Abstract
The number of peer-reviewed articles published during the 2016 solar year and retrieved using the “autoimmunity” key word remained stable while gaining a minimal edge among the immunology articles. Nonetheless, the quality of the publications has been rising significantly and, importantly, acquisitions have become available through scientific journals dedicated to immunology or autoimmunity. Major discoveries have been made in the fields of systemic lupus erythematosus, rheumatoid arthritis, autoimmunity of the central nervous system, vasculitis, and seronegative spondyloarthrithritides. Selected examples include the role of IL17-related genes and long noncoding RNAs in systemic lupus erythematosus or the effects of anti-pentraxin 3 (PTX3) in the treatment of this paradigmatic autoimmune condition. In the case of rheumatoid arthritis, there have been reports of the role of induced regulatory T cells (iTregs) or fibrocytes and T cell interactions with exciting implications. The large number of studies dealing with neuroimmunology pointed to Th17 cells, CD56(bright) NK cells, and low-level TLR2 ligands as involved in multiple sclerosis, along with a high salt intake or the micriobiome-derived Lipid 654. Lastly, we focused on the rare vasculitides to which numerous studies were devoted and suggested that unsuspected cell populations, including monocytes, mucosal-associated invariant T cells, and innate lymphoid cells, may be crucial to ANCA-associated manifestations. This brief and arbitrary discussion of the findings published in 2016 is representative of a promising background for developments that will enormously impact the work of laboratory scientists and physicians at an exponential rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
2016 and Autoimmunity
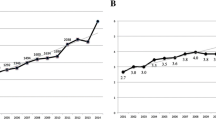
As we did for the previous years [1,2,3,4,5,6,7,8], we provide an overview of the publications dedicated to autoimmunity over the past solar year. In this view, 2016 was a stable year (+0.3%) in the absolute number of publications compared to 2015, with 2341 papers retrieved on PubMed (Fig. 1). The ratio of autoimmunity over immunology papers increased slightly in 2016, with a 5.1% prevalence (+0.1%) (Fig. 2).
To retrieve the most important publications regarding autoimmunity in 2016, we performed a literature research on PubMed in May 2017 among the major journals in the areas of immunology (Nature Immunology, Journal of Immunology, Nature Medicine, Clinical Reviews in Allergy and Immunology) and autoimmunity (Autoimmunity Reviews, Journal of Autoimmunity) and divided the articles in the most important clinical topics: systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), nervous system autoimmunity, vasculitis, and seronegative spondyloarthropathies. Indeed, this approach leads to an underrepresentation of other important diseases, such as inflammatory myopathies [9,10,11,12,13,14,15,16,17], antiphospholipid antibodies and syndrome [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33], Sjogren syndrome [34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49], and systemic sclerosis [50,51,52,53,54,55,56,57,58,59,60,61,62]. Similarly, we overlooked papers regarding bone homeostasis, which were well represented in 2016 [63,64,65,66,67,68]. Taken altogether, we should be aware that the choice of the articles to be briefly discussed is arbitrary and will lead to some missing references. Nonetheless, we will discuss the most recent findings regarding SLE [61, 69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113], RA [114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144], seronegative spondyloarthritis [121, 145,146,147,148,149,150,151,152,153,154,155,156,157,158,159], neuroimmunology [160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195], and vasculitis [196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224].
Systemic Lupus Erythematosus
Each year, systemic lupus erythematosus remains one of the most important topics for research, and we selected 42 articles dedicated to the disease in 2016, mostly dealing with the SLE pathophysiology and clinical manifestations. Last year, however, other original papers investigated the co-occurrence of other autoimmune diseases with SLE, in particular thyroid autoimmunity [104, 105], gastrointestinal [72, 98] as well as asthma and rhinitis [99].
It is well known that SLE is a multifactorial disease, where genetics and environmental factors interact. In the last year, the genetic factors predisposing to SLE were reviewed [101], as well as new polymorphisms conferring susceptibility to SLE were reported, in particular within the interleukin (IL)-17 family. In a Polish population of patients with SLE has been reported an increased frequency of the AG genotype as well as the G allele of the IL-17F rs763780 (OR = 3.947; p = 0.001 and OR = 3.538; p = 0.002, respectively) and in the rs1884444 TT genotype (OR = 138.1) and the rs1884444 T allele (OR = 2.176), p < 0.001 in both [92]. Moreover, the GGAGAA combined genotype and the GGA haplotype of IL-17A rs2275913, IL-17F rs763780, and rs2397084 can be considered risk factors for the development of SLE in Egyptian children [81]. Interestingly, it has been reported that having any children who carry DRB1*04:01 alleles inherited from the father influences a mother’s subsequent risk of SLE (OR 1.9; 95% CI, 1.1–3.2) [71].
Epigenetics may reflect the interaction between genetics and the environment, and last year, this hypothesis was brilliantly reviewed [84, 86, 107]. Long noncoding RNAs (lncRNAs) have recently been identified to be tightly linked to diverse human diseases; lncRNA NEAT1 has been shown last year to contribute to the pathogenesis of SLE and to be abnormally increased in SLE patients and predominantly expressed in human monocytes, while being involved in the TLR4-mediated inflammatory process through affecting the activation of the late MAPK signaling pathway. NEAT1 furthermore correlated with clinical disease activity [108]. 5-Hydroxymethylcytosine (5-hmC), a newly discovered modified form of cytosine, is suspected to be an important epigenetic modification in embryonic development, cell differentiation, and cancer. Last year, it has been shown that increased 5-hmC levels are present in genomic DNA in CD4(+) T cells of patients with SLE compared with healthy controls, accompanied by the upregulated expression of the ten-eleven translocation TET2 and TET3, which can enzymatically convert 5-methylcytosine (5-mC) to 5-hmC [109].
The progression from tolerance breakdown and autoantibodies to SLE is not clear; however, it is supposed that an immune dysregulation involving multiple pathways contributes to SLE pathogenesis. The interferon (IFN) pathways are dysregulated in preclinical SLE, and multiple soluble mediators, including IL-5, IL-6, and IFN-gamma, are significantly elevated in SLE compared to controls more than 3.5 years before classification criteria are met, prior to or concurrent with autoantibody positivity. Moreover, innate cytokines, IFN-associated chemokines, and soluble tumor necrosis factor (TNF) superfamily mediators increase longitudinally in SLE patients approaching SLE classification, but not in controls. In particular, levels of B lymphocyte stimulator (BLyS) and a proliferation-inducing ligand (APRIL) are comparable in cases and controls until less than 10 months before the diagnosis is made [85]. From a clinical point of view, several reviews critically discussed the current understanding of lupus nephritis (LN) [78, 87] and neuropsychiatric SLE (NPSLE) [80, 82, 97, 100, 106]. More importantly, 2016 was the year in which positive pregnancy outcomes and SLE were extensively reported, resulting in long-awaited recommendations for patients with LN [83, 88, 89, 225].
SLE treatment remains a major area of interest, and an elegant study showed how anti-pentraxin 3 (PTX3) antibodies, which were associated with the absence of LN, delay LN and prolong survival of NZB/NZW F1 mice. In vitro observations suggest anti-PTX3 antibodies may dampen complement activation via their Fc fragment, likely hindering renal inflammation [77].
Rheumatoid Arthritis
Rheumatoid arthritis also remained a major research trend in 2016, with most articles dedicated to old and new treatment. In particular, biologic dose reduction risk was reviewed as cessation in established disease usually leads to disease flare; dose-tapering approaches for those achieving low disease activity often appear to be successful in the short term; however, tapering can be associated with a higher risk of losing disease control. Over relatively short periods of follow-up, a number of studies have shown no statistical difference in radiographic progression in patients tapering or discontinuing biologics. However, a Cochrane review found that radiographic and functional outcomes may be worse after TNF inhibitor discontinuation, and over long-term disease follow-up, flares have been associated with radiographic progression and worse patient-reported outcomes [117]. It is increasingly clear that obesity may modify the course of a rheumatologic disease and the clinical response to biotherapies based on a complex relationship of cytokines, hormones, growth factors, and intracellular regulators. A 2016 review article highlighted how obesity impairs the clinical response of RA to anti-TNF-alpha treatment, and this might be an effect limited to TNF-blocking agents, as preliminary studies are not confirming these findings for abatacept or tocilizumab [127].
In the case of new treatments, both positive and negative results have been achieved in 2016 [226]. The adoptive transfer of induced regulatory T cells (iTregs) may be effective in treating collagen-induced arthritis (CIA). Recently, it has been shown that the adoptive transfer of LAG3(+) Treg-of-B cells alleviates the joint severity as well as local and systemic inflammation, promoting IL-10 production in lymphocytes isolated from the spleen and draining lymph nodes [118]. Connective tissue growth factor (CTGF) contains four distinct modules connected in tandem, namely insulin-like growth factor-binding protein (IGFBP)-like, von Willebrand factor (vWF) type C repeat, thrombospondin type 1 (TSP-1) repeat, and carboxyl-terminal (CT) modules. In RA, it has been shown that the inhibition of each CTGF module affects M-CSF/RANKL-mediated osteoclastogenesis, furthermore, the angiogenesis of RA synoviocytes, and lastly that mAbs against CTGF neutralize the TNF-alpha enhanced the expression of CTGF and matrix metalloproteinase-3 (MMP3) in MH7A cells. Thus, a mAb against CTGF but also mAbs against each specific module of CTGF might serve as potential therapeutic agents in the treatment of RA [135]. RA-derived adipose-derived mesenchymal stem cell (ASC) secretory and proliferative activities are similar to those of osteoarthritis ASCs, derived from the infrapatellar fat pad of the knee. Both RA and osteoarthritis ASCs inhibit PBMC proliferation and induce IL-10 production but upregulate IL-17A secretion and fail to limit the release of other proinflammatory mediators (TNF, IFN-gamma, and CCL5) by PBMCs. RA and osteoarthritis ASCs do not suppress activation marker expression on T cells and do not trigger Tregs expansion [141]. Thus, RA ASCs may have less immunomodulatory properties compared to previous data.
New single-nucleotide polymorphisms (SNPs) have been associated with the risk of RA, in particular the IL-6-174G > C SNP in the Asian population [122], thus stressing once again the role of IL6 in chronic inflammation and its consequences. Moreover, the CD247 gene SNP (rs858554) was found to be associated with RA Chinese patients, especially with ACCP+ and RF+ phenotype [132]. Conversely to what is reported in the Caucasian population, the PTPN22 1858C/T polymorphism is not associated with RA risk in Asian populations [136]. Genetic susceptibility to RA is often defined by the presence of a shared epitope (QKRAA, QRRAA, or RRRAA) at positions 70–74 in HLA-DRbeta1. However, the DRbeta1*01:01 and 01:02 contain the same QRRAA epitope, but differ considerably in their susceptibility to RA. A recent study shows that DRbeta1*01:01 and *01:02 both exhibited a 6.5-fold preference for citrullinated vimentin (66–78) compared to native vimentin, while DRbeta1*01:01 also exhibited a 1.7-fold preference for citrullinated alpha-enolase (11–25) and bound collagen (258–272), while DRbeta1*01:02 bound neither of these peptides. Conversely, DRbeta1*01:03 preferentially bound native vimentin (66–78) and alpha-enolase (11–25) over the citrullinated forms of these peptides, and also failed to bind collagen (258–272). When site-directed mutagenesis was performed to determine which amino acid residues were responsible for the differences between these alleles, mutating position 86 in DRbeta1*01:01 from glycine to the valine residue found in DRbeta1*01:02 eliminated binding of both citrullinated alpha-enolase (11–25) and collagen (258–272), thereby recapitulating the peptide-binding profile of DRbeta1*01:02 [139].
Recent findings support the role of fibrocytes and T cell interactions as one of the initiating factors in RA. A recent study shows that RA fibrocytes exhibit increased activation, denoted as elevated levels of phosphorylation of STAT3 and NF-kappaB, with a direct correlation with the number of circulating activated Th17 cells and Tregs [125]. A novel transcription factor, YY1, has been identified as a potential key player in RA pathogenesis, since YY1 was over-expressed in RA patients and CIA mice, and the blocking of YY1 action with YY1 shRNA lentivirus ameliorated disease progression in CIA mice [133].
One of the major issues in RA management is the recognition and treatment of comorbidities, of which cardiovascular disease remains one of the most important. A recent study on 11,782 patients with RA and 57,973 age- and sex-matched controls confirms that the prevalence of ischemic heart disease in RA patients is increased compared to that in controls (16.6 and 12.8% respectively, p < 0.001) [126]. Moreover, heart impairment as a result of chronic inflammation and secondarily myocardial fibrosis markedly participates in heart failure development. Early detection of heart dysfunction is based on echocardiographic detection of diastolic dysfunction resulting from myocardial inflammation and fibrosis. The impact of biological treatment on the progression of atherosclerosis and heart failure is still controversial [131]. Overall, cardiovascular risk assessment should be performed routinely in RA patients, since cardiovascular disease is the most common cause of mortality. However, current cardiovascular screening and management strategies underestimate the real risk associated with RA [134].
Seronegative Spondyloarthritis
The increasing knowledge of psoriatic arthritis (PsA) pathogenesis [146, 148, 156] and the emerging treatments [151] currently approved for the disease have caused a significant increase of papers devoted to PsA, as well as psoriasis [145] and other spondyloarthrithritides, i.e., ankylosing spondylitis. Albeit psoriasis and PsA are considered more an autoinflammatory than autoimmune disease, PsA and psoriasis are mainly T cell-driven diseases, and the IL-23/IL-17 axis plays a critical pathogenic role for both PsA and psoriasis, while biologics neutralizing IL-17A or IL-23/IL-12 are effective therapies for both diseases [153, 155]. Growing evidence shows that albeit PsA is considered and classified as a seronegative disease, autoantibodies may be present in patient sera; in particular, anti-CarP antibodies have been reported in sera from patients with active PsA [121]. Moreover, autoantibodies have been detected also in ankylosing spondylitis patients, particularly anti-CD74 antibodies [150].
Biomarkers are of pivotal importance in spondyloarthritis to determine disease severity and prognosis. CRP currently appears to be the best circulating measure for assessing disease activity, predicting structural progression and therapeutic response, while key molecules in the pathogenesis of the disease and essential therapeutic targets show only limited association with disease characteristics or disease progression [149]. Spondyloarthritis is classically associated with the HLA-B27 allele, which may play a role also in disease pathogenesis, and more recently, nonconventional heavy chain forms of B27 expression in joints and lymphoid tissues from B27 TG(1) rats prior to the onset of arthritis are consistent with the hypothesis that they play a pathogenic role in spondyloarthritis [152].
Similarly to RA, also spondyloarthritis is associated with a significantly increased cardiovascular risk. The impact of treatment of psoriasis and PsA with anti-TNF on cardiovascular risk has been recently investigated in a meta-analysis, which included almost 50,000 patients. The results show that anti-TNF are associated with a lower risk of cardiovascular events, both compared to topical/photo treatment and methotrexate, and also to a decreased rate of mortality [157].
Neuroimmunology
As in previous years, the field of neuroimmunology has enormously increased in the number and quality of publications [191], with multiple sclerosis (MS) representing the most investigated disease [167, 174, 183, 189]. The importance of B cells in the pathogenesis [172, 173, 178] of the disease is supported by large lines of evidence also regarding B cell-depleting therapy [172, 185] and modulation of B cells by other treatments, as fingolimod [164]. However, increasing evidence shows that also T cells and especially Th17 [193] have a pivotal role in MS pathogenesis [160, 161, 187] and may represent possible future treatment strategies. It has been recently shown that mast cell-T cell co-localization in the meninges and CNS resident meningeal mast cells are an early source of caspase-1-dependent IL-1beta that licenses Th cells to produce GM-CSF and become encephalitogenic [168, 190]. Tregs have been reported to be defective in MS and correlate with disease phase, i.e., remission and relapse. Injections of expanded ex vivo autologous Tregs (eTregs) could be helpful in bringing up the level of Tregs in patients’ blood, and could be applied as immunotherapy for MS [182].
CD56(bright) NK cells may have immunoregulatory functions, and the expansion of CD56(bright) NK cells has been associated with successful response to different treatments and to remission of MS during pregnancy. In MS, CD56(bright) NK cells, albeit being in equal number as healthy subjects, have significantly lower ability to inhibit autologous T cell proliferation, due to increased HLA-E expression on T cells from MS/CIS subjects, which could enhance the inhibitory effect mediated by NKG2A that is homogeneously expressed on CD56(bright) NK cells [180].
Recent studies investigated the role of toll-like receptors (TLRs) in MS and animal models. In the EAE model, increased IFN-gamma production and presence of NK cells and reduced expression of TLR-3 and TLR-9 were observed [170]. A microbiome-derived TLR2 ligand, Lipid 654 (L654), which is present in healthy human serum, is significantly decreased in the serum of MS patients. The administration of low-level TLR2 ligands in adoptive transfer EAE induces TLR2 tolerance and attenuates disease [162]. Novel mechanisms increasing MS susceptibility have been identified, in particular salt (NaCl) intake, which promotes pathogenic T cell responses contributing to CNS autoimmunity [177].
Vasculitis
A large amount of publications was devoted during 2016 to systemic vasculitides [196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221], despite the cumulative rare prevalence of these conditions. Most of the published articles were dedicated to vasculitis associated with anti-neutrophil cytoplasm antibody (ANCA) [196, 197, 199, 201, 207,208,209,210, 212, 220] and giant cell arteritis [204, 205, 213, 214, 216, 218].
In the field of ANCA-associated vasculitis, the most recent findings highlight the importance of new cell types, i.e., monocytes, which contain the major autoantibody antigens (i.e., PR3 and MPO) in lysosomes, can express these at the cell surface, and can respond to ANCA by producing pro-inflammatory and chemotactic cytokines and reactive oxygen species and by upregulating CD14 [197]. Other previously unsuspected cell types such as the mucosal-associated invariant T cells (MAIT) and the innate lymphoid cells (ILCs) have been studied in vasculitis based on their involvement in various inflammatory and autoimmune diseases, via their immunoregulatory functions at mucosal sites. Importantly, MAIT cells are significantly decreased in ANCA-associated vasculitis during both acute and remission phases [196]. The pathogenesis of ANCA-associated vasculitis was investigated using phenotyping, transcriptome and functional analyses of T cell populations to evaluate triggers of memory T cell expansion. The data showed an increased percentage of circulating CD4+CD28−, CD8+CD28−, and CD4+CD161+ single-positive and CD4+CD8+ double-positive T cells in ANCA-associated vasculitis, while transcriptomic profiling of sorted T cell populations showed major differences between ANCA-associated vasculitis and healthy controls reflecting antigen- (bacteria, viruses, fungi) and cytokine-driven impact on T cell populations [209]. From a semantics standpoint, the name of eosinophilic granulomatosis with polyangiitis, previously known as Churg Strauss syndrome, was proposed to be refined, suggesting hypereosinophilic asthma with systemic (nonvasculitic) manifestations, as ANCA alone were reported to be insufficient to categorize patients with vasculitis features [199, 210].
One of the most important achievement of 2016 was the identification of IL-6 and IL-6 receptor inhibition as an effective therapy in giant cell arteritis [205], but also nonbiologic DMARDs have important beneficial effects, despite the limited evidence [213]. However, it was recently reported that long-term remission after glucocorticoids withdrawal in an Italian cohort of patients with biopsy-proven GCA is achieved in 56% of patients [216].
A Wishlist for 2017
During 2016, new scenarios were proposed in the autoimmunity field and spanned from new pathogenetic mechanisms to biomarkers to treatment optimization. What results is ultimately the possibility that basic science and clinics do indeed concur to make a difference in the understanding and management of patients with autoimmune diseases, often by connecting lines of evidence which are not intuitively close. In the oncology field, this is ideally represented by the checkpoint inhibitors and the birth of a new avenue of treatments. It is quite obvious that the number of published articles may not well represent the activity and success in any research area, but we are convinced that new developments are expected to stem from these reports, as in previous years for the microbiota, microRNA, or monoclonal antibodies.
References
Selmi C (2010) Autoimmunity in 2009. Autoimmun Rev 9(12):795–800. doi:10.1016/j.autrev.2010.08.008
Selmi C (2011) Autoimmunity in 2010. Autoimmun Rev 10(12):725–732. doi:10.1016/j.autrev.2011.06.004
Selmi C (2012) Autoimmunity in 2011. Clin Rev Allergy Immunol 43(1–2):194–206. doi:10.1007/s12016-012-8330-2
Selmi C (2013) Autoimmunity in 2012. Clin Rev Allergy Immunol 45(2):290–301. doi:10.1007/s12016-013-8386-7
Selmi C (2014) Autoimmunity in 2013. Clin Rev Allergy Immunol 47(1):100–109. doi:10.1007/s12016-014-8426-y
Selmi C (2015) Autoimmunity in 2014. Clin Rev Allergy Immunol 49(2):93–99. doi:10.1007/s12016-015-8504-9
Selmi C (2016) Autoimmunity in 2015. Clin Rev Allergy Immunol 51(1):110–119. doi:10.1007/s12016-016-8576-1
Selmi C (2007) One year in autoimmunity. Autoimmun Rev 7(1):85–93. doi:10.1016/j.autrev.2007.09.001
Musset L, Allenbach Y, Benveniste O, Boyer O, Bossuyt X, Bentow C, Phillips J, Mammen A, Van Damme P, Westhovens R, Ghirardello A, Doria A, Choi MY, Fritzler MJ, Schmeling H, Muro Y, Garcia-De La Torre I, Ortiz-Villalvazo MA, Bizzaro N, Infantino M, Imbastaro T, Peng Q, Wang G, Vencovsky J, Klein M, Krystufkova O, Franceschini F, Fredi M, Hue S, Belmondo T, Danko K, Mahler M (2016) Anti-HMGCR antibodies as a biomarker for immune-mediated necrotizing myopathies: a history of statins and experience from a large international multi-center study. Autoimmun Rev 15(10):983–993. doi:10.1016/j.autrev.2016.07.023
Danieli MG, Gelardi C, Guerra F, Cardinaletti P, Pedini V, Gabrielli A (2016) Cardiac involvement in polymyositis and dermatomyositis. Autoimmun Rev 15(5):462–465. doi:10.1016/j.autrev.2016.01.015
Muro Y, Sugiura K, Akiyama M (2016) Cutaneous manifestations in dermatomyositis: key clinical and serological features—a comprehensive review. Clin Rev Allergy Immunol 51(3):293–302. doi:10.1007/s12016-015-8496-5
Day J, Otto S, Proudman S, Hayball JD, Limaye V (2017) Dysregulated innate immune function in the aetiopathogenesis of idiopathic inflammatory myopathies. Autoimmun Rev 16(1):87–95. doi:10.1016/j.autrev.2016.09.019
Lu X, Tang Q, Lindh M, Dastmalchi M, Alexanderson H, Popovic Silwerfeldt K, Agerberth B, Lundberg IE, Wick C (2017) The host defense peptide LL-37 a possible inducer of the type I interferon system in patients with polymyositis and dermatomyositis. J Autoimmun 78:46–56. doi:10.1016/j.jaut.2016.12.003
Bhattarai S, Ghannam K, Krause S, Benveniste O, Marg A, de Bruin G, Xin BT, Overkleeft HS, Spuler S, Stenzel W, Feist E (2016) The immunoproteasomes are key to regulate myokines and MHC class I expression in idiopathic inflammatory myopathies. J Autoimmun 75:118–129. doi:10.1016/j.jaut.2016.08.004
Cherin P, Belizna C, Cartry O, Lascu-Dubos G, de Jaeger C, Delain JC, Crave JC, Hachulla E (2016) Long-term subcutaneous immunoglobulin use in inflammatory myopathies: a retrospective review of 19 cases. Autoimmun Rev 15(3):281–286. doi:10.1016/j.autrev.2015.12.003
Hosono Y, Nakashima R, Serada S, Murakami K, Imura Y, Yoshifuji H, Ohmura K, Naka T, Mimori T (2017) Splicing factor proline/glutamine-rich is a novel autoantigen of dermatomyositis and associated with anti-melanoma differentiation-associated gene 5 antibody. J Autoimmun 77:116–122. doi:10.1016/j.jaut.2016.11.006
Alvarado-Cardenas M, Marin-Sanchez A, Martinez MA, Martinez-Martinez L, Pinal-Fernandez I, Labrador-Horrillo M, Balada E, Mundet-Tuduri X, Gonzalez-Mera L, Casademont J, Acebes EM, Moreno PJ, Juarez C, Grau-Junyent JM, Pujol-Borrell R, Selva-O’Callaghan A (2016) Statin-associated autoimmune myopathy: a distinct new IFL pattern can increase the rate of HMGCR antibody detection by clinical laboratories. Autoimmun Rev 15(12):1161–1166. doi:10.1016/j.autrev.2016.09.005
Ruffatti A, Hoxha A, Favaro M, Tonello M, Colpo A, Cucchini U, Banzato A, Pengo V (2016) Additional treatments for high-risk obstetric antiphospholipid syndrome: a comprehensive review. Clin Rev Allergy Immunol. doi:10.1007/s12016-016-8571-6
Binder SR, Litwin CM (2016) Anti-phospholipid antibodies and smoking: an overview. Clin Rev Allergy Immunol. doi:10.1007/s12016-016-8565-4
Pengo V, Bison E, Zoppellaro G, Padayattil Jose S, Denas G, Hoxha A, Ruffatti A, Banzato A (2016) APS—diagnostics and challenges for the future. Autoimmun Rev 15(11):1031–1033. doi:10.1016/j.autrev.2016.07.028
Rodriguez-Pinto I, Moitinho M, Santacreu I, Shoenfeld Y, Erkan D, Espinosa G, Cervera R, Group CRP (2016) Catastrophic antiphospholipid syndrome (CAPS): descriptive analysis of 500 patients from the International CAPS Registry. Autoimmun Rev 15(12):1120–1124. doi:10.1016/j.autrev.2016.09.010
Yelnik CM, Kozora E, Appenzeller S (2016) Cognitive disorders and antiphospholipid antibodies. Autoimmun Rev 15(12):1193–1198. doi:10.1016/j.autrev.2016.09.002
Oku K, Nakamura H, Kono M, Ohmura K, Kato M, Bohgaki T, Horita T, Yasuda S, Amengual O, Atsumi T (2016) Complement and thrombosis in the antiphospholipid syndrome. Autoimmun Rev 15(10):1001–1004. doi:10.1016/j.autrev.2016.07.020
Bertolaccini ML, Contento G, Lennen R, Sanna G, Blower PJ, Ma MT, Sunassee K, Girardi G (2016) Complement inhibition by hydroxychloroquine prevents placental and fetal brain abnormalities in antiphospholipid syndrome. J Autoimmun 75:30–38. doi:10.1016/j.jaut.2016.04.008
Hoxha A, Banzato A, Ruffatti A, Pengo V (2017) Detection of lupus anticoagulant in the era of direct oral anticoagulants. Autoimmun Rev 16(2):173–178. doi:10.1016/j.autrev.2016.12.010
Sebastiani GD, Iuliano A, Cantarini L, Galeazzi M (2016) Genetic aspects of the antiphospholipid syndrome: an update. Autoimmun Rev 15(5):433–439. doi:10.1016/j.autrev.2016.01.005
Chighizola CB, de Jesus GR, Branch DW (2016) The hidden world of anti-phospholipid antibodies and female infertility: a literature appraisal. Autoimmun Rev 15(6):493–500. doi:10.1016/j.autrev.2016.01.018
Tenti S, Cheleschi S, Guidelli GM, Galeazzi M, Fioravanti A (2016) Intravenous immunoglobulins and antiphospholipid syndrome: how, when and why? A review of the literature. Autoimmun Rev 15(3):226–235. doi:10.1016/j.autrev.2015.11.009
Simonin L, Pasquier E, Leroyer C, Cornec D, Lemerle J, Bendaoud B, Hillion S, Pers JO, Couturaud F, Renaudineau Y (2016) Lymphocyte disturbances in primary antiphospholipid syndrome and application to venous thromboembolism follow-up. Clin Rev Allergy Immunol. doi:10.1007/s12016-016-8568-1
Mayer-Pickel K, Eberhard K, Lang U, Cervar-Zivkovic M (2016) Pregnancy outcome in women with obstetric and thrombotic antiphospholipid syndrome—a retrospective analysis and a review of additional treatment in pregnancy. Clin Rev Allergy Immunol. doi:10.1007/s12016-016-8569-0
Meroni PL (2016) Prevention & treatment of obstetrical complications in APS: is hydroxychloroquine the holy grail we are looking for? J Autoimmun 75:1–5. doi:10.1016/j.jaut.2016.07.003
Moulis G, Audemard-Verger A, Arnaud L, Luxembourger C, Montastruc F, Gaman AM, Svenungsson E, Ruggeri M, Mahevas M, Gerfaud-Valentin M, Brainsky A, Michel M, Godeau B, Lapeyre-Mestre M, Sailler L (2016) Risk of thrombosis in patients with primary immune thrombocytopenia and antiphospholipid antibodies: a systematic review and meta-analysis. Autoimmun Rev 15(3):203–209. doi:10.1016/j.autrev.2015.11.001
Patsouras MD, Sikara MP, Grika EP, Moutsopoulos HM, Tzioufas AG, Vlachoyiannopoulos PG (2015) Elevated expression of platelet-derived chemokines in patients with antiphospholipid syndrome. J Autoimmun 65:30–37. doi:10.1016/j.jaut.2015.08.001
Skarstein K, Aqrawi LA, Oijordsbakken G, Jonsson R, Jensen JL (2016) Adipose tissue is prominent in salivary glands of Sjogren’s syndrome patients and appears to influence the microenvironment in these organs. Autoimmunity 49(5):338–346. doi:10.1080/08916934.2016.1183656
Cornec D, Costa S, Devauchelle-Pensec V, Jousse-Joulin S, Marcorelles P, Berthelot JM, Chiche L, Hachulla E, Hatron PY, Goeb V, Vittecoq O, Saraux A, Pers JO (2016) Blood and salivary-gland BAFF-driven B-cell hyperactivity is associated to rituximab inefficacy in primary Sjogren’s syndrome. J Autoimmun 67:102–110. doi:10.1016/j.jaut.2015.11.002
Konsta OD, Le Dantec C, Charras A, Cornec D, Kapsogeorgou EK, Tzioufas AG, Pers JO, Renaudineau Y (2016) Defective DNA methylation in salivary gland epithelial acini from patients with Sjogren’s syndrome is associated with SSB gene expression, anti-SSB/LA detection, and lymphocyte infiltration. J Autoimmun 68:30–38. doi:10.1016/j.jaut.2015.12.002
Bose T, Diedrichs-Mohring M, Wildner G (2016) Dry eye disease and uveitis: a closer look at immune mechanisms in animal models of two ocular autoimmune diseases. Autoimmun Rev 15(12):1181–1192. doi:10.1016/j.autrev.2016.09.001
Xu C, Wu F, Mao C, Wang X, Zheng T, Bu L, Mou X, Zhou Y, Yuan G, Wang S, Xiao Y (2016) Excess iodine promotes apoptosis of thyroid follicular epithelial cells by inducing autophagy suppression and is associated with Hashimoto thyroiditis disease. J Autoimmun 75:50–57. doi:10.1016/j.jaut.2016.07.008
Roca F, Dominique S, Schmidt J, Smail A, Duhaut P, Levesque H, Marie I (2017) Interstitial lung disease in primary Sjogren’s syndrome. Autoimmun Rev 16(1):48–54. doi:10.1016/j.autrev.2016.09.017
Szyszko EA, Aqrawi LA, Jonsson R, Brokstad KA, Skarstein K (2016) Non-proliferating plasma cells detected in the salivary glands and bone marrow of autoimmune NOD.B10.H2b mice, a model for primary Sjogren’s syndrome. Autoimmunity 49(1):41–49. doi:10.3109/08916934.2015.1079820
Goules AV, Tzioufas AG (2016) Primary Sjogren’s syndrome: clinical phenotypes, outcome and the development of biomarkers. Autoimmun Rev 15(7):695–703. doi:10.1016/j.autrev.2016.03.004
Barrera MJ, Aguilera S, Castro I, Cortes J, Bahamondes V, Quest AF, Molina C, Gonzalez S, Hermoso M, Urzua U, Leyton C, Gonzalez MJ (2016) Pro-inflammatory cytokines enhance ERAD and ATF6alpha pathway activity in salivary glands of Sjogren’s syndrome patients. J Autoimmun 75:68–81. doi:10.1016/j.jaut.2016.07.006
Uomori K, Nozawa K, Ikeda K, Doe K, Yamada Y, Yamaguchi A, Fujishiro M, Kawasaki M, Morimoto S, Takamori K, Sekigawa I, Chan EK, Takasaki Y (2016) A re-evaluation of anti-NA-14 antibodies in patients with primary Sjogren’s syndrome: significant role of interferon-gamma in the production of autoantibodies against NA-14. Autoimmunity 49(5):347–356. doi:10.1080/08916934.2016.1196676
Fujimura T, Fujimoto T, Itaya-Hironaka A, Miyaoka T, Yoshimoto K, Sakuramoto-Tsuchida S, Yamauchi A, Takeda M, Tsujinaka H, Tanaka Y, Takasawa S (2017) Significance of interleukin-6/STAT pathway for the gene expression of REG Ialpha, a new autoantigen in Sjogren’s syndrome patients, in salivary duct epithelial cells. Clin Rev Allergy Immunol 52(3):351–363. doi:10.1007/s12016-016-8570-7
Masi G, Annunziata P (2016) Sjogren’s syndrome and multiple sclerosis: two sides of the same coin? Autoimmun Rev 15(5):457–461. doi:10.1016/j.autrev.2016.01.013
Espitia-Thibault A, Masseau A, Neel A, Espitia O, Toquet C, Mussini JM, Hamidou M (2017) Sjogren’s syndrome-associated myositis with germinal centre-like structures. Autoimmun Rev 16(2):154–158. doi:10.1016/j.autrev.2016.12.006
Nezos A, Gravani F, Tassidou A, Kapsogeorgou EK, Voulgarelis M, Koutsilieris M, Crow MK, Mavragani CP (2015) Type I and II interferon signatures in Sjogren’s syndrome pathogenesis: contributions in distinct clinical phenotypes and Sjogren’s related lymphomagenesis. J Autoimmun 63:47–58. doi:10.1016/j.jaut.2015.07.002
Haacke EA, Bootsma H, Spijkervet FK, Visser A, Vissink A, Kluin PM, Kroese FG (2017) FcRL4+ B-cells in salivary glands of primary Sjogren’s syndrome patients. J Autoimmun. doi:10.1016/j.jaut.2017.03.012
Chaigne B, Lasfargues G, Marie I, Huttenberger B, Lavigne C, Marchand-Adam S, Maillot F, Diot E (2015) Primary Sjogren’s syndrome and occupational risk factors: a case-control study. J Autoimmun 60:80–85. doi:10.1016/j.jaut.2015.04.004
Riemekasten G, Cabral-Marques O (2016) Antibodies against angiotensin II type 1 receptor (AT1R) and endothelin receptor type A (ETAR) in systemic sclerosis (SSc)-response. Autoimmun Rev 15(9):935. doi:10.1016/j.autrev.2016.04.004
Klein M, Schmalzing M, Almanzar G, Benoit S, Hamm H, Tony HP, Goebeler M, Prelog M (2016) Contribution of CD8+ T cells to inflammatory cytokine production in systemic sclerosis (SSc). Autoimmunity 49(8):532–546. doi:10.1080/08916934.2016.1217997
Furnari M, Savarino V, Savarino E (2016) Fecal calprotectin in systemic sclerosis: light and shade of a promising tool. Autoimmun Rev 15(12):1206–1207. doi:10.1016/j.autrev.2016.09.026
Murdaca G, Contatore M, Gulli R, Mandich P, Puppo F (2016) Genetic factors and systemic sclerosis. Autoimmun Rev 15(5):427–432. doi:10.1016/j.autrev.2016.01.016
Maria AT, Toupet K, Maumus M, Fonteneau G, Le Quellec A, Jorgensen C, Guilpain P, Noel D (2016) Human adipose mesenchymal stem cells as potent anti-fibrosis therapy for systemic sclerosis. J Autoimmun 70:31–39. doi:10.1016/j.jaut.2016.03.013
Furnari M, Savarino V, de Bortoli N, Savarino E (2016) Interstitial lung disease in systemic sclerosis patients may benefit more from anti-reflux therapies than from immunosuppressants. Autoimmun Rev 15(12):1208–1209. doi:10.1016/j.autrev.2016.09.025
Morales-Cardenas A, Perez-Madrid C, Arias L, Ojeda P, Mahecha MP, Rojas-Villarraga A, Carrillo-Bayona JA, Anaya JM (2016) Pulmonary involvement in systemic sclerosis. Autoimmun Rev 15(11):1094–1108. doi:10.1016/j.autrev.2016.07.025
Desbois AC, Cacoub P (2016) Systemic sclerosis: an update in 2016. Autoimmun Rev 15(5):417–426. doi:10.1016/j.autrev.2016.01.007
Mehta H, Goulet PO, Nguyen V, Perez G, Koenig M, Senecal JL, Sarfati M (2016) Topoisomerase I peptide-loaded dendritic cells induce autoantibody response as well as skin and lung fibrosis. Autoimmunity 49(8):503–513. doi:10.1080/08916934.2016.1230848
Cabral-Marques O, Riemekasten G (2016) Vascular hypothesis revisited: role of stimulating antibodies against angiotensin and endothelin receptors in the pathogenesis of systemic sclerosis. Autoimmun Rev 15(7):690–694. doi:10.1016/j.autrev.2016.03.005
Wallukat G, Muller J, Schimke I (2016) Vascular hypothesis revisited: role of stimulating antibodies against angiotensin and endothelin receptors in the pathogenesis of systemic sclerosis: Cabral-Marques O, Riemekasten G., Autoimmun Rev. 2016 Mar 10. Pii: S1568-9972(16)30053-2. Doi: 10.1016/j.autrev.2016.03.005. [Epub ahead of print]. Autoimmun Rev 15(8):856–858. doi:10.1016/j.autrev.2016.04.005
Baranska M, Rychlik-Sych M, Kaszuba A, Dziankowska-Bartkowiak B, Skretkowicz J, Waszczykowska E (2016) Genetic polymorphism of CYP2D6 in patients with systemic lupus erythematosus and systemic sclerosis. Autoimmunity:1–6. doi:10.3109/08916934.2015.1134508
Bossini-Castillo L, Lopez-Isac E, Martin J (2015) Immunogenetics of systemic sclerosis: defining heritability, functional variants and shared-autoimmunity pathways. J Autoimmun 64:53–65. doi:10.1016/j.jaut.2015.07.005
Alves CH, Farrell E, Vis M, Colin EM, Lubberts E (2016) Animal models of bone loss in inflammatory arthritis: from cytokines in the bench to novel treatments for bone loss in the bedside-a comprehensive review. Clin Rev Allergy Immunol 51(1):27–47. doi:10.1007/s12016-015-8522-7
Wu DJ, Gu R, Sarin R, Zavodovskaya R, Chen CP, Christiansen BA, Adamopoulos IE (2016) Autophagy-linked FYVE containing protein WDFY3 interacts with TRAF6 and modulates RANKL-induced osteoclastogenesis. J Autoimmun 73:73–84. doi:10.1016/j.jaut.2016.06.004
Baum R, Gravallese EM (2016) Bone as a target organ in rheumatic disease: impact on osteoclasts and osteoblasts. Clin Rev Allergy Immunol 51(1):1–15. doi:10.1007/s12016-015-8515-6
Humphrey MB, Nakamura MC (2016) A comprehensive review of immunoreceptor regulation of osteoclasts. Clin Rev Allergy Immunol 51(1):48–58. doi:10.1007/s12016-015-8521-8
Horwood NJ (2016) Macrophage polarization and bone formation: a review. Clin Rev Allergy Immunol 51(1):79–86. doi:10.1007/s12016-015-8519-2
Sabokbar A, Mahoney DJ, Hemingway F, Athanasou NA (2016) Non-canonical (RANKL-independent) pathways of osteoclast differentiation and their role in musculoskeletal diseases. Clin Rev Allergy Immunol 51(1):16–26. doi:10.1007/s12016-015-8523-6
Al Kindi MA, Colella AD, Chataway TK, Jackson MW, Wang JJ, Gordon TP (2016) Secreted autoantibody repertoires in Sjogren’s syndrome and systemic lupus erythematosus: a proteomic approach. Autoimmun Rev 15(4):405–410. doi:10.1016/j.autrev.2016.01.008
Balada E, Selva-O’Callaghan A, Felip L, Ordi-Ros J, Simeon-Aznar CP, Solans-Laque R, Vilardell-Tarres M (2016) Sequence analysis of TMEM173 exon 5 in patients with systemic autoimmune diseases. Autoimmunity 49(1):12–16. doi:10.3109/08916934.2015.1113404
Cruz GI, Shao X, Quach H, Ho KA, Sterba K, Noble JA, Patsopoulos NA, Busch MP, Triulzi DJ, Wong WS, Solomon BD, Niederhuber JE, Criswell LA, Barcellos LF (2016) A child’s HLA-DRB1 genotype increases maternal risk of systemic lupus erythematosus. J Autoimmun 74:201–207. doi:10.1016/j.jaut.2016.06.017
Dahan S, Shor DB, Comaneshter D, Tekes-Manova D, Shovman O, Amital H, Cohen AD (2016) All disease begins in the gut: celiac disease co-existence with SLE. Autoimmun Rev 15(8):848–853. doi:10.1016/j.autrev.2016.06.003
Doria A, Gershwin ME, Selmi C (2016) From old concerns to new advances and personalized medicine in lupus: the end of the tunnel is approaching. J Autoimmun 74:1–5. doi:10.1016/j.jaut.2016.08.007
Durcan L, Petri M (2016) Immunomodulators in SLE: clinical evidence and immunologic actions. J Autoimmun 74:73–84. doi:10.1016/j.jaut.2016.06.010
Duron L, Cohen-Aubart F, Diot E, Borie R, Abad S, Richez C, Banse C, Vittecoq O, Saadoun D, Haroche J, Amoura Z (2016) Shrinking lung syndrome associated with systemic lupus erythematosus: a multicenter collaborative study of 15 new cases and a review of the 155 cases in the literature focusing on treatment response and long-term outcomes. Autoimmun Rev 15(10):994–1000. doi:10.1016/j.autrev.2016.07.021
Floris A, Piga M, Cauli A, Mathieu A (2016) Predictors of flares in systemic lupus erythematosus: preventive therapeutic intervention based on serial anti-dsDNA antibodies assessment. Analysis of a monocentric cohort and literature review. Autoimmun Rev 15(7):656–663. doi:10.1016/j.autrev.2016.02.019
Gatto M, Ghirardello A, Luisetto R, Bassi N, Fedrigo M, Valente M, Valentino S, Del Prete D, Punzi L, Doria A (2016) Immunization with pentraxin 3 (PTX3) leads to anti-PTX3 antibody production and delayed lupus-like nephritis in NZB/NZW F1 mice. J Autoimmun 74:208–216. doi:10.1016/j.jaut.2016.07.002
Gatto M, Iaccarino L, Ghirardello A, Punzi L, Doria A (2016) Clinical and pathologic considerations of the qualitative and quantitative aspects of lupus nephritogenic autoantibodies: a comprehensive review. J Autoimmun 69:1–11. doi:10.1016/j.jaut.2016.02.003
Gatto M, Saccon F, Zen M, Bettio S, Iaccarino L, Punzi L, Doria A (2016) Success and failure of biological treatment in systemic lupus erythematosus: a critical analysis. J Autoimmun 74:94–105. doi:10.1016/j.jaut.2016.06.014
Govoni M, Bortoluzzi A, Padovan M, Silvagni E, Borrelli M, Donelli F, Ceruti S, Trotta F (2016) The diagnosis and clinical management of the neuropsychiatric manifestations of lupus. J Autoimmun 74:41–72. doi:10.1016/j.jaut.2016.06.013
Hammad A, Mosaad YM, Hammad EM, Elhanbly S, El-Bassiony SR, Al-Harrass MF, Eid R, Sharaf Eldein OA, Alsawah GA, Yahia S, Fawzy IM (2016) Interleukin-17A rs2275913, interleukin-17F rs763780 and rs2397084 gene polymorphisms as possible risk factors in juvenile lupus and lupus related nephritis. Autoimmunity 49(1):31–40. doi:10.3109/08916934.2015.1101071
Jeltsch-David H, Muller S (2016) Autoimmunity, neuroinflammation, pathogen load: a decisive crosstalk in neuropsychiatric SLE. J Autoimmun 74:13–26. doi:10.1016/j.jaut.2016.04.005
Lazzaroni MG, Dall’Ara F, Fredi M, Nalli C, Reggia R, Lojacono A, Ramazzotto F, Zatti S, Andreoli L, Tincani A (2016) A comprehensive review of the clinical approach to pregnancy and systemic lupus erythematosus. J Autoimmun 74:106–117. doi:10.1016/j.jaut.2016.06.016
Long H, Yin H, Wang L, Gershwin ME, Lu Q (2016) The critical role of epigenetics in systemic lupus erythematosus and autoimmunity. J Autoimmun 74:118–138. doi:10.1016/j.jaut.2016.06.020
Lu R, Munroe ME, Guthridge JM, Bean KM, Fife DA, Chen H, Slight-Webb SR, Keith MP, Harley JB, James JA (2016) Dysregulation of innate and adaptive serum mediators precedes systemic lupus erythematosus classification and improves prognostic accuracy of autoantibodies. J Autoimmun 74:182–193. doi:10.1016/j.jaut.2016.06.001
Meroni PL, Penatti AE (2016) Epigenetics and systemic lupus erythematosus: unmet needs. Clin Rev Allergy Immunol 50(3):367–376. doi:10.1007/s12016-015-8497-4
Moroni G, Depetri F, Ponticelli C (2016) Lupus nephritis: when and how often to biopsy and what does it mean? J Autoimmun 74:27–40. doi:10.1016/j.jaut.2016.06.006
Moroni G, Doria A, Giglio E, Imbasciati E, Tani C, Zen M, Strigini F, Zaina B, Tincani A, Gatto M, de Liso F, Grossi C, Meroni PL, Cabiddu G, Messa P, Ravani P, Mosca M (2016) Maternal outcome in pregnant women with lupus nephritis. A prospective multicenter study. J Autoimmun 74:194–200. doi:10.1016/j.jaut.2016.06.012
Moroni G, Doria A, Giglio E, Tani C, Zen M, Strigini F, Zaina B, Tincani A, de Liso F, Matinato C, Grossi C, Gatto M, Castellana P, Limardo M, Meroni PL, Messa P, Ravani P, Mosca M (2016) Fetal outcome and recommendations of pregnancies in lupus nephritis in the 21st century. A prospective multicenter study. J Autoimmun 74:6–12. doi:10.1016/j.jaut.2016.07.010
Nielsen CT, Rasmussen NS, Heegaard NH, Jacobsen S (2016) “Kill” the messenger: targeting of cell-derived microparticles in lupus nephritis. Autoimmun Rev 15(7):719–725. doi:10.1016/j.autrev.2016.03.009
Novak GV, Marques M, Balbi V, Gormezano NW, Kozu K, Sakamoto AP, Pereira RM, Terreri MT, Magalhaes CS, Guariento A, Sallum AM, Marini R, Ferriani VP, Barbosa CM, de Castro TC, Ramos VC, Bonfa E, Silva CA (2017) Anti-RO/SSA and anti-La/SSB antibodies: association with mild lupus manifestations in 645 childhood-onset systemic lupus erythematosus. Autoimmun Rev 16(2):132–135. doi:10.1016/j.autrev.2016.12.004
Paradowska-Gorycka A, Sowinska A, Stypinska B, Grobelna MK, Walczyk M, Olesinska M, Piotrowski P, Jagodzinski PP (2016) Impact of the IL-17F, IL-23 and IL-23R on susceptibility and phenotype of systemic lupus erythematosus. Autoimmunity 49(6):373–382. doi:10.1080/08916934.2016.1196678
Pons-Estel GJ, Andreoli L, Scanzi F, Cervera R, Tincani A (2017) The antiphospholipid syndrome in patients with systemic lupus erythematosus. J Autoimmun 76:10–20. doi:10.1016/j.jaut.2016.10.004
Qian J, Wang Y, Huang C, Yang X, Zhao J, Wang Q, Tian Z, Li M, Zeng X (2016) Survival and prognostic factors of systemic lupus erythematosus-associated pulmonary arterial hypertension: a PRISMA-compliant systematic review and meta-analysis. Autoimmun Rev 15(3):250–257. doi:10.1016/j.autrev.2015.11.012
Sahebkar A, Rathouska J, Derosa G, Maffioli P, Nachtigal P (2016) Statin impact on disease activity and C-reactive protein concentrations in systemic lupus erythematosus patients: a systematic review and meta-analysis of controlled trials. Autoimmun Rev 15(4):344–353. doi:10.1016/j.autrev.2015.12.007
Schneider M (2016) Pitfalls in lupus. Autoimmun Rev 15(11):1089–1093. doi:10.1016/j.autrev.2016.07.033
Shimizu Y, Yasuda S, Kako Y, Nakagawa S, Kanda M, Hisada R, Ohmura K, Shimamura S, Shida H, Fujieda Y, Kato M, Oku K, Bohgaki T, Horita T, Kusumi I, Atsumi T (2016) Post-steroid neuropsychiatric manifestations are significantly more frequent in SLE compared with other systemic autoimmune diseases and predict better prognosis compared with de novo neuropsychiatric SLE. Autoimmun Rev 15(8):786–794. doi:10.1016/j.autrev.2016.03.017
Shor DB, Dahan S, Comaneshter D, Cohen AD, Amital H (2016) Does inflammatory bowel disease coexist with systemic lupus erythematosus? Autoimmun Rev 15(11):1034–1037. doi:10.1016/j.autrev.2016.07.027
Sin E, Anand P, Frieri M (2016) A link: allergic rhinitis, asthma & systemic lupus erythematosus. Autoimmun Rev 15(5):487–491. doi:10.1016/j.autrev.2016.02.003
Tay SH, Fairhurst AM, Mak A (2017) Clinical utility of circulating anti-N-methyl-d-aspartate receptor subunits NR2A/B antibody for the diagnosis of neuropsychiatric syndromes in systemic lupus erythematosus and Sjogren’s syndrome: an updated meta-analysis. Autoimmun Rev 16(2):114–122. doi:10.1016/j.autrev.2016.12.002
Teruel M, Alarcon-Riquelme ME (2016) The genetic basis of systemic lupus erythematosus: what are the risk factors and what have we learned. J Autoimmun 74:161–175. doi:10.1016/j.jaut.2016.08.001
Velo-Garcia A, Castro SG, Isenberg DA (2016) The diagnosis and management of the haematologic manifestations of lupus. J Autoimmun 74:139–160. doi:10.1016/j.jaut.2016.07.001
Vivero F, Gonzalez-Echavarri C, Ruiz-Estevez B, Maderuelo I, Ruiz-Irastorza G (2016) Prevalence and predictors of valvular heart disease in patients with systemic lupus erythematosus. Autoimmun Rev 15(12):1134–1140. doi:10.1016/j.autrev.2016.09.007
Watad A, Cohen AD, Comaneshter D, Tekes-Manova D, Amital H (2016) Hyperthyroidism association with SLE, lessons from real-life data—a case-control study. Autoimmunity 49(1):17–20. doi:10.3109/08916934.2015.1090985
Watad A, Mahroum N, Whitby A, Gertel S, Comaneshter D, Cohen AD, Amital H (2016) Hypothyroidism among SLE patients: case-control study. Autoimmun Rev 15(5):484–486. doi:10.1016/j.autrev.2016.01.019
Wen J, Stock AD, Chalmers SA, Putterman C (2016) The role of B cells and autoantibodies in neuropsychiatric lupus. Autoimmun Rev 15(9):890–895. doi:10.1016/j.autrev.2016.07.009
Wu H, Zhao M, Tan L, Lu Q (2016) The key culprit in the pathogenesis of systemic lupus erythematosus: aberrant DNA methylation. Autoimmun Rev 15(7):684–689. doi:10.1016/j.autrev.2016.03.002
Zhang F, Wu L, Qian J, Qu B, Xia S, La T, Wu Y, Ma J, Zeng J, Guo Q, Cui Y, Yang W, Huang J, Zhu W, Yao Y, Shen N, Tang Y (2016) Identification of the long noncoding RNA NEAT1 as a novel inflammatory regulator acting through MAPK pathway in human lupus. J Autoimmun 75:96–104. doi:10.1016/j.jaut.2016.07.012
Zhao M, Wang J, Liao W, Li D, Li M, Wu H, Zhang Y, Gershwin ME, Lu Q (2016) Increased 5-hydroxymethylcytosine in CD4(+) T cells in systemic lupus erythematosus. J Autoimmun 69:64–73. doi:10.1016/j.jaut.2016.03.001
Ghodke-Puranik Y, Niewold TB (2015) Immunogenetics of systemic lupus erythematosus: a comprehensive review. J Autoimmun 64:125–136. doi:10.1016/j.jaut.2015.08.004
Chen K, Liu J, Cao X (2017) Regulation of type I interferon signaling in immunity and inflammation: a comprehensive review. J Autoimmun. doi:10.1016/j.jaut.2017.03.008
Figgett WA, Deliyanti D, Fairfax KA, Quah PS, Wilkinson-Berka JL, Mackay F (2015) Deleting the BAFF receptor TACI protects against systemic lupus erythematosus without extensive reduction of B cell numbers. J Autoimmun 61:9–16. doi:10.1016/j.jaut.2015.04.007
Coit P, Renauer P, Jeffries MA, Merrill JT, McCune WJ, Maksimowicz-McKinnon K, Sawalha AH (2015) Renal involvement in lupus is characterized by unique DNA methylation changes in naive CD4+ T cells. J Autoimmun 61:29–35. doi:10.1016/j.jaut.2015.05.003
Barreira SC, Fonseca JE (2016) The impact of conventional and biological disease modifying antirheumatic drugs on bone biology. Rheumatoid arthritis as a case study. Clin Rev Allergy Immunol 51(1):100–109. doi:10.1007/s12016-016-8547-6
Cambridge G, Leandro MJ, Lahey LJ, Fairhead T, Robinson WH, Sokolove J (2016) B cell depletion with rituximab in patients with rheumatoid arthritis: multiplex bead array reveals the kinetics of IgG and IgA antibodies to citrullinated antigens. J Autoimmun 70:22–30. doi:10.1016/j.jaut.2016.03.010
Cao H, Lin J, Chen W, Xu G, Sun C (2016) Baseline adiponectin and leptin levels in predicting an increased risk of disease activity in rheumatoid arthritis: a meta-analysis and systematic review. Autoimmunity 49(8):547–553. doi:10.1080/08916934.2016.1230847
Chan CK, Holroyd CR, Mason A, Zarroug J, Edwards CJ (2016) Are there dangers in biologic dose reduction strategies? Autoimmun Rev 15(7):742–746. doi:10.1016/j.autrev.2016.03.013
Chen SY, Hsu WT, Chen YL, Chien CH, Chiang BL (2016) Lymphocyte-activation gene 3(+) (LAG3(+)) forkhead box protein 3(-) (FOXP3(-)) regulatory T cells induced by B cells alleviates joint inflammation in collagen-induced arthritis. J Autoimmun 68:75–85. doi:10.1016/j.jaut.2016.02.002
Chevalier N, Tan JK, Mason LJ, Robert R, McKenzie CI, Lim F, Wong CH, Macia L, Thorburn AN, Russ BE, Masters SL, Mackay CR (2016) Avenues to autoimmune arthritis triggered by diverse remote inflammatory challenges. J Autoimmun 73:120–129. doi:10.1016/j.jaut.2016.06.018
Clavarino G, Adriouach S, Quesada JL, Clay M, Chevreau M, Trocme C, Grange L, Gaudin P, Gatti E, Pierre P, Cesbron JY, Dumestre-Perard C (2016) Unfolded protein response gene GADD34 is overexpressed in rheumatoid arthritis and related to the presence of circulating anti-citrullinated protein antibodies. Autoimmunity 49(3):172–178. doi:10.3109/08916934.2016.1138220
Conigliaro P, Chimenti MS, Triggianese P, Sunzini F, Novelli L, Perricone C, Perricone R (2016) Autoantibodies in inflammatory arthritis. Autoimmun Rev 15(7):673–683. doi:10.1016/j.autrev.2016.03.003
Dar SA, Haque S, Mandal RK, Singh T, Wahid M, Jawed A, Panda AK, Akhter N, Lohani M, Areeshi MY, Rai G, Datt S, Bhattacharya SN, Ramachandran VG, Das S (2016) Interleukin-6-174G > C (rs1800795) polymorphism distribution and its association with rheumatoid arthritis: a case-control study and meta-analysis. Autoimmunity:1–12. doi:10.1080/08916934.2016.1261833
Elshabrawy HA, Essani AE, Szekanecz Z, Fox DA, Shahrara S (2017) TLRs, future potential therapeutic targets for RA. Autoimmun Rev 16(2):103–113. doi:10.1016/j.autrev.2016.12.003
Fragoulis GE, Fragkioudaki S, Reilly JH, Kerr SC, McInnes IB, Moutsopoulos HM (2016) Analysis of the cell populations composing the mononuclear cell infiltrates in the labial minor salivary glands from patients with rheumatoid arthritis and sicca syndrome. J Autoimmun 73:85–91. doi:10.1016/j.jaut.2016.06.008
Galligan CL, Keystone EC, Fish EN (2016) Fibrocyte and T cell interactions promote disease pathogenesis in rheumatoid arthritis. J Autoimmun 69:38–50. doi:10.1016/j.jaut.2016.02.008
Houri Levi E, Watad A, Whitby A, Tiosano S, Comaneshter D, Cohen AD, Amital H (2016) Coexistence of ischemic heart disease and rheumatoid arthritis patients—a case control study. Autoimmun Rev 15(4):393–396. doi:10.1016/j.autrev.2016.01.006
Iannone F, Lopalco G, Rigante D, Orlando I, Cantarini L, Lapadula G (2016) Impact of obesity on the clinical outcome of rheumatologic patients in biotherapy. Autoimmun Rev 15(5):447–450. doi:10.1016/j.autrev.2016.01.010
Ishikawa LLW, Colavite PM, Fraga-Silva TFC, Mimura LAN, Franca TGD, Zorzella-Pezavento SFG, Chiuso-Minicucci F, Marcolino LD, Penitenti M, Ikoma MRV, Sartori A (2017) Vitamin D deficiency and rheumatoid arthritis. Clin Rev Allergy Immunol 52(3):373–388. doi:10.1007/s12016-016-8577-0
Kalyoncu U, Solmaz D, Emmungil H, Yazici A, Kasifoglu T, Kimyon G, Balkarli A, Bes C, Ozmen M, Alibaz-Oner F, Erten S, Cagatay Y, Cetin GY, Yilmaz S, Yildiz F, Pamuk ON, Kucuksahin O, Kilic L, Yazisiz V, Karadag O, Koca SS, Hayran M, Akar S, Aksu K, Akkoc N, Keser G, Gonullu E, Kisacik B, Onat AM, Soy M, Inanc N, Direskeneli H, Sayarlioglu M, Erken E, Turgay M, Cefle A, Ertenli I, Pay S (2016) Response rate of initial conventional treatments, disease course, and related factors of patients with adult-onset Still’s disease: data from a large multicenter cohort. J Autoimmun 69:59–63. doi:10.1016/j.jaut.2016.02.010
Komaki F, Komaki Y, Micic D, Ido A, Sakuraba A (2017) Outcome of pregnancy and neonatal complications with anti-tumor necrosis factor-alpha use in females with immune mediated diseases; a systematic review and meta-analysis. J Autoimmun 76:38–52. doi:10.1016/j.jaut.2016.11.004
Lazurova I, Tomas L (2017) Cardiac impairment in rheumatoid arthritis and influence of anti-TNFalpha treatment. Clin Rev Allergy Immunol 52(3):323–332. doi:10.1007/s12016-016-8566-3
Li P, Wang X, Zhao MQ, Li LJ, Zhang C, Li BZ, Liu J, Yang XK, Leng RX, Fan YG, Pan HF, Ye DQ (2016) TCR-CD3zeta gene polymorphisms and expression profile in rheumatoid arthritis. Autoimmunity 49(7):466–471. doi:10.1080/08916934.2016.1174855
Lin J, He Y, Chen J, Zeng Z, Yang B, Ou Q (2017) A critical role of transcription factor YY1 in rheumatoid arthritis by regulation of interleukin-6. J Autoimmun 77:67–75. doi:10.1016/j.jaut.2016.10.008
Lopez-Mejias R, Castaneda S, Gonzalez-Juanatey C, Corrales A, Ferraz-Amaro I, Genre F, Remuzgo-Martinez S, Rodriguez-Rodriguez L, Blanco R, Llorca J, Martin J, Gonzalez-Gay MA (2016) Cardiovascular risk assessment in patients with rheumatoid arthritis: the relevance of clinical, genetic and serological markers. Autoimmun Rev 15(11):1013–1030. doi:10.1016/j.autrev.2016.07.026
Miyashita T, Morimoto S, Fujishiro M, Hayakawa K, Suzuki S, Ikeda K, Miyazawa K, Morioka M, Takamori K, Ogawa H, Sekigawa I, Takasaki Y (2016) Inhibition of each module of connective tissue growth factor as a potential therapeutic target for rheumatoid arthritis. Autoimmunity 49(2):109–114. doi:10.3109/08916934.2015.1113405
Nabi G, Akhter N, Wahid M, Bhatia K, Mandal RK, Dar SA, Jawed A, Haque S (2016) Meta-analysis reveals PTPN22 1858C/T polymorphism confers susceptibility to rheumatoid arthritis in Caucasian but not in Asian population. Autoimmunity 49(3):197–210. doi:10.3109/08916934.2015.1134514
Nevius E, Gomes AC, Pereira JP (2016) Inflammatory cell migration in rheumatoid arthritis: a comprehensive review. Clin Rev Allergy Immunol 51(1):59–78. doi:10.1007/s12016-015-8520-9
Rizzi M, Lorenzetti R, Fischer K, Staniek J, Janowska I, Troilo A, Strohmeier V, Erlacher M, Kunze M, Bannert B, Kyburz D, Voll RE, Venhoff N, Thiel J (2017) Impact of tofacitinib treatment on human B-cells in vitro and in vivo. J Autoimmun 77:55–66. doi:10.1016/j.jaut.2016.10.005
Roark CL, Anderson KM, Aubrey MT, Rosloniec EF, Freed BM (2016) Arthritogenic peptide binding to DRB1*01 alleles correlates with susceptibility to rheumatoid arthritis. J Autoimmun 72:25–32. doi:10.1016/j.jaut.2016.04.006
Seaman A, Darrah E, Infantino M, Meacci F, Manfredi M, Benucci M, Mahler M (2016) Anti-peptidyl-arginine deaminase 3 (PAD3) antibodies as a promising marker to measure joint damage in patients with rheumatoid arthritis. Autoimmun Rev 15(7):776–780. doi:10.1016/j.autrev.2016.03.016
Skalska U, Kontny E (2016) Adipose-derived mesenchymal stem cells from infrapatellar fat pad of patients with rheumatoid arthritis and osteoarthritis have comparable immunomodulatory properties. Autoimmunity 49(2):124–131. doi:10.3109/08916934.2015.1113267
Stofkova A, Krskova K, Vaculin S, Jurcovicova J (2016) Enhanced activity of hormone sensitive lipase (HSL) in mesenteric but not epididymal fat correlates with higher production of epinephrine in mesenteric adipocytes in rat model of cachectic rheumatoid arthritis. Autoimmunity 49(4):268–276. doi:10.3109/08916934.2016.1164145
Wen W, He M, Liang X, Gao SS, Zhou J, Yuan ZY (2016) Accelerated transformation of macrophage-derived foam cells in the presence of collagen-induced arthritis mice serum is associated with dyslipidemia. Autoimmunity 49(2):115–123. doi:10.3109/08916934.2015.1118761
Messemaker TC, Huizinga TW, Kurreeman F (2015) Immunogenetics of rheumatoid arthritis: understanding functional implications. J Autoimmun 64:74–81. doi:10.1016/j.jaut.2015.07.007
Deng Y, Chang C, Lu Q (2016) The inflammatory response in psoriasis: a comprehensive review. Clin Rev Allergy Immunol 50(3):377–389. doi:10.1007/s12016-016-8535-x
Karczewski J, Dobrowolska A, Rychlewska-Hanczewska A, Adamski Z (2016) New insights into the role of T cells in pathogenesis of psoriasis and psoriatic arthritis. Autoimmunity 49(7):435–450. doi:10.3109/08916934.2016.1166214
Marre ML, Profozich JL, Coneybeer JT, Geng X, Bertera S, Ford MJ, Trucco M, Piganelli JD (2016) Inherent ER stress in pancreatic islet beta cells causes self-recognition by autoreactive T cells in type 1 diabetes. J Autoimmun 72:33–46. doi:10.1016/j.jaut.2016.04.009
Pollock RA, Abji F, Gladman DD (2017) Epigenetics of psoriatic disease: a systematic review and critical appraisal. J Autoimmun 78:29–38. doi:10.1016/j.jaut.2016.12.002
Prajzlerova K, Grobelna K, Pavelka K, Senolt L, Filkova M (2016) An update on biomarkers in axial spondyloarthritis. Autoimmun Rev 15(6):501–509. doi:10.1016/j.autrev.2016.02.002
Quaden DH, De Winter LM, Somers V (2016) Detection of novel diagnostic antibodies in ankylosing spondylitis: an overview. Autoimmun Rev 15(8):820–832. doi:10.1016/j.autrev.2016.06.001
Raychaudhuri SP, Wilken R, Sukhov AC, Raychaudhuri SK, Maverakis E (2017) Management of psoriatic arthritis: early diagnosis, monitoring of disease severity and cutting edge therapies. J Autoimmun 76:21–37. doi:10.1016/j.jaut.2016.10.009
Rysnik O, McHugh K, van Duivenvoorde L, van Tok M, Guggino G, Taurog J, Kollnberger S, Ciccia F, Baeten D, Bowness P (2016) Non-conventional forms of HLA-B27 are expressed in spondyloarthritis joints and gut tissue. J Autoimmun 70:12–21. doi:10.1016/j.jaut.2016.03.009
Sakkas LI, Bogdanos DP (2017) Are psoriasis and psoriatic arthritis the same disease? The IL-23/IL-17 axis data. Autoimmun Rev 16(1):10–15. doi:10.1016/j.autrev.2016.09.015
Sgambelluri F, Diani M, Altomare A, Frigerio E, Drago L, Granucci F, Banfi G, Altomare G, Reali E (2016) A role for CCR5(+)CD4 T cells in cutaneous psoriasis and for CD103(+) CCR4(+) CD8 Teff cells in the associated systemic inflammation. J Autoimmun 70:80–90. doi:10.1016/j.jaut.2016.03.019
Sticherling M (2016) Psoriasis and autoimmunity. Autoimmun Rev 15(12):1167–1170. doi:10.1016/j.autrev.2016.09.004
Sukhov A, Adamopoulos IE, Maverakis E (2016) Interactions of the immune system with skin and bone tissue in psoriatic arthritis: a comprehensive review. Clin Rev Allergy Immunol 51(1):87–99. doi:10.1007/s12016-016-8529-8
Yang ZS, Lin NN, Li L, Li Y (2016) The effect of TNF inhibitors on cardiovascular events in psoriasis and psoriatic arthritis: an updated meta-analysis. Clin Rev Allergy Immunol 51(2):240–247. doi:10.1007/s12016-016-8560-9
Harden JL, Krueger JG, Bowcock AM (2015) The immunogenetics of psoriasis: a comprehensive review. J Autoimmun 64:66–73. doi:10.1016/j.jaut.2015.07.008
Isailovic N, Daigo K, Mantovani A, Selmi C (2015) Interleukin-17 and innate immunity in infections and chronic inflammation. J Autoimmun 60:1–11. doi:10.1016/j.jaut.2015.04.006
Aggelakopoulou M, Kourepini E, Paschalidis N, Panoutsakopoulou V (2016) ERbeta in CD4+ T cells is crucial for ligand-mediated suppression of central nervous system autoimmunity. J Immunol 196(12):4947–4956. doi:10.4049/jimmunol.1600246
Aggelakopoulou M, Kourepini E, Paschalidis N, Simoes DC, Kalavrizioti D, Dimisianos N, Papathanasopoulos P, Mouzaki A, Panoutsakopoulou V (2016) ERbeta-dependent direct suppression of human and murine Th17 cells and treatment of established central nervous system autoimmunity by a neurosteroid. J Immunol 197(7):2598–2609. doi:10.4049/jimmunol.1601038
Anstadt EJ, Fujiwara M, Wasko N, Nichols F, Clark RB (2016) TLR tolerance as a treatment for central nervous system autoimmunity. J Immunol 197(6):2110–2118. doi:10.4049/jimmunol.1600876
Aricha R, Reuveni D, Fuchs S, Souroujon MC (2016) Suppression of experimental autoimmune myasthenia gravis by autologous T regulatory cells. J Autoimmun 67:57–64. doi:10.1016/j.jaut.2015.09.005
Blumenfeld S, Staun-Ram E, Miller A (2016) Fingolimod therapy modulates circulating B cell composition, increases B regulatory subsets and production of IL-10 and TGFbeta in patients with multiple sclerosis. J Autoimmun 70:40–51. doi:10.1016/j.jaut.2016.03.012
Chen Q, Liu Y, Lu A, Ni K, Xiang Z, Wen K, Tu W (2017) Influenza virus infection exacerbates experimental autoimmune encephalomyelitis disease by promoting type I T cells infiltration into central nervous system. J Autoimmun 77:1–10. doi:10.1016/j.jaut.2016.10.006
De Virgilio A, Greco A, Fabbrini G, Inghilleri M, Rizzo MI, Gallo A, Conte M, Rosato C, Ciniglio Appiani M, de Vincentiis M (2016) Parkinson’s disease: autoimmunity and neuroinflammation. Autoimmun Rev 15(10):1005–1011. doi:10.1016/j.autrev.2016.07.022
Duraes FV, Lippens C, Steinbach K, Dubrot J, Brighouse D, Bendriss-Vermare N, Issazadeh-Navikas S, Merkler D, Hugues S (2016) pDC therapy induces recovery from EAE by recruiting endogenous pDC to sites of CNS inflammation. J Autoimmun 67:8–18. doi:10.1016/j.jaut.2015.08.014
Elieh-Ali-Komi D, Cao Y (2017) Role of mast cells in the pathogenesis of multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Rev Allergy Immunol 52(3):436–445. doi:10.1007/s12016-016-8595-y
Esposito S, Longo MR (2017) Guillain-Barre syndrome. Autoimmun Rev 16(1):96–101. doi:10.1016/j.autrev.2016.09.022
Evangelista MG, Castro SB, Alves CC, Dias AT, Souza VW, Reis LB, Silva LC, Castanon MC, Farias RE, Juliano MA, Ferreira AP (2016) Early IFN-gamma production together with decreased expression of TLR3 and TLR9 characterizes EAE development conditional on the presence of myelin. Autoimmunity 49(4):258–267. doi:10.3109/08916934.2016.1141898
Fraune J, Gerlach S, Rentzsch K, Teegen B, Lederer S, Affeldt K, Fechner K, Danckwardt M, Voigt J, Probst C, Komorowski L, Stocker W (2016) Multiparametric serological testing in autoimmune encephalitis using computer-aided immunofluorescence microscopy (CAIFM). Autoimmun Rev 15(10):937–942. doi:10.1016/j.autrev.2016.07.024
Fraussen J, de Bock L, Somers V (2016) B cells and antibodies in progressive multiple sclerosis: contribution to neurodegeneration and progression. Autoimmun Rev 15(9):896–899. doi:10.1016/j.autrev.2016.07.008
Gao Q, Zhang Y, Han C, Hu X, Zhang H, Xu X, Tian J, Liu Y, Ding Y, Liu J, Wang C, Guo Z, Yang Y, Cao X (2016) Blockade of CD47 ameliorates autoimmune inflammation in CNS by suppressing IL-1-triggered infiltration of pathogenic Th17 cells. J Autoimmun 69:74–85. doi:10.1016/j.jaut.2016.03.002
Goncalves ED, Souza PS, Lieberknecht V, Fidelis GS, Barbosa RI, Silveira PC, de Pinho RA, Dutra RC (2016) Low-level laser therapy ameliorates disease progression in a mouse model of multiple sclerosis. Autoimmunity 49(2):132–142. doi:10.3109/08916934.2015.1124425
Guptill JT, Juel VC, Massey JM, Anderson AC, Chopra M, Yi JS, Esfandiari E, Buchanan T, Smith B, Atherfold P, Jones E, Howard JF Jr (2016) Effect of therapeutic plasma exchange on immunoglobulins in myasthenia gravis. Autoimmunity 49(7):472–479. doi:10.1080/08916934.2016.1214823
Haggmark-Manberg A, Zandian A, Forsstrom B, Khademi M, Lima Bomfim I, Hellstrom C, Arnheim-Dahlstrom L, Hallbook T, Darin N, Lundberg IE, Uhlen M, Partinen M, Schwenk JM, Olsson T, Nilsson P (2016) Autoantibody targets in vaccine-associated narcolepsy. Autoimmunity 49(6):421–433. doi:10.1080/08916934.2016.1183655
Hucke S, Eschborn M, Liebmann M, Herold M, Freise N, Engbers A, Ehling P, Meuth SG, Roth J, Kuhlmann T, Wiendl H, Klotz L (2016) Sodium chloride promotes pro-inflammatory macrophage polarization thereby aggravating CNS autoimmunity. J Autoimmun 67:90–101. doi:10.1016/j.jaut.2015.11.001
Kok LF, Marsh-Wakefield F, Marshall JE, Gillis C, Halliday GM, Byrne SN (2016) B cells are required for sunlight protection of mice from a CNS-targeted autoimmune attack. J Autoimmun 73:10–23. doi:10.1016/j.jaut.2016.05.016
Koneczny I, Stevens JA, De Rosa A, Huda S, Huijbers MG, Saxena A, Maestri M, Lazaridis K, Zisimopoulou P, Tzartos S, Verschuuren J, van der Maarel SM, van Damme P, De Baets MH, Molenaar PC, Vincent A, Ricciardi R, Martinez-Martinez P, Losen M (2017) IgG4 autoantibodies against muscle-specific kinase undergo Fab-arm exchange in myasthenia gravis patients. J Autoimmun 77:104–115. doi:10.1016/j.jaut.2016.11.005
Laroni A, Armentani E, Kerlero de Rosbo N, Ivaldi F, Marcenaro E, Sivori S, Gandhi R, Weiner HL, Moretta A, Mancardi GL, Uccelli A (2016) Dysregulation of regulatory CD56(bright) NK cells/T cells interactions in multiple sclerosis. J Autoimmun 72:8–18. doi:10.1016/j.jaut.2016.04.003
Laurent C, Capron J, Quillerou B, Thomas G, Alamowitch S, Fain O, Mekinian A (2016) Steroid-responsive encephalopathy associated with autoimmune thyroiditis (SREAT): characteristics, treatment and outcome in 251 cases from the literature. Autoimmun Rev 15(12):1129–1133. doi:10.1016/j.autrev.2016.09.008
Lifshitz GV, Zhdanov DD, Lokhonina AV, Eliseeva DD, Lyssuck EY, Zavalishin IA, Bykovskaia SN (2016) Ex vivo expanded regulatory T cells CD4+CD25+FoxP3+CD127Low develop strong immunosuppressive activity in patients with remitting-relapsing multiple sclerosis. Autoimmunity 49(6):388–396. doi:10.1080/08916934.2016.1199020
Lippens C, Duraes FV, Dubrot J, Brighouse D, Lacroix M, Irla M, Aubry-Lachainaye JP, Reith W, Mandl JN, Hugues S (2016) IDO-orchestrated crosstalk between pDCs and Tregs inhibits autoimmunity. J Autoimmun 75:39–49. doi:10.1016/j.jaut.2016.07.004
Lucchese G, Kanduc D (2016) Zika virus and autoimmunity: from microcephaly to Guillain-Barre syndrome, and beyond. Autoimmun Rev 15(8):801–808. doi:10.1016/j.autrev.2016.03.020
Milo R (2016) Therapeutic strategies targeting B-cells in multiple sclerosis. Autoimmun Rev 15(7):714–718. doi:10.1016/j.autrev.2016.03.006
Montagna G, Imperiali M, Agazzi P, D’Aurizio F, Tozzoli R, Feldt-Rasmussen U, Giovanella L (2016) Hashimoto’s encephalopathy: a rare proteiform disorder. Autoimmun Rev 15(5):466–476. doi:10.1016/j.autrev.2016.01.014
Paterka M, Voss JO, Werr J, Reuter E, Franck S, Leuenberger T, Herz J, Radbruch H, Bopp T, Siffrin V, Zipp F (2017) Dendritic cells tip the balance towards induction of regulatory T cells upon priming in experimental autoimmune encephalomyelitis. J Autoimmun 76:108–114. doi:10.1016/j.jaut.2016.09.008
Ramanathan S, Dale RC, Brilot F (2016) Anti-MOG antibody: the history, clinical phenotype, and pathogenicity of a serum biomarker for demyelination. Autoimmun Rev 15(4):307–324. doi:10.1016/j.autrev.2015.12.004
Rolf L, Damoiseaux J, Hupperts R, Huitinga I, Smolders J (2016) Network of nuclear receptor ligands in multiple sclerosis: common pathways and interactions of sex-steroids, corticosteroids and vitamin D3-derived molecules. Autoimmun Rev 15(9):900–910. doi:10.1016/j.autrev.2016.07.002
Russi AE, Walker-Caulfield ME, Guo Y, Lucchinetti CF, Brown MA (2016) Meningeal mast cell-T cell crosstalk regulates T cell encephalitogenicity. J Autoimmun 73:100–110. doi:10.1016/j.jaut.2016.06.015
Selmi C, Barin JG, Rose NR (2016) Current trends in autoimmunity and the nervous system. J Autoimmun 75:20–29. doi:10.1016/j.jaut.2016.08.005
St Charles JL, Bell JA, Gadsden BJ, Malik A, Cooke H, Van de Grift LK, Kim HY, Smith EJ, Mansfield LS (2017) Guillain Barre syndrome is induced in non-obese diabetic (NOD) mice following Campylobacter jejuni infection and is exacerbated by antibiotics. J Autoimmun 77:11–38. doi:10.1016/j.jaut.2016.09.003
Teniente-Serra A, Grau-Lopez L, Mansilla MJ, Fernandez-Sanmartin M, Ester Condins A, Ramo-Tello C, Martinez-Caceres E (2016) Multiparametric flow cytometric analysis of whole blood reveals changes in minor lymphocyte subpopulations of multiple sclerosis patients. Autoimmunity 49(4):219–228. doi:10.3109/08916934.2016.1138271
van Sonderen A, Schreurs MW, Wirtz PW, Sillevis Smitt PA, Titulaer MJ (2016) From VGKC to LGI1 and Caspr2 encephalitis: the evolution of a disease entity over time. Autoimmun Rev 15(10):970–974. doi:10.1016/j.autrev.2016.07.018
Wiwanitkit V (2016) Zika virus, autoimmunity and microcephaly: other things for consideration. Autoimmun Rev 15(8):855. doi:10.1016/j.autrev.2016.04.002
Braudeau C, Amouriaux K, Neel A, Herbreteau G, Salabert N, Rimbert M, Martin JC, Hemont C, Hamidou M, Josien R (2016) Persistent deficiency of circulating mucosal-associated invariant T (MAIT) cells in ANCA-associated vasculitis. J Autoimmun 70:73–79. doi:10.1016/j.jaut.2016.03.015
Brunini F, Page TH, Gallieni M, Pusey CD (2016) The role of monocytes in ANCA-associated vasculitides. Autoimmun Rev 15(11):1046–1053. doi:10.1016/j.autrev.2016.07.031
Chan E, Sangle SR, Coghlan JG, D’Cruz DD (2016) Pulmonary artery aneurysms in Behcet’s disease treated with anti-TNFalpha: a case series and review of the literature. Autoimmun Rev 15(4):375–378. doi:10.1016/j.autrev.2016.01.003
Cottin V, Bel E, Bottero P, Dalhoff K, Humbert M, Lazor R, Sinico RA, Sivasothy P, Wechsler ME, Groh M, Marchand-Adam S, Khouatra C, Wallaert B, Taille C, Delaval P, Cadranel J, Bonniaud P, Prevot G, Hirschi S, Gondouin A, Dunogue B, Chatte G, Briault C, Pagnoux C, Jayne D, Guillevin L, Cordier JF, Groupe d’Etudes et de Recherche sur les Maladies Orphelines P (2017) Revisiting the systemic vasculitis in eosinophilic granulomatosis with polyangiitis (Churg-Strauss): a study of 157 patients by the Groupe d’Etudes et de Recherche sur les Maladies Orphelines Pulmonaires and the European Respiratory Society Taskforce on eosinophilic granulomatosis with polyangiitis (Churg-Strauss). Autoimmun Rev 16(1):1–9. doi:10.1016/j.autrev.2016.09.018
Criado PR, Marques GF, Morita TC, de Carvalho JF (2016) Epidemiological, clinical and laboratory profiles of cutaneous polyarteritis nodosa patients: report of 22 cases and literature review. Autoimmun Rev 15(6):558–563. doi:10.1016/j.autrev.2016.02.010
Csernok E, Damoiseaux J, Rasmussen N, Hellmich B, van Paassen P, Vermeersch P, Blockmans D, Cohen Tervaert JW, Bossuyt X (2016) Evaluation of automated multi-parametric indirect immunofluorescence assays to detect anti-neutrophil cytoplasmic antibodies (ANCA) in granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA). Autoimmun Rev 15(7):736–741. doi:10.1016/j.autrev.2016.03.010
Cui Z, Zhao MH, Jia XY, Wang M, Hu SY, Wang SX, Yu F, Brown KL, Hudson BG, Pedchenko V (2016) Antibodies to alpha5 chain of collagen IV are pathogenic in Goodpasture’s disease. J Autoimmun 70:1–11. doi:10.1016/j.jaut.2016.04.001
De Virgilio A, Greco A, Magliulo G, Gallo A, Ruoppolo G, Conte M, Martellucci S, de Vincentiis M (2016) Polyarteritis nodosa: a contemporary overview. Autoimmun Rev 15(6):564–570. doi:10.1016/j.autrev.2016.02.015
Espitia O, Samson M, Le Gallou T, Connault J, Landron C, Lavigne C, Belizna C, Magnant J, de Moreuil C, Roblot P, Maillot F, Diot E, Jego P, Durant C, Masseau A, Brisseau JM, Pottier P, Espitia-Thibault A, Santos AD, Perrin F, Artifoni M, Neel A, Graveleau J, Moreau P, Maisonneuve H, Fau G, Serfaty JM, Hamidou M, Agard C (2016) Comparison of idiopathic (isolated) aortitis and giant cell arteritis-related aortitis. A French retrospective multicenter study of 117 patients. Autoimmun Rev 15(6):571–576. doi:10.1016/j.autrev.2016.02.016
Ferfar Y, Mirault T, Desbois AC, Comarmond C, Messas E, Savey L, Domont F, Cacoub P, Saadoun D (2016) Biotherapies in large vessel vasculitis. Autoimmun Rev 15(6):544–551. doi:10.1016/j.autrev.2016.02.012
Huart A, Josse AG, Chauveau D, Korach JM, Heshmati F, Bauvin E, Cointault O, Kamar N, Ribes D, Pourrat J, Faguer S, French Society of H (2016) Outcomes of patients with Goodpasture syndrome: a nationwide cohort-based study from the French Society of Hemapheresis. J Autoimmun 73:24–29. doi:10.1016/j.jaut.2016.05.015
Iannella G, Greco A, Granata G, Manno A, Pasquariello B, Angeletti D, Didona D, Magliulo G (2016) Granulomatosis with polyangiitis and facial palsy: literature review and insight in the autoimmune pathogenesis. Autoimmun Rev 15(7):621–631. doi:10.1016/j.autrev.2016.02.005
Jarrot PA, Kaplanski G (2016) Pathogenesis of ANCA-associated vasculitis: an update. Autoimmun Rev 15(7):704–713. doi:10.1016/j.autrev.2016.03.007
Kerstein A, Schuler S, Cabral-Marques O, Fazio J, Hasler R, Muller A, Pitann S, Moosig F, Klapa S, Haas C, Kabelitz D, Riemekasten G, Wolters S, Lamprecht P (2017) Environmental factor and inflammation-driven alteration of the total peripheral T-cell compartment in granulomatosis with polyangiitis. J Autoimmun 78:79–91. doi:10.1016/j.jaut.2016.12.004
Lefevre G, Ackermann F, Kahn JE (2017) Hypereosinophilia with asthma and systemic (non-vasculitic) manifestations: eosinophilic granulomatosis with polyangiitis or hypereosinophilic syndrome? Autoimmun Rev 16(2):208–209. doi:10.1016/j.autrev.2016.11.001
Legendre P, Regent A, Thiebault M, Mouthon L (2017) Anti-endothelial cell antibodies in vasculitis: a systematic review. Autoimmun Rev 16(2):146–153. doi:10.1016/j.autrev.2016.12.012
Lionaki S, Mavragani CP, Karras A, Liapis G, Somarakis G, Boletis JN, Drosos A, Tzioufas AG, Guillevin L, Moutsopoulos HM (2016) Predictors of renal histopathology in antineutrophil cytoplasmic antibody associated glomerulonephritis. J Autoimmun 72:57–64. doi:10.1016/j.jaut.2016.05.004
Misra DP, Sharma A, Kadhiravan T, Negi VS (2017) A scoping review of the use of non-biologic disease modifying anti-rheumatic drugs in the management of large vessel vasculitis. Autoimmun Rev 16(2):179–191. doi:10.1016/j.autrev.2016.12.009
Muratore F, Boiardi L, Cavazza A, Aldigeri R, Pipitone N, Restuccia G, Bellafiore S, Cimino L, Salvarani C (2016) Correlations between histopathological findings and clinical manifestations in biopsy-proven giant cell arteritis. J Autoimmun 69:94–101. doi:10.1016/j.jaut.2016.03.005
Renauer P, Coit P, Sawalha AH (2016) Epigenetics and Vasculitis: a comprehensive review. Clin Rev Allergy Immunol 50(3):357–366. doi:10.1007/s12016-015-8495-6
Restuccia G, Boiardi L, Cavazza A, Catanoso M, Macchioni P, Muratore F, Soriano A, Cimino L, Aldigeri R, Crescentini F, Pipitone N, Salvarani C (2017) Long-term remission in biopsy proven giant cell arteritis: a retrospective cohort study. J Autoimmun 77:39–44. doi:10.1016/j.jaut.2016.10.002
Rizzo G, Licchetta L, Scaglione C, Buttiglione M, Capellari S, Martinelli P, Martino D (2016) Behcet disease presenting with movement disorders and antibasal ganglia antibodies. Autoimmun Rev 15(3):287–288. doi:10.1016/j.autrev.2015.11.011
Samson M, Ly KH, Tournier B, Janikashvili N, Trad M, Ciudad M, Gautheron A, Devilliers H, Quipourt V, Maurier F, Meaux-Ruault N, Magy-Bertrand N, Manckoundia P, Ornetti P, Maillefert JF, Besancenot JF, Ferrand C, Mesturoux L, Labrousse F, Fauchais AL, Saas P, Martin L, Audia S, Bonnotte B (2016) Involvement and prognosis value of CD8(+) T cells in giant cell arteritis. J Autoimmun 72:73–83. doi:10.1016/j.jaut.2016.05.008
Sawalha AH, Dozmorov MG (2016) Epigenomic functional characterization of genetic susceptibility variants in systemic vasculitis. J Autoimmun 67:76–81. doi:10.1016/j.jaut.2015.10.002
Weiner M, Segelmark M (2016) The clinical presentation and therapy of diseases related to anti-neutrophil cytoplasmic antibodies (ANCA). Autoimmun Rev 15(10):978–982. doi:10.1016/j.autrev.2016.07.016
Xue LJ, Wu R, Du GL, Xu Y, Yuan KY, Feng ZC, Pan YL, Hu GY (2017) Effect and safety of TNF inhibitors in immunoglobulin-resistant Kawasaki disease: a meta-analysis. Clin Rev Allergy Immunol 52(3):389–400. doi:10.1007/s12016-016-8581-4
Takeuchi M, Kastner DL, Remmers EF (2015) The immunogenetics of Behcet’s disease: a comprehensive review. J Autoimmun 64:137–148. doi:10.1016/j.jaut.2015.08.013
Vallet H, Riviere S, Sanna A, Deroux A, Moulis G, Addimanda O, Salvarani C, Lambert M, Bielefeld P, Seve P, Sibilia J, Pasquali J, Fraison J, Marie I, Perard L, Bouillet L, Cohen F, Sene D, Schoindre Y, Lidove O, Le Hoang P, Hachulla E, Fain O, Mariette X, Papo T, Wechsler B, Bodaghi B, Rigon MR, Cacoub P, Saadoun D, French Behcet N (2015) Efficacy of anti-TNF alpha in severe and/or refractory Behcet’s disease: multicenter study of 124 patients. J Autoimmun 62:67–74. doi:10.1016/j.jaut.2015.06.005
Quartuccio L, Zuliani F, Corazza L, Scaini P, Zani R, Lenzi M, Tavoni A, Sebastiani M, Baldovino S, Urraro T, Saccardo F, Sbreglia C, Mazzaro C, Pioltelli P, Fraticelli P, Filippini D, Gabrielli A, Perrella O, Scarpato S, Roccatello D, Zignego AL, Ferri C, Bombardieri S, Pietrogrande M, Monti G, Galli M, De Vita S (2015) Retreatment regimen of rituximab monotherapy given at the relapse of severe HCV-related cryoglobulinemic vasculitis: long-term follow up data of a randomized controlled multicentre study. J Autoimmun 63:88–93. doi:10.1016/j.jaut.2015.07.012
Bundhun PK, Soogund MZ, Huang F (2017) Impact of systemic lupus erythematosus on maternal and fetal outcomes following pregnancy: a meta-analysis of studies published between years 2001-2016. J Autoimmun 79:17–27. doi:10.1016/j.jaut.2017.02.009
Zampeli E, Vlachoyiannopoulos PG, Tzioufas AG (2015) Treatment of rheumatoid arthritis: unraveling the conundrum. J Autoimmun 65:1–18. doi:10.1016/j.jaut.2015.10.003
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares that he has no conflict of interest.
Funding
No funding supported this paper.
Ethical Approval and Informed Consent
The present article is a review; thus, no informed consent was obtained.
Rights and permissions
About this article
Cite this article
Selmi, C. Autoimmunity in 2016. Clinic Rev Allerg Immunol 53, 126–139 (2017). https://doi.org/10.1007/s12016-017-8615-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-017-8615-6