Abstract
Our previous study revealed that 3T3-L1 preadipocytes can differentiate to either osteoblasts or adipocytes in response to bone morphogenic protein 9 (BMP9). In the present study, we try to further investigate whether the Wnt/β-catenin signaling plays a crucial role in this process. It was found that BMP9 effectively activated the Wnt/β-catenin signaling, and induced the expression levels of certain canonical Wnt ligands and their receptors in preadipocytes. Exogenous expression of β-catenin, Wnt1, Wnt3a, and Wnt10b potentiated BMP9-induced alkaline phosphatase (ALP) activity, while β-catenin knockdown or Dickkopf 1 (Dkk1) diminished BMP9-induced ALP activity. Moreover, it was demonstrated that β-catenin overexpression promoted BMP9-induced mineralization, and increased the expression levels of late osteogenic markers osteopontin and osteocalcin. Furthermore, β-catenin inhibited BMP9-induced lipid accumulation and the adipogenic marker adipocyte fatty acid binding protein (aP2). The cell-implantation assay results identified that β-catenin not only augmented BMP9-induced ectopic bone formation, but also blocked adipogenesis in vivo. Mechanistically, it was found that β-catenin and BMP9 synergistically stimulated the osteogenic transcription factors runt-related transcription factor 2 (Runx2) and Osterix (OSX). However, BMP9-induced adipogenic transcription factors, peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT enhancer-binding protein α (C/EBPα), were inhibited by β-catenin. Therefore, these findings suggested that the Wnt/β-catenin signaling, potentially via the modulation of osteogenic and adipogenic transcriptional factors, exerts an opposite effect on BMP9-induced osteogenic and adipogenic differentiation in preadipocytes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a systemic and metabolic bone disorder that is progressively more common in old age. Moreover, it occurs as a result of bone loss and degradation of the bone microstructure, thus leading to fragile bones that are prone to fracture. This reduction of bone mass is partly due to the fact that bone marrow mensenchymal stem cells (MSCs) from older patients have an attenuated capacity to differentiate into osteocytes and an enhanced capacity to differentiate into adipocytes, which leads to an excess of bone marrow fat [1,2,3,4,5]. Therefore, the imbalance in the osteogenesis and adipogenesis contributes to the occurrence and deterioration of osteoporosis.
Both osteogenesis and adipogenesis of MSCs are controlled by specific transcriptional factors. During adipogenesis, MSCs firstly differentiate to preadipocytes and subsequently mature to adipocytes under the influence of adipogenic transcription factors. Therefore, differentiation induction of preadipocytes to osteocytes may be a feasible treatment option of osteoporosis, to not only suppress extra marrow adipose tissue formation, but also promote preadipocytes to a more osteoblastic phenotype. While a large plasticity exists between preadipocytes and osteocytes [6,7,8,9], the underlying molecular mechanism is not yet fully elucidated.
Wnts are a group of secreted glycoproteins that are associated with several biological and pathological processes [10,11,12]. Moreover, Wnts can bind to transmembrane receptors, such as low density lipoprotein receptor related protein 5/6 (LRP5/6) and Frizzleds (Fzs), to activate specific pathways, including the canonical Wnt/β-catenin signaling pathway, which is important for bone formation [13,14,15,16]. In our previous study, it was shown that BMP9 may activate the Wnt/β-catenin signaling via the inactivation of glycogen synthase kinase 3β (GSK3β) in 3T3-L1 preadipocytes [17]. Therefore, suggesting that Wnt/β-catenin signaling may regulate BMP9-stimulated osteogenic and adipogenic differentiation of preadipocytes [17]. However, the mechanism of the interaction between BMP9 and Wnt/β-catenin signaling, and the precise function of Wnt/β-catenin signaling in BMP9-induced differentiation of preadipocytes are not fully understood.
In this study, we investigated the role of the Wnt/β-catenin signaling in BMP9-induced osteogenesis and adipogenesis of preadipocytes. It was found that BMP9 can stimulate the expression levels of several canonical Wnt ligands and receptors, result in activation of Wnt/β-catenin signaling. Furthermore, Wnt/β-catenin signaling activation promoted BMP9-mediated osteogenesis and inhibited BMP9-mediated adipogenesis in 3T3-L1 preadipocytes. Thus, the present results indicated that the Wnt/β-catenin signaling may be an important regulator for BMP9-induced osteogenesis and adipogenesis of preadipocytes.
Materials and Methods
Cell Culture
3T3-L1 preadipocytes cell line was obtained from ATCC. Cells were maintained under conditions as described previously [18]. Unless indicated otherwise, all chemicals were purchased from Sigma-Aldrich.
Construction of Recombinant Adenoviruses
Recombinant adenoviruses expressing Wnt1 (AdWnt1), Wnt3a (AdWnt3a), Wnt10b (AdWnt10b), BMP9 (AdBMP9), Dkk1 (AdDkk1), β-catenin (AdBC), and small interference RNA (siRNA) of β-catenin (AdR-simBC) were constructed by AdEasy technology [17, 18]. AdBMP9, AdWnt1, AdWnt3a, AdWnt10b, AdDkk1, AdBC also express green fluorescent protein (GFP) as marker for tracking viruses. Adenoviruses expressing only GFP (AdGFP) was used as control.
ALP Assay
ALP activity was assessed with a Great Escape SEAP Chemiluminescence assay (BD Clontech, Mountain View, CA) and/or histochemical staining assay as described [17]. ALP activity was normalized to the level of total cellular protein.
RNA Isolation and Semiquantitative RT-PCR Analysis
Total RNA was isolated from cells using TRIZOL Reagents (Invitrogen, Carlsbad, CA, USA). Reverse transcription of 1 μg was conducted with the instructions of TaKaRa RT kit (TaKaRa Biotechnology, Dalian, China). The cDNA products were diluted tenfold and then used as PCR templates. Semiquantitative RT-PCR was performed as described [19]. In order to examine the genes of interest, the Primer3 program was used to design PCR primers. The specific primers used are shown in Table 1. All samples were normalized to the expression of GAPDH.
Western Blotting Analysis
For total protein level assay, preadipocytes were collected and lysed in Laemmli buffer which consisted of 60 mM Tris-HCl, 10% glycerol, 0.002% bromphenol blue, 2% SDS, and 5% β-mercaptoethanol. The nucleus fraction protein was extracted by using Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime Institute of Biotechnology, Jiangsu, China). Western blotting was carried out as described previously [17]. All the antibodies were purchased from Santa Cruz, as follows: anti-BMP9 (sc-514211), anti- osteopontin (OPN) (sc-21742), anti- osteocalcin (OC) (sc-365797), anti-β-catenin (sc-7963), anti-Histone H2A.X (sc-517336), anti-aP2 (sc-217529), anti-Runx2 (sc-390351), anti-OSX (sc-393325), anti-PPARγ (sc-81152), anti-C/EBPα (sc-7962), and anti-GAPDH (sc-365062). The ImageJ software was used to quantify protein bands.
Alizarin Red S Staining
Preadipocytes infected with AdGFP, AdBMP9, AdBC and/or AdR-simBC were cultured in the presence of ascorbic acid (50 μg/mL) and β-glycerophosphate (10 mM). After treatment for 14 days, cells were fixed with 0.05% (vol/vol) glutaraldehyde for 10 min. Cells were washed with PBS and incubated with 0.4% Alizarin Red S for 5 min, followed by washing with distilled water. The stained preadipocytes were photographed under bright field microscopy. The cultured cells were then extracted with cetylpyridinium chloride, and the Alizarin Red S concentration was determined by measuring the absorbance at 562 nm.
Oil Red O Staining
On day eight preadipocytes were fixed with 10% formalin for 1 h and stained with Oil Red O solution at 37 °C for 15 min. The stained Oil Red O was extracted with isopropanol and the concentration was quantified through measuring the absorbance at 570 nm.
Transfection and Luciferase Reporter Assay
Preadipocytes were seeded in T25 flasks and transfected with 2 μg per flask of β-catenin/Tcf4-responsive luciferase reporter pTOP-Luc [18], using Lipofectamine (Invitrogen). 16 h later, preadipocytes were replated to 24-well plates and transduced with different combinations of recombinant adenoviruses at 4 h after replating. At 1 day post infection, preadipocytes were lysed and cell lysates were collected for luciferase assays using Promega’s Luciferase Assay Kit (Promega, Madison, WI, USA).
Subcutaneous Cells Implantation
The animal experiments were approved by Ethics Committee of the second affiliated hospital of Chongqing Medical University (Chongqing, China). Preadipocytes were treated with AdBMP9, AdBC and/or AdR-simBC for 24 h. Then cells were harvested and subcutaneously injected (5 × 106 cells/injection) into the both flanks of athymic nude mice (five animals per group, 4–6 weeks old, female, Harlan Sprague Dawley). At 5 weeks after injection, all the mice were euthanized, and the implantation sites were collected for micro-computed tomography (μCT) scanning and histological evaluation.
Histological Evaluation
Retrieved tissues were decalcified, fixed in 10% formalin, and embedded in paraffin. Serial sections of the embedded specimens were stained with haematoxylin and eosin (H&E). Masson Trichrome and Alcian Blue stains were also conducted as described [17]. The ImageJ software was used to analyze the regions of interest and/or intensity of the acquired image data.
Micro-Computed Tomography Analysis
Bone masses were analyzed using a cone-beam-type desktop μCT system (Explore Locus SP, GE Healthcare, USA). The three-dimensional reconstruction processing software within the scanner (Micview V2.1.2) was used to complete the image data analysis.
Statistical Analysis
The experimental data were presented as the mean ± standard deviation. Differences between groups were calculated by Student’s t test and one-way analysis of variance. A p value of < 0.05 was defined statistically significance. Each assay condition was performed in triplicate, and the assays were repeated in at least three independent experiments.
Results
BMP9 Activates Wnt/β-Catenin Signaling in Preadipocytes
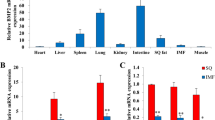
The present study examined whether BMP9 activated canonical Wnt signaling activity in preadipocytes. Firstly, AdBMP9 was shown to be capable of inducing BMP9 expression in preadipocytes (Fig. 1a). Exogenous expression of BMP9 significantly increased the β-catenin protein level not only in the nucleus, but also in the cytoplasm and the whole cell. (Fig. 1b). Moreover, BMP9 significantly stimulated luciferase activities of pTOP-Luc reporter (Fig. 1c). As Wnt/β-catenin signaling is initiated through the binding of secreted Wnt molecules to their receptors, we assessed the effect of BMP9 on the expression of Wnt ligands and receptors. The present results suggested that the mRNA expression levels of several canonical Wnt ligands were upregulated by BMP9 (Fig. 1d). Furthermore, while BMP9 had on effect on LRP5 and LRP6 mRNA levels, the mRNA levels of the Wnt receptors, Fz1, Fz3, Fz4, Fz5, Fz7, and Fz8, were increased after BMP9 treatment (Fig. 1e). Collectively, the present findings suggested that BMP9 activated canonical Wnt signaling via the upregulation of ligands and receptors in preadipocytes.
Wnt/β-catenin signaling is activated by BMP9 in preadipocytes. a AdBMP9 significantly stimulates BMP9 expression in preadipocytes. Preadipocytes were infected with AdBMP9. Fluorescence images of the cells were recorded at 2 days after infection, and AdBMP9 markedly induced BMP9 expression at 1 day (D1) and 2 days (D2) after infection. **p < 0.01 vs. BMP9(D0) group. b BMP9 increases β-catenin expression in preadipocytes. Preadipocytes were stimulated with AdBMP9 or AdGFP for 24 h, western blotting analysis was conducted to test β-catenin protein. N, nucleus; C, cytoplasm; W, whole cell. **p < 0.01 vs. control group. c The positive effect of BMP9 on luciferase activities of pTOP-Luc reporter. **p < 0.01 vs. control group. d, e Expression of canonical Wnt ligands and receptors. Preadipocytes were treated with AdBMP9. At the indicated time point, semiquantitative RT-PCR was performed to detect the mRNA levels of Wnt ligands and receptors
Wnt/β-Catenin Signaling Modulates BMP9 Induction of ALP Activity
We also examined the effect of Wnt/β-catenin signaling on BMP9-stimulated early stage of osteogenesis of preadipocytes. Preadipocytes were infected with AdBC, Wnt1, AdWnt3a, AdWnt10b, AdsimBC, AdDkk1, and/or AdBMP9. It was found that BMP9-activated luciferase activities of pTOP-luc reporter were enhanced by overexpression of β-catenin, Wnt1, Wnt3a, or Wnt10b, but decreased by β-catenin knockdown and Dkk1 (Fig. 2a). While the exogenous expression of β-catenin alone had no effect on ALP activity, it significantly enhanced BMP9 induction of ALP activity (Fig. 2b). Moreover, similar to β-catenin, overexpression of Wnt1, Wnt3a, or Wnt10b also promoted ALP activity induced by BMP9 (Fig. 2c–e). However, β-catenin knockdown significantly inhibited ALP activity stimulated by BMP9 (Fig. 2f). It was demonstrated that overexpression of Dkk1, an inhibitor of the Wnt/β-catenin signaling, also blocked BMP9-induced ALP activity (Fig. 2g). These results demonstrated that the Wnt/β-catenin pathway may have a positive role in the BMP9-induced early stage of osteogenesis in preadipocytes.
The ability of Wnt/β-catenin signaling to promote BMP9-stimulated early osteogenic marker ALP in preadipocytes. a Verification of the effect of β-catenin, Wnt ligands and Dkk1 on BMP9-activated pTOP-Luc luciferase reporter activities. *p < 0.05 vs. BMP9 group; **p < 0.01 vs.BMP9 group. b–e ALP histochemical staining (upper panel) and ALP activity (lower panel) showed β-catenin and Wnt ligands promoted BMP9-induced ALP activity. ∇∇p < 0.01 vs. control group; **p < 0.01 vs. BMP9 group. f, g ALP histochemical staining (upper panel) and ALP activity (lower panel) showed Wnt/β-catenin inhibition decreased BMP9-induced ALP. ∇∇p < 0.01 vs. control group; **p < 0.01 vs. BMP9 group
β-Catenin Promotes BMP9 Induction of Late Osteogenic Differentiation and Osteogenic Transcriptional Factors in Preadipocytes
OPN and OC are specific markers for late osteogenesis. Thus, we examined the effect of β-catenin on OPN and OC stimulated by BMP9. It was identified that BMP9 increased protein expression levels of OPN and OC, and that preadipocytes stimulated with both AdBMP9 and AdBC presented higher expression levels of these factors than cells treated with only BMP9 (Fig. 3a). However, β-catenin knockdown significantly inhibited the potentiation effect of BMP9 on the induction of OPN and OC expression in preadipocytes (Fig. 3a). It was also found that β-catenin significantly promoted BMP9-induced mineralization, whereas this function of BMP9 was prevented by knockdown of β-catenin using siRNA in preadipocytes (Fig. 3b). Furthermore, β-catenin was identified to act synergistically with BMP9 to activate the osteogenic transcription factors Runx2 and OSX in preadipocytes (Fig. 3c). Thus, while β-catenin may not induce late osteogenesis, it was found to promote BMP9-mediated late osteogenic differentiation of preadipocytes by increasing the activities of osteogenic transcription factors.
β-catenin augments BMP9-mediated late stage of osteogenesis in preadipocytes. a β-catenin overexpression (BC) and silencing (SimBC) exert an opposite effect on BMP9-mediated late osteogenic markers OPN and OC. Preadipocytes were transfected with AdBMP9, AdBC and/or AdR-simBC for 12 days and afterwards protein expression levels of OPN and OC were measured.**p < 0.01 vs. control group; ∇p < 0.05 vs. BMP9 group; ∇∇p < 0.01 vs. BMP9 group. All experiments were performed in triplicates. b Alizarin Red S staining. 3T3-L1 preadipocytes were transfected with AdBMP9, AdBC and/or AdR-simBC. After 2 weeks, cells were stained with Alizarin Red S solution. The mineralized nodule was extracted and quantified via measuring absorbance at 562 nm. ∇∇p < 0.01 vs. control group; **p < 0.01 vs. BMP9 group. c Potentiation effect of β-catenin on BMP9-induced Runx2 and OSX expression. 3T3-L1 preadipocytes were coinfected with AdBMP9 and/or AdBC for 3 days. Afterwards, the expression levels of Runx2 and OSX were detected by Western blotting. *p < 0.05 vs. control group; **p < 0.01 vs. control group; ∇p < 0.05 vs. BMP9 group; ∇∇p < 0.01 vs. BMP9 group
β-Catenin Inhibits BMP9-Mediated Adipose Differentiation of Preadipocytes
Next, the present study investigated whether Wnt/β-catenin signaling affected BMP9-mediated adipose differentiation of preadipocytes. Preadipocytes were stimulated with AdBMP9 and/or AdBC for 8 days, and then Oil Red O staining was conducted to detect lipid synthesis. A large level of lipid was observed in cells stimulated by BMP9, while preadipocytes infected with the combination of AdBMP9 and AdBC showed less lipid formation (Fig. 4a). Moreover, Wnt1, Wnt3a and Wnt10b also had an attenuated effect on BMP9-mediated lipid accumulation in preadipocytes (Fig. 4b–d). Furthermore, it was found that the BMP9-induced adipogenic marker aP2 was reduced in response to overexpression of β-catenin (Fig. 4e). In addition, the BMP9-induced adipogenic transcriptional factors PPARγ and C/EBPα, were significantly suppressed by β-catenin (Fig. 4f). Thus, the present study identified an inhibitory function of Wnt/β-catenin signaling in BMP9-induced adipogenesis in preadipocytes.
Wnt/β-catenin signaling suppresses BMP9-stimulated adipogenic differentiation of preadipocytes. a–d Adipogenic differentiation. Preadipocytes were infected with AdBMP9 and AdBC, AdWnt1, AdWnt3a or AdWnt10b for 8 days, followed by Oil red O staining (upper panel). Quantitative analysis was performed by measuring absorbance at 570 nm (lower panel). ∇∇p < 0.01 vs. control group; **p < 0.01 vs. BMP9 group. e Protein expression level of adipogenic marker aP2. 3T3-L1 cells were infected with AdBMP9 and/or AdBC. At day 7, aP2 protein expression was detected by Western blotting ∇p < 0.05 vs. BMP9 + BC group; **p < 0.01 vs. control group. All experiments were performed in triplicates. f β-catenin overexpression decreases BMP9-stimulated PPARγ and C/EBPα. Preadipocytes were treated with AdBMP9 and/or AdBC. At 3 days post treatment, Western blotting was used to examine the expression of PPARγ and C/EBPα. ∇p < 0.05 vs. BMP9 + BC group; ∇∇p < 0.01 vs. BMP9+BC group; **p < 0.01 vs. control group. All experiments were performed in triplicates
β-Catenin Regulates BMP9-Mediated Osteogenic and Adipogenic Differentiation of Preadipocytes In Vivo
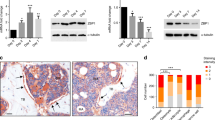
The in vitro results indicated that the canonical Wnt signaling exerted a contrary effect on BMP9-mediated osteogenesis and adipogenesis of 3T3-L1 cells. A cell implantation experiment was used to assess these findings in vivo. Athymic mice were subcutaneously injected with preadipocytes that were stimulated with AdBMP9, AdBC and/or AdR-simBC. Then, these injection sites were collected for histological evaluation and μCT analysis after 5 weeks. The gross appearance of these retrieved specimens demonstrated that β-catenin promoted the formation of ectopic bony masses (Fig. 5a), which were also identified by μCT scanning (Fig. 5b, c). However, preadipocytes infected with the combination of AdBMP9 and AdR-simBC formed relatively bulky and soft masses, rather than detectable bony masses (Fig. 5a).
β-catenin promotes BMP9-stimulated ectopic bone formation. a Representative retrieved bone masses showed the effect of β-catenin overexpression (BC) and silencing (SimBC) on BMP9-stimulated ectopic bone formation. b, c μCT scanning and analysis showed the 3D surface images and volume of bone mass. **p < 0.01 vs. BMP9 group. d–f Histological stains showed the effect of β-catenin on BMP9-stimulated ectopic bone formation. (AC, adipocyte; OB, osteoblast; OC, osteocyte; MBM, mineralized bone matrix; BM, bone matrix; UPA, Undifferentiated preadipocytes; CM, Cartilage Matrix) g Quantitative analysis of trabecular and osteoid matrix area was performed by using ImageJ software. **p < 0.01 vs. BMP9 group
H&E staining indicated that the combination of AdBMP9 and AdBC resulted to mature bone matrices and thicker trabeculae, with less undifferentiated preadipocytes and mature adipocytes (Fig. 5d, g). Furthermore, Masson’s Trichrome staining identified that β-catenin dramatically promoted BMP9-stimulated matrix mineralization (Fig. 5e). However, β-catenin impaired BMP9 induction of chondrogenesis (Fig. 5f). However, despite the presence of mature adipocytes and a large number of undifferentiated preadipocytes, no focal ossification and osteoid, or cartilaginous collagen matrix were observed in specimens retrieved from preadipocytes transfected with AdBMP9 and AdR-simBC (Fig. 5d–f). Collectively, the in vivo results further indicated that Wnt/β-catenin signaling may potentiate BMP9-induced osteogenesis and block BMP9-induced adipogenesis in preadipocytes.
Discussion
The precise function of Wnt/β-catenin signaling in BMP9-mediated osteogenesis and adipogenesis of preadipocytes has remained elusive. As the Wnt/β-catenin signaling is important for BMPs-induced bone formation and can be activated by BMP9 [18, 20, 21], it was speculated that it may also be related to BMP9-induced differentiation of preadipocytes. To assess this hypothesis, the effect of BMP9 on the Wnt/β-catenin activity was examined, and it was found that BMP9 effectively stimulated Wnt/β-catenin activity in preadipocytes. Moreover, BMP9 or BMP2 can also activate canonical Wnt signaling in other different cell lines [21,22,23,24]. However, previous studies using human primary periosteal cells and mice primary osteoblasts showed that BMP signaling blocked Wnt/β-catenin activity via the activation of Dkk1 [25, 26], which was inconsistent with the present results. This discrepancy may be attributed to the use of various types of cells, the cellular conditions, such as proliferation, differentiation and cell environment, or the supraphysiologic levels of in vitro BMP treatment. Despite these factors, the present results evidenced that BMP9 significantly activated the Wnt/β-catenin signaling, thus indicating the potential importance of this pathway in BMP9-mediated differentiation of preadipocytes.
Our previous study revealed that BMP9 can promote nuclear translocation of β-catenin through blocking GSK3β activity in preadipocytes [17], but the specific mechanism by which BMP9 modulates Wnt/β-catenin activity remains unknown. Based on the fact that the signal transduction of the Wnt/β-catenin pathway is initiated by canonical Wnt ligands binding to Fzs and LRP5/6 co-receptors [27], and that BMP9 is able to induce Wnt ligand Wnt11 expression in MSCs [28], the present study examined whether BMP9 had an effect on Wnt ligands and receptors in preadipocytes. We found that BMP9 significantly increased several canonical Wnt ligands and receptors mRNA levels. In addition to BMP9, BMP2 also has an similar effect on the activities of Wnt ligands and receptors. Zhang et al. [23] reported that BMP2 can stimulate LRP5 expression to modulate β-catenin signaling activity in osteoblasts. Moreover, BMP2 also promotes LRP5 expression in ST-2 cells [29]. Furthermore, BMP2 effectively increases mRNA levels of specific Wnt ligands and receptors in a dose-dependent way in osteoblasts [21]. For keratinocytes, BMP2 promotes cellular expression levels of Wnt2b, Wnt5b, Wnt7b, Wnt13, Fz6, Fz8, and Fz10 [24]. While the mechanism via which BMPs affect Wnt ligands and receptors remains unknown, the present results indicated that regulation of Wnt ligands and receptors by BMP9 may be related to Wnt/β-catenin activation in preadipocytes.
Further analyses were performed to assess the role of Wnt/β-catenin signaling in BMP9 induction of preadipocytes osteogenic and adipocytic differentiation. The present results revealed that Wnt/β-catenin signaling potentiated BMP9-induced bone formation and suppressed BMP9-induced adipocytic commitment in preadipocytes. Moreover, these findings are in line with other studies, which revealed that the canonical Wnt pathway may act as a crucial modulator for BMPs-induced osteogenesis and adipogenesis in different cell types [18, 21, 30,31,32]. Furthermore, our results indicated that Wnt/β-catenin signaling plays contrary roles in BMP9-mediated osteogenic and adipocytic differentiation in preadipocytes.
The present study found that only Wnt/β-catenin activation had no influence on differentiation of preadipocytes, thus suggesting that the Wnt/β-catenin pathway exerts biological function possibly by enhancing preadipocytes responsiveness to BMP9. However, the crosstalk between BMP9 and Wnt/β-catenin signal pathways is not yet understood. It is known that Runx2, a master regulator of osteogenesis, is essential for osteoinductive effect of Wnt/β-catenin pathway [18, 33, 34]. In addition, Runx2 is highly expressed in preadipocytes and is capable of promoting preadipocytes differentiation into osteoblasts [35, 36]. Our previous study revealed that BMP9 can increase Runx2 expression in preadipocytes [17]. In addition, Runx2 can also act as an inhibitor for adipogenesis both in MSCs and preadipocytes [36,37,38]. Therefore, it was hypothesized that the Wnt/β-catenin signaling may involve crosstalk with BMP9 via Runx2 in preadipocytes. It was demonstrated that β-catenin significantly increased BMP9-induced Runx2 in preadipocytes, thus indicating that Runx2 may be involved in the regulatory effect of Wnt/β-catenin signaling on BMP9-mediated commitment of preadipocytes. β-catenin also increased the expression of the BMP9-induced osteogenic transcription factor OSX, and inhibited BMP9-induced adipogenic transcription factors PPARγ and C/EBPα, which are targets of Wnt/β-catenin signaling for stimulating osteogenesis, rather than adipogenesis, in mesenchymal precursors [39]. Therefore, the present preliminarily results identified the possible mechanism via which the Wntβ-catenin signaling pathway interacts with BMP9 in preadipocytes.
In conclusion, we showed that Wnt/β-catenin signaling can promote BMP9-induced bone formation, and suppress BMP9-mediated adipogenesis in preadipocytes, by regulating osteogenic and adipogenic transcriptional factors. Therefore, our study provides a novel insight into the role of BMP9 in preadipocytes differentiation, and identified the possible mechanism via which BMP9 interacts with Wnt/β-catenin pathway in preadipocytes.
References
Meunier, P., Aaron, J., Edouard, C., & Vignon, G. (1971). Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clinical Orthopaedics and Related Research, 80, 147–154. https://doi.org/10.1097/00003086-197110000-00021.
Verma, S., Rajaratnam, J. H., Denton, J., Hoyland, J. A., & Byers, R. J. (2002). Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. Journal of Clinical Pathology, 55, 693–698. https://doi.org/10.1136/jcp.55.9.693.
Justesen, J., Stenderup, K., Ebbesen, E. N., Li, M., Steiniche, T., & Kassem, M. (2001). Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology, 2, 165–171. https://doi.org/10.1023/A:1011513223894.
Justesen, J., Stenderup, K., Eriksen, E. F., Kassem, M., Justesen, J., Stenderup, K., Eriksen, E. F., & Kassem, M. (2002). Maintenance of osteoblastic and adipocytic differentiation potential with age and osteoporosis in human marrow stromal cell cultures. Calcified Tissue International, 71, 36–44. https://doi.org/10.1007/s00223-001-2059-x.
Gimble, J. M., Zvonic, S., Floyd, Z. E., Kassem, M., & Nuttall, M. E. (2006). Playing with bone and fat. Journal of Cellular Biochemistry, 98, 251. https://doi.org/10.1002/jcb.20777.
Park, S. R., Oreffo, R. O., & Triffitt, J. T. (1999). Interconversion potential of cloned human marrow adipocytes in vitro. Bone, 24, 549. https://doi.org/10.1016/s8756-3282(99)00084-8.
Justesen, J., Pedersen, S. B., Stenderup, K., & Kassem, M. (2004). Subcutaneous adipocytes can differentiate into bone-forming cells in vitro and in vivo. Tissue Engineering, 10, 381–391. https://doi.org/10.1089/107632704323061744.
Park, J. G., Lee, D. H., Moon, Y. S., & Kim, K. H. (2014). Reversine increases the plasticity of lineage-committed preadipocytes to osteogenesis by inhibiting adipogenesis through induction of TGF-β pathway in vitro. Biochemical & Biophysical Research Communications, 446, 30–36. https://doi.org/10.1016/j.bbrc.2014.02.036.
Skillington, J., Choy, L., & Derynck, R. (2002). Bone morphogenetic protein and retinoic acid signaling cooperate to induce osteoblast differentiation of preadipocytes. Journal of Cell Biology, 159, 135–146. https://doi.org/10.1083/jcb.200204060.
MacDonald, B. T., Tamai, K., & Xi, H. (2009). Wnt/β-catenin signaling: components, mechanisms, and diseases. Developmental Cell, 17, 0–26. https://doi.org/10.1016/j.devcel.2009.06.016.
Congdon, K. L., Voermans, C., Ferguson, E. C., Dimascio, L. N., & Reya, T. (2008). Activation of Wnt signaling in hematopoietic regeneration. Stem Cells, 26, 1202–1210. https://doi.org/10.1634/stemcells.2007-0768.
Zhan, T., Rindtorff, N., & Boutros, M. (2016). Wnt signaling in cancer. Oncogene, 36, 1461–1473. https://doi.org/10.1038/onc.2016.304.
Nd, G. D., & Karsenty, G. (2007). In vivo analysis of Wnt signaling in bone. Endocrinology, 148, 2630–2634. https://doi.org/10.1210/en.2006-1372.
Cadigan, K. M., & Nusse, R. (1997). Wnt signaling: a common theme in animal development. Genes Development, 11, 3286–3305. https://doi.org/10.1101/gad.11.24.3286.
Karsenty, G., & Wagner, E. F. (2002). Reaching a genetic and molecular understanding of skeletal development. Developmental Cell, 2, 389–406. https://doi.org/10.1016/S1534-5807(02)00157-0.
Kim, J. H., Liu, X., Wang, J., Chen, X., Zhang, H., Kim, S. H., Cui, J., Li, R., Zhang, W., & Kong, Y. (2013). Wnt signaling in bone formation and its therapeutic potential for bone diseases. Therapeutic Advances in Musculoskelet Disease, 5, 13–31. https://doi.org/10.1177/1759720X12466608.
Liu, Y., Liu, Y. Y., Zhang, R. X., Wang, X., Huang, F., Yan, Z. J., Mao, N., Huang, J., Wang, Y. Z., Wang, Y., Chen, L., Yin, L. J., He, B. C., & Deng, Z. L. (2014). All-trans retinoic acid modulates bone morphogenic protein 9-induced osteogenesis and adipogenesis of preadipocytes through BMP/Smad and Wnt/β-catenin signaling pathways. International Journal of Biochemistry & Cell Biology, 47, 47–56. https://doi.org/10.1016/j.biocel.2013.11.018.
Tang, N., Song, W. X., Luo, J., Luo, X., Jin, C., Sharff, K. A., Yang, B., He, B. C., Huang, J. Y., & Zhu, G. H. (2009). BMP-9-induced osteogenic differentiation of mesenchymal progenitors requires functional canonical Wnt/beta-catenin signalling. Journal of Cellular & Molecular Medicine, 13, 2448–2464. https://doi.org/10.1111/j.1582-4934.2008.00569.x.
Pi, C. J., Liang, K. L., Ke, Z. Y., Chen, F., Cheng, Y., Yin, L. J., Deng, Z. L., He, B. C., & Chen, L. (2016). Adenovirus-mediated expression of vascularendothelial growth factor-a potentiates bone morphogenetic protein 9-induced osteogenic differentiation and bone formation. Biological Chemistry, 397, 765–775. https://doi.org/10.1515/hsz-2015-0296.
Lin, L., Qiu, Q., Zhou, N., Dong, W., Shen, J., Jiang, W., Fang, J., Hao, J., & Hu, Z. (2016). Dickkopf-1 is involved in BMP9-induced osteoblast differentiation of C3H10T1/2 mesenchymal stem cells. BMB Reports, 49, 179–184. https://doi.org/10.5483/BMBRep.2016.49.3.206.
Chen, Y., Whetstone, H. C., Youn, A., Nadesan, P., Chow, E. C., Lin, A. C., & Alman, B. A. (2007). Beta-catenin signaling pathway is crucial for bone morphogenetic protein 2 to induce new bone formation. Journal of Biological Chemistry, 282, 526–533. https://doi.org/10.1074/jbc.m602700200.
Papathanasiou, I., Malizos, K. N., & Tsezou, A. (2012). Bone morphogenetic protein-2-induced Wnt/β-catenin signaling pathway activation through enhanced low-density-lipoprotein receptor-related protein 5 catabolic activity contributes to hypertrophy in osteoarthritic chondrocytes.Arthritis Research & Therapy, 14(2), 1–14. https://doi.org/10.1186/ar3805.
Zhang, M., Yan, Y., Lim, Y. B., Tang, D., Xie, R., Chen, A., Tai, P., Harris, S. E., Xing, L., & Qin, Y. X. (2009). BMP-2 modulates β-catenin signaling through stimulation of Lrp5 expression and inhibition of β-TrCP expression in osteoblasts. Journal of Cellular Biochemistry, 108, 896–905. https://doi.org/10.1002/jcb.22319.
Yang, L., Yamasaki, K., Shirakata, Y., Dai, X., Tokumaru, S., Yahata, Y., Tohyama, M., Hanakawa, Y., Sayama, K., & Hashimoto, K. (2006). Bone morphogenetic protein-2 modulates Wnt and frizzled expression and enhances the canonical pathway of Wnt signaling in normal keratinocytes. Journal of Dermatological Science, 42, 111–119. https://doi.org/10.1016/j.jdermsci.2005.12.011.
Kim, H. K. W., Oxendine, I., & Kamiya, N. (2013). High-concentration of BMP2 reduces cell proliferation and increases apoptosis via DKK1 and SOST in human primary periosteal cells. Bone, 54, 141–150. https://doi.org/10.1016/j.bone.2013.01.031.
Kamiya, N., Kobayashi, T., Mochida, Y., Yu, P. B., Yamauchi, M., Kronenberg, H. M., & Mishina, Y. (2010). Wnt inhibitors Dkk1 and Sost are downstream targets of BMP signaling through the type IA receptor (BMPRIA) in osteoblasts. Journal of Bone & Mineral Research, 25, 200–210. https://doi.org/10.1359/jbmr.090806.
Clevers, H. (2006). Wnt/beta-catenin signaling in development and disease. Cell, 127, 469–480. https://doi.org/10.1016/j.cell.2006.10.018.
Zhu, J. H., Liao, Y. P., Li, F. S., Hu, Y., Li, Q., Ma, Y., Wang, H., Zhou, Y., He, B. C., & Su, Y. X. (2018). Wnt11 promotes BMP9-induced osteogenic differentiation through BMPs/Smads and p38 MAPK in mesenchymal stem cells. Journal of Cellular Biochemistry, 119, 9462–9473. https://doi.org/10.1002/jcb.27262.
Gong, Y., Slee, R. B., Fukai, N., Rawadi, G., Romanroman, S., Reginato, A. M., Wang, H., Cundy, T., Glorieux, F. H., & Lev, D. (2001). LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell, 107, 513–523. https://doi.org/10.1016/S0092-8674(01)00571-2.
Georges, R., Béatrice, V., Fred, D., Roland, B., & Sergio, R. R. (2010). BMP-2 controls alkaline phosphatase expression and osteoblast mineralization by a Wnt autocrine loop. Journal of Bone & Mineral Research, 18, 1842–1853. https://doi.org/10.1359/jbmr.2003.18.10.1842.
Zhang, H., Wang, J., Deng, F., Huang, E., Yan, Z., Wang, Z., Deng, Y., Zhang, Q., Zhang, Z., & Ye, J. (2015). Canonical Wnt signaling acts synergistically on BMP9-induced osteo/odontoblastic differentiation of stem cells of dental apical papilla (SCAPs). Biomaterials, 39, 145–154. https://doi.org/10.1016/j.biomaterials.2014.11.007.
Shen, J., James, A. W., Zhang, X., Shen, P., Zara, J. N., Asatrian, G., Chiang, M., Min, L., Khadarian, K., & Nguyen, A. (2016). Novel Wnt regulator NEL-Like molecule-1 antagonizes adipogenesis and augments osteogenesis induced by bone morphogenetic protein 2. American Journal of Pathology, 186, 419–434. https://doi.org/10.1016/j.ajpath.2015.10.011.
Ducy, P., Zhang, R., Geoffroy, V., Ridall, A. L., & Karsenty, G. (1997). Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell, 89, 747–754. https://doi.org/10.1016/s0092-8674(00)80257-3.
Tripti, G., Lengner, C. J., Hayk, H., Bhat, R. A., Bodine, P. V. N., Komm, B. S., Amjad, J., Wijnen, A. J. V., Stein, J. L., & Stein, G. S. (2005). Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. Journal of Biological Chemistry, 280, 33132–33140. https://doi.org/10.1074/jbc.M500608200.
Takahashi, T. (2011). Overexpression of Runx2 and MKP-1 stimulates transdifferentiation of 3T3-L1 preadipocytes into bone-forming osteoblasts in vitro. Calcified Tissue International, 88, 336–347. https://doi.org/10.1007/s00223-011-9461-9.
Zhang, Y., Li, X., Qian, S., Guo, L., Huang, H., He, Q., Liu, Y., Ma, C., & Tang, Q. Q. (2012). Down-regulation of Type I Runx2 mediated by dexamethasone is required for 3T3-L1 adipogenesis. Molecular Endocrinology, 26, 798–808.
Enomoto, H., Furuichi, T. A., Yamana, K., Yoshida, C., Sumitani, S., Yamamoto, H., Enomoto-Iwamoto, M., Iwamoto, M., & Komori, T. (2004). Runx2 deficiency in chondrocytes causes adipogenic changes in vitro. Journal of Cell Science, 117, 417 https://doi.org/10.1242/jcs.00866.
Gori, F., Thomas, T., Hicok, K. C., Spelsberg, T. C., & Riggs, B. L. (2010). Differentiation of human marrow stromal precursor cells: bone morphogenetic protein-2 increases OSF2/CBFA1, enhances osteoblast commitment, and inhibits late adipocyte maturation. Journal of Bone & Mineral Research, 14, 1522–1535. https://doi.org/10.1359/jbmr.1999.14.9.1522.
Kang, S., Bennett, C. N., Gerin, I., Rapp, L. A., & Macdougald, O. A. (2007). WNT signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing c/EBPα and PPARγ. Journal of Biological Chemistry, 282, 14515–14524. https://doi.org/10.1074/jbc.m700030200.
Acknowledgements
We thank Dr. TC He (University of Chicago Medical Center, USA) for providing recombinant adenoviruses and pTOP-luc reporter plasmid. The present study was supported by Natural Science Foundation of China (grant no. 81601895).
Author information
Authors and Affiliations
Contributions
Y.L., K.L.L., and L.C. conceived and designed the study. K.L.L., R.D.L., Z.J.Y., and L.Y.W. performed the experiments. Y.L. and K.L.L. collected and analyzed the data. Y.L. wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
All authors have no conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liang, K., Du, Y., Chen, L. et al. Contrary Roles of Wnt/β-Catenin Signaling in BMP9-Induced Osteogenic and Adipogenic Differentiation of 3T3-L1 Preadipocytes. Cell Biochem Biophys 78, 347–356 (2020). https://doi.org/10.1007/s12013-020-00935-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-020-00935-0