic stroke: overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol Biochem Behav 87(1):179–197
Fisher M, Albers GW (2013) Advanced imaging to extend the therapeutic time window of acute ischemic stroke. Ann Neurol 73(1):4–9
Gao X, Zhang H, Steinberg G, Zhao H (2010) The Akt pathway is involved in rapid ischemic tolerance in focal ischemia in rats. Transl Stroke Res 1(3):202–209
Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D (2008) Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 359(26):1317–1329
Jones NM, Bergeron M (2004) Hypoxia-induced ischemic tolerance in neonatal rat brain involves enhanced ERK1/2 signaling. J Neurochem 89(1):157–167
Kilic E, Kilic U, Soliz J, Bassetti CL, Gassmann M, Hermann DM (2005) Brain-derived erythropoietin protects from focal cerebral ischemia by dual activation of ERK-1/-2 and Akt pathways. FASEB J 19(14):2026–2028
Kinsey GR, Huang L, Vergis AL, Li L, Okusa MD (2010) Regulatory T cells contribute to the protective effect of ischemic preconditioning in the kidney. Kidney Int 77(9):771–780
Kleindorfer D, Lindsell CJ, Brass L, Koroshetz W, Broderick JP (2008) National US estimates of recombinant tissue plasminogen activator use: ICD-9 codes substantially underestimate. Stroke 39(3):924–928
Lee JC, Kim IH, Cho GS, Park JH, Ahn JH, Yan BC, Kwon HM, Kim YM, Cheon SH, Cho JH, Lee HY, Won MH, Seo JY (2014) Ischemic preconditioning-induced neuroprotection against transient cerebral ischemic damage via attenuating ubiquitin aggregation. J Neurol Sci 336(1–2):74–82
Liesz A, Zhou W, Na SY, Hämmerling GJ, Garbi N, Karcher S, Mracsko E, Backs J, Rivest S, Veltkamp R (2013) Boosting regulatory T cells limits neuroinflammation in permanent cortical stroke. J Neurosci 33(44):17350–17362
Lin TN, He YY, Wu G, Khan M, Hsu CY (1993) Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke 24(1):117–121
Mansour Z, Charles AL, Bouitbir J, Pottecher J, Kindo M, Mazzucotelli JP, Zoll J, Geny B (2012) Remote and local ischemic postconditioning further impaired skeletal muscle mitochondrial function after ischemia-reperfusion. J Vasc Surg 56(3):774–82.e1
Prasad SS, Russell M, Nowakowska M (2011) Neuroprotection induced in vitro by ischemic preconditioning and postconditioning: modulation of apoptosis and PI3K-Akt pathways. J Mol Neurosci 43(3):428–442
Romanque P, Díaz A, Tapia G, Uribe-Echevarría S, Videla LA, Fernandez V (2010) Delayed ischemic preconditioning protects against liver ischemia-reperfusion injury in vivo. Transplant Proc 42(5):1569–1575
Shi SS, Yang WZ, Tu XK, Wang CH, Chen CM, Chen Y (2013) 5-Lipoxygenase inhibitor zileuton inhibits neuronal apoptosis following focal cerebral ischemia. Inflammation 36(6):1209–1217
Sirén AL, Fratelli M, Brines M, Goemans C, Casagrande S, Lewczuk P, Keenan S, Gleiter C, Pasquali C, Capobianco A, Mennini T, Heumann R, Cerami A, Ehrenreich H, Ghezzi P (2001) Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci U S A 98(7):4044–4049
Smith WS (2004) Pathophysiology of focal cerebral ischemia: a therapeutic perspective. J Vasc Interv Radiol 15(1 Pt 2):S3–S12
Sommer C (2008) Ischemic preconditioning: postischemic structural changes in the brain. J Neuropathol Exp Neurol 67(2):85–92
Thompson JW, Narayanan SV, Perez-Pinzon MA (2012) Redox signaling pathways involved in neuronal ischemic preconditioning. Curr Neuropharmacol 10(4):354–369
Thompson JW, Dave KR, Young JI, Perez-Pinzon MA (2013) Ischemic preconditioning alters the epigenetic profile of the brain from ischemic intolerance to ischemic tolerance. Neurotherapeutics 10(4):789–797
Tu XK, Yang WZ, Shi SS, Wang CH, Chen CM (2009) Neuroprotective effect of baicalin in a rat model of permanent focal cerebral ischemia. Neurochem Res 34(9):1626–1634
Tu XK, Yang WZ, Wang CH, Shi SS, Zhang YL, Chen CM, Yang YK, Jin CD, Wen S (2010a) Zileuton reduces inflammatory reaction and brain damage following permanent cerebral ischemia in rats. Inflammation 33(5):344–352
Tu XK, Yang WZ, Shi SS, Wang CH, Zhang GL, Ni TR, Chen CM, Wang R, Jia JW, Song QM (2010b) Spatio-temporal distribution of inflammatory reaction and expression of TLR2/4 signaling pathway in rat brain following permanent focal cerebral ischemia. Neurochem Res 35(8):1147–1155
Tu XK, Yang WZ, Shi SS, Chen Y, Wang CH, Chen CM, Chen Z (2011) Baicalin inhibits TLR2/4 signaling pathway in rat brain following permanent cerebral ischemia. Inflammation 34(5):463–470
Wang Z, Zhang H, Xu X, Shi H, Yu X, Wang X, Yan Y, Fu X, Hu H, Li X, Xiao J (2012) bFGF inhibits ER stress induced by ischemic oxidative injury via activation of the PI3K/Akt and ERK1/2 pathways. Toxicol Lett 212(2):137–146
Warzecha Z, Dembiński A, Ceranowicz P, Dembiński M, Cieszkowski J, Kuśnierz-Cabala B, Naskalski JW, Jaworek J, Konturek SJ, Pawlik WW, Tomaszewska R (2007) Influence of ischemic preconditioning on blood coagulation, fibrinolytic activity and pancreatic repair in the course of caerulein-induced acute pancreatitis in rats. J Physiol Pharmacol 58(2):303–319
Xia DY, Li W, Qian HR, Yao S, Liu JG, Qi XK (2013) Ischemia preconditioning is neuroprotective in a rat cerebral ischemic injury model through autophagy activation and apoptosis inhibition. Braz J Med Biol Res 46(7):580–588
Zhan L, Yan H, Zhou H, Sun W, Hou Q, Xu E (2013) Hypoxic preconditioning attenuates neuronal cell death by preventing MEK/ERK signaling pathway activation after transient global cerebral ischemia in adult rats. Mol Neurobiol 48(1):109–119
Zhang F, Chen J (2008) Leptin protects hippocampal CA1 neurons against ischemic injury. J Neurochem 107(2):578–587
Zhang FY, Chen XC, Ren HM, Bao WM (2006) Effects of ischemic preconditioning on blood–brain barrier permeability and MMP-9 expression of ischemic brain. Neurol Res 28(1):21–24
Zhou R, Yang Z, Tang X, Tan Y, Wu X, Liu F (2013) Propofol protects against focal cerebral ischemia via inhibition of microglia-mediated proinflammatory cytokines in a rat model of experimental stroke. PLoS ONE 8(12):e82729
Zhu YM, Wang CC, Chen L, Qian LB, Ma LL, Yu J, Zhu MH, Wen CY, Yu LN, Yan M (2013) Both PI3K/Akt and ERK1/2 pathways participate in the protection by dexmedetomidine against transient focal cerebral ischemia/reperfusion injury in rats. Brain Res 1494:1–8
Zubakov D, Hoheisel JD, Kluxen FW, Brändle M, Ehring T, Hentsch B, Frohme M (2003) Late ischemic preconditioning of the myocardium alters the expression of genes involved in inflammatory response. FEBS Lett 547(1–3):51–55
Acknowledgments
The National Natural Science Foundation of China (81100987), the Natural Science Foundation of Fujian Province of China (2011J05066), the Doctoral Program Foundation of Institutions of Higher Education of China (20113518120005), the Clinical Key Subject (Neurosurgery) Funding of Fujian Medical University, and the Key Laboratory (Neurosurgical Department) Funding from the Affiliated Union Hospital of Fujian Medical University supported this work.
Competing Interests
The authors declare that they have no competing interests.
Authors’ Contributions
Xian-kun Tu, Song-sheng Shi, Quan Chen, and Ping-ping Chen performed all experimental studies and data acquisition and contributed to the study conception, design, analysis, and data interpretation. Xian-kun Tu, Song-sheng Shi, Jian-ping Chen, and Yan Chen collected samples, performed data analysis, and drafted the manuscript. Wei-zhong Yang revised the manuscript. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Tu, Xk., Yang, Wz., Chen, Jp. et al. Repetitive Ischemic Preconditioning Attenuates Inflammatory Reaction and Brain Damage After Focal Cerebral Ischemia in Rats: Involvement of PI3K/Akt and ERK1/2 Signaling Pathway. J Mol Neurosci 55, 912–922 (2015). https://doi.org/10.1007/s12031-014-0446-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-014-0446-9