Abstract
Recent work from our laboratory demonstrated that baicalin attenuates inflammatory reaction and cerebral ischemia injury in rats. Toll-like receptor 2 and 4 (TLR2/4) and the downstream nuclear factor-kappa B (NF-κB) signaling pathway, which mediate the inflammatory reaction, are involved in the pathophysiological processes of cerebral ischemia. In this study, we investigated whether baicalin inhibits TLR2/4 signaling pathway in a rat model of permanent focal cerebral ischemia. Adult Sprague–Dawley rats underwent permanent middle cerebral artery occlusion (MCAO). Baicalin was administered by intraperitoneally injected twice at 2 and 12 h after the onset of ischemia. Cerebral infarct area and infarct volume were measured 24 h after MCAO. Expression of TLR2/4, NF-κB, inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2) were determined by RT-PCR or western blot. NO and PGE2 production in rat brain were measured 24 h after MCAO. Serum content of tumor necrosis factor-alpha (TNF-α) and interleukin-1β (IL-1β) were detected by ELISA. Baicalin reduced cerebral infarct area and infarct volume. Baicalin reduced the expression of TLR2/4 and NF-κB, decreased the expression and activity of iNOS and COX-2 in rat brain. Baicalin also attenuated the serum content of TNF-α and IL-1β. Our results suggest that baicalin inhibits the TLR2/4 signaling pathway in cerebral ischemia, which may be a mechanism underlying the baicalin’s neuroprotection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Stroke is one of the leading causes of morbidity and mortality worldwide in adults [1]. Ischemia-induced brain injury can be separated into three serial phases: excitotoxicity and blood–brain barrier leak (acute phase, minutes to hours), inflammatory reaction and neuronal apoptosis (subacute phase, hours to days), and repair and regeneration (chronic phase, days to months) [2, 3]. Accumulating evidence showed that post-ischemic inflammation in the subacute phase of cerebral ischemia contributes to the expansion of cerebral infarction [4, 5].
Toll-like receptors (TLRs), which mediate the inflammatory reaction [6], are involved in the pathophysiological processes of ischemic brain injury [7–11]. Among the family of Toll-like receptors (TLRs), TLR2, and TLR4 are the focus of particular interest because they have been demonstrated to initiate the cerebral inflammation related to Alzheimer’s disease, Parkinson’s disease, brain injury, and ischemic stroke [12–14]. TLR2 and TLR4 (TLR2/4) activate a common signaling pathway leading to the activation of nuclear factor-kappa B (NF-κB) transcription factor [6]. As a downstream transcription factor of TLR2/4 signaling pathway, NF-κB is a key regulator involved the inducible expression of proinflammatory mediators such as inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), tumor necrosis factor-α (TNF-α), and interleukin-1β (IL-1β) [15]. Proinflammatory enzymes iNOS and COX-2, and their reaction product nitric oxide (NO) and prostaglandin E2 (PGE2), and proinflammatory cytokines TNF-α and IL-1β are the major mechanisms by which post-ischemic inflammation contributes to ischemic brain injury.
Recent studies have shown that baicalin plays a neuroprotective role in cerebral ischemia [16]. Our previous studies demonstrated that baicalin could reduce neurological deficit scores and cerebral infarct volume in rats after permanent focal cerebral ischemia [17]. Baicalin protects cerebral ischemia injury in rats via attenuating inflammatory reaction, which may be associated with the inhibition of the mRNA expression of iNOS and COX-2 [17]. In the present study, we, for the first time, demonstrated that baicalin inhibits TLR2/4 signaling pathway in rats after focal cerebral ischemia.
MATERIALS AND METHODS
Animals and Focal Cerebral Ischemia Models
Male Sprague–Dawley rats (Shanghai Laboratory Animal Center, Chinese Academy of Sciences, Shanghai, China), weighing 250–300 g, were housed in a colony room under controlled temperature (22°C), humidity, and a 12:12 light–dark cycle, with food and water available. All animal experiments were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals. Animals were anesthetized with an intraperitoneal injection of chloral hydrate (300 mg/kg). Permanent middle cerebral artery occlusion (MCAO) was induced by inserting a silicone-coated filament via the internal carotid artery as described in the previous report [17]. Briefly, the right common carotid artery (CCA), external carotid artery (ECA), and internal carotid artery (ICA) were carefully exposed. A nylon monofilament suture (0.24 mm in diameter) with a distal cylinder (0.32 mm in diameter) was gently inserted from the ECA into the ICA to occlude the origin of the right middle cerebral artery (MCA). The suture was inserted 18–20 mm from the carotid bifurcation. For sham-operated animals, the suture was inserted 10 mm and withdrawn a minute later. Rectal temperature of all animals before, during and after surgery was maintained at 37.0°C with a heating pad and warm light.
Experimental Groups
Baicalin (Sigma, with a purity >95%), was dissolved in normal saline and intraperitoneally injected twice at 2 and 12 h after the onset of ischemia. Rats were divided into four groups as follows: (1) sham + vehicle group, which underwent sham operation and received vehicle; (2) sham + baicalin group, which underwent sham operation and received baicalin 100 mg/kg; (3) MCAO + vehicle group, which was subjected to MCAO and treated with vehicle; (4) MCAO + baicalin group, which was subjected to MCAO and treated with baicalin 100 mg/kg. The dosage of baicalin was determined according to the previous report [17], in which showed that the baicalin at 100 mg/kg could produce the best protective effect on the brain of rats subjected to permanent focal cerebral ischemia. Rats were treated with normal saline as the vehicle control at the same volume and time point as baicalin.
Measurement of Infarct Area and Infarct Volume
Twenty-four hours after induction of MCAO, the rats (n = 8 for each group) were killed and the brain was removed and sliced into 2.0 mm-thick sections. All slices were incubated in 2% 2,3,5-triphenyltetrazolium chloride (TTC, Amresco) at 37°C for 20 min. Then, the infarcted brain tissue appeared white, whereas the non-infarcted region appeared red. The sections were digitized, and the infarct areas including cortex and striatum were measured using Photoshop software, by tracing around the white area in each brain section. Infarct volume was determined by integrating the cross-sectional area of infarction at each stereotaxic level and the thickness of slice according to the following formula: V = t× (A 1 + A 2 + …An). V is the infarct volume (mm3), t is the thickness of slice, and A is the infarct area (mm2). Correction for edema of infarct area was performed as described by Lin et al [18].
Preparation of Ischemic Brain Tissue
Experimental rats were given an overdose of chloral hydrate and sacrificed at 24 h after permanent MCAO (n = 8 per time point). Brain slice respect to bregma +2.0 mm~bregma +0.0 mm was stained with TTC to confirm the success of MCAO. Tissue samples from ischemic cortex respect to bregma +0.0 mm~bregma −4.0 mm were quickly dissected, collected and stored at −80°C for latter experiments, including reverse transcription-polymerase chain reaction (RT-PCR), western blot and biochemical analysis.
Reverse Transcription-Polymerase Chain Reaction
Total RNA (eight rats for each group) was extracted from ischemic brain tissue using Trizol reagents (Invitrogen, USA) and reverse-transcribed to obtain single-strand cDNA using a Reverse Transcription System (Promega, USA) according to the manufacturer’s protocol. Single-strand cDNA was amplified by PCR in a 100 μl reaction mixture containing 50 mM KCl, 10 mM Tris-HCl (pH 9.0), 2 mM MgCl2, 200 μM dNTPs, 0.5 μM sense and antisense primers and 2.5 units Taq DNA polymerase (Promega, USA). The primer sequences were as follows: TLR2 (primers, sense 5′-GAA AGA TGC GCT TCC TGA AC-3′; antisense 5′-CGC CTA AGA GCA GGA TCA AC-3′, product size 513 bp), TLR4 (primers, sense 5′-GTG GGT CAA GGA CCA GAA AA-3′; antisense 5′-GAA ACT GCC ATG TCT GAG CA-3′, product size 506 bp), β-actin as an internal standard (primers, sense 5′-CAC CCT GTG CTG CTC ACC GAG GCC-3′; antisense 5′-CCA CAC AGA TGA CTT GCG CTC AGG-3′, product size 720 bp). The reactions were initially heated at 94°C for 4 min; then at 94°C for 40 s, 57°C for 40 s and 72°C for 50 s, totally 35 cycles; finally stopped at 72°C for 7 min. PCR products were separated by 2% agarose gel electrophoresis and visualized by ethidium bromide staining. The optical density of DNA band was measured by UVP gel analysis system (Quantity one, Bio-Rad).
Western Blot
Total proteins (eight rats for each group) were purified using protein extraction reagents according to the manufacturer’s instructions (Beyotime Biotech. CO., China). Different samples with an equal amount of protein (100 μg) were separated on 10% SDS polyacrylamide gels, transferred to nitrocellulose membranes and blocked in 5% nonfat dry milk buffer. The membranes were incubated overnight at 4°C with a rabbit polyclonal antibody against TLR2 (1:1,000, Santa Cruz, USA), or a rabbit polyclonal antibody against TLR4 (1:1,000, Santa Cruz, USA), or a mouse monoclonal antibody against NF-κB p65 (1:200, Santa Cruz, USA), or a rabbit polyclonal antibody against iNOS (1:500, Santa Cruz, USA), or a rabbit polyclonal antibody against COX-2 (1:200, Wuhan Boster Biological Technology, LTD, Wuhan, Chian), followed by incubation with horseradish-peroxidase conjugated secondary antibodies (1:2,000, KPL Inc, USA). Protein expression was detected with an ECL detection system (KPL Inc, USA) and exposed on an X-ray film. GAPDH was used as a loading control. The optical densities of protein bands on the X-ray film were quantitatively analyzed with Quantity one software (Bio-Rad).
Brain NO and PGE2 Production
To determine the enzymatic activity of iNOS in ischemic brain, iNOS reaction product NO (eight rats for each group) was measured according to the manufacturer’s instructions from the assay kit (Nanjing Jiancheng Bioengineering Institute, China). To determine the enzymatic activity of COX-2 in ischemic brain, COX-2 reaction product PGE2 (eight rats for each group) was detected using a commercially available enzyme-linked immunosorbent assay (ELISA) kits (Shanghai Xitang Biological Technology, LTD, Shanghai, China) according to the manufacturer’s instructions.
Serum Concentration of TNF-α and IL-1β
Twenty-four hours after the onset of ischemia, blood samples (1 ml, eight rats for each group) were drawn from femoral vein of rats. After centrifugation at 3,000 r/min for 15 min, the supernatant was collected and stored at −80°C in refrigerator until analysis. Serum content of proinflammatory cytokines (TNF-α and IL-1β) were measured using a rat TNF-α or IL-1β immunoassay ELISA kits (R&D Systems) according to manufacturer’s instructions.
Statistical Analysis
Experimental data were presented as mean ± SD. Statistical analysis was performed using ANOVA followed by LSD test for individual comparisons between group means. A value P < 0.05 was considered statistically significant.
RESULTS
Effect of Baicalin on Brain Damage of Infarct Volume and Infarct Area
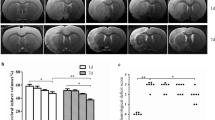
Baicalin reduced the total cerebral infarct volume in rats of permanent MCAO, which was proved in our previous report [17]. The infarct volume in the cortex and striatum were respectively measured in this study, as illustrated in Fig. 1b, the infarct volume of both the cortex and striatum were significantly reduced by the intraperitoneal administration of baicalin (100 mg/kg). In addition to the infarct volume, results of further analysis from the present study showed that baicalin could also significantly reduce the infarct area of cortex in each brain section, and that of striatum in section 2 and 4 (Fig. 1c and d).
Effect of baicalin on cerebral infarct area and infarct volume in rats of permanent MCAO. Representative TTC-stained sections following permanent MCAO at 24 h were presented in (a), showing reduced cerebral infarction from vehicle-treated control rats following baicalin administration. Cerebral infarct volume of both the cortex and striatum were significantly reduced by baicalin (b). Infarct area of cortex in each brain section (c), and striatum in brain section 2 and 4 (d) were significantly reduced by baicalin; n = 8, *p < 0.05 and #p < 0.01 versus vehicle-treated control rats.
Baicalin Reduces the mRNA and Protein Expression of TLR2/4
Expression of TLR2 mRNA and TLR4 mRNA in ischemic rat brain were significantly increased 24 h after MCAO in comparison to sham control group. Baicalin (100 mg/kg) significantly downregulated the expression of TLR2 mRNA and TLR4 mRNA (Fig. 2a and c). Expression of TLR2 protein and TLR4 protein in ischemic rat brain were significantly increased 24 h after MCAO in comparison to sham control group. Baicalin (100 mg/kg) significantly downregulated the expression of TLR2 protein and TLR4 protein (Fig. 2b and d).
Effect of baicalin on the expression of TLR2 and TLR4 in rat brain after MCAO. Representative mRNA expression bands of TLR2 and TLR4 in rat brain after MCAO were detected by RT-PCR (a), and the data were summarized in (c). Representative protein expression bands of TLR2 and TLR4 in rat brain after MCAO were determined by western blot (b), and the data were summarized in (d); n = 8, #p < 0.01 versus sham + vehicle and sham + baicalin groups, *p < 0.05, and **p < 0.01 versus MCAO + vehicle group.
Baicalin Decreases the Expression of NF-κB p65, iNOS, and COX-2
MCAO caused an increased protein expression of NF-κB p65, iNOS, and COX-2 in ischemic rat brain in comparison to sham control group, which were significantly decreased by baicalin administration (Fig. 3a and b).
Effect of baicalin on the activation of NF-κB, the expression and activaton of iNOS and COX-2 in rat brain after MCAO. Representative protein expression bands of NF-κB p65, iNOS, and COX-2 in rat brain after MCAO were detected by western blot (a), and the data were summarized in (b). NO (c) and PGE2 (d), the indicator of enzymatic activity of iNOS and COX-2, were significantly increased in rat brain after MCAO, which were decreased by baicalin administration. n = 8, #p < 0.01 versus sham + vehicle and sham + baicalin groups, *p < 0.05 and **p < 0.01 versus MCAO + vehicle group.
Baicalin Inhibits the Enzymatic Activity of iNOS and COX-2 in Rat Brain
NO and PGE2, produced by iNOS and COX-2, were respectively used as an indicator of the enzymatic activity of iNOS and COX-2 [19]. MCAO caused an obvious increase of NO and PGE2 production in ischemic rat brain in comparison to sham control group, which were significantly inhibited by baicalin administration (Fig. 3c and d).
Baicalin Attenuates the Release of TNF-α and IL-1β in Serum
MCAO caused an increased release of TNF-α and IL-1β in serum in comparison to sham control group, both of which were significantly attenuated by baicalin administration (Fig. 4).
Effect of baicalin on the release of proinflammatory cytokines in rats of permanent MCAO. MCAO caused an increased concentration of TNF-α and IL-1β in serum, which were significantly attenuated by baicalin administration. n = 8, #p < 0.01 versus sham + vehicle and sham + baicalin groups, *p < 0.05, and **p < 0.01 versus MCAO + vehicle group.
DISCUSSION
Our previous study demonstrated that baicalin possesses a neuroprotective effect on cerebral ischemia, which may be attributed to its potential anti-inflammatory and anti-apoptotic properties [17]. The anti-inflammatory effect of baicalin on cerebral ischemia may be associated with the inhibition of the mRNA expression of iNOS and COX-2 [17]. In addition, recent studies showed that baicalin could inhibit the expression of NF-κB and TNF-α in severe acute pancreatitis [20–22]. In the present study, we first demonstrated that baicalin at the dose of 100 mg/kg is capable of attenuating not only the total cerebral infarct volume, but also the infarct volume and infarct area of both the cortex and striatum in rats of permanent MCAO. Then, we further explored the effect of baicalin on TLR2/4 and the downstream inflammatory mediators in rats following permanent cerebral ischemia. This is the first paper, to our best knowledge, indicating that baicalin could inhibit TLR2/4 signaling pathway in rats of permanent MCAO.
TLR2/4 can activate a common signaling pathway that culminates the activation of NF-κB transcription factor [6], which initiates the expression of proinflammatory mediators and leads to the induction of inflammatory reaction [6, 15]. The expression of TLR2 and TLR4 were demonstrated to be elevated in mouse brain after focal cerebral ischemia [23, 24]. A considerable amount of research demonstrated that there was an obvious reduction of ischemic brain injury in TLR2 or TLR4 knockout mice compared with wild-type mice following an experimental stroke, indicating a detrimental role of TLR2/4 [7–10, 23, 24]. Our results showed that baicalin downregulated the mRNA and protein expression of TLR2/4 in rats of permanent MCAO; it also inhibited the activation of NF-κB transcription factor. The inhibitory effect of baicalin on the expression of TLR2/4 and the activation of NF-κB may partially explain the baicalin’s anti-inflammatory property in cerebral ischemia.
Furthmore, mice that lacked TLR4 had minor expression of ischemia-induced iNOS, COX-2, interferon regulatory factor-1, TNF-α, IL-6, and other mediators implicated in brain injury following an experimental stroke [7, 8], which suggested that TLR4 may be an upstream target of inflammatory cascade reaction. Lee et al. [25] found that necrotic neuronal cells-induced expression of various inflammatory mediators such as iNOS and TNF-α were decreased in TLR2-deficient Schwann cells compared to wide-type Schwann cells, demonstrating that TLR2 may be also the upstream target of inflammatory cascade reaction. In the previous study, we have shown that baicalin could decrease the mRNA expression of iNOS and COX-2 in rats of permanent MCAO [17]. In the present study, we demonstrated that baicalin could not only decrease the protein expression of iNOS and COX-2, but also inhibit the enzymatic activites of iNOS and COX-2 in rats of permanent MCAO. NO and PGE2, which are the reaction products of proinflammatory enzymes iNOS and COX-2 respectively, are usually used as an indicator of the enzymatic activity of iNOS and COX-2 [19]. The inhibitory effect of baicalin on the overexpression of iNOS and COX-2 and the overproduction of NO and PGE2 in cerebral ischemia may also explain the anti-inflammatory property of baicalin.
Several studies have reported that proinflammatory cytokines, including TNF-α and IL-1β are elevated in serum in the early phase of acute ischemic stroke [26, 27]. In our study, we demonstrated that permanent cerebral ischemia causes an elevation of TNF-α and IL-1β in serum, which are significantly attenuated by baicalin administration. The anti-inflammatory effect of baicalin may also be associated with the attenuation of proinflammatory mediators TNF-α and IL-1β.
In conclusion, baicain exerts the inhibitory effect on TLR2/4 signaling pathway, including the expression of TLR2/4, the activation of NF-κB, the expression and activation of iNOS and COX-2, and the release of TNF-α and IL-1β, which might explain the neuroprotective role and the anti-inflammatory mechanism of baicalin in cerebral ischemia. Our results further support that baicalin may be used as a neuroprotective agent for treatment of patients suffering from acute ischemic stroke. This study also suggests that TLR2/4 signaling pathway may be a potential and promising therapeutic target for acute ischemic stroke in future.
REFERENCES
Durukan, A., and T. Tatlisumak. 2007. Acute ischemic stroke: overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacology, Biochemistry and Behavior 87: 179–197.
Fagan, S.C., D.C. Hess, E.J. Hohnadel, D.M. Pollock, and A. Ergul. 2004. Targets for vascular protection after acute ischemic stroke. Stroke 35: 2220–2225.
Zhou, Y., E.Q. Wei, S.H. Fang, L.S. Chu, M.L. Wang, W.P. Zhang, G.L. Yu, Y.L. Ye, S.C. Lin, and Z. Chen. 2006. Spatio-temporal properties of 5-lipoxygenase expression and activation in the brain after focal cerebral ischemia in rats. Life Sciences 79: 1645–1656.
Iadecola, C., and M. Alexander. 2001. Cerebral ischemia and inflammation. Current Opinion in Neurology 14: 89–94.
Yilmaz, G., and D.N. Granger. 2008. Cell adhesion molecules and ischemic stroke. Neurological Research 30: 783–793.
Barton, G.M., and R. Medzhitov. 2003. Toll-like receptor signaling pathways. Science 300: 1524–1525.
Cao, C.X., Q.W. Yang, F.L. Lv, J. Cui, H.B. Fu, and J.Z. Wang. 2007. Reduced cerebral ischemia-reperfusion injury in Toll-like receptor 4 deficient mice. Biochemical and Biophysical Research Communications 353: 509–514.
Caso, J.R., J.M. Pradillo, O. Hurtado, P. Lorenzo, M.A. Moro, and I. Lizasoain. 2007. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation 115: 1599–1608.
Tang, S.C., T.V. Arumugam, X. Xu, A. Cheng, M.R. Mughal, D.G. Jo, J.D. Lathia, D.A. Siler, S. Chigurupati, X. Ouyang, T. Magnus, S. Camandola, and M.P. Mattson. 2007. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proceedings of the National Academy of Sciences of the United States of America 104: 13798–13803.
Ziegler, G., D. Harhausen, C. Schepers, O. Hoffmann, C. Rohr, V. Prinz, J. König, H. Lehrach, W. Nietfeld, and G. Trendelenburg. 2007. TLR2 has a detrimental role in mouse transient focal cerebral ischemia. Biochemical and Biophysical Research Communications 359: 574–579.
Tu, X.K., W.Z. Yang, S.S. Shi, C.H. Wang, G.L. Zhang, T.R. Ni, C.M. Chen, R. Wang, J.W. Jia, and Q.M. Song. 2010. Spatio-temporal distribution of inflammatory reaction and expression of TLR2/4 signaling pathway in rat brain following permanent focal cerebral ischemia. Neurochemical Research 35: 1147–1155.
Hoffmann, O., J.S. Braun, D. Becker, A. Halle, D. Freyer, E. Dagand, E. Dagand, S. Lehnardt, and J.R. Weber. 2007. TLR2 mediates neuroinflammation and neuronal damage. Journal of Immunology 178: 6476–6481.
Lee, S.J., and S. Lee. 2002. Toll-like receptors and inflammation in the CNS. Current Drug Targets. Inflammation and Allergy 1: 181–191.
Zwagerman, N., C. Plumlee, M. Guthikonda, and Y. Ding. 2009. Toll-like receptor-4 and cytokine cascade in stroke after exercise. Neurological Research 32: 123–126.
Ridder, D.A., and M. Schwaninger. 2009. NF-kappaB signaling in cerebral ischemia. Neuroscience 158: 995–1006.
Zhang, Z.J., Z. Wang, X.Y. Zhang, K. Ying, J.X. Liu, and Y.Y. Wang. 2005. Gene expression profile induced by oral administration of baicalin and gardenin after focal brain ischemia in rats. Acta Pharmacologica Sinica 26: 307–314.
Tu, X.K., W.Z. Yang, S.S. Shi, C.H. Wang, and C.M. Chen. 2009. Neuroprotective effect of baicalin in a rat model of permanent focal cerebral ischemia. Neurochemical Research 34: 1626–1634.
Lin, T.N., Y.Y. He, G. Wu, M. Khan, and C.Y. Hsu. 1993. Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke 24: 117–121.
Chou, T.C., L.P. Chang, C.Y. Li, C.S. Wong, and S.P. Yang. 2003. The antiinflammatory and analgesic effects of baicalin in carrageenan-evoked thermal hyperalgesia. Anesthesia and Analgesia 97: 1724–1729.
Zhang, X., J. Zhang, Q. Xu, G. Feng, Y. Cai, T. Ju, and Q. Xie. 2009. Influence of baicalin and octreotide on NF-kappaB and p-selectin expression in liver and kidney of rats with severe acute pancreatitis. Inflammation 32: 1–11.
Tian, H., X. Zhang, C. Wu, L. Chen, R. Ying, J. Ye, B. Yu, Q. Ye, Y. Pan, M. Ma, and F. Zhu. 2009. Effects of baicalin and octreotide on the serum TNF-alpha level and apoptosis in multiple organs of rats with severe acute pancreatitis. Inflammation 32: 191–201.
Zhang, X., G. Feng, J. He, W. Weng, R. Xu, W. Zhu, J. Ye, Q. Yang, M. Yuan, Q. Wang, and L. Fang. 2010. Baicalin protects thymus of rats with severe acute pancreatitis. Inflammation 33: 157–165.
Lehnardt, S., S. Lehmann, D. Kaul, K. Tschimmel, O. Hoffmann, S. Cho, C. Kruegor, R. Nitsch, A. Meisel, and J.R. Weber. 2007. Toll-like receptor 2 mediates CNS injury in focal cerebral ischemia. Journal of Neuroimmunology 190: 28–33.
Hua, F., J. Ma, T. Ha, Y. Xia, J. Kelley, D.L. Williams, R.L. Kao, I.W. Browder, J.B. Schweitzer, J.H. Kalbfleisch, and C. Li. 2007. Activation of Toll-like receptor 4 signaling contributes to hippocampal neuronal death following global cerebral ischemia/reperfusion. Journal of Neuroimmunology 190: 101–111.
Lee, H., E.K. Jo, S.Y. Choi, S.B. Oh, K. Park, J.S. Kim, and S.J. Lee. 2006. Necrotic neuronal cells induce inflammatory Schwann cell activation via TLR2 and TLR3: implication in Wallerian degeneration. Biochemical and Biophysical Research Communications 350: 742–747.
Oto, J., A. Suzue, D. Inui, Y. Fukuta, K. Hosotsubo, M. Torii, S. Nagahiro, and M. Nishimura. 2008. Plasma proinflammatory and anti-inflammatory cytokine and catecholamine concentrations as predictors of neurological outcome in acute stroke patients. Journal of Anesthesia 22: 207–212.
Tu, X.K., W.Z. Yang, C.H. Wang, S.S. Shi, Y.L. Zhang, C.M. Chen, Y.K. Yang, C.D. Jin, and S. Wen. 2010. Zileuton reduces inflammatory reaction and brain damage following permanent cerebral ischemia in rats. Inflammation 33: 344–352.
ACKNOWLEDGEMENTS
The Key Laboratory (Neurosurgical Department) Fund from the Affiliated Union Hospital of Fujian Medical University, and the Professor Academic Development Fund of Fujian Medical University (JS0610) supported this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tu, Xk., Yang, Wz., Shi, Ss. et al. Baicalin Inhibits TLR2/4 Signaling Pathway in Rat Brain Following Permanent Cerebral Ischemia. Inflammation 34, 463–470 (2011). https://doi.org/10.1007/s10753-010-9254-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-010-9254-8