Abstract
Background
Minimally invasive liver surgery is increasing worldwide. The benefit of the robot in this scenario is currently controversially discussed. We compared our robotic cases vs. laparoscopic and open minor hepatic resections and share the experience.
Material and methods
From 2011 to 2015, ten patients underwent robotic and 19 patients underwent laparoscopic minor liver resections in the Department of Surgery, University Hospital Erlangen. These patients were compared to a case-matched control group of 53 patients. The perioperative prospectively collected data were analyzed retrospectively.
Results
Blood loss was significantly decreased in the robotic (306 ml) and laparoscopic (356 ml) vs. the open (903 ml) surgery group (p = 0.001). Mean tumor size was 4.1–4.8 cm in all groups (p = 0.571). Negative surgical margins were present in 94 % of the open and 100 % of the laparoscopic and robotic group (p = 0.882). Time for surgery was enlarged for robotic (321 min) vs. laparoscopic (242 min) and open (186 min) surgery (p = 0.001). Postoperative hospitalization was decreased after robotic (7 days) and laparoscopic (8 days) vs. open (10 days) surgery (p = 0.004). Total morbidity was 17 % for open, 16 % for laparoscopic, and 1 % for robotic cases (p = 0.345). Postoperative pain medication and elevation of liver enzymes were remarkably lower after minimally invasive vs. open procedures.
Conclusion
Minimally invasive liver surgery can be performed safely for minor hepatic resections and should be considered whenever possible. Minor liver resections can be performed by standard laparoscopy equivalent to robotic procedures. Nevertheless, the robot adds a technical upgrade which may have benefits for challenging cases and major liver surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Minimally invasive liver surgery is increasing worldwide. It is used for benign and malignant liver diseases. Initially, series recommended laparoscopic approaches for left lateral and anterior liver segments with minor tumor size (≤5 cm) only [1–3]. But currently, the left lateral resection is recommended as a standard of care for laparoscopic procedures [4, 5]. The technique has improved, and now, all liver segments can be reached by minimally invasive procedures [6–8]. In experienced hands, even major resections can be performed safely [9, 10]. Patients benefit from laparoscopic technique with less blood loss, less postoperative pain, and less hospitalization, and the oncologic results are equivalent to open liver resections [5, 11]. In the case of liver metastases, they can be transferred to adjuvant chemotherapy earlier compared to open procedures [12].
The use of robots is increasing worldwide for various indications. It may add some new technical innovations to improve the quality of operations in surgical oncology. Especially when precise vessel dissection or major sewing is needed, the robot adds an upgrade to conventional laparoscopy. But it still has several limitations like missing haptic feedback, long operation times, and the currently high costs. These facts lead to ongoing discussions about its value for clinical routine procedures. There are several series published which demonstrate that minor and major liver resections can be performed safely by robotic assistance [13]. This was also confirmed for hepatic donor resections in liver transplantation [14]. Encouraged by these data, we were the first center in Germany starting with robotic-assisted liver resections [15]. We present here our initial experience in minor liver resections compared to laparoscopic and case-matched open procedures.
Material and methods
Patients
We selected patients which underwent minor liver resections in the Department of Surgery, University Hospital Erlangen, in 2011–2015. Criteria for minimally invasive liver surgery were ≤2 lesions during clinical imaging (CT scan, MRI) and resection ≤3 segments required. The decision was made by a multidisciplinary hepatobiliary tumor board. Tumor size, neoadjuvant treatment, liver disease, malignant lesion, or prior abdominal surgery was no exclusion criteria. Cases which underwent minor open liver resection during the same time period were selected from our database, and a case-matched comparison between open, laparoscopic, and robotic-assisted procedures was performed. To evaluate perioperative morbidity, all complications were collected during the patients’ hospital stay. Mortality was defined as dead within postoperative hospitalization. Postoperative pain was assessed by a numeric rating scale reaching from 1 (less pain) to 10 (severe pain). The data of patients were collected prospectively and analyzed in a retrospective study. Patient demographics are listed in Table 1.
Surgery
For open surgery, a right subcostal incision (∼15 cm) was performed which was extended to the midline. The liver was mobilized and the affected liver segments were exposed. Parenchyma dissection was carried out with Cavitron Ultrasonic Surgical Aspirator (CUSA) (Valleylab Boulder, CO, USA). Intrahepatic vessels and bile ducts were ligated or clipped selectively. Intraoperative ultrasound was performed not routinely during open surgery. Three surgeons performed all open procedures.
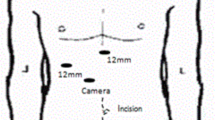
Laparoscopic liver resections were performed in a three- or four-trocar technique depending on the demand. Ultrasound was used in each case to determine resection margins and intraoperative liver screening. Parenchyma dissection was performed with a harmonic scalpel as recently described [8]. Intrahepatic vessels and bile ducts were clipped. Major bile ducts were closed by laparoscopic sutures. The specimens were removed via a recovery bag through a Pfannenstil incision or in the case of prior surgery during a reincision of a preexisting scar.
Robotic-assisted liver resections were performed using the da Vinci robotic system (Intuitive Surgical, Inc., CA, USA) in a recently described fashion [15]. The robot was located coming from the patient’s right shoulder. Trocars were placed in the left and right upper abdomen. Intraoperative ultrasound was used in every case to determine the resection margin and for liver screening. Parenchyma dissection was performed using the harmonic scalpel or the monopolar scissors. Minor intrahepatic vessels and bile ducts were clipped while major bile ducts and vessels in selected cases underwent robotic sewing. Specimens were removed in the same way as described for laparoscopy. All minimally invasive liver resections were performed by one surgeon only.
Perioperative cost analysis
To evaluate perioperative costs for the different procedures, representative cases from open, laparoscopic, and robotic-assisted liver resections were compared. For the open technique, costs for the CUSA hand sampler were included. For laparoscopic and robotic procedures, a harmonic scalpel and one stapler was calculated. For each procedure, a clipping device was part of the calculation. In the robotic group, the proportional maintenance charges for the robot and the specific coverage were included in the cost analysis.
Statistics
The chi-square test was used for comparison of categorical data, and the Kruskal-Wallis test or ANOVA was used to compare quantitative data. A p value of less than 0.05 was considered to be significant. Analyses were performed using the statistical software package SPSS version 21.0 (SPSS Inc., Chicago, USA).
Results
Perioperative findings
Ten patients underwent robotic-assisted, 19 patients laparoscopic, and 53 patients open liver resections regarding our selection criteria. No significant differences in age, body mass index (BMI), or ASA scoring were identified between the groups. Although prior abdominal surgery was no exclusion criteria for minimally invasive procedures, significantly more patients were referred to open surgery than to laparoscopic procedures (p = 0.003). There was no significant difference between open and robotic case selection (p = 0.233) or laparoscopic vs. robotic patients (p = 0.151) in this manner. No differences in adhesions were found intraoperatively between all groups. In the open surgery group, 49 patients, in the laparoscopic group, 5 patients, and in the robotic group, 10 patients, suffered from malignancies. In four patients of the open and laparoscopic group but in no patient of the robotic group, benign liver tumors (Adenoma, FNH, hemangioma) were an indication for surgery. No significant differences regarding the indication were identified between the groups (p = 0.118). Preoperative laboratory levels showed no signs of anemia, progressed liver disease, or severe inflammation within or between the groups (Table 1).
An average of two liver segments was removed in each group with a higher range in the open and laparoscopic group (range 1–3 liver segments) compared to the robotic group (range 1–2 liver segments). But this difference was not statistically significant (p = 0.838). There was no significant difference regarding the weight of the removed specimens (p = 0.409). The operative time was significantly increased in the robotic vs. the open group (p < 0.001). But there were no statistical differences between the open vs. laparoscopic (p = 0.075) and the robotic vs. laparoscopic (p = 0.104) groups. Perioperative estimated blood loss (ESBL) was significantly increased in the open surgery group vs. the laparoscopic (p = 0.001) and the robotic surgery group (p = 0.007). No differences in ESBL between laparoscopic and robotic surgeries were identified (p = 0.897). Perioperative red blood cell transfusion did not differ between the groups. Mean required units were 0.81 in the open, 0.11 in the laparoscopic, and 0.2 in the robotic group (p = 0.271). Drains were significantly more often placed in the open vs. laparoscopic surgery group (p = 0.006). No significant differences were identified between the open vs. robotic (p = 0.075) or laparoscopic vs. robotic (p = 0.104) groups. Although only patients with ≤2 liver lesions identified during clinical imaging were selected for minimally invasive procedures, in 5 % of the laparoscopic and 20 % of the robotic group, more ≥3 lesions were identified during histopathological workup of the specimens. In the open surgery group in 13 %, ≥3 lesions were found. There was no significant difference regarding lesion side (p = 0.264) and tumor size (p = 0.571) between the groups. Complete tumor removal with negative histopathological margin was achieved in 94 % of the open and 100 % of the laparoscopic and robotic groups (p = 0.427). Mean margin was 0.7 cm in the open, 0.76 cm in the laparoscopic, and 0.57 cm in the robotic group (p = 0.882). The percentual distribution of steatosis was similar in the three groups (p = 1.000). Liver cirrhosis was more frequent in the laparoscopic (21 %) and the robotic (20 %) vs. the open surgery group (8 %) without any statistical significance (p = 0.220) (Table 2).
Morbidity and mortality
Total morbidity was 32 % in the open, 16 % in the laparoscopic, and 11 % in the robotic group (p = 0.345). Most patients suffered from minor postoperative complications (Clavien-Dindo I–II). These problems were identified in 25 % of patients in the open, 11 % in the laparoscopic, and 10 % in the robotic group. One patient of each group developed small-bowel fistula after surgery. In the case of robotic-assisted surgery, this was a minor small-bowel fistula which could be handled by drainage. Five patients in the open and two patients in the laparoscopic surgery group suffered from postoperative infections (pneumonia, wound infection). One patient in the open vs. no patient in the laparoscopic or robotic group developed cholangitis. Biliary fistula after surgery was identified in one patient of the open and one patient of the laparoscopic group which could be treated by nasobiliary tube. Eight percent of patients in the open group suffer from postoperative morbidity Clavien-Dindo IIIB. One of these patients developed small-intestine fistula which needed reoperation; two patients suffered from postoperative bleeding and one patient needed osteosynthesis after postoperative trauma. One patient (5 %) in the laparoscopic group developed duodenal perforation after surgery postoperative day 2, suffered from peritonitis, and died after open revision (Clavien-Dindo V). No patient died in the robotic and open surgery group (Table 3).
Postoperative course
The postoperative time for oral died was prolonged after open surgery. This took a mean period of 3.5 days (range 1–10) for patients after open, 2.2 days (range 1–4) after laparoscopic, and 3.3 days (range 2–9) after robotic surgery. This was statistically significant between open vs. laparoscopic (p = 0.001), but not for open vs. robotic (p = 0.275) or laparoscopic vs. robotic (p = 0.081) procedures. The time course for postoperative pain medication was decreased after minimally invasive procedures. Especially in the robotic group, there was less intake for morphine and non-steroidal pain medication observed. But there was no statistical significance between the groups (p = 0.270) (Table 3). Subjective pain rating was evidently lower after minimally invasive procedures (Fig. 1). Postoperative pain scale levels below 2 at a numeric rating scale were reached on postoperative day 2 after robotic, postoperative day 3 after laparoscopic, and postoperative day 4 after open surgery. Lowest pain scale levels which were observed on postoperative day 5 after open procedures could be identified at postoperative day 2 for robotic and postoperative day 3 for laparoscopic surgery (Fig. 1). Nevertheless, the pain scale data failed to reach statistical significance between the groups. Postoperative liver enzymes (ALT, AST) were evidently but not statistically significantly evaluated after open vs. minimally invasive procedures (p = 0.092) (Fig. 2, Table 3). They decreased postoperatively after surgery in all groups and developed similar levels on postoperative day 4. But the open surgery group reached the initial postoperative level of the laparoscopic group at day 2 and the level of the robotic group at day 3 (Fig. 2a).
Perioperative costs
Calculated perioperative costs for open surgery were 2.672 Euro, for laparoscopic surgery 3.437 Euro, and for robotic procedures 8.765 Euro. These are 36 % of the total costs for open surgery, 42 % for laparoscopic, and 64 % for robotic-assisted cases.
Discussion
Our study is a further indicator that minimally invasive liver resections can be performed safely. This is in concordance with various previous studies which evaluated similar results [1, 2, 5, 16–18]. Patients benefit from less blood loss, less postoperative pain, and less hospitalization with equivalent morbidity, mortality, and oncological results compared to open procedures which were even the results of our analysis [5, 12, 16, 17, 19]. Of course we even followed selection criteria for minimally invasive resections, and only one experienced surgeon performed robotic and laparoscopic cases. But we consider ourselves being still in a learning curve. Therefore, our results represent preliminary experiences which need validation in a bigger series. More experienced centers demonstrated the value of minimally invasive approaches even for major liver resections, reoperations, and more challenging cases [4, 8–11, 19–25]. Nevertheless, even in our series, prior abdominal surgery and preexisting liver disease were not exclusion criteria for minimally invasive procedures, and this did not result in increased morbidity or mortality. One major benefit for patients which underwent minimally invasive liver resection for colorectal liver metastases was recently reported by the Pittsburgh group. They figured out that these patients underwent postoperative chemotherapy significantly sooner compared to open procedures (median 42 vs. 63 days, p < 0.001) [12]. This could become a major argument pro minimally invasive procedures. We described a decreased level of measured postoperative liver enzymes after minimally invasive procedures which were lowest after robotic surgery. This is an indicator that the liver is much less traumatized by the gentle handling during minimally invasive surgery compared to open technique. During open procedures, the liver is usually mobilized to get access to the affected segments which are more traumatic for the liver. In open surgery, parenchymal dissection was performed with a CUSA. Usually bigger vessels and bile ducts are preserved by this technique. They can be ligated separately very safely to prevent bleeding or bile leaks. During minimally invasive procedures, the parenchymal dissection was carried out with a harmonic scalpel. Hereby smaller intrahepatic vessels and bile ducts can be closed effectively. Problems occur if bigger vessels are captured tangentially and sealed not completely by the dissector. Then bleeding can arise. Detailed intraoperative ultrasound with landscaping of intrahepatic vessels prior to liver dissection can help to prevent this failure. The dissection with the monopolar scissors which was used during robotic procedures is another technique to approximate to intrahepatic vessels and to preserve them if anatomic situations are unclear. Nevertheless, it must be considered that the tip of the minimally invasive instruments become hot during surgery. This can damage neighboring organs if a crucial distance is not respected. But our data demonstrate that parenchymal dissection can be performed safely during minimally invasive procedures and are not behind open techniques.
The R1 resection rate in the open procedure group was higher compared to the minimally invasive cases. But it has to be considered that the patients in this cohort had a tumor size up to 16 cm, up to nine lesion sides, and a higher incidence of adhesions with more often prior surgery. Therefore, they represent a more complex type of unselected cases which leads to these results.
The operative time for minimally invasive cases and here especially for the robotic part was higher compared to open surgery. Furthermore, no significant differences between laparoscopic and robotic procedures could be identified. These findings are in concordance with recently published findings which did not evaluate superiority of the robot against standard laparoscopy [13] [26]. The main problem of the robot is the added high cost to the procedures. They cannot be reimbursed even by decreased postoperative hospitalization in Germany. At least 6 days of patients’ hospital stay are needed to achieve complete reimbursement by the current system. The increased time needed in the operation room which is partially required for preparing and setting the robot accelerates these effects.
Although robotic liver surgery was performed in a small group of selected patients, we demonstrated, as the first center starting robotic liver surgery in Germany with our initial experience, that robotic minor liver resections can be performed safely and equivalent to standard laparoscopic procedures. Minimally invasive liver surgery is not very widespread in Germany. Our data may encourage other groups to consider these techniques. The robot adds without any question a technical upgrade to common laparoscopy. Especially when precise vessel dissection or advanced sewing is necessary, using the robot is much more comfortable for the surgeon. The EndoWrist, three movable arms, and excellent visualization are the major benefits which become evident in these situations. The whole technology is really convincing, but it must find its indications during critical comparisons with already established procedures in surgical oncology. It must be realized that we are still at the beginning in the era of robotics in surgery. The technology will persist. It will be improved over time, and it is the surgeons’ decision being part of the decision-making for useful indications or to act as critical observers only.
References
Buell JF et al (2009) The international position on laparoscopic liver surgery: the Louisville Statement, 2008. Ann Surg 250(5):825–830
Buell JF et al (2008) Experience with more than 500 minimally invasive hepatic procedures. Ann Surg 248(3):475–486
Gigot JF et al (2002) Laparoscopic liver resection for malignant liver tumors: preliminary results of a multicenter European study. Ann Surg 236(1):90–97
Belli G et al (2013) Laparoscopic left hemihepatectomy a consideration for acceptance as standard of care. Surg Endosc 27(8):2721–2726
Wakabayashi G et al (2015) Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 261(4):619–629
Cardinal JS et al (2013) Laparoscopic major hepatectomy: pure laparoscopic approach versus hand-assisted technique. J Hepatobiliary Pancreat Sci 20(2):114–119
Gayet B et al (2007) Totally laparoscopic right hepatectomy. Am J Surg 194(5):685–689
Ishizawa T et al (2012) Laparoscopic segmentectomy of the liver: from segment I to VIII. Ann Surg 256(6):959–964
Gumbs AA, Gayet B (2007) Totally laparoscopic left hepatectomy. Surg Endosc 21(7):1221
Gumbs AA, Gayet B (2008) Totally laparoscopic central hepatectomy. J Gastrointest Surg 12(7):1153
Koffron AJ et al (2007) Evaluation of 300 minimally invasive liver resections at a single institution: less is more. Ann Surg 246(3):385–392, discussion 392–4
Tohme S et al (2015) Minimally invasive resection of colorectal cancer liver metastases leads to an earlier initiation of chemotherapy compared to open surgery. J Gastrointest Surg 19(12):2199–2206
Ho CM et al (2013) Systematic review of robotic liver resection. Surg Endosc 27(3):732–739
Giulianotti PC et al (2012) Robot-assisted right lobe donor hepatectomy. Transpl Int 25(1):e5–e9
Croner RS et al (2015) Pioneering robotic liver surgery in Germany: first experiences with liver malignancies. Front Surg 2:18
Belli G et al (2015) Laparoscopic liver resection for hepatocellular carcinoma in cirrhosis: long-term outcomes. Dig Surg 28(2):134–140
Croome KP, Yamashita MH (2010) Laparoscopic vs open hepatic resection for benign and malignant tumors: an updated meta-analysis. Arch Surg 145(11):1109–1118
Cho SW et al (2011) Safety of liver resection in the elderly: how important is age? Ann Surg Oncol 18(4):1088–1095
Geller DA, Tsung A (2015) Long-term outcomes and safety of laparoscopic liver resection surgery for hepatocellular carcinoma and metastatic colorectal cancer. J Hepatobiliary Pancreat Sci 22(10):728–730
Belli G et al (2009) Laparoscopic redo surgery for recurrent hepatocellular carcinoma in cirrhotic patients: feasibility, safety, and results. Surg Endosc 23(8):1807–1811
Belli G et al (2005) Laparoscopic hepatic resection for completely exophytic hepatocellular carcinoma on cirrhosis. J Hepatobiliary Pancreat Surg 12(6):488–493
Conrad C et al (2012) Laparoscopic portal vein ligation with in situ liver split for failed portal vein embolization. Ann Surg 256(3):E14–E15, author reply e16-7
Costi R et al (2003) Laparoscopic right posterior hepatic bisegmentectomy (segments VII-VIII). Surg Endosc 17(1):162
Dagher I et al (2009) Laparoscopic major hepatectomy: an evolution in standard of care. Ann Surg 250(5):856–860
Lin NC, Nitta H, Wakabayashi G (2013) Laparoscopic major hepatectomy: a systematic literature review and comparison of 3 techniques. Ann Surg 257(2):205–213
Tsung A et al (2014) Robotic versus laparoscopic hepatectomy: a matched comparison. Ann Surg 259(3):549–555
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the University Hospital Erlangen and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Croner, R.S., Perrakis, A., Hohenberger, W. et al. Robotic liver surgery for minor hepatic resections: a comparison with laparoscopic and open standard procedures. Langenbecks Arch Surg 401, 707–714 (2016). https://doi.org/10.1007/s00423-016-1440-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-016-1440-1