Abstract
Mesenchymal stem cells (MSCs) are multipotent stem cells that are able to differentiate into several cell types, including cartilage, fat, and bone. It has been reported that the decision process of MSCs into fat and bone cells is competing and reciprocal. Interleukin (IL)-35 is an important effector protein in the Wnt/β-catenin signaling pathway that acts as a bone metabolism regulator. However, it is unclear whether IL-35 is also important for regulating MSC differentiation to fat and bone. In the current study, we evaluated the role of IL-35 in C3H10T1/2 cells, which are a good cell model for investigating osteogenesis and adipogenesis in bone marrows. The role of IL-35 on osteoblast proliferation and apoptosis was assessed using cell counting kit-8 assay and flow cytometry, respectively. Extracellular matrix mineralization and lipid accumulation were measured by Alizarin Red S staining and Oil Red O staining, respectively. The most important transcription factor of the process of osteogenesis Runx2 and Wnt/β-catenin signaling pathway components β-catenin and Axin2 were investigated in response to IL-35 treatment. Furthermore, the adipogenic markers PPAR-γ and C/EBPα were also investigated. Our observations showed that IL-35 could promote the proliferation of MSCs and inhibit the apoptosis of MSCs. We found that IL-35 treatment resulted in a dramatic stimulation of osteogenesis and inhibition of adipogenesis. Moreover, IL-35 enhanced Wnt/β-catenin pathway key component β-catenin as well as Axin2 expression during MSCs differentiated to osteoblasts. Our findings suggested that IL-35 might control the balance between osteogenic and adipogenic differentiation of progenitor cells through the Wnt/β-catenin-PPARγ signaling pathway, suggesting its potential application in providing an intervention in osteoporosis and obesity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The lineage commitment of mesenchymal stem cells (MSCs), which is a common progenitor, plays a crucial role in the maintenance of bone homeostasis [1]. MSCs can differentiate into different cell types. However, the competitive differentiation of MSCs to adipocytes and osteoblasts has been drawn much attention [2]. The dysregulation between osteogenic and adipogenic differentiation is often observed in multiple human diseases, such as osteoporosis and obesity [3]. Thus, it is important to explore the mechanisms that control the balance between osteoblasts and adipocytes and the commitment to differentiate to either lineage. Osteogenesis and adipogenesis are mediated through multiple signal pathways and a harmonious cascade of downstream transcription factors. Previous studies have demonstrated that Wnt/β-catenin signaling, β-catenin, and Runt-related transcription factor 2 (Runx2) are closely linked to osteogenesis, and peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT/enhancer binding proteins (C/EBPs) are closely related to adipogenesis.
Interleukin (IL)-35 is a heterodimer which exhibits growth factor-like activities on diverse cell types, including osteoblasts, osteoclasts, and synovial fibroblasts [4,5,6]. IL-35 was a new member of the IL-12 family which was identified as an immunosuppressive and anti-inflammatory cytokine and was observed to mitigate collagen-induced arthritis (CIA) in mice [7]. IL-35 is mainly produced by Tregs, which played a crucial role in exerting the maximal immune-suppressive function of Tregs. Tregs exerted immune-stimulatory function mainly via EBI3, which could stimulate macrophages to synthesize macrophage inflammatory protein-1 and further commit B and T lymphocytes to inflammatory local sites [8]. Further study showed that IL-35 could stimulate Tregs proliferation in turn, which is named iTR35. This type of iTR35 exhibited inhibition of inflammation by switching initial T cells into a new type of Foxp3-Treg [9].
Recent research has demonstrated that IL-35 plays physiological and pathophysiological roles in different cells and tissues including osteoblasts, osteoclasts, liver, intestine, and lung [5, 10,11,12,13]. IL-35 is necessary for in vitro bone formation and bone resorption. In the MC3T3E1 cell line, IL-35 could upregulate the expression of osteoprotegerin (OPG) and downregulate the expression of receptor activator of nuclear factor-κB ligand (RANKL) through the Wnt/β-catenin signaling pathway, the pre-osteoblast cell line [10]. In RAW264 mouse monocytic cell line, IL-35 collaborated with RANKL to stimulate osteoclastogenesis via signal-regulated kinase (ERK) and p38 mitogen-activated protein kinase pathway. Furthermore, IL-35-MSCs exerted a stronger immunosuppressive function and further decreased the percentage of Th17 cells, increased the proportion of CD4 + Foxp3 + T cells, and controlled Th1/Th2 balance in heart transplant mice. To date, it is unknown whether and how IL-35 regulates osteogenic and adipogenic differentiation from mesenchymal progenitor cells.
However, it has not yet fully been investigated whether IL-35 have an impact on MSCs. In the present study, the biological changes in C3H10T1/2 cells after IL-35 treatment are measured, which are a good cell model for investigating osteogenesis and adipogenesis in bone marrows. We demonstrated that IL-35 could promote osteogenesis and inhibit adipogenesis in MSCs in vitro, which is likely to contribute to the activation of the crosstalk between the Wnt/β-catenin signaling pathway and the PPARγ signaling pathway.
MATERIALS AND METHODS
Cells
C3H10T1/2 cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS). For osteoblast differentiation, C3H10T1/2 cells of 80% confluence were exposed to an osteogenic medium (a-MEM containing 10% FBS, 50 μg/mL ascorbic acid, and 5 mM β-glycerophosphate) for 14 days followed by 21 days for Alizarin Red staining. For adipogenic differentiation, C3H10T1/2 cells of 100% confluence were exposed to an adipogenic medium (a-MEM containing 10% FBS, 0.5 μM dexamethasone, 0.25 mM methylisobutylxanthine, 5 μg/mL insulin, and 50 μM indomethacin) for 3 days, then with insulin alone for an additional 2 days followed by Oil Red O staining. The medium was refreshed every 3 days.
CCK-8 Assay
The effect of IL-35 on cell proliferation was measured by CCK-8 assay. Briefly, C3H10T1/2 cells were seeded in 96-well plates at a density of 3 × 103 cells per well. Then, cells were treated with IL-35 at various concentrations (0, 25, 50, and 100 ng/mL) for 0, 24, 48, and 72 h. Then, 10 μL CCK-8 was added to each well and the mixture was incubated for 4 h at 37 °C. After shaking for 15 min, absorbance was measured on a microplate reader at 450 nm.
Flow Cytometry
Flow cytometry was used to detect apoptosis frequency in MSCs. Briefly, 1 × 106 MSCs were seeded into 6-well plates. Briefly, after incubation with IL-35 at various concentrations (0, 25, 50, and 100 ng/mL) for 48 h, MSCs were collected by trypsin digestion. Discard the supernatant and gently resuspend the cells by adding 195μL of Annexin V-FITC binding solution. Then, 5μL Annexin V-FITC and 10μL propidium iodide staining solution were added to each well and mix gently 15 min in darkness at room. The MSC apoptosis frequency was analyzed by a flow cytometer.
Alizarin Red Staining
After 14 days of culture of MSCs in the presence of the osteogenic medium, the differentiated osteoblasts were gently washed twice with PBS and fixed in 4% paraformaldehyde for 15 min. For Alizarin Red staining, MSCs were cultured in the presence of the osteogenic medium for 21 days, then washed twice with PBS buffer, fixed with 70% ethanol, and incubated for 1 h at 37 ℃. Then, the cells were stained with 40 mM Alizarin Red S for 30 min. The calcium salt nodules were visualized by microscopy.
Oil Red O Staining
Fully differentiated adipocytes were gently washed twice with PBS and fixed in 4% paraformaldehyde for 15 min. The samples were washed twice with deionized water and then stained for 5 min with 0.3% Oil Red O in 60% saturated isopropanol. The stained fat droplets in the adipocytes were visualized under a light microscope and photographed. Spectrophotometric analysis of the stain was performed by re-dissolving the stained lipid droplets with isopropanol and measuring the light absorbance at 520 nm.
Quantitative RT-PCR
RNA was extracted from cells using a total RNA isolation kit. The reverse transcription was performed by using MMLV reverse transcriptase. The quantitative real-time PCR was implemented by using a SYBR Green fluorescence PCR kit on a real-time PCR cycler with gene-specific primers. The cycling scheme consisted of 40 cycles (95 ℃ for 10 s, 57 ℃ for 10 s, and 72 ℃ for 10 s) after an initial denaturing step (95 ℃ for 2 min). The expression levels were normalized against β-actin and measured by the ΔΔCt method. The primers used are listed as follows:
β-catenin forward: GCAACCCTGAGGAAGAAGA;
β-catenin reverse: GGATGAGCAGCGTCAAACT;
Runx2 forward: GCAGCACTCCATATCTCTACT;
Runx2 reverse: TTCCGTCAGCGTCAACAC;
Axin2 forward: CAACGACAGCGAGTTATCC;
Axin2 reverse: GTTCCACAGGCGTCATCT;
PPARγ forward: ACCACTCGCATTCCTTT;
PPARγ reverse: CACAGACTCGGCACTCA;
C/EBPα forward: GAGGAGGACGAGGCGAAGCA;
C/EBPα reverse: CGATCTGGAACTGCAAGTGGG;
β-actin forward: CTGTGCCCATCTACGAGGGCTAT;
β-actin reverse: TTTGATGTCACGCACGATTTCC.
Western Blot Analysis
Cells were lysed using RIPA buffer, supplemented with protease inhibitors and phosphatase inhibitors, and proteins were separated by SDS–polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes. The membranes were probed overnight with primary antibodies including rabbit mAbs by Abcam (Cambridge, Massachusetts): anti-β-catenin, anti-Runx, anti-Axin2, anti-PPARγ, and anti-C/EBPα. Finally, an enhanced chemiluminescence reagent was used to visualize the results. β-actin was used as a loading control.
Adipogenesis Analysis After Blockage with DKK-1
The cell line of 100% confluence was exposed to adipogenic medium as previously described. After treatment with 50 ng/mL IL-35 for 48 h, the cell line was blocked with 100 ng/mL DKK-1 for 24 h, which is a classic Wnt/β-catenin pathway inhibitor. The experimental procedures for real-time PCR and western blot on adipogenic expressions including PPARγ and C/EBPα were conducted as previously described.
Statistical Analysis
All data were analyzed by SPSS 17.0 (SPSS Inc., Chicago, IL) and GraphPad Prism 6 software. All values were presented as mean ± standard deviation (SD) from at least three independent experiments. Differences between groups were assessed by Student’s t-tests and one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons post-test. Differences of p < 0.05 were considered significant.
RESULTS
IL-35 Promoted the Proliferation of MSCs
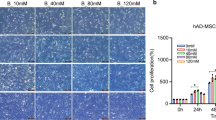
To identify the effect of IL-35 on MSC growth, we tested the MSC viability rate using the CCK-8 assay. As shown in Fig. 1, the results of the CCK-8 assay showed that IL-35 promoted the proliferation of MSCs in a time- and dose-dependent manner.
IL-35 Inhibited Apoptosis of MSCs
MSC apoptosis in each group was measured by flow cytometry. In the control group, the frequency of apoptotic MSCs was 8.43 ± 1.06%. In the 100 ng/mL IL-35 group, the frequency of apoptotic MSCs was only 2.03 ± 0.35%, which was significantly lower (all p < 0.05) than that in the 50 ng/mL IL-35 group (4.01 ± 0.54%) and 25 ng/mL IL-35 group (5.71 ± 0.57%). This result suggested that IL-35 could inhibit MSC apoptosis in a dose-dependent manner (Fig. 2).
The effects of IL-35 at different concentrations (0, 25, 50, 100 ng/mL) for 48 h on the percentage of apoptotic MSCs. *p < 0.05 vs. IL-35 (0 ng/mL). a–d Representative flow cytometric analysis of FLS apoptosis. e Percentages of apoptotic FLS after IL-35 treatment. Data are expressed as mean ± SD, n = 3. **p < 0.01 versus the control group. The right upper quadrant represents the late stage of MSC apoptosis, and the right lower quadrant represents the early stage of MSC apoptosis.
IL-35 Regulates Osteogenic-Adipogenic Gene Expressions in Progenitor Cells
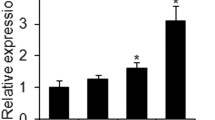
First, we examined the effect of IL-35 on Runx2 expression, which was the most important transcription factor of the process of osteogenesis. The C3H10T1/2 cells initiated their differentiation to osteoblasts upon treatment with the osteogenic medium. At the same time, Wnt/β-catenin signaling components were changed. Interestingly, we found that the IL-35 treatment resulted in a significant increase in the β-catenin and Axin2 expression (Fig. 3a–c). Next, to explore whether IL-35 influences the adipogenesis of MSCs, we investigated the effect of IL-35 on adipocyte differentiation. The C3H10T1/2 cells initiated their differentiation to adipocytes upon treatment with the adipogenic medium. Compared to control cells, the expression levels of the adipogenic markers PPAR-γ and C/EBPα were also reduced in mature adipocytes in response to the treatment with IL-35 (Fig. 3d, e).
IL-35 Regulates Osteogenic-Adipogenic Protein Expressions in Progenitor Cells
Consistent with real-time PCR results, western blot analysis showed that exogenous IL-35 increased the protein levels of osteogenic transcription factors and Wnt/β-catenin components, including β-catenin, Runx2, and Axin2, after 14 days of osteogenic induction dose dependently (Fig. 4a–c). However, exogenous IL-35 decreased the protein levels of the two master regulators of adipocyte differentiation including PPARγ and C/EBPα dose dependently (Fig. 4d, e).
IL-35 Promoted Mineralization and Inhibited Lipid Accumulation in C3H10T1/2 Cells
Last, we explored what effect of IL-35 on matrix mineralization and lipid accumulation in MSCs. The C3H10T1/2 cell lines at confluence were treated with IL-35 for 48 h. Alizarin Red staining showed that IL-35 could increase the area and the density of mineralized nodules in MSCs. Furthermore, Oil Red O images revealed that IL-35 could enhance the lipid accumulation in a dose-dependent manner in MSCs (Fig. 5).
a–c Osteogenic transcription factor Runx2 and Wnt/β-catenin component including β-catenin and Axin2 mRNA expression in MSCs in response to IL-35 at different concentrations (0, 25, 50, and 100 ng/mL) when MSCs were cultured in an osteogenic medium. d, e The two master regulators of adipocyte differentiation including PPARγ and C/EBPα mRNA expression in MSCs in response to IL-35 at different concentrations (0, 25, 50, and 100 ng/mL) when MSCs were cultured in an adipogenic medium. *p < 0.05, **p < 0.01 vs. the control group (IL-35 (0 ng/mL)). Comparison between the two groups was conducted by the Student t-test. p-values were calculated using one-way ANOVA, followed by Tukey’s multiple comparisons post-test.
IL-35 Inhibited Adipogenic Differentiation via the Wnt/β-catenin Signaling Pathway in Progenitor Cells
To explore the participation of the Wnt/β-catenin signaling pathway in the adipogenic differentiation of IL-35, exogenous DKK-1 was utilized in this work, which was a specific inhibitor to block the Wnt/β-catenin signaling pathway. With DKK-1 treatment, the mRNA and protein expression of β-catenin was significantly decreased, suggesting the Wnt/β-catenin signaling pathway was blocked (Fig. 6a, e). After blocking the Wnt/β-catenin signaling pathway with DKK-1, the mRNA and protein expression of PPARγ and C/EBPα was significantly increased by DKK-1 in progenitor cells (Fig. 6b, c, f, g), respectively. These results demonstrated DKK-1 could reverse the effect of anti-adipogenic differentiation of IL-35, validating that IL-35 inhibited adipogenic differentiation via the Wnt/β-catenin signaling pathway in progenitor cells.
a–c Osteogenic transcription factors and Wnt/β-catenin components including Runx2, β-catenin, and Axin2 protein expression in MSCs in response to IL-35 at different concentrations (0, 25, 50, and 100 ng/mL) when MSCs were cultured in an osteogenic medium. d, e The two master regulators of adipocyte differentiation including PPARγ and C/EBPα protein expression in MSCs in response to IL-35 at different concentrations (0, 25, 50, and 100 ng/mL) when MSCs were cultured in an adipogenic medium. *p < 0.05, **p < 0.01 vs. the control group (IL-35 (0 ng/mL)). Comparison between the two groups was conducted by the Student t-test. p-values were calculated using one-way ANOVA, followed by Tukey’s multiple comparisons post-test.
Effect of IL-35 on lipid accumulation and mineralization in MSCs. a–d With the increased concentration of IL-35, the area and the density of the mineralized nodule in MSCs were enhanced. e–h With the increased concentration of IL-35, lipid droplet accumulation in MSCs was prohibited. i, j Evaluation of MSC matrix mineralization and lipid accumulation by Alizarin Red S and Oil Red O staining, respectively, using a microplate reader at 520 nm. *p < 0.05, **p < 0.01 vs. the control group (IL-35 (0 ng/mL)); ns, non-significant. Comparison between the two groups was conducted by the Student t-test. p-values were calculated using one-way ANOVA, followed by Tukey’s multiple comparisons post-test.
DKK-1 reversed the anti-adipogenic effect in response of IL-35 including PPARγ and C/EBPα mRNA in MSCs. The C3H10T1/2 cells were treated with IL-35 (50 ng/mL) and DKK-1 (100 ng/mL) either alone or in combination as indicated. a–c β-catenin, PPARγ, and C/EBPα mRNA expression in each group. e–g β-catenin, PPARγ, and C/EBPα protein expression in each group. *p < 0.05, **p < 0.01 vs. the control group (IL-35 (0 ng/mL)). p-values were calculated using one-way ANOVA, followed by Tukey’s multiple comparisons post-test.
DISCUSSION
IL-35 is a novel member of the IL-12 family, which is composed of a p35 subunit and an Epstein-Barr virus-induced gene 3 (EBI3) subunit. IL-35 is an immune-suppressive cytokine, mostly derived from CD3 + CD4 + CD25 + Foxp3 + regulatory T cells (Tregs). Previous studies have demonstrated that IL-35 was significantly associated with multiple autoimmune diseases including rheumatoid arthritis (RA) [14, 15], periodontitis [16], ulcerative colitis [12], and idiopathic inflammatory myopathies [17]. Additionally, elevated IL-35 levels in allografts may reduce the occurrence of acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation [18]. Cell-based remedy using MSCs has a great promising prospect in immunosuppression. These multipotent cells have immunomodulatory and paracrine properties, which have shown promise in the treatment of multiple diseases such as multiple sclerosis [19] and graft-versus-host disease. MSCs can differentiate into different cell types including adipocytes, osteoblasts, myocytes, and chondrocytes [20]. Several signaling pathways have been confirmed to be involved in the lineage commitment and differentiation of MSCs, including the cooperation of Wnt, BMP, Notch, Hedgehog, and PPAR [21]. A previous study demonstrated that marrow fat volume was increased in animal models of ovariectomy and in aging in humans [22]. This phenomenon exists partly because MSCs have a decreased capacity to differentiate into osteoblasts and an increased capacity to differentiate into adipocytes [23]. This observation might suggest that the differentiation pathways of osteoblasts and adipocytes are mediated jointly and indicated that an inverse relationship occurs between the two lineages.
In the present study, IL-35 promoted MSC proliferation and inhibited MSC apoptosis. These results may suggest that IL-35 exerts its immunosuppressive functions through increasing the number of MSCs. MSCs have been demonstrated to act by proliferating the circulating Treg cell population [24]. IL-35-transduced Ad-MSCs could promote the expansion of CD4 + CD25 + Tregs and refrain the function of effector T cells including T helper (Th) 1, Th2, and Th17 cells and may delay the development of allograft rejection [25]. In bone metabolism, the role of IL-35 is widely investigated. IL-35 could promote the expression of OPG and inhibited the expression of RANKL in osteoblasts through the Wnt/β-catenin signaling pathway [10]. In this study, IL-35 could promote the expression of Runx2, which was the most important transcription factor of the process of osteogenesis. At the same time, IL-35 suppressed the expression of PPARγ, which was best known for its crucial role in mediating adipogenic differentiation in MSCs. MSCs derived from many adult tissues have the potential to differentiate into cells of different lineages, including osteoblasts, chondrocytes, adipocytes, and myocytes [26]. Among these plausible fates, differentiation to the osteoblast and adipocyte lineages has a specific effect on the maintenance of normal bone metabolism. A previous study demonstrated that a shift in MSC differentiation to favor the adipocyte lineage over the osteoblast lineage can lead to imbalances in bone formation and resorption, finally contributing to bone loss. The reduction in bone volume related to age-related osteopenia and osteoporosis was followed with a rise in marrow adipose tissue [27]. Therefore, another mechanism on osteoblastic roles of IL-35 could be explained that IL-35 could control the balance between osteogenic and adipogenic differentiation of progenitor cells.
At the same time, we also linked this phenomenon to the increased expression of the members of the Wnt/β-catenin signaling pathway. Wnt/β-catenin signaling components such as β-actin and Axin2 were also elevated in response to IL-35. The activation of β-catenin can be mediated when the expression and/or phosphorylation of the Wnt signaling components was changed. Interestingly, we found that the IL-35 treatment resulted in a significant increase in the β-catenin and Axin2 expression. Furthermore, we also found that IL-35 treatment downregulated the expression of PPAR-γ and C/EBPα, which are considered as the crucial factors in the adipogenesis of MSCs. Previous studies demonstrated that the activation of PPAR-γ led to a decrease of β-catenin expression during adipogenesis; this reduction of β-catenin is involved via a proteasome-dependent degradation mechanism [28]. In the current study, the IL-35 treatment resulted in an increase of β-catenin and Axin2 with a concomitant suppression of adipogenesis, indicating that activation of the Wnt/β-catenin signaling pathway and the inhibition of the PPARγ signaling pathway are not parallel and independent events caused by IL-35. Thus, it is likely that the IL-35-induced activation of osteogenesis contributes to the inhibition of adipogenesis in MSCs. However, it is not clear whether Wnt/β-catenin signaling and PPARγ signaling pathway exist “cross-talk” and what the specific interaction factor during the IL-35-induced promotion of osteogenesis and inhibition of adipogenesis during MSC differentiation. Molecular study during the past decades has confirmed that the Wnt/β-catenin signaling components act as a negative mediator of adipogenesis. In our study, Axin2 was upregulated by IL-35 in MSCs. We further screened out PPARγ that may be transcriptionally activated by Axin2 according to the JASPAR database (Supplementary Fig. 1). We could speculate that IL-35 stimulate osteogenesis and inhibit adipogenesis via PPARγ-Axin2-Wnt/β-catenin crosstalk during MSC differentiation. However, further studies are needed to be verified. There are still some limitations in this work. MSCs were first discovered in the bone marrow and were widely distributed in the human body, such as umbilical cord, adipose tissue, umbilical cord blood, amniotic membrane, (placental) chorion, dental pulp, thymus, synovium, fetal blood and liver, etc. In the mechanism of osteoporosis, especially the unbalanced differentiation of bone marrow-derived mesenchymal stem cells (BMSCs) (decreased osteogenic differentiation and increased adipogenic differentiation) is the key factor. Therefore, using bone marrow-derived MSCs (BMSCs) as a research model can provide a more valuable direction for the prevention and treatment of osteoporosis. In addition, in this study, MSCs were treated by IL-35 alone without osteogenesis or induction needed.
CONCLUSION
The balance between adipogenesis and osteogenesis within the bone marrow microenvironment offers a promising target for medical intervention. We confirmed that IL-35 could promote MSC proliferation and inhibit MSC apoptosis. Furthermore, IL-35 is the key regulator of adipo-osteogenic differentiation in MSCs. Our findings suggested that IL-35 might prevent the development of bone loss through shifting adipo-osteogenic differentiation, indicating a promising treatment target for bone loss in the future.
Availability of Data and Material
The data used to support the findings of this study are available from the corresponding author upon request.
References
Gimble, J.M., S. Zvonic, Z.E. Floyd, M. Kassem, and M.E. Nuttall. 2006. Playing with bone and fat. Journal of cellular biochemistry 98 (2): 251–266.
Chen, Q., P. Shou, C. Zheng, M. Jiang, G. Cao, Q. Yang, J. Cao, N. Xie, T. Velletri, X. Zhang, C. Xu, L. Zhang, H. Yang, J. Hou, Y. Wang, and Y. Shi. 2016. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell death and differentiation 23 (7): 1128–1139.
Hu, L., C. Yin, F. Zhao, A. Ali, J. Ma, and A, Qian. 2018. Mesenchymal stem cells: Cell fate decision to osteoblast or adipocyte and application in osteoporosis treatment. International journal of molecular sciences 19 (2).
Liu, S., Y. Li, L. Xia, H. Shen, and J. Lu. 2019. IL-35 prevent bone loss through promotion of bone formation and angiogenesis in rheumatoid arthritis. Clinical and experimental rheumatology 37 (5): 820–825.
Kamiya, Y., T. Kikuchi, H. Goto, I. Okabe, Y. Takayanagi, Y. Suzuki, N. Sawada, T. Okabe, Y. Suzuki, S. Kondo, J.I. Hayashi, and A. Mitani. 2020. IL-35 and RANKL synergistically induce osteoclastogenesis in RAW264 mouse monocytic cells. International journal of molecular sciences 21 (6).
Peng, M., L. Qiang, Y. Xu, C. Li, T. Li, and J. Wang. 2019. IL-35 ameliorates collagen-induced arthritis by promoting TNF-α-induced apoptosis of synovial fibroblasts and stimulating M2 macrophages polarization. The FEBS journal 286 (10): 1972–1985.
Wu, S., Y. Li, Y. Li, L. Yao, T. Lin, S. Jiang, H. Shen, L. Xia, and J. Lu. 2016. Interleukin-35 attenuates collagen-induced arthritis through suppression of vascular endothelial growth factor and its receptors. International immunopharmacology 34: 71–77.
Liu, J.Q., Z. Liu, X. Zhang, Y. Shi, F. Talebian, J.W. Carl Jr., C. Yu, F.D. Shi, C.C. Whitacre, J. Trgovcich, and X.F. Bai. 2012. Increased Th17 and regulatory T cell responses in EBV-induced gene 3-deficient mice lead to marginally enhanced development of autoimmune encephalomyelitis. The Journal of Immunology 188 (7): 3099–3106.
Collison, L.W., V. Chaturvedi, A.L. Henderson, P.R. Giacomin, C. Guy, J. Bankoti, D. Finkelstein, K. Forbes, C.J. Workman, S.A. Brown, J.E. Rehg, M.L. Jones, H.T. Ni, D. Artis, M.J. Turk, and D.A. Vignali. 2010. IL-35-mediated induction of a potent regulatory T cell population. Nature immunology 11 (12): 1093–1101.
Li, Y., L. Yuan, S. Jiang, S. Liu, L. Xia, H. Shen, and J. Lu. 2019. Interleukin-35 stimulates tumor necrosis factor-α activated osteoblasts differentiation through Wnt/β-catenin signaling pathway in rheumatoid arthritis. International immunopharmacology 75: 105810.
Long, J., H. Guo, S. Cui, H. Zhang, X. Liu, D. Li, Z. Han, L. Xi, W. Kou, J. Xu, T.S. Li, and Y. Ding. 2016. IL-35 expression in hepatocellular carcinoma cells is associated with tumor progression. Oncotarget 7 (29): 45678–45686.
Wetzel, A., B. Scholtka, F. Schumacher, H. Rawel, B. Geisendörfer, and B. Kleuser. 2021. Epigenetic DNA Methylation of EBI3 modulates human interleukin-35 formation via NFkB signaling: A promising therapeutic option in ulcerative colitis. International journal of molecular sciences, 22 (10).
Zhang, T., J. Nie, X. Liu, Z. Han, N. Ding, K. Gai, Y. Liu, L. Chen, and C. Guo. 2021. Correlation analysis among the level of IL-35, microvessel density, lymphatic vessel density, and prognosis in non-small cell lung cancer. Clinical and translational science 14 (1): 389–394.
Kam, N.W., D. Liu, Z. Cai, W.Y. Mak, C.K. Wong, K.H. Chiu, K.Y. Wong, W.L. Tsang, and L.S. Tam. 2018. Synoviocytes-derived interleukin 35 potentiates B cell response in patients with osteoarthritis and rheumatoid arthritis. The Journal of rheumatology 45 (4): 563–573.
Li, Y., L. Yao, S. Liu, W. Jisheng, L. Xia, H. Shen, and J. Lu. 2019. Correlation between serum IL-35 levels and bone loss in postmenopausal women with rheumatoid arthritis. Mediators of Inflammation 9139145.
Han, Y., C. Yu, Y. Yu, and L. Bi. 2021. CD25+ B cells produced IL-35 and alleviated local inflammation during experimental periodontitis. Oral diseases.
Mann, H., O. Kryštůfková, J. Zámečník, J. Háček, H. Hulejová, M. Filková, J. Vencovský, and L. Šenolt. 2021. Interleukin-35 in idiopathic inflammatory myopathies. Cytokine 137: 155350.
Zhang, X., Y. Zhou, L. Xu, W. Han, H. Chen, Y. Chen, H. Fu, S. Zhou, J. Zhao, Q. Wang, F. Feng, X. Zhu, K. Liu, and X. Huang. 2015. Reduced IL-35 levels are associated with increased platelet aggregation and activation in patients with acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Annals of hematology 94 (5): 837–845.
Ferrara, G., F. Ivaldi, G. Mancardi, N. Kerlero de Rosbo, and A. Uccelli. 2021. Bone marrow transfer in relapsing-remitting EAE ameliorates disease at first remission, with no synergistic effect upon co-transplantation with mesenchymal stem cells. Vaccines 9 (7).
Shi, Y., L. Du, L. Lin, and Y. Wang. 2017. Tumour-associated mesenchymal stem/stromal cells: Emerging therapeutic targets. Nature reviews Drug discovery 16 (1): 35–52.
Lin, G.L., and K.D. Hankenson. 2011. Integration of BMP, Wnt, and notch signaling pathways in osteoblast differentiation. Journal of cellular biochemistry 112 (12): 3491–3501.
Abdallah, B.M. 2017. Marrow adipocytes inhibit the differentiation of mesenchymal stem cells into osteoblasts via suppressing BMP-signaling. Journal of biomedical science 24 (1): 11.
Zhang, L., M. Liu, X. Zhou, Y. Liu, B. Jing, X. Wang, Q. Zhang, and Y. Sun. 2016. Role of osteoprotegerin (OPG) in bone marrow adipogenesis. Cellular Physiology and Biochemistry 40 (3–4): 681–692.
Tasso, R., C. Ilengo, R. Quarto, R. Cancedda, R.R. Caspi, and G. Pennesi. 2012. Mesenchymal stem cells induce functionally active T-regulatory lymphocytes in a paracrine fashion and ameliorate experimental autoimmune uveitis. Investigative ophthalmology & visual science 53 (2): 786–793.
Guo, H., N. Zhao, H. Gao, and X. He. 2017. Mesenchymal stem cells overexpressing interleukin-35 propagate immunosuppressive effects in mice. Scandinavian journal of immunology 86 (5): 389–395.
Arthur, A., A. Zannettino, and S. Gronthos. 2009. The therapeutic applications of multipotential mesenchymal/stromal stem cells in skeletal tissue repair. Journal of cellular physiology 218 (2): 237–245.
Burkhardt, R., G. Kettner, W. Böhm, M. Schmidmeier, R. Schlag, B. Frisch, B. Mallmann, W. Eisenmenger, and T. Gilg. 1987. Changes in trabecular bone, hematopoiesis and bone marrow vessels in aplastic anemia, primary osteoporosis, and old age: A comparative histomorphometric study. Bone 8 (3): 157–164.
Moldes, M., Y. Zuo, R.F. Morrison, D. Silva, B.H. Park, J. Liu, and S.R. Farmer. 2003. Peroxisome-proliferator-activated receptor gamma suppresses Wnt/beta-catenin signalling during adipogenesis. The Biochemical journal 376 (Pt 3): 607–613.
Funding
This study was funded by the China Postdoctoral Science Foundation (2019M661173) and the 345 Talent Project of Shengjing Hospital of China Medical University.
Author information
Authors and Affiliations
Contributions
XF W and J.L. designed the study and revised the manuscript. YX L performed the experiments. YX L drafted the manuscript. YX L analyzed the data.
Corresponding author
Ethics declarations
Consent for Publication
Written informed consent for publication was obtained from all participants.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10753_2022_1749_MOESM1_ESM.tif
Supplementary file1 JASPAR website was employed to predict the binding site of Axin2 and PPARγ. The promoter sequence of Axin2 was found and the score value was greater than 80%, suggesting the transcription of Axin2 could upregulate the PPARγ expression during the MSCs differentiate to adipocytes. (TIF 8512 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Wang, X. & Lu, J. Interleukin-35 Promote Osteogenesis and Inhibit Adipogenesis: Role of Wnt/β-Catenin and PPARγ Signaling Pathways. Inflammation 46, 522–533 (2023). https://doi.org/10.1007/s10753-022-01749-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-022-01749-3