Abstract
Toll-like receptors (TLR) that belong to the group of protein recognition receptor (PPR) provide an innate immune response following the sensing of conserved pathogen-associated microbial patterns (PAMPs) and changes in danger-associated molecular patterns (DAMPs) that are generated as a consequence of cellular injury. Analysis of the TLR pathway has moreover offered new insights into the pathogenesis of rheumatoid arthritis (RA). Indeed, a dysfunctional TLR-mediated response characterizes RA patients and participates in establishment of a chronic inflammatory state. Such an inappropriate TLR response has been attributed (i) to the report of important alterations in the microbiota and abnormal responses to infectious agents as part of RA; (ii) to the abnormal presence of TLR-ligands in the serum and synovial fluid of RA patients; (iii) to the overexpression of TLR molecules; (iv) to the production of a large panel of pro-inflammatory cytokines downstream of the TLR pathway; and (v) to genetic variants and epigenetic factors in susceptible RA patients promoting a hyper TLR response. As a consequence, the development of promising therapeutic strategies targeting TLRs for the treatment and prevention of RA is emerging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With a prevalence of 0.5 ± 0.2% in the general population and a twofold female predominance, rheumatoid arthritis (RA) represents the most common chronic systemic autoimmune rheumatism [1]. RA clinical presentation typically retrieves morning stiffness, fatigue, poly-articular pain, and joint and bone inflammation and destruction. A more severe evolution is encountered in RA patients presenting early bone erosion, an age at diagnosis lower than 50 years, and an elevated level of autoantibodies (Abs) targeting immunoglobulin G, referred to as rheumatoid factor (RF), and/or anti-cyclic citrullinated peptide autoantibodies (ACPA) [2, 3].
RA presents a strong environmental factor component in addition to genetic, epigenetic, and female bias risk factors [4]. Environmental risk factors include smoking, silica exposure, education level, vitamin D deficiency, obesity, change in microbiota, and infectious agents [5]. As reported in Fig. 1 and although the primary events are suspected to occur outside the joint at mucosal surfaces (mouth, pulmonary, gut), leading to a primary immunization in secondary lymphoid organs, there are consistent information from human and animal models supporting a critical role of the TLR (Toll-like receptor)/IL-1R (interleukin-1 receptor) pathway, at least as a second hit signal in the synovium. Indeed, the TLR pathway amplifies the abnormal crosstalk existing between antigen-presenting cells (APCs), T cells, and B cells, leading to production of high amounts of pro-inflammatory cytokines, the expansion of autoreactive lymphocytes, and local detection of Abs including ACPA and RF. After several months or years, persistent immune activation can lead to FLS (fibroblast-like synoviocyte) hyperplasia, neutrophil recruitment, complement activation resulting in cartilage destruction, bone erosion, and joint damage.
A “two hits” model for the development of RA: critical role of TLRs. The first events for the occurrence of RA are supposed to take place outside the joint on the mucosal surfaces, leading to a primary immunization in secondary lymphoid organs. Nonetheless, a critical role of TLR/IL-1R as a second hit signal for the induction of an immune response directly inside the joint has to be taken into account, as a TLR-dependant second hit. Activation of TLRs induces a perpetual cycle of inflammation with the increased production of pro-inflammatory molecules like IL6, TNF alpha, or VEGF. A micro-environment is created within the joint, thanks to local (MLS, FLS) and immune-activated cells (monocytes, macrophages, B and T cells, PMN), inflammatory cytokines and death cell components, triggering a positive feedback for further TLR-mediated inflammation. PADI, peptidyl arginin deiminase; RF, rheumatoid factor; ACPA, anti-cyclic citrullinated peptide autoantibodies; FLS, fibroblast-like synoviocytes; MLS, macrophage-like synoviocytes; PMN, polymorphonuclear neutrophils
The TLR and IL-1 Receptor Signaling Pathway
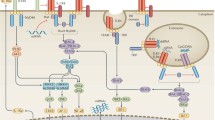
The TLR family is evolutionarily conserved and, in humans, it is composed of 10 members, TLR1 to TLR10 [6]. TLR can be dichotomized into two groups based on their localization either on the cell membrane for TLR1/2/4/5/6/10, or on the membranes of intracellular compartments such as endosomes and endolysosomes for TLR3/7/8/9 (Fig. 2). TLRs are predominantly expressed by immune cells, as well as cells exposed to the external environment such as in the mouth, lungs, and gut (e.g., mast cells, epithelial cells) [7]. Their expression profiles vary among tissues and cell types (Table 1).
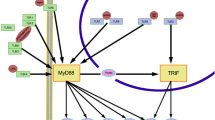
The TLR family and signaling pathway. TLRs are activated through the binding of exogenous or endogenous ligands. Each of the ten members of the TLR family is classified into extracellular or intracellular subtypes. MyD88 (myeloid differentiation primary-response protein 88) and TRIF (Tir-domain-containing adapter protein inducing IFN-beta) are the two main adapter pathways for the transduction of TLR signaling. Predominantly, receptor activation leads to the association of MyD88. Therefore, two major downstream signaling pathways are induced: the NF-KB and IRF (interferon regulator factor) pathways. Regarding the NFKB pathway, the MyD88 fixation causes the phosphorylation of IRAK (interleukin-1-receptor-associated kinase), which results in the recruitment of TRAF (TNF receptor-associated factor). Some TLRs, like TLR3 and TLR4, can produce a MyD88-independent signal. Upon activation, TRIF protein is associated, enabling IRF3 and IRF7. All of these activating pathways promote the production of inflammatory proteins like interferons or inflammatory cytokines
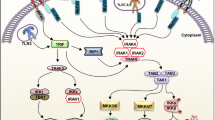
By sensing highly conserved structural motifs known as PAMPs (pathogen-associated microbial patterns), which are expressed exclusively by microbial pathogens, or DAMPs (danger-associated molecular patterns) that are endogenous molecules released from necrotic or dying cells, TLRs play a critical role in the early innate immune response [8]. TLRs are type I transmembrane proteins characterized by an extracellular domain-containing LRR (leucine-rich repeats) and a cytoplasmic tail that contains a conserved region named TIR (Toll/IL-1 receptor) and able to recruit the adapter molecule MYD88 (myeloid differentiation primary response protein 88) after homodimerization or heterodimerization of the TLRs (e.g., TLR1/2 and TLR2/6) [9] (Fig. 3). The TIR domain is present on both IL-1R (interleukin-1 receptor) and all TLRs with the exception of TLR3. MYD88 carries a death domain which helps in interacting with IRAK1/4 (interleukin-1/4-receptor-activating kinase) [10]. Subsequently, IRAK4 activates IRAK2 by phosphorylation. After dimerization, both IRAK2 and IRAK4 leave the TLR-MYD88 complex to associate with TRAF6 (tumor necrosis factor-receptor-associated protein 6). As a consequence, the recruitment and phosphorylation of TAK1 (TGF-b activated kinase 1) and TRAF6 are ubiquitinated by interacting with Bcl10 and MALT1 [11]. In addition to MYD88, there are four other TLR adapters that can further orchestrate the inflammatory response: TRIF/TICAM1 (TIR domain-containing adapter molecule 1), TRAM/TICAM2 (TIR domain-containing adapter molecule 2), TIRAP (TIR domain-containing adapter protein), and Mal (MYD88 adapter-like protein); each of them interact with a specific set of TLR [12]. The TLR intracellular signaling is also controlled by endogenous inhibitors such as IL-1R8, a transmembrane molecule acting on TLRs, and the short-MYD88, acting on MYD88 [13]. TLR4 presents several characteristics and, in particular, the necessity to recruit the adapter MD2 (myeloid differentiation protein-2) and the accessory molecule CD14 in order to form a complex with LPS leading to a MYD88-dependent pro-inflammatory signal. The second particularity is related to the capacity of the TLR4/MD2/CD14 complex to translocate from the plasma membrane to the endosomes, which is associated with recruitment of TRIF, as observed with TLR3, which triggers production of IFN-β in an MYD88-independent manner [14].
Effects of microorganisms on IL1/TLR pathway and RA development. Several infectious agents have been related to the development of RA. The most famous is Porphyromonas gingivalis, responsible for periodontitis infection. The bacterium is well-known for producing an enzyme, the PPAD (prokaryotic peptidylarginine deiminase), able to convert the arginine of various peptides to citrulline. The citrullinated proteins are then recognized by the immune system and especially when presented by the HLA-DR4. This germ also activates the TLR2 on a chronic basis, leading to the expression of many inflammation markers and stimulating osteoclasts for bone destruction in the RA. Some particular intestinal microbiota profiles have also been described in RA patients, characterized by the presence of Prevotella copri among others, and leading to auto-immune diseases like inflammatory bowel disease and colitis, but also RA, through the activation of IL-1R/TLR pathway. Some protective bacteria have also been identified, such as lactobacilli, reducing inflammation and restoring healthy intestinal microbiota. RA patients are concerned as well by a higher frequency of HSV infections or reactivations, causing a TLR2 and a TLR9 activation which results in the production of inflammatory molecules
Stimulation of TLRs by the corresponding PAMPs or DAMPs initiates MYD88 dependent signaling cascades leading to the activation of TAK1 which in turn phosphorylates the IKK complex (IκB kinase, IKK-α, IKK-β, and IKK-γ) that leads to activation of the NF-κB (nuclear factor kappa B) pathway and the MAPK (mitogen-activated protein kinases) pathway including ERK1/2 (extracellular signal-regulated kinase), JNK (C-Jun N-terminal kinase), and P38 necessary to induce pro-inflammatory cytokines (TNF-α, IL-1, and IL-12) [12]. Following viral nucleic acid binding to the endosomal TLR3/7/8/9, the activated IRF (interferon regulatory factor) induces the production of type I interferon [15]. Type I IFN production stimulated by TLR3 and TLR4 involves IRF3 and IRF7, while TLR7, TLR8, and TLR9 involve IRF5 and IRF7. Last but not least, TLR expression is tightly regulated by several means including pro-inflammatory cytokines such as IFN-γ.
TLR and Rheumatoid Arthritis
Lessons from Clinical Studies
In healthy individuals, the synovium is important for providing nutrients to the cartilage and lubricants to allow cartilage mobility. With RA initiation, important changes in the synovium are observed including expansion of the synovial intimal lining composed of FLS and MLS (macrophage-like synoviocytes). Such expansion is associated with FLS with overexpression of TLR2/3/4/7 and production of a high amount of IL-6 and MMP3 (metalloproteinase 3). Regarding MLS, they produce a large panel of pro-inflammatory cytokines in response to TLR overexpression and hyper response. In addition to being overexpressed at the FLS and MLS cell surface, an abnormal presence of bacterial DNA and bacterial peptidoglycans has been reported in joints of patients with RA [16, 17] as well as demonstration that active TLR-4 ligands are increased in the serum and synovial fluid of RA patients [18].
Analysis of T and B cells present in the synovial sublining with APC (DC, macrophages, mastocytes) reveals CD4+ memory T cells that can be diffusively organized or associated with mature B cells and antibody-producing plasmablasts to form ectopic germinal centers. Peripheral memory T cells have been proposed as an interesting biomarker associated with the biological response to disease-modifying antirheumatic drugs (DMARDs) [19, 20]. Present in the synovial fluid space, neutrophils express TLR and contribute to joint damage by the release of pro-inflammatory cytokines and MMPs.
Lessons from Mouse Models
In RA animal models, activation of the TLR pathway is used to induce the disease in susceptible strains (Table 2). The disease can be reproduced in IL-1 receptor antagonist (Ra)-deficient mice that spontaneously develop autoimmune arthritis due to excessive IL-1R/TLR signaling [21]. Autoimmune arthritis in this model is dependent on the microbial flora since germ-free mice do not develop arthritis. Coupling IL-1Ra and TLR2 knockdown revealed a more severe arthritis with Treg reduction, while the IL-1Ra and TLR4 knockdown had a markedly lower capacity to produce IL-17 [22, 23]. Analysis of the microbiota in the IL-1Ra-deficient mice revealed an aberrant intestinal flora and, when the fecal microbiota was transferred into wild-type mice, they reproduce IL-17 production by the lamina propria and T helper (TH)17 expansion [23].
Mice expressing both the T cell receptor (TCR) transgene KRN and the MHC class II molecule A(g7) (K/BxN mice) develop a severe arthritis, and sera from these mice cause a similar arthritis in a wide range of mouse strains, due to Abs recognizing glucose-6-phosphate isomerase. This mechanism is dependent on the IL-1R/TLR pathway since neither IL-1R nor MYD88 knockdown mice develop synovitis after the transfer of the arthritogenic sera [24]. The functional significance of TLR2 and TLR4 was tested after serum transfer revealing the protective role of TLR2 on joint inflammation and bone erosion by controlling the FcγR (Fc gamma receptor) response in macrophages [25], while TLR4 mediated pro-inflammatory cytokine production by joint macrophages and mast cells [26]. The mechanisms by which the gut microbiota affects arthritis development were further explored revealing the importance of follicular helper T cell differentiation, instead of a TH17-dependent mechanism [27].
Lessons from Genetic and Epigenetic Studies
More than 100 genetic variants have been characterized for RA, and among them, several involve the TLR pathway (Table 3). Regarding TLR2, a dinucleotide polymorphism present in intron 2 is suspected to confer susceptibility to RA in a Korean population [28], while it is the TLR3 rs3775291 A allele that is significantly associated with RA in sero-negative Danish patients [29]. Several groups have further evaluated TLR4 polymorphisms supporting roles for a TLR4 Asp299Gly mutation (rs4986790) in RA pathogenesis, in preventing chronic periodontal disease mediated by Porphyromonas gingivalis, and in providing a more effective response following anti-TNF biotherapy [30,31,32]. TLR4 rs1927911 is associated with disease activity [33]. For TLR8 rs5741883, a moderate association with RF positivity has been reported by a Danish group [34]. TLR9 rs187084 presents regional variations with a susceptibility to RA, and an anti-TNF therapy response is reported in RA patients from Turkey and Poland [30, 35]. With regard to downstream TLRs, TRAF1 rs7021206 is associated with RA susceptibility in those patients positive for RF and ACPA, and TRAF5 rs7514863 represents another RA susceptibility risk factor [36, 37].
Among the epigenetic factors associated with RA, increasing evidence supports a role for miRNAs in the regulation of the TLR pathway [38]. MiRNAs are defined as short non-coding RNAs capable of gene expression modulation via direct binding to the 3′-UTR (untranslated region) of target mRNAs [39]. One way in which miRNAs can affect the IL-1R/TLR pathway is by controlling TLRs and IL-1R, and this includes miR19a and miR140-5p/miR6089 which regulate TLR2 and TLR4, respectively [40,41,42]. A second way is to act as an endogenous ligand for TLR as demonstrated with Let-7b which possesses a GU-rich domain able to stimulate TLR7 in myeloid cells leading to pro-inflammatory M1 macrophage differentiation [43]. A third way is to target TLR/IL-1R adapter molecules, and the best example is miR146a, demonstrated to be overexpressed in RA, which controls IRAK1 and TRAF6 except when the C allele is present since it is considered to be protective for RA development [44]. Another example is miR10a which is downregulated in RA FLS with IRAK4 and TAK1 as targets and which is upregulated in those patients responding to methotrexate [45, 46].
Infections, TLR, and Rheumatoid Arthritis
Among potential infectious sources of PAMPs, oral microbiota and commensal intestinal microbiota are suspected. In this regard, epidemiologic data have been reported pointing to a positive association between RA and upper respiratory tract infection on one hand [47], while, on the other hand, it is a negative association that was observed relative to gastrointestinal and urogenital tract infections [48].
Microbiota
Recent studies suggest that alteration of intestinal microbiota, known as gut dysbiosis, contributes to the occurrence or development of RA through an impaired balance between pro- and anti-inflammatory immune responses [49]. In particular, Prevotella copri, a Gram-negative anaerobic bacterial member of the Bacteriodetes phylum, defined the microbiome of RA patients and is implicated in other autoimmune diseases including inflammatory bowel disease and colitis [50]. The consequential effects of these shifts include alterations in the metabolic composition of the gut, hyperactivation of the IL-1R/TLR pathway, upregulation of pro-inflammatory cytokines, increased intestinal permeability, and increased inflammation [51]. Differential microbiome compositions exist between males and females [52]. Moreover, intervention at the level of the microbiota appears to attenuate symptoms as reported with lactobacilli, playing a positive role in restoring intestinal health, and decreasing inflammation [53].
Porphyromonas gingivalis
Several arguments support P. gingivalis as an important etiological factor in RA. First, P. gingivalis is associated with periodontitis, an inflammatory disorder of the mouth, and it is a well-known environmental risk factor associated with RA [54]. Second, P. gingivalis has the particularity to express a prokaryotic peptidylarginine deiminase (PPAD) able to convert arginine to citrulline, thereby becoming a target for ACPA [55]. Such capacity appears to be unique and not shared with other common oral prokaryotic organisms. Further evidential support that LPS (lipopolysaccharide) from P. gingivalis activates TLR2 leading to the upregulation of the extracellular matrix protein TSP1 (thrombospondin-1) and IL-33 in monocytes. IL-33 is an IL-1 family cytokine that is important in regulating T helper type 2 anti-inflammatory cytokines and mast cell development to the production of calprotectin by neutrophils and to the bone mineral release and matrix degradation by increasing osteoclast differentiation in response to RANKL (receptor activator of NF-KB ligand) overexpression [56,57,58,59]. Furthermore, the TLR2 response to P. gingivalis is reduced in the presence of cigarette smoke extract, another important RA risk factor. This supports the assumption that periodontitis is increased in tobacco smokers and also that smokers have fewer signs of inflammation [60, 61]. Third, elevated levels of P. gingivalis DNA have been isolated in the synovial fluids of inflamed joints from patients with RA and, in particular, in those harboring the RA susceptibility, HLA-DR shared epitope DR4 [62]. Fourth, treating RA patients with anti-TNF mAb reduces P. gingivalis oral colonization and periodontal disease but a persistent periodontal disease hampers the treatment response [63, 64]. In the SKG RA mouse model, P. gingivalis extra-articular injection in the peritoneum enhances the severity of the disease, and this is dependent on the TH17/IL-7 signaling pathway [65].
Herpes Simplex and TLR
For a long time, HSV (herpes simplex virus) is suspected of being involved in RA although the debate is still open as to whether or not the increasing reports of HSV reactivation during RA results, in fact, from an alteration in the immune system which then increases the susceptibility to infection or if the infectious events predispose one to RA [66]. Furthermore, innate resistance to HSV relies on the activation of TLR2 and TLR9, two TLRs overexpressed in monocytes from active RA and which display higher production of pro-inflammatory cytokines in response to TLR agonists [67] (Table 4). This then supports a role for HSV and other HHV (human herpes virus) family members in the exacerbation of RA symptoms [47]. Indeed, HSV genomic DNA can engage TLR9 and result in the secretion of IFN-α by pDCs [68], while the HSV envelope glycoprotein gB and dUTPase are both recognized by TLR2, which leads to the activation of NF-κB and secretion of pro-inflammatory cytokines [69, 70].
Conclusions
The discovery of TLRs has opened up new perspectives in autoimmune diseases and, in particular, in RA. As a consequence, blocking TLR signals represents an attractive therapeutic approach as demonstrated with an anti-TLR2 mAb able to decrease spontaneous pro-inflammatory cytokine release from RA synovial tissue explant cultures [71], or with hydroxychloroquine—a DMARD that suppresses the TLR9-mediated human B cell capacity to differentiate to plasmablasts [72]. Hence, controlling TLR activation in RA, as well as identifying RA patients who will respond to these therapies, and a better knowledge of the innate immune mechanisms as reviewed in this special issue [49, 73,74,75,76,77,78,79,80] open new therapeutic perspectives.
References
Smolen JS, Aletaha D, Barton A, Burmester GR, Emery P, Firestein GS, Kavanaugh A, McInnes IB, Solomon DH, Strand V, Yamamoto K (2018) Rheumatoid arthritis. Nat Rev Dis Primers 4:18001
Renaudineau Y, Jamin C, Saraux A, Youinou P (2005) Rheumatoid factor on a daily basis. Autoimmunity 38:11–16
Smolen JS, Aletaha D, McInnes IB (2016) Rheumatoid arthritis. Lancet 388:2023–2038
Brooks WH, Le Dantec C, Pers JO, Youinou P, Renaudineau Y (2010) Epigenetics and autoimmunity. J Autoimmun 34:J207–J219
Arleevskaya MI, Kravtsova OA, Lemerle J, Renaudineau Y, Tsibulkin AP (2016) How rheumatoid arthritis can result from provocation of the immune system by microorganisms and viruses. Front Microbiol 7:1296
Imler JL, Hoffmann JA (2001) Toll receptors in innate immunity. Trends Cell Biol 11:304–311
Takeda K, Kaisho T, Akira S (2003) Toll-like receptors. Annu Rev Immunol 21:335–376
Cook DN, Pisetsky DS, Schwartz DA (2004) Toll-like receptors in the pathogenesis of human disease. Nat Immunol 5:975–979
Medzhitov R (2001) Toll-like receptors and innate immunity. Nat Rev Immunol 1:135–145
Wesche H, Henzel WJ, Shillinglaw W, Li S, Cao Z (1997) MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity 7:837–847
Strickson S, Emmerich CH, Goh ETH, Zhang J, Kelsall IR, Macartney T, Hastie CJ, Knebel A, Peggie M, Marchesi F, Arthur JSC, Cohen P (2017) Roles of the TRAF6 and Pellino E3 ligases in MyD88 and RANKL signaling. Proc Natl Acad Sci U S A 114:E3481–E3489
Narayanan KB, Park HH (2015) Toll/interleukin-1 receptor (TIR) domain-mediated cellular signaling pathways. Apoptosis 20:196–209
Boraschi D, Italiani P, Weil S, Martin MU (2018) The family of the interleukin-1 receptors. Immunol Rev 281:197–232
Rajaiah R, Perkins DJ, Ireland DD, Vogel SN (2015) CD14 dependence of TLR4 endocytosis and TRIF signaling displays ligand specificity and is dissociable in endotoxin tolerance. Proc Natl Acad Sci U S A 112:8391–8396
Severa M, Fitzgerald KA (2007) TLR-mediated activation of type I IFN during antiviral immune responses: fighting the battle to win the war. Curr Top Microbiol Immunol 316:167–192
Kempsell KE, Cox CJ, Hurle M, Wong A, Wilkie S, Zanders ED, Gaston JSH, Crowe JS (2000) Reverse transcriptase-PCR analysis of bacterial rRNA for detection and characterization of bacterial species in arthritis synovial tissue. Infect Immun 68:6012–6026
van der Heijden IM, Wilbrink B, Tchetverikov I, Schrijver IA, Schouls LM, Hazenberg MP, Breedveld FC, Tak PP (2000) Presence of bacterial DNA and bacterial peptidoglycans in joints of patients with rheumatoid arthritis and other arthritides. Arthritis Rheum 43:593–598
Roelofs MF, Joosten LA, Abdollahi-Roodsaz S et al (2005) The expression of toll-like receptors 3 and 7 in rheumatoid arthritis synovium is increased and costimulation of toll-like receptors 3, 4, and 7/8 results in synergistic cytokine production by dendritic cells. Arthritis Rheum 52:2313–2322
Gazeau P, Alegria GC, Devauchelle-Pensec V, Jamin C, Lemerle J, Bendaoud B, Brooks WH, Saraux A, Cornec D, Renaudineau Y (2017) Memory B cells and response to abatacept in rheumatoid arthritis. Clin Rev Allergy Immunol 53:166–176
Gazeau P, Devauchelle-Pensec V, Pochard P, Pers JO, Saraux A, Renaudineau Y, Cornec D (2016) Abatacept efficacy in rheumatoid arthritis is dependent upon baseline blood B-cell levels. Rheumatology (Oxford) 55:1138–1140
Horai R, Saijo S, Tanioka H, Nakae S, Sudo K, Okahara A, Ikuse T, Asano M, Iwakura Y (2000) Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist-deficient mice. J Exp Med 191:313–320
Abdollahi-Roodsaz S, Joosten LA, Koenders MI et al (2008) Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J Clin Invest 118:205–216
Rogier R, Ederveen THA, Boekhorst J, Wopereis H, Scher JU, Manasson J, Frambach SJCM, Knol J, Garssen J, van der Kraan PM, Koenders MI, van den Berg WB, van Hijum SAFT, Abdollahi-Roodsaz S (2017) Aberrant intestinal microbiota due to IL-1 receptor antagonist deficiency promotes IL-17- and TLR4-dependent arthritis. Microbiome 5:63
Choe JY, Crain B, Wu SR, Corr M (2003) Interleukin 1 receptor dependence of serum transferred arthritis can be circumvented by toll-like receptor 4 signaling. J Exp Med 197:537–542
Abdollahi-Roodsaz S, Koenders MI, Walgreen B, Bolscher J, Helsen MM, van den Bersselaar L, van Lent P, van de Loo F, van den Berg W (2013) Toll-like receptor 2 controls acute immune complex-driven arthritis in mice by regulating the inhibitory Fcgamma receptor IIB. Arthritis Rheum 65:2583–2593
Kim HS, Chung DH (2012) TLR4-mediated IL-12 production enhances IFN-gamma and IL-1beta production, which inhibits TGF-beta production and promotes antibody-induced joint inflammation. Arthritis Res Ther 14:R210
Block KE, Zheng Z, Dent AL, Kee BL, Huang H (2016) Gut microbiota regulates K/BxN autoimmune arthritis through follicular helper T but not Th17 cells. J Immunol 196:1550–1557
Lee EY, Yim JJ, Lee HS, Lee YJ, Lee EB, Song YW (2006) Dinucleotide repeat polymorphism in intron II of human toll-like receptor 2 gene and susceptibility to rheumatoid arthritis. Int J Immunogenet 33:211–215
Laska MJ, Hansen B, Troldborg A, Lorenzen T, Stengaard-Pedersen K, Junker P, Nexø BA, Lindegaard HM (2014) A non-synonymous single-nucleotide polymorphism in the gene encoding toll-like receptor 3 (TLR3) is associated with sero-negative rheumatoid arthritis (RA) in a Danish population. BMC Res Notes 7:716
Gębura K, Świerkot J, Wysoczańska B et al (2017) Polymorphisms within genes involved in regulation of the NF-κB pathway in patients with rheumatoid arthritis. Int J Mol Sci 18. https://doi.org/10.3390/ijms18071432
Wang Y, Chen L, Li F, Bao M, Zeng J, Xiang J, Luo H, Li J, Tang L (2017) TLR4 rs41426344 increases susceptibility of rheumatoid arthritis (RA) and juvenile idiopathic arthritis (JIA) in a central south Chinese Han population. Pediatr Rheumatol Online J 15:12
Sellers RM, Payne JB, Yu F, LeVan TD, Walker C, Mikuls TR (2016) TLR4 Asp299Gly polymorphism may be protective against chronic periodontitis. J Periodontal Res 51:203–211
Davis MLR, LeVan TD, Yu F et al (2015) Associations of toll-like receptor (TLR)-4 single nucleotide polymorphisms and rheumatoid arthritis disease progression: an observational cohort study. Int Immunopharmacol 24:346–352
Enevold C, Radstake TR, Coenen MJ et al (2010) Multiplex screening of 22 single-nucleotide polymorphisms in 7 toll-like receptors: an association study in rheumatoid arthritis. J Rheumatol 37:905–910
Etem EO, Elyas H, Ozgocmen S, Yildirim A, Godekmerdan A (2011) The investigation of toll-like receptor 3, 9 and 10 gene polymorphisms in Turkish rheumatoid arthritis patients. Rheumatol Int 31:1369–1374
Han TU, Bang SY, Kang C, Bae SC (2009) TRAF1 polymorphisms associated with rheumatoid arthritis susceptibility in Asians and in Caucasians. Arthritis Rheum 60:2577–2584
Potter C, Eyre S, Cope A, Worthington J, Barton A (2007) Investigation of association between the TRAF family genes and RA susceptibility. Ann Rheum Dis 66:1322–1326
Sujitha S, Rasool M (2017) MicroRNAs and bioactive compounds on TLR/MAPK signaling in rheumatoid arthritis. Clin Chim Acta 473:106–115
Zare-Shahabadi A, Renaudineau Y, Rezaei N (2013) MicroRNAs and multiple sclerosis: from physiopathology toward therapy. Expert Opin Ther Targets 17:1497–1507
Philippe L, Alsaleh G, Suffert G, Meyer A, Georgel P, Sibilia J, Wachsmann D, Pfeffer S (2012) TLR2 expression is regulated by microRNA miR-19 in rheumatoid fibroblast-like synoviocytes. J Immunol 188:454–461
Li H, Guan SB, Lu Y, Wang F (2017) MiR-140-5p inhibits synovial fibroblasts proliferation and inflammatory cytokines secretion through targeting TLR4. Biomed Pharmacother 96:208–214
Xu D, Song M, Chai C et al (2019) Exosome-encapsulated miR-6089 regulates inflammatory response via targeting TLR4. J Cell Physiol 234:1502–1511
Kim SJ, Chen Z, Essani AB, Elshabrawy HA, Volin MV, Volkov S, Swedler W, Arami S, Sweiss N, Shahrara S (2016) Identification of a novel toll-like receptor 7 endogenous ligand in rheumatoid arthritis synovial fluid that can provoke arthritic joint inflammation. Arthritis Rheum 68:1099–1110
Ayeldeen G, Nassar Y, Ahmed H, Shaker O, Gheita T (2018) Possible use of miRNAs-146a and -499 expression and their polymorphisms as diagnostic markers for rheumatoid arthritis. Mol Cell Biochem 449:145–156
Hong H, Yang H, Xia Y (2018) Circulating miR-10a as predictor of therapy response in rheumatoid arthritis patients treated with methotrexate. Curr Pharm Biotechnol 19:79–86
Mu N, Gu J, Huang T, Zhang C, Shu Z, Li M, Hao Q, Li W, Zhang W, Zhao J, Zhang Y, Huang L, Wang S, Jin X, Xue X, Zhang W, Zhang Y (2016) A novel NF-kappaB/YY1/microRNA-10a regulatory circuit in fibroblast-like synoviocytes regulates inflammation in rheumatoid arthritis. Sci Rep 6:20059
Arleevskaya MI, Albina S, Larionova RV, Gabdoulkhakova AG, Lemerle J, Renaudineau Y (2018) Prevalence and incidence of upper respiratory tract infection events are elevated prior to the development of rheumatoid arthritis in first-degree relatives. Front Immunol 9:2771
Sandberg ME, Bengtsson C, Klareskog L, Alfredsson L, Saevarsdottir S (2015) Recent infections are associated with decreased risk of rheumatoid arthritis: a population-based case-control study. Ann Rheum Dis 74:904–907
Arleevskaya MI, Aminov R, Brooks WH, Manukyan G, Renaudineau Y (2019) Editorial: shaping oh human immune system and metabolic processes by viruses and microorganisms. Front Microbiol 10:816
Scher JU, Sczesnak A, Longman RS, Segata N, Ubeda C, Bielski C, Rostron T, Cerundolo V, Pamer EG, Abramson SB, Huttenhower C, Littman DR (2013) Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife 2:e01202
Kasselman LJ, Vernice NA, DeLeon J, Reiss AB (2018) The gut microbiome and elevated cardiovascular risk in obesity and autoimmunity. Atherosclerosis 271:203–213
Bolnick DI, Snowberg LK, Hirsch PE, Lauber CL, Org E, Parks B, Lusis AJ, Knight R, Caporaso JG, Svanbäck R (2014) Individual diet has sex-dependent effects on vertebrate gut microbiota. Nat Commun 5:4500
Badsha H (2018) Role of diet in influencing rheumatoid arthritis disease activity. Open Rheumatol J 12:19–28
Mikuls TR, Payne JB, Yu F, Thiele GM, Reynolds RJ, Cannon GW, Markt J, McGowan D, Kerr GS, Redman RS, Reimold A, Griffiths G, Beatty M, Gonzalez SM, Bergman DA, Hamilton BC III, Erickson AR, Sokolove J, Robinson WH, Walker C, Chandad F, O’Dell JR (2014) Periodontitis and Porphyromonas gingivalis in patients with rheumatoid arthritis. Arthritis Rheum 66:1090–1100
Quirke AM, Lugli EB, Wegner N, Hamilton BC, Charles P, Chowdhury M, Ytterberg AJ, Zubarev RA, Potempa J, Culshaw S, Guo Y, Fisher BA, Thiele G, Mikuls TR, Venables PJW (2014) Heightened immune response to autocitrullinated Porphyromonas gingivalis peptidylarginine deiminase: a potential mechanism for breaching immunologic tolerance in rheumatoid arthritis. Ann Rheum Dis 73:263–269
Kassem A, Henning P, Lundberg P, Souza PPC, Lindholm C, Lerner UH (2015) Porphyromonas gingivalis stimulates bone resorption by enhancing RANKL (receptor activator of NF-kappaB ligand) through activation of toll-like receptor 2 in osteoblasts. J Biol Chem 290:20147–20158
Gokyu M, Kobayashi H, Nanbara H, Sudo T, Ikeda Y, Suda T, Izumi Y (2014) Thrombospondin-1 production is enhanced by Porphyromonas gingivalis lipopolysaccharide in THP-1 cells. PLoS One 9:e115107
Nile CJ, Barksby E, Jitprasertwong P, Preshaw PM, Taylor JJ (2010) Expression and regulation of interleukin-33 in human monocytes. Immunology 130:172–180
Kido J, Kido R, Suryono, Kataoka M, Fagerhol MK, Nagata T (2003) Calprotectin release from human neutrophils is induced by Porphyromonas gingivalis lipopolysaccharide via the CD-14-toll-like receptor-nuclear factor kappaB pathway. J Periodontal Res 38:557–563
Mahanonda R, Sa-Ard-Iam N, Eksomtramate M, Rerkyen P, Phairat B, Schaecher KE, Fukuda MM, Pichyangkul S (2009) Cigarette smoke extract modulates human beta-defensin-2 and interleukin-8 expression in human gingival epithelial cells. J Periodontal Res 44:557–564
Bagaitkar J, Demuth DR, Daep CA, Renaud DE, Pierce DL, Scott DA (2010) Tobacco upregulates P. gingivalis fimbrial proteins which induce TLR2 hyposensitivity. PLoS One 5:e9323
Totaro MC, Cattani P, Ria F, Tolusso B, Gremese E, Fedele A, D'Onghia S, Marchetti S, Sante G, Canestri S, Ferraccioli G (2013) Porphyromonas gingivalis and the pathogenesis of rheumatoid arthritis: analysis of various compartments including the synovial tissue. Arthritis Res Ther 15:R66
Savioli C, Ribeiro AC, Fabri GM, Calich AL, Carvalho J, Silva CA, Viana VS, Bonfá E, Siqueira JT (2012) Persistent periodontal disease hampers anti-tumor necrosis factor treatment response in rheumatoid arthritis. J Clin Rheumatol 18:180–184
Mayer Y, Balbir-Gurman A, Machtei EE (2009) Anti-tumor necrosis factor-alpha therapy and periodontal parameters in patients with rheumatoid arthritis. J Periodontol 80:1414–1420
Yamakawa M, Ouhara K, Kajiya M, Munenaga S, Kittaka M, Yamasaki S, Takeda K, Takeshita K, Mizuno N, Fujita T, Sugiyama E, Kurihara H (2016) Porphyromonas gingivalis infection exacerbates the onset of rheumatoid arthritis in SKG mice. Clin Exp Immunol 186:177–189
Arleevskaya MI, Shafigullina AZ, Filina YV, Lemerle J, Renaudineau Y (2017) Associations between viral infection history symptoms, granulocyte reactive oxygen species activity, and active rheumatoid arthritis disease in untreated women at onset: results from a longitudinal cohort study of Tatarstan women. Front Immunol 8:1725
Lacerte P, Brunet A, Egarnes B, Duchêne B, Brown JP, Gosselin J (2016) Overexpression of TLR2 and TLR9 on monocyte subsets of active rheumatoid arthritis patients contributes to enhance responsiveness to TLR agonists. Arthritis Res Ther 18:10
Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A (2003) Toll-like receptor 9-mediated recognition of herpes simplex virus-2 by plasmacytoid dendritic cells. J Exp Med 198:513–520
Cai M, Li M, Wang K, Wang S, Lu Q, Yan J, Mossman KL, Lin R, Zheng C (2013) The herpes simplex virus 1-encoded envelope glycoprotein B activates NF-kappaB through the toll-like receptor 2 and MyD88/TRAF6-dependent signaling pathway. PLoS One 8:e54586
Ariza ME, Glaser R, Williams MV (2014) Human herpesviruses-encoded dUTPases: a family of proteins that modulate dendritic cell function and innate immunity. Front Microbiol 5:504
Ultaigh SN, Saber TP, McCormick J et al (2011) Blockade of toll-like receptor 2 prevents spontaneous cytokine release from rheumatoid arthritis ex vivo synovial explant cultures. Arthritis Res Ther 13:R33
Torigoe M, Sakata K, Ishii A, Iwata S, Nakayamada S, Tanaka Y (2018) Hydroxychloroquine efficiently suppresses inflammatory responses of human class-switched memory B cells via toll-like receptor 9 inhibition. Clin Immunol 195:1–7
Hillion S, Arleevskaya MI, Brooks WH et al (2019) The innate part of the adaptive immune system. Clin Rev Allergy Immunol
Guia S, Vivier E, Narni-Mancinelli E (2019) Helper-like innate lymphoid cells: definition, functions and clinical implications in inflammatory diseases and cancer. Clin Rev Allergy Immunol
Grasseau A, Boudigou M, Le Pottier L et al (2019) Innate B-cells: the archetype of protective immune cells. Clin Rev Allergy Immunol
Brilland B, Scherlinger M, Khoryati L et al (2019) Platelets and IgE: shaping the innate immune response in systemic lupus erythematosus. Clin Rev Allergy Immunol
Maddur MS, Lacroix-Desmazes S, Dimitrov JD et al (2019) Natural antibodies: from first line defense against pathogens to perpetual immune homeostasis. Clin Rev Allergy Immunol
Defendia F, Thielensb NM, Clavarinoa G, Cesbron JY, Dumestre-Pérard C (2019) Autoantibodies targeting complement components and associated diseases. Clin Rev Allergy Immunol
Bordron A, Bagacean C, Tempescul A et al (2019) Complement system: a neglected pathway in immunotherapy. Clin Rev Allergy Immunol
Charras A, Arvaniti P, Le Dantec C et al (2019) JAK Inhibitors Suppress innate epigenetic reprogramming: a promise for patients with Sjögren’s syndrome. Clin Rev Allergy Immunol. https://doi.org/10.1007/s12016-019-08743-y
Shaker OG, El Boghdady NA, El Sayed AE (2018) Association of MiRNA-146a, MiRNA-499, IRAK1 and PADI4 polymorphisms with rheumatoid arthritis in Egyptian population. Cell Physiol Biochem 46:2239–2249
Hu F, Li Y, Zheng L, Shi L, Liu H, Zhang X, Zhu H, Tang S, Zhu L, Xu L, Yang Y, Li Z (2014) Toll-like receptors expressed by synovial fibroblasts perpetuate Th1 and th17 cell responses in rheumatoid arthritis. PLoS One 9:e100266
Chovanova L, Vlcek M, Krskova K, Penesova A, Radikova Z, Rovensky J, Cholujova D, Sedlak J, Imrich R (2013) Increased production of IL-6 and IL-17 in lipopolysaccharide-stimulated peripheral mononuclears from patients with rheumatoid arthritis. Gen Physiol Biophys 32:395–404
Acknowledgments
We are thankful to Servier Medical for providing free art for the figures and to Simone Forest and Genevieve Michel for secretarial help.
Funding
This study was supported by research funding from the “Russian Science Foundation” (no. 17-15-01099) and the “Association Française de Gougerot Sjögren et des syndromes secs”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arleevskaya, M.I., Larionova, R.V., Brooks, W.H. et al. Toll-Like Receptors, Infections, and Rheumatoid Arthritis. Clinic Rev Allerg Immunol 58, 172–181 (2020). https://doi.org/10.1007/s12016-019-08742-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-019-08742-z