Abstract
The aim of the study was to examine the protective effects and possible mechanism of gossypin against isoproterenol (ISO)-mediated myocardial damage in vivo and H9c2 cell damage in vitro. H9c2 cells were categorized into five groups. Viability was evaluated with MTT and LDH release in H9c2 cells. Apoptotic parameter analysis was performed with cytochrome c (Cyt-c), caspase-3 (CASP-3), and BCL2/Bax mRNA expression levels. In vivo, gossypin was administered orally to mice at doses of 5, 10, and 20 mg/kg for 7 days. ISO groups were injected with isoproterenol (150 mg/kg) subcutaneously (on 8th and 9th) for 2 days. Afterward, lactate dehydrogenase (LDH), creatine kinase-MB (CK-MB) levels and Troponin-I (Tn-I) amount from their serum, oxidative stress parameters superoxide dismutase (SOD) activity, glutathione (GSH) and malondialdehyde (MDA) levels, and tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), interleukin-1β (IL-1 β), and NF-kB mRNA expression levels with inflammatory markers from heart tissue were evaluated. In addition, IL-1B, BCL-2, and cas-3 immunohistochemical staining was performed from heart tissue and TNF-a level was measured by ELISA method. Administration of Gossypin protected the cells by dose-dependent, eliminating the reduced cell viability and increased LDH release of ISO in H9c2 cells. In mice serum analyses, increased LDH, CK-MB levels, and Tn-I levels were normalized by gossypin. ISO administration in heart tissue is regulated by gossypin with increased SOD activity, GSH amount, TNF-α, IL-6, IL-1β, and NF-kB mRNA expression levels and decreased MDA amount. Overall, the present results demonstrated that gossypin has a potential cardioprotective treatment for ischemic heart disease on in vivo and in vitro.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Ischemic heart diseases resulting from coronary spasm and/or occlusion are among the most common diseases on the World with high risk for morbidity and mortality [1]. As a result of decreased and/or completely blocked coronary blood flow myocardial infarction (MI) occurs. As a result of short- and long-term injuries occurred in the heart ventricles MI occurs. The level of acute damage during MI is related to the level of inflammatory responses such as the release of various inflammatory cytokines, formation of molecular patterns, such as immune cell infiltration, and level of reactive oxygen species [2, 3]. Inflammation and oxidative damage are the main physiopathological changes occurred during MI [4,5,6].
Isoproterenol(L-b-(3,4-dihydroxyphenyl)-α-isopropyl amino ethanol hydrochloride) is a β-adrenergic receptor agonist drug. In several studies, the state of initiation and/or maintenance of apoptosis between Bcl-2, Bax, Cyt-C, and CAS-3 has been noted as both a balance and a collaboration [2]. Although adult cardiomyocytes have lost their ability to divide, cardiomyocyte apoptosis has a very important role in heart disease and this situation has another potential mechanism in heart disease [7]. Finding effective drugs as a therapeutic tool that slows myocyte loss can also be considered as a novel approach to reducing and/or preventing inappropriate heart cell death. The other mechanism is inflammation. Proinflammatory cytokines, which are associated with severe congestive heart disease [8, 9]. During myocardial movements, proinflammatory cytokines such as TNF‐α and IL‐6 are elevated by activation of the NF‐kB signaling mechanism [10]. Activation of NF-kB can regulate genes in vascular cells that include IL‐1β, IL‐6, and TNF‐α expression, depending on its invasive function as well as NF‐kB [11].
Recently, flavonoids from various plant species have been noted for their antioxidant nature as potential therapeutic agents in the prevention and treatment of cardiovascular diseases [12, 13].
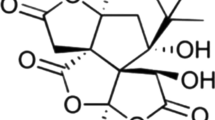
Gossypin (GOS) (3,5,8,3,4-pentahidroxy-7-o-glucosyl flavone 8-glucosyl) (GOS), originally isolated from Hibiscus vitifolius, is a flavonoid with anticancer [14], analgesic [15], antioxidant and anti-inflammatory features [16]. Gossypin activates both aminolevulinate dehydratase and antioxidant defense enzymes, demonstrating its supporting effects against defense-induced oxidative stress. [17]. In the present study, when we combine the damage caused by the MI process and its associated inflammatory development, apoptosis, and physiopathology, it comes to mind that gossypin may be an important agent for preventing ISO-related damage.
Thus, this study was designed to investigate the possible effects of gossypin on ISO-induced MI in vitro and in vivo and to evaluate the possible contribution of oxidative and inflammatory markers by biochemical, molecular, and histopathological analyses.
Materials and Methods
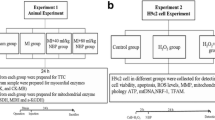
Cell Culture and Treatment
H9c2 (rat cardiac myoblasts, a cardiomyoblast cell line derived from the heart tissue of embryonic rats) cell line American Type Culture Collection (ATCC, USA) was provided. The cell lines in Cryotube in the liquid nitrogen were removed from the tank and allowed to dissolve in the water bath at 37 °C for a short time. Soluble cells were transferred to flasks with T75 cm2. After 48 h, H9c2 cells were counted at 2 × 105 cells/well in DMEM containing 10% FBS and seeded in a 96-well plate and incubated at 37 °C in a humid atmosphere containing 5% CO2. Cells were exposed to gossypin [purchased from Sigma Aldrich (CAS Number 652-78-8)] at different concentrations (0.1–200 µg/ml). The experimental groups are consisted of Control, ISO, ISO + GOS25, ISO + GOS50, and ISO + GOS100. H9c2 cells were categorized into five groups based on treatment: (1) Control group (cells were cultured in DMEM for 24 h and then treated with saline at 37 °C for 24 h); (2) ISO group (cells were cultured in DMEM for 24 h and then treated with 10 µM ISO at 37 °C for 24 h); (3) ISO + GOS groups [cells were pre-incubated with different concentrations (25, 50, or 100 µg/ml) of gossypin [Sigma Aldrich (CAS Number 652-78-8)] at 37 °C for 24 h and then treated with 10 µM ISO at 37 °C for 24 h.
MTT Assays
The proliferation of H9c2 cells cultured at a density of 1 × 104 cells/well in 96‑well plates was measured using the MTT method and was applied to the cells at 24, 48, and 72 h; the absorbance values of 570 nm were measured 3 times with microplate reader spectrophotometer (Epoch Microplate Spectrophotometer, BioTek, USA) according to the manufacturer's protocol [14]. Inhibition of cell growth was calculated as the percentage survival of treated cells over control cells × 100 (T/C %).
LDH Activity
The extent of cellular injury was monitored by LDH release. H9c2 cells were cultured at a density of 1 × 106 cells/well in 6‑well plates. Following treatment, the culture medium was analyzed to determine LDH activity using a LDH assay kit (Roche, Cat. No. 04 744 926 001, Mannheim, Germany) with a microplate reader according to the manufacturer's protocol.
Animals
Thirty male Balb/C mice weighing 25–30 g were used in the study. The animal experiments and procedures were performed in accordance with national guidelines for the use and care of laboratory animals and approved by Kafkas University’s Local Animal Care Committee (No: KAÜ-HADYEK/2020-056). The mice were obtained from the Medicinal and Experimental Application and Research Center, Erzurum, Turkey (ATADEM). They were kept in standard laboratory conditions under a natural cycle of light and dark.
Experimental Protocols of İsoproterenol-İnduced MI
After a week of acclimatization, the experimental animals were divided into five groups, (comprising six mice each) as follows:
-
1.
Healthy.
-
2.
Healthy + ISO (150 mg/kg).
-
3.
Healthy + ISO (150 mg/kg) + GOS 5 (5 mg/kg).
-
4.
Healthy + ISO (150 mg/kg) + GOS 10 (10 mg/kg).
-
5.
Healthy + ISO (150 mg/kg) + GOS 20 (20 mg/kg).
Mice in group I (normal control) received standard diet for a period of 7 days. To group II animals (ISO) were injected with isoproterenol (150 mg/kg body weight/day) dissolved in physiological saline subcutaneously (on 8th and 9th) for 2 days at 24-h interval for the induction of myocardial infarction. In group III, IV, and V animals (GOS 5, GOS 10, and GOS 20) [Sigma Chemical Company (Germany)] received 5, 10, and 20 mg/kg of gossypin (5, 10, and 20 mg/kg body weight/day), respectively, for 7 days and then injected with isoproterenol at the dose mentioned above. GOS is insoluble in water, and the drug was dissolved in a mixture with one part DMSO (0.1%DMSO) per one thousand parts of normal saline that is administered to control and ISO groups as vehicle. At the end of the experimental period, that is, 12 h after last injection of isoproterenol, all procedure was performed according to our previous study [6].
Biochemical Analysis
The separated serums were used for the determination of LDH and CK-MB (creatine kinase-MB) levels with an autoanalyzer (AU 5800, BECKMAN-COULTER diagnostic system, California, USA) and for determination of Tn-I (troponin-I) levels with another autoanalyzer (Access2, BECKMAN-COULTER diagnostic system, California, USA). And each heart tissue sample stored at − 80 °C for biochemical and molecular analyses was pulverized using liquid nitrogen and stainless steel jars and balls in a TissueLyser II device (Qiagen, Hilden, Germany). Superoxide dismutase (SOD) activity and glutathione (GSH) and malondialdehyde (MDA) levels were measured manually from the supernatants, as described in our previous studies [18, 19]. The tissue homogenate was centrifuged (10,000 rpm, 4 °C, 5 min) and the TNF-α was detected in the supernatant using ELISA kits for TNF-α, according to the manufacturer's instructions (R&D Systems, MN, USA). Results are expressed as picograms of TNF-α per milligram of protein.
Reverse Transcription‑Quantitative Polymerase Chain Reaction (RT‑qPCR)
Total RNA extraction and cDNA synthesis were performed according to the methods described in our previous studies [14].
Histological Procedure
Heart tissues are designed according to the procedure in the direction of previous literature and our studies [6, 20]. Cell infiltration, necrosis, and hemorrhage. A minimum of 5 fields for each heart section at 200 × magnification were evaluated and assigned for severity of changes using scores on a scale of none or rare represented as (−/+), mild (+), moderate (++), and severe (+++) (Table 1).
Immunohistochemical Procedure
For the immunohistochemical staining, the sections were deparaffinized and rehydrated. Citrate buffer solution was used under heat and pressure for inhibition of masking of antigens. To block endogenous peroxidase activity, the sections were treated with 0.3% H2O2 and then incubated with a blocking solution (Thermo Scientific, TA-060-PBQ). After blocking solution removed, the sections were incubated overnight at + 40 °C with primary antibodies anti-Bcl-2 (1:100 dilution—sc-7382), anti-IL1β (1:100 dilution—sc-32294) and anti-Caspase 3 (1:100 dilution—sc-7272). The antibodies against anti-Bcl-2, anti-IL1β, and anti-Caspase 3 were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). After primary antibody incubation, tissues were incubated with secondary antibody, Streptavidin-peroxidase complex, and 3,3'-diaminobenzidine (DAB) solution (Thermo Scientific, TL-060-HD), respectively, in accordance with the manufacturer's procedure. The sections were counterstained with Harris hematoxylin and coated with entellan. For immunohistochemical analysis, the sections were photographed different five fields for each heart section at 200 × magnification. Micrographs were analyzed in ImageJ software (ImageJ1,51j8, National Institutes of Health, USA) to determine staining intensities. H-SCORE rates were calculated using H-SCORE = Σ Pi (i + 1) formula.
Statistical Analysis
Biochemical and molecular data were subjected to one-way analysis of variance (ANOVA) using the IBM SPSS 20.0 statistical program. The differences between the groups were determined by the non-parametric Kruskal–Wallis test (P < 0.05). Bonferroni correction was applied for multiple comparisons. All results are expressed as mean ± SD for each group.
Results
H9c2 cells Viability Results
The effects of gossypin on ISO-induced H9c2 cells have been investigated. No significant changes up to 50 µg/ml concentration were observed in the dose-dependent study of gossypin. However, an increase in gossypin groups of 50 and 100 µg/ml has occurred. Administration of 200 µg/ml gossypin has been toxic to H9c2 cells (Fig. 1A). Therefore, concentrations of 25, 50, and 100 µg/ml were used in subsequent cellular studies. There was a significant increase in the ISO group in terms of LDH release (P < 0.001). There is also a significant increase in the GOS 25 Group (P < 0.01). However, there is an increase in the GOS 50 group (P < 0.05). In the GOS 100 Group, a significant decrease is observed in terms of LDH release according to control (P < 0.05) (Fig. 1B). If we look at the effects of gossypin on ISO-treated H9c2 cells, ISO groups show a significant reduction in control at all times (P < 0.001). Gossypin, on the other hand, although the dose of 25 µg/ml was not sufficient in the treatment groups with ISO, 50 and 100 µg/ml doses eliminated the negative effect of ISO and brought it closer to the control group. In particular, the effect of GOS 100 µg/ml at 72 h not only eliminated the ISO effect in H9c2 cells but also significantly increased the number of cells (P < 0.05) (Fig. 1C, D, E).
Assessment of H9c2 cells viability after treatment with different concentrations of gossypin. Untreated cells served as control. (A) The effects of gossypin(0.1-200 μM) on proliferation in H9c2 cells were measured by MTT test. (B) LDH levels in H9c2 cells. (C) Proliferation of H9c2 cells at 24h (D) Proliferation of H9c2 cells at 48h (E) Proliferation of H9c2 cells at 72h. Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01 and ***P < 0.001 vs. control
Biochemical Results
Effect of Gossypin on ISO-İnduced Elevation of Serum Cardiac İnjury Biomarkers
Isoproterenol administration significantly increased the activity of CK-MB and LDH (P < 0.001), which are enzymatic biomarkers of myocardial injury, and level of Tn-I (P < 0.001). Preliminary treatment with 5 and 10 mg/kg doses of gossypin showed no significant difference in LDH, CK-MB, and Tn-I levels; however, 20 mg/kg dose of gossypin decreased the ISO-induced changes at LDH, CK-MB (Fig. 2A, B), and Tn-I levels (Fig. 2C).
Effects of gossypin on ISO-induced myocardial injury in vivo. The Effects of gossypin and ISO on LDH (A), CK activities (B) and Tn-I levels (C) were measured. The levels of SOD activity (D), GSH (E) MDA levels (F) and Tumor necrosis factor-α (TNF-α) in the heart tissues were measured by the corresponding kits. Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01 and ***P < 0.001 vs. control
Oxidative Stress Markers in Heart Tissues
As shown in Fig. 2D–F SOD activity and GSH level decreased significantly in the ISO group compared to the control group and MDA level increased significantly (P < 0.001). SOD activity and GSH levels increased, and mda levels decreased significantly depending on the dose of gossypin compared to the control group. It is even close to control. Gossypin exerted an immunomodulatory response in the heart tissue (Fig. 2G) by reducing the level of inflammatory cytokine TNF-α (P < 0.01).
Molecular Results
H9c2 cells mRNA Expression Results
To assess the effectiveness of Gossypin in ISO-induced H9c2 cells, we analyzed the apoptotic parameters Cyty-c, CASP-3, and BCL2/Bax mRNA expression levels in cdnas obtained from H9c2 lysates using real-time PCR. The Cyty-c, CASP-3, and Bcl2/Bax mRNA expressions of the groups are shown in Fig. 3. Cyty-c and CASP-3 mRNA expressions were significantly increased in ISO-applied group compared to healthy control group (P < 0.05). Similarly, cyty-c and CASP-3 mRNA expression were significantly increased in the GOS25 and gos 50 groups (P < 0.01 and P < 0.05, respectively). But the GOS100 group has increased these values to the same level as control. BCL2/Bax mRNA expression was significantly reduced in ISO group and gos 25 Group (P < 0.001 and P < 0.01 respectively). A significant increase is observed in the GOS100 group compared to the control group (P < 0.01).
mRNA levels of apoptotic genes in H9c2 cells. mRNA levels in isoproterenol (ISO) - induced H9c2 cells were evaluated in the presence or absence of different concentrations of gossypin (GOS). Untreated cells were considered Control (C). (A) Bcl-2/BAX, (B) Caspase-3 (CASP3) and (C) Cytochrome-c (Cyt-c). Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01 and ***P < 0.001 vs. control
Heart Tissues mRNA Expression Results
TNF-α, IL-6, IL-1b, and NF-kB mRNA expression results are seen in Fig. 4. Levels of cytokines were significantly higher in the ISO group than in the control group (P < 0.001). TNF-α, IL-6, IL-1b, and NF-kB mRNA expression levels showed a dose-dependent decrease in the ISO + GOS 10 and ISO + GOS 20 groups compared to the sepsis group. No significant difference was observed between TNF-α mRNA expression levels and the control group in the ISO + GOS 20 group.
mRNA levels of inflammatory genes in heart tisue. mRNA levels in isoproterenol (ISO)-induced heart tisue were evaluated in the presence or absence of different concentrations of gossypin (GOS). Untreated mice was considered Control (C). (A) NF-kB, (B) TNF-α, (C) IL-1β and (D) IL-6. Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01 and ***P < 0.001 vs. control
Histopathology of Heart Tissues
According to the histopathology assessment, normal-looking muscle fibers and connective tissue areas were monitored in the healthy group. In the ISO Group, highly degenerate muscle fibers and inflammatory cells with Polymorph nuclei were found in the connective tissue between them. Moderate degenerate muscle fibers were observed in the ISO + Gos5 group, while no inflammatory cells were found. In the ISO + Gos10 there were no signs of inflammation, while slightly degenerate novelized muscle fibers were observed in the group. In the ISO + Gos20 group, degenerate muscle fibers were rarely seen, while no signs of inflammation were found Nov. (Fig. 6).
Immunohistochemical Analyses of Heart Tissues
When the markings made with BCL-2 primary antibody were examined, there was a significant difference among the groups in H-SCORE averages (P = 0.0005). Isoproterenol administration significantly decreased the expression of BCL-2 (P < 0.005). After treatment with gossypin at doses of 5, 10, and 20 mg/kg, expression levels of BCL-2 were increased compared to the isoproterenol group (respectively; P < 0.0001, P < 0.05, P < 0.05). When the control group and ISO + Gos10 group were compared in terms of BCL-2 expressions, no statistically significant difference was found between them (P = 0.5). When the control group and ISO + Gos5 and ISO + Gos20 groups were compared, a statistically significant decrease was observed (respectively; P < 0.05, P < 0.05) (Figs. 5B and 6).
H-score rates (Mean±Std) of immunolabeling with IL-1β (A) BCL-2 (B) primary antibody in heart tissue. *P < 0.05; comparison between control and ISO-ISO+Gos5-ISO+Gos20 groups. #P < 0.05; comparison between ISO and ISO+Gos5- ISO+Gos10-ISO+Gos20 groups. And the number of positive cells (Mean ± Std) of immunolabeling with Caspase 3 (C) primary antibody in heart tissue. *P < 0.05; comparison between control and ISO-ISO+Gos5-ISO+Gos10-ISO+Gos20 groups. #P < 0.05; comparison between ISO and ISO+Gos5-ISO+Gos10-ISO+Gos20 groups
Hematoxylene and eosin staining histopathology findings (A: Control, B: MI, C: MI+ GOS5, D: MI+GOS10, E: MI+GOS20 - arrows: PMNL cells, Triangle: degenerate novelized muscle fibers). Representative micrograps of immunohistochemical analysis of IL1β, BCL-2, and Caspase3 in sections of heart tissue. In IL1β and BCL2 micrograps, brown staining indicates positive immune-reactivity. In Caspase 3 micrograph, → indicates positive staining cells
When the H-SCORE averages of the micrographs of the IL1β primary antibody were compared, a statistically significant difference was found among the groups (P < 0.0001). Isoproterenol administration significantly increased the expression of IL1β (P = 0.0006). It was observed that IL1β expression levels decreased after the administration of gossypin to the ISO groups. When the IL1β expression levels of the ISO group and gossypin groups were compared, although a decrease was observed among the gossypin groups, a statistically significant difference was found only in the ISO + Gos20 group (ISO via ISO + Gos5, P = 0.3405; ISO via ISO + Gos10, P = 0.0565; ISO via ISO + Gos20, P = 0.0096) (Figs. 5A and 6).
A statistically significant difference was found among the groups in terms of the number of positive cells in the labeling with Caspase-3 primary antibody, which is an indicator of apoptosis (P < 0.0001). There was a significant increase in the number of positive cells in the ISO group compared to the control group (P = 0.0001). After treatment with gossypin at doses of 5, 10, and 20 mg/kg, the number of positive cells decreased compared to the ISO group (respectively; P = 0.0235, P = 0.0048, P = 0.0022) (Figs. 5C and 6).
Discussion
The current study is the first to examine the cardioprotective effects of gossypin on ISO-induced experimental MI in H9c2 cell line and Balb/c mice.
Hypoxia, ischemia, and β-adrenergic receptor stimulation are among the methods that cause cardiac damage. Of these, the application of ISO is a well-known standard model for evaluating cardioprotective drugs [21, 22]. Because the pathophysiological changes in the supramaximal dose ISO administration model in mice are quite similar to the changes observed in MI in humans [23, 24], ISO application stimulates severe oxidative stress in the myocardium, which has been reported as the main mechanism of myocardial necrosis [6]. Therefore, in the treatment of these diseases, clearing excess intracellular ROS and reducing apoptosis, especially due to oxidative stress, can serve important functions. In our study, the cardioprotective potential of gossypin against ISO-induced damage was demonstrated in H9c2 cells and BALB/c mice. This study showed that ISO led to decreased cell viability and increased LDH activities in H9c2 cells, two reliable markers for cardiac myocyte death [25]. Treatment with Gossypin greatly reversed the loss of cell viability and reduced LDH activities in H9c2 cells in a dose-dependent manner. In addition, gossypin showed anti-apoptotic action in apoptotic genes in H9c2 cells, creating regulation on apoptotic genes.
Clinically, the endocardium enzymes and proteins released by cardiomyocytes, membrane damage, and heart problems can be used to identify—most of them favorites, LDH, CK-MB enzyme activities, and Tn-I. These biochemical markers are very sensitive and specific diagnostic markers in the diagnosis of patients with myocardial damage where ECG is normal [26]. CK-MB, which is highly specific to the heart, can also increase due to ISO-enzymatic skeletal injury. Our results confirmed previous studies that reported that ISO caused significant increases in serum Tn-i levels and CK-MB and LDH enzyme activity, which are indicative of severe damage to myocardial tissue [27, 28]. Administration of Gossypin returned dose-dependent increases in LDH, CK-MB enzyme activities, and Tn-I levels. In this way, our serum marker results supported the results showing that flavonoid-rich nutrients prevent heart disease [29].
Oxidative stress is caused by a pathophysiological process characterized by ROS production and/or antioxidant enzyme dysfunction. Excessive ROS production and lipid peroxidation contribute to tissue damage occurred during ISO-induced cardiotoxicity [30]. The factors leading to lipid peroxidation are ROS and cellular damage resulting from tissue GSH depletion [31, 32]. In the detoxification of excess ROS, non-enzymatic and enzymatic antioxidants take the main role [33]. Therefore, to evaluate the protective role of Gossypin on oxidative stress in ISO-induced cardiotoxicity, we measured GSH levels, MDA content, and SOD enzyme activity in heart tissue as different markers of oxidative stress. High levels of MDA may be due to increased ROS production (superoxide radicals, hydrogen peroxide, and hydroxyl radicals) and low GSH levels and ROS excretion because GSH is a large intracellular redox buffer and also has the ability to detoxify through direct interaction with ROS [34]. In this study, a significant decrease in SOD and GSH activity and a significant increase in MDA levels were observed in heart tissue exposed to oxidative stress in an in vivo mice model, but these changes were regulated in a dose-dependent manner when gossypin pre-treated. The tissue TNF-α level measured by the ELISA method also supported our current findings and dose dependently reduced ISO damage. In previous studies with Gossypin, it has also been shown to regulate these markers, which increase for various conditions [35]. These results suggest that the cardioprotective effects of gossypin may be due to its antioxidative properties.
The relationship between oxidative stress and inflammatory responses is known. Under inflammatory conditions, ROS can trigger activation of NF‐kB and MAPKs signaling pathways in different cell types. ISO coordinates the activation of a large network between the chemokines and cytokines in the heart. We observed that ISO treatment increased TNF-α, IL-1β, and IL-6 based on these previous findings, and treatment with gossypin significantly reduced ISO-induced cytokines, demonstrating the protective effect of gossypin on heart tissue [36, 37]. Our data showed that Gossypin inhibits ISO-induced NF-kB mRNA expression. In our previous study, gossypin exhibited a regulatory effect on inflammatory genes [16].
Apoptosis is a form of programmed cell death with certain signaling cascades that represent a regular process of cell death [38]. And the application of ISO has been shown by studies to activate the apoptotic pathway [30]. Under various stimulation factors, the balance between Bcl-2 and Bax increases the permeability of mitochondrial membranes and finally Cyt-c is released from the mitochondria to the cytoplasm to induce apoptosis. Bax is a member of the Bcl-2 family. It is a critical factor in regulating cell apoptosis and has been widely accepted [39, 40]. The mitochondrial apoptotic pathway is known to be one of the apoptotic pathways regulated by Bcl-2 family proteins. Mitochondrial apoptosis occurs by increasing the pro-apoptotic protein Bax and reducing the anti-apoptotic protein Bcl-2 [41]. The ratio of Bcl-2 to Bax is a very important finding in the analysis of cell apoptosis and survival. But the factor that initiates apoptosis in myocardial cells is CASP-3 [42]. Application of ISO inhibited the ISO‐induced release of cytochrome‐c from mitochondria and as a result ISO induced a significant reduction of the BCL-2/Bax ratio followed by activation of CASP-3 cleavage. Previous studies have shown the anti-apoptotic activity of gossypin [14, 43,44,45]. In our results supporting all these findings, gossypin significantly suppressed the apoptosis of cardiomyocytes by up-regulating Bcl-2, down-regulating Bax, and inhibiting CASP-3 mRNA levels. Beneficial effects of GOSSYPIN against ISO-induced apoptosis, the determination of gossypin both at the mRNA level and immunohistochemically in this study, and when we combine our available data with in vitro results provides quite strong evidence of the cardioprotective effect of gossypin.
Our histopathological findings brought advanced degenerate muscle fibers formed in the ISO group with gossypin administration and Polymorph-nucleated inflammatory cells in the connective tissue between them closer to healthy heart tissues. Immunohistochemical analysis of IL-1β, BCL-2, and Caspase-3 was also performed in our study. These data also supported biochemical and molecular cardiac tissues and serum findings of oral administration of gossypin, confirming its cardioprotective effect.
Conclusion
This study shows that gossypin attenuates ISO-induced damage in H9c2 cardiomyocytes and mice heart tissue by reducing proinflammatory cytokines and NF-kB activation involved in inflammatory responses. In addition, gossypin reduced oxidative stress and increased the activity of antioxidant enzymes in heart tissue. Furthermore, gossypin freed H9c2 cardiomyocytes from ISO-induced apoptosis. All these findings have shown that gossypin exhibits cardioprotective effects through anti-inflammatory, antioxidant, and anti-apoptotic activities. Gossypin may therefore be a promising therapeutic agent for heart diseases. However, more clinical and experimental studies are necessary to develop and disseminate this knowledge.
Data Availability
Data that support the plots within this publication and other findings of this study are available from the corresponding author upon reasonable request.
References
Yang, H., Carasso, S., Woo, A., Jamorski, M., Nikonova, A., Wigle, E. D., & Rakowski, H. (2010). Hypertrophy pattern and regional myocardial mechanics are related in septal and apical hypertrophic cardiomyopathy. Journal of the American Society of Echocardiography, 23, 1081–1089.
Chiong, M., Wang, Z. V., Pedrozo, Z., Cao, D. J., Troncoso, R., Ibacache, M., Criollo, A., Nemchenko, A., Hill, J. A., & Lavandero, S. (2011). Cardiomyocyte death: Mechanisms and translational implications. Cell Death Disease, 2, e244.
Ong, S. B., Hernandez-Resendiz, S., Crespo-Avilan, G. E., Mukhametshina, R. T., Kwek, X. Y., Cabrera-Fuentes, H. A., & Hausenloy, D. J. (2018). Inflammation following acute myocardial infarction: Multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacology & Therapeutics, 186, 73–87.
Sawyer, D. B., Siwik, D. A., Xiao, L., Pimentel, D. R., Singh, K., & Colucci, W. S. (2002). Role of oxidative stress in myocardial hypertrophy and failure. Journal of Molecular and Cellular Cardiology, 34, 379–388.
Neri, M., Fineschi, V., Di Paolo, M., Pomara, C., Riezzo, I., Turillazzi, E., & Cerretani, D. (2015). Cardiac oxidative stress and inflammatory cytokines response after myocardial infarction. Current Vascular Pharmacology, 13, 26–36.
Cinar, I., Halici, Z., Dincer, B., Sirin, B., & Cadirci, E. (2020). The role of 5-HT7 receptors on isoproterenol-induced myocardial infarction in rats with high-fat diet exacerbated coronary endothelial dysfunction. Human and Experimental Toxicology, 1, 96032712.
Krishnamurthy, P., Subramanian, V., Singh, M., & Singh, K. (2007). Beta1 integrins modulate beta-adrenergic receptor-stimulated cardiac myocyte apoptosis and myocardial remodeling. Hypertension, 49, 865–872.
Gabriel, A. S., Martinsson, A., Wretlind, B., & Ahnve, S. (2004). IL-6 levels in acute and post myocardial infarction: Their relation to CRP levels, infarction size, left ventricular systolic function, and heart failure. European Journal of Internal Medicine, 15, 523–528.
Yang, J., Wang, Z., & Chen, D. L. (2017). Shikonin ameliorates isoproterenol (ISO)-induced myocardial damage through suppressing fibrosis, inflammation, apoptosis and ER stress. Biomedicine & Pharmacotherapy, 93, 1343–1357.
Karin, M., & Delhase, M. (2000). The I kappa B kinase (IKK) and NF-kappa B: Key elements of proinflammatory signalling. Seminars in Immunology, 12, 85–98.
Xu, F., Sun, S., Wang, X., Ni, E., Zhao, L., & Zhu, W. (2017). GRK2 mediates arginine vasopressin-induced interleukin-6 production via nuclear factor-kappaB signaling neonatal rat cardiac fibroblast. Molecular Pharmacology, 92, 278–284.
Du, G., Sun, L., Zhao, R., Du, L., Song, J., Zhang, L., He, G., Zhang, Y., & Zhang, J. (2016). Polyphenols: Potential source of drugs for the treatment of ischaemic heart disease. Pharmacology & Therapeutics, 162, 23–34.
Sedighi, M., Sewell, R. D. E., Nazari, A., Abbaszadeh, S., Cheraghi, M., Amini, A., Heydari, Z., & Rafieian-Kopaei, M. (2019). A review on the most important medicinal plants effective in cardiac ischemia-reperfusion injury. Current Pharmaceutical Design, 25, 352–358.
Cinar, I. (2020). Apoptosis-inducing activity and antiproliferative effect of gossypin on PC-3 prostate cancer cells. Anti-Cancer Agents in Medicinal Chemistry, 21(4), 445–450.
Viswanathan, S., Thirugnanasambantham, P., Ramaswamy, S., & Bapna, J. S. (1993). A study on the role of cholinergic and gamma amino butyric acid systems in the anti-nociceptive effect of gossypin. Clinical and Experimental Pharmacology and Physiology, 20, 193–196.
Cinar, I., Sirin, B., Aydin, P., Toktay, E., Cadirci, E., Halici, I., & Halici, Z. (2019). Ameliorative effect of gossypin against acute lung injury in experimental sepsis model of rats. Life Sciences, 221, 327–334.
Gautam, P., & Flora, S. J. (2010). Oral supplementation of gossypin during lead exposure protects alteration in heme synthesis pathway and brain oxidative stress in rats. Nutrition, 26, 563–570.
Ugan, R. A., Cadirci, E., Halici, Z., Toktay, E., & Cinar, I. (2018). The role of urotensin-II and its receptors in sepsis-induced lung injury under diabetic conditions. European Journal of Pharmacology, 818, 457–469.
Cadirci, E., Ugan, R. A., Dincer, B., Gundogdu, B., Cinar, I., Akpinar, E., & Halici, Z. (2019). Urotensin receptors as a new target for CLP induced septic lung injury in mice. Naunyn-Schmiedeberg’s Archives of Pharmacology, 392, 135–145.
Lobo Filho, H. G., Ferreira, N. L., Sousa, R. B., Carvalho, E. R., Lobo, P. L., & Lobo Filho, J. G. (2011). Experimental model of myocardial infarction induced by isoproterenol in rats. Revista Brasileira de Cirurgia Cardiovascular, 26, 469–476.
Granata, R., Trovato, L., Gallo, M. P., Destefanis, S., Settanni, F., Scarlatti, F., Brero, A., Ramella, R., Volante, M., Isgaard, J., Levi, R., Papotti, M., Alloatti, G., & Ghigo, E. (2009). Growth hormone-releasing hormone promotes survival of cardiac myocytes in vitro and protects against ischaemia-reperfusion injury in rat heart. Cardiovascular Research, 83, 303–312.
Zaugg, M., Xu, W., Lucchinetti, E., Shafiq, S. A., Jamali, N. Z., & Siddiqui, M. A. (2000). Beta-adrenergic receptor subtypes differentially affect apoptosis in adult rat ventricular myocytes. Circulation, 102, 344–350.
Ning, B. B., Zhang, Y., Wu, D. D., Cui, J. G., Liu, L., Wang, P. W., Wang, W. J., Zhu, W. L., Chen, Y., & Zhang, T. (2017). Luteolin-7-diglucuronide attenuates isoproterenol-induced myocardial injury and fibrosis in mice. Acta Pharmacologica Sinica, 38, 331–341.
Sahu, B. D., Putcha, U. K., Kuncha, M., Rachamalla, S. S., & Sistla, R. (2014). Carnosic acid promotes myocardial antioxidant response and prevents isoproterenol-induced myocardial oxidative stress and apoptosis in mice. Molecular and Cellular Biochemistry, 394, 163–176.
Zhang, H. J., Chen, R. C., Sun, G. B., Yang, L. P., Zhu, Y. D., Xu, X. D., & Sun, X. B. (2018). Protective effects of total flavonoids from Clinopodium chinense (Benth.) O. Ktze on myocardial injury in vivo and in vitro via regulation of Akt/Nrf2/HO-1 pathway. Phytomedicine, 40, 88–97.
Meeran, M. F., & Prince, P. S. (2012). Protective effects of thymol on altered plasma lipid peroxidation and nonenzymic antioxidants in isoproterenol-induced myocardial infarcted rats. Journal of Biochemical and Molecular Toxicology, 26, 368–373.
Kannan, M. M., & Quine, S. D. (2013). Ellagic acid inhibits cardiac arrhythmias, hypertrophy and hyperlipidaemia during myocardial infarction in rats. Metabolism Clinical and Experimental, 62, 52–61.
Evran, B., Karpuzoglu, H., Develi, S., Kalaz, E. B., Soluk-Tekkesin, M., Olgac, V., Dogru-Abbasoglu, S., & Uysal, M. (2014). Effects of carnosine on prooxidant-antioxidant status in heart tissue, plasma and erythrocytes of rats with isoproterenol-induced myocardial infarction. Pharmacological Reports, 66, 81–86.
Fernandez, S. P., Nguyen, M., Yow, T. T., Chu, C., Johnston, G. A., Hanrahan, J. R., & Chebib, M. (2009). The flavonoid glycosides, myricitrin, gossypin and naringin exert anxiolytic action in mice. Neurochemical Research, 34, 1867–1875.
Thangaiyan, R., Robert, B. M., Arjunan, S., Govindasamy, K., & Nagarajan, R. P. (2018). Preventive effect of apigenin against isoproterenol-induced apoptosis in cardiomyoblasts. Journal of Biochemical and Molecular Toxicology, 32, e22213.
Konukoglu, D., Serin, O., Demiriz Kemerli, G., Serin, E., Hayirhoglu, A., & Oner, B. (1998). A study on the carotid artery intima-media thickness and its association with lipid peroxidation. Clinica Chimica Acta, 277, 91–98.
Hanukoglu, I. (2006). Antioxidant protective mechanisms against reactive oxygen species (ROS) generated by mitochondrial P450 systems in steroidogenic cells. Drug Metabolism Reviews, 38, 171–196.
Birben, E., Sahiner, U. M., Sackesen, C., Erzurum, S., & Kalayci, O. (2012). Oxidative stress and antioxidant defense. World Allergy Organization Journal, 5, 9–19.
Yilmaz, S., Atessahin, A., Sahna, E., Karahan, I., & Ozer, S. (2006). Protective effect of lycopene on adriamycin-induced cardiotoxicity and nephrotoxicity. Toxicology, 218, 164–171.
Gautam, A., & Vijayaraghavan, R. (2007). Prophylactic effect of gossypin against percutaneously administered sulfur mustard. Biomedical and Environmental Sciences, 20, 250–259.
Zhao, L., Wu, D., Sang, M., Xu, Y., Liu, Z., & Wu, Q. (2017). Stachydrine ameliorates isoproterenol-induced cardiac hypertrophy and fibrosis by suppressing inflammation and oxidative stress through inhibiting NF-kappaB and JAK/STAT signaling pathways in rats. International Immunopharmacology, 48, 102–109.
Baldissera, M. D., Souza, C. F., Grando, T. H., Stefani, L. M., & Monteiro, S. G. (2017). beta-caryophyllene reduces atherogenic index and coronary risk index in hypercholesterolemic rats: The involvement of cardiac oxidative damage. Chemico-Biological Interactions, 270, 9–14.
Hu, K., Gong, X., Ai, Q., Lin, L., Dai, J., Cai, L., Jiang, R., Ge, P., & Zhang, L. (2017). Endogenous AMPK acts as a detrimental factor in fulminant hepatitis via potentiating JNK-dependent hepatocyte apoptosis. Cell Death Disease, 8, e2637.
Campbell, K. J., & Tait, S. W. G. (2018). Targeting BCL-2 regulated apoptosis in cancer. Open Biology, 8, 15002.
Maes, M. E., Schlamp, C. L., & Nickells, R. W. (2017). BAX to basics: How the BCL2 gene family controls the death of retinal ganglion cells. Progress in Retinal and Eye Research, 57, 1–25.
Cui, C., Cui, N., Wang, P., Song, S., Liang, H., & Ji, A. (2015). Sulfated polysaccharide isolated from the sea cucumber Stichopus japonicus against PC12 hypoxia/reoxygenation injury by inhibition of the MAPK signaling pathway. Cellular and Molecular Neurobiology, 35, 1081–1092.
Yan, S. H., Zhao, N. W., Geng, Z. R., Shen, J. Y., Liu, F. M., Yan, D., Zhou, J., Nie, C., Huang, C. C., & Fang, Z. Y. (2018). Modulations of Keap1-Nrf2 signaling axis by TIIA ameliorated the oxidative stress-induced myocardial apoptosis. Free Radical Biology & Medicine, 115, 191–201.
Wang, L., Wang, X., Chen, H., Zu, X., Ma, F., Liu, K., Bode, A. M., Dong, Z., & Kim, D. J. (2019). Gossypin inhibits gastric cancer growth by direct targeting of AURKA and RSK2. Phytotherapy Research, 33, 640–650.
Bhaskaran, S., Dileep, K. V., Deepa, S. S., Sadasivan, C., Klausner, M., Krishnegowda, N. K., Tekmal, R. R., VandeBerg, J. L., & Nair, H. B. (2013). Gossypin as a novel selective dual inhibitor of v-raf murine sarcoma viral oncogene homolog B1 and cyclin-dependent kinase 4 for melanoma. Molecular Cancer Therapeutics, 12, 361–372.
Kunnumakkara, A. B., Nair, A. S., & Ahn, K. S. (2013). Gossypin, a pentahydroxy glucosyl flavone, inhibits the transforming growth factor beta-activated kinase-1-mediated NF-kappa B activation pathway, leading to potentiation of apoptosis, suppression of invasion, and abrogation of osteoclastogenesis (vol 109, pg 5112, 2007). Blood, 122, 1327–1328.
Author information
Authors and Affiliations
Contributions
IC and MY conceptualized and designed the research. TT, ETPB, RAU, and HH performed most of the experiments and analyzed the data with the help from IC and MY. IC and MY wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Handling Editor: Jianyong Ma.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cinar, I., Yayla, M., Tavaci, T. et al. In Vivo and In Vitro Cardioprotective Effect of Gossypin Against Isoproterenol-Induced Myocardial Infarction Injury. Cardiovasc Toxicol 22, 52–62 (2022). https://doi.org/10.1007/s12012-021-09698-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-021-09698-3