Abstract
Myocardial infarction (MI) is an extremely severe cardiovascular disease, which ranks as the leading cause of sudden death worldwide. Studies have proved that cardiac injury following MI can cause cardiomyocyte apoptosis and myocardial fibrosis. Bilobalide (Bilo) from Ginkgo biloba leaves have been widely reported to possess excellent cardioprotective effects. However, concrete roles of Bilo in MI have not been investigated yet. We here designed both in vitro and in vivo experiments to explore the effects of Bilo on MI-induced cardiac injury and the underlying mechanisms of its action. We conducted in vitro experiments using oxygen–glucose deprivation (OGD)-treated H9c2 cells. Cell apoptosis in H9c2 cells was assessed by conducting flow cytometry assay and evaluating apoptosis-related proteins with western blotting. MI mouse model was established by performing left anterior descending artery (LAD) ligation. Cardiac function of MI mice was determined by assessing ejection fraction (EF), fractional shortening (FS), left ventricular end-systolic diameter (LVESD), and left ventricular end-diastolic diameter (LVEDD). Histological changes were analyzed, infarct size and myocardial fibrosis were measured by hematoxylin and eosin (H&E) and Masson staining in cardiac tissues from the mice. The apoptosis of cardiomyocytes in MI mice was assessed by TUNEL staining. Western blotting was applied to detect the effect of Bilo on c-Jun N-terminal kinase (JNK)/p38 mitogen-activated protein kinases (p38 MAPK) signaling both in vitro and in vivo. Bilo inhibited OGD-induced cell apoptosis and lactate dehydrogenase (LDH) release in H9c2 cells. The protein levels of p-JNK and p-p38 were significantly downregulated by Bilo treatment. SB20358 (inhibitor of p38) and SP600125 (inhibitor of JNK) suppressed OGD-induced cell apoptosis as Bilo did. In MI mouse model, Bilo improved the cardiac function and significantly reduced the infarct size and myocardial fibrosis. Bilo inhibited MI-induced cardiomyocytes apoptosis in mice. Bilo suppressed the protein levels of p-JNK and p-p38 in cardiac tissues from MI mice. Bilo alleviated OGD-induced cell apoptosis in H9c2 cells and suppressed MI-induced cardiomyocyte apoptosis and myocardial fibrosis in mice via the inactivation of JNK/p38 MAPK signaling pathways. Thus, Bilo may be an effective anti-MI agent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemic heart disease has become a leading cause of morbidity and mortality worldwide [1]. Myocardial infarction (MI) is the most common manifestation of ischemic heart disease [2], and the progression of MI begins with myocardial injury induced by sudden interruption of the blood supply to the heart, which can subsequently lead to cardiac dysfunction and massive myocardial cell death [3]. Increasing patients with MI have received revascularization treatment in time. However, the myocardial ischemia–reperfusion injury after reperfusion treatment increased the risk of arrhythmia, heart failure and death [4]. Apoptosis is a typical form of myocyte loss [5]. Numerous studies have confirmed that MI leads to accumulated apoptosis of the myocardial cells in response to prolonged ischemia, eventually contributing to the MI development [6,7,8]. More importantly, long term myocardial apoptosis can result in the deposition of fibrillar collagens in infarcted and non-infarcted areas and induce myocardial fibrosis [9, 10]. Inhibition of cardiomyocytes apoptosis and myocardial fibrosis has been shown to prominently alleviate ventricular remodeling and heart failure after MI [11, 12]. Therefore, finding more effective target to improve MI-induced apoptosis and fibrosis and further understanding the underlying mechanisms of it is of great importance.
Numerous natural compounds have been recognized as therapeutic candidates for MI treatment [13, 14]. Notably, AM Rabie et al. have revealed some hydroxylated oxygenous heterocyclic class of compounds such as cordycepin and oxadiazole antioxidants that have potential to improve human cardiac functions and treat cardiac diseases like MI [15,16,17]. Thus, other similar compounds with cardioprotective effects are well worth exploring. Ginkgo biloba L. is one of the oldest living tree species, which has long been used as a Traditional Chinese medicine (TCM) in China to treat lung ailments and cardiovascular diseases. Ginkgo biloba L. includes two types of major constituents: ginkgolides (A, B, C, and J) and Bilobalide (Bilo; Fig. 1) [15]. Bilo is a sesquiterpene trilactone constituent with various beneficial effects, including neuroprotective effect on ischemic strokes [16], anti‐inflammatory effect on Cuprizone-induced demyelination [17], anti-hyperalgesic effect [18], and the anti-apoptotic activity in gastric carcinoma [19], Type 1 diabetes mellitus [20], and Ischemic stroke [21]. In addition, the cardioprotective effects of Bilo also has been widely discussed in previous studies [22,23,24]. Particularly, Bilo inhibits MI development by rescuing impaired tricarboxylic acid cycle flux in isoproterenol (ISO)-induced ischemia-like cardiomyocytes. [25]. Besides, Cao et al. revealed the mitigating effects of Bilo on myocardial ischemia. Specifically, it was shown to improve oxygen–glucose deprivation (OGD)-induced cell injury by enhancing miR-27a expression and activating PI3K/AKT and Wnt/β-catenin pathways [26]. We thus wondered whether Bilo alleviated OGD-induced cell injury through different mechanisms or exerted similar cardioprotective effects in vivo.
The c-Jun N-terminal kinases (JNKs) and the p38 enzymes (p38 MAPKs) are members of the mitogen-activated protein kinase (MAPK) family, which have been reported to regulate various cellular functions including cardiomyocyte apoptosis [27]. Previous study has revealed that JNK can activate the apoptotic pathway by enhancing the expression of apoptotic gene [28]. In addition, activation of p38 MAPK triggers the cardiomyocytes apoptosis, while the inactivation of it prevents cardiomyocyte apoptosis [29]. Therefore, understanding the roles of the JNK/p38 MAPK signaling pathway in MI-induced myocardial injury has become significant.
In this study, we further explored the effects of Bilo on OGD-induced cell apoptosis in H9c2 myocytes. We established MI mouse model by performing left anterior descending artery (LAD) ligation to determine the cardioprotective effect of Bilo in vivo. Furthermore, the involvement of JNK/p38 MAPK signaling was detected to confirm the underlying mechanisms of Bilo both in vitro and in vivo.
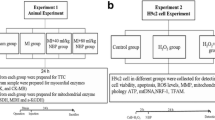
Materials and Methods
Cell culture and Treatment
H9c2 myocytes were obtained from the American Type Culture Collection (ATCC, Manassas, VA). After being washed with phosphate-buffered saline (PBS) for three times, all the cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) encompassing fetal bovine serum (FBS; 10%) and penicillin–streptomycin (100 U/ml). The culture conditions contained 95% humidified air and 5% CO2 at 37 °C.
The oxygen–glucose deprivation (OGD) cell model was established as previously described [30]. Briefly, H9c2 cells were placed in an anoxia chamber (1% O2, 5% CO2, and 94% N2) at 37 °C for 2 h and then glucose (33 mM) was added and incubated with the cells for 6 h. Control cells were cultured in the normal medium (5% CO2 and 95% air) with equal volume of vehicle control. The cell experiments were conducted in four groups: Control + PBS; OGD + PBS; OGD + Bilo 20 ng/ml; and OGD + Bilo 50 ng/ml. PBS was used as the vehicle control. Cells in OGD + Bilo 20 ng/ml; and OGD + Bilo 50 ng/ml were pretreated with 20 or 50 ng/ml of Bilo for 2 h before the OGD induction.
For inhibiting the JNK/ p38 MAPK signaling, H9c2 cells were pretreated with SB20358 (inhibitor of p38, 25 nM) or SP600125 (inhibitor of JNK, 25 nM) followed by OGD induction, and the flow cytometry was conducted in four groups: OGD + PBS; OGD + SB20358; OGD + SP600125; and OGD + Bilo 50 ng/ml. Cells in OGD + Bilo 50 ng/ml were pretreated with 50 ng/ml of Bilo for 2 h before the OGD induction.
Flow Cytometry
After the indicated treatment of OGD and Bilo, H9c2 cells (1 × 106 cells/mL) were centrifuged at 300×g for 5 min. After washing with PBS, the cells were resuspended with binding buffer (200 μL), added with Annexin V-FITC (5 μL) and PI staining solution (10 μL), and incubated in the darkness for 30 min. Annexin V-FITC-positive cells and PI-negative cells were identified as apoptotic cells. Finally, after washing, the cells were analyzed by BD Facscalibur flow cytometry, and the data was analyzed by FlowJo software.
Cell Viability Assay
The cell Counting Kit-8 assay (CCK-8) assay was used to determine the effect of OGD stimulation and Bilo pretreatment on H9c2 cell viability. Briefly, H9c2 cells (1 × 104 cells/well) were placed in 96-well plates. After the indicated treatment of OGD and Bilo, H9c2 cells were added with CCK-8 reagent (10 μL, Beyotime, Shanghai, China) and incubated at 37 °C for 2 h. A microplate reader was used to detect the absorbance at 450 nm.
Lactate Dehydrogenase (LDH) Secretion
To determine the effect of OGD stimulation and Bilo pretreatment on H9c2 cell damage, cytotoxicity was measured by using the lactate dehydrogenase (LDH) Assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions. H9c2 cells (1 × 104 cells/well) were seeded in 96-well culture plates. After the indicated treatment of OGD and Bilo, cells were lysed and centrifuged at 600×g for 10 min to collect the cell supernatant. Then the LDH reaction agent (100 μL) was incubated with the cell supernatant for 30 min at room temperature. Finally, absorbance was measured at 490 nm using a microplate reader.
Animal Model
C57BL/6 mice (male, 10–12 weeks old, 20–22 g, Beijing Vital River Laboratory Animal Technology, Beijing, China) were housed in standard conditions (24 ± 2 °C, 50–60% of humidity, and 12 h light/darkness) with ad libitum access to water and food. After the acclimatization, mice were randomly divided into three groups: Sham (n = 10); MI (n = 10); MI + Bilo (n = 10). MI mice model was established using LAD ligation. Specifically, All the mice were anesthetized using pentobarbital sodium (60 mg/kg) and then ventilated using a mouse ventilator. The chests of the mice were opened, and LAD was visualized and then ligated using a 7–0 nylon suture, then the electrocardiogram (ECG) confirmed the ischemia. The same procedure without ligation was conducted in the mice in Sham group. One week after the surgery, the survival rate of the mice was 65.0% (n = 13). They were randomly treated with Bilo (10 mg/kg) or saline subcutaneously once a day for 4 weeks. The dose of Bilo was determined by previous study [31]. The sham group was given equal saline. All procedures were approved by the Animal Protection and Utilization Committee of the Institute at Heart Center of Henan Provincial People’s Hospital.
Echocardiography
Cardiac function of the mice were evaluated under anesthesia at 24 h post-LAD ligation using an in vivo ultrasound system for small animal models (Visual Sonics, Toronto, Canada). left ventricular (LV) ejection fraction (EF), LV fractional shortening (FS), LV end-systolic diameter (LVESD), LV end-diastolic dimension (LVEDD) were calculated. A pressure transducer (Taimeng, Chengdu, China) was then used for measuring LV end-systolic pressure (LVESP), and LV end-diastolic pressure (LVEDP).
Hematoxylin and Eosin (H&E) and Masson Staining
At the end of the in vivo treatments, all the mice were euthanized under anesthesia, and the hearts were isolated immediately, fixed overnight in formaldehyde (10%), and embedded in paraffin. After cutting the hearts into Sects. (5 μm), hematoxylin and eosin were used to stain the heart sections and evaluate the histological changes. To directly observe the collagen fibers in the heart, Masson trichrome staining was conducted using a corresponding detection kit (Nanjing Jiancheng Bioengineering Institute). Sections in each group were photographed using an imaging microscope (Nikon, Japan). The relative LV fibrosis area was measured, and the infarct size was determined.
Terminal Deoxynucleotidyl Transferase dUTP Nick End Labelling (TUNEL) Staining
The One-Step TUNEL Apoptosis Assay Kit (Beyotime) was used to perform the TUNEL assay. Specifically, hearts were obtained from the mice after euthanasia, and the paraffin Sects. (7–10 μm) of the cardiac tissue were stained with the TUNEL staining kit. The TUNEL-positive cells were imaged using a fluorescent microscopy (Nikon).
Western Blotting
Cardiac tissues and H9c2 cells were lysed using RIPA lysis buffer (Beyotime). Total protein was quantified with the assistance of the bicinchoninic acid protein assay kit. After the quantification, the separation of the equal amount (20 μg) of protein was conducted on sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) gels (10%). Then, the proteins were transferred onto the polyvinylidene difluoride (PVDF) membranes, which were blocked with non-fat milk (5%). Primary antibodies (Abcam, Beijing, China) against Bcl-2 (ab182858), Bax (ab32503), p-JNK (ab124956), JNK (ab110724), p-p38 (ab195049), p38 (ab170099), and GAPDH (ab8245) were incubated with the membranes overnight at 4 °C. After being washed with PBS, the membranes were continually incubated for 1 h with the corresponding secondary antibodies at room temperature. To visualize the protein bands, membranes were immersed in enhanced chemiluminescent (ECL) reagent. The intensity of the protein bands was analyzed by ImageJ.
Statistical Analysis
All experiments were conducted at least three times. Data were shown as means ± standard deviation (SD) and were analyzed with GraphPad Prism 6.0. To compare the differences between two groups, unpaired Student's t test was used. One-way ANOVA followed by a Tukey’s post hoc test was used for statistical analysis. The differences were considered as significant when p < 0.05.
Results
Bilo Inhibits OGD-Induced Cell Apoptosis in H9c2 Cardiomyocytes
OGD is a stimulation model that induces ischemia/hypoxia at the cellular level. Flow cytometry was conducted to analyze the effect of Bilo on cell apoptosis in OGD-treated H9c2 cardiomyocytes. The results showed that the apoptosis rate was increased after the induction of OGD and then significantly reduced by Bilo at different concentrations (20 or 50 ng/ml). Particularly, the higher concentration of Bilo exerted the more significant effect on decreasing the apoptosis rate (Fig. 2A). Western blotting further revealed that the level of anti-apoptotic protein Bcl-2 was downregulated by OGD stimulation, but partially upregulated by Bilo treatments, and 50 ng/ml of Bilo had the most significant effect. Inversely, OGD elevated the protein level of pro-apoptotic Bax, which was then obviously reduced by Bilo treatment. (Fig. 2B). Consistently, the viability of H9c2 cardiomyocytes was impaired by OGD, compared with that in control group. While Bilo treatment promoted the viability of H9c2 cardiomyocytes significantly and concentration-dependently (Fig. 2C). The LDH release can reflect the degree of cell damage. As expected, OGD group showed a higher level of LDH release, which was then diminished by Bilo treatment (Fig. 2D).
Bilo inhibits OGD-induced cell apoptosis in H9c2 cardiomyocytes. A Flow cytometry revealed the cell apoptosis in H9c2 cardiomyocytes. B Western blotting showed the levels of Bcl-2 and Bax proteins. C CCK-8 revealed H9c2 cell viability. D LDH release was evaluated by corresponding kit in Control + PBS; OGD + PBS; OGD + Bilo 20 ng/ml; and OGD + Bilo 50 ng/ml groups. ** p < 0.01, *** p < 0.001
Bilo Suppresses OGD-Induced Activation of JNK/p38 MAPK Signaling in H9c2 Cardiomyocytes
In this part, western blotting was conducted to identify the effect of Bilo on JNK/p38 MAPK signaling in H9c2 cardiomyocytes. In OGD group, an increased phosphorylation of JNK and p38 was observed. However, the activation of JNK and p38 was subsequently inhibited by Bilo treatment, suggesting that Bilo inhibited cell apoptosis via inactivating JNK/p38 MAPK signaling in H9c2 (Fig. 3A). To confirm this result, we treated cells with SB20358 (inhibitor of p38) and SP600125 (inhibitor of JNK) to further analyze the apoptotic rate in H9c2 cells using flow cytometry. As expected, SB20358 and SP600125 significantly weakened the apoptotic ability of H9c2 cells, which was consistent with the effect of 50 ng/ml of Bilo (Fig. 3B).
Bilo suppresses OGD-induced activation of JNK/p38 MAPK signaling in H9c2 cardiomyocytes. A Western blotting showed the levels of signaling-related proteins in Control + PBS; OGD + PBS; OGD + Bilo 20 ng/ml; and OGD + Bilo 50 ng/ml groups. B Flow cytometry assessed cell apoptosis in OGD + PBS; OGD + SB20358; OGD + SP600125; and OGD + Bilo 50 ng/ml groups. **p < 0.01; ***p < 0.001
Bilo Improves the Cardiac Function in MI Mouse Model
To induce MI, mice underwent the LAD ligation. After the surgery, cardiac function was evaluated using echocardiography. Compared with the sham-operated group, the EF and FS were significantly decreased in MI mice, and LVESD and LVEDD were obviously increased, suggesting that MI induction substantially damaged cardiac function. After the administration of Bilo (10 mg/kg) to the MI mice, the levels of EF, FS, LVESD and LVEDD were all almost reversed to the levels in sham-operated groups (Fig. 4A). Then we found MI induction decreased LVESP and increased LVEDP, while Bilo reversed the changes (Fig. 4B).
Bilo Reduces Infarct Size and Ameliorates Cardiac Fibrosis in MI Mouse Model
After the surgery and the indicated treatment of Bilo, mice were euthanized under anesthesia and hearts were quickly removed for the histological analysis. The results of H&E staining showed the formation of myocardial fibers and infiltration of inflammatory cells in MI group, which were all significantly reduced by Bilo treatment. The results of Masson's trichrome staining showed increased collagen fibers that stained blue in MI group, which were subsequently improved by Bilo (Fig. 5A). Cardiac infarct size and fibrosis were quantified and the levels of them were drastically pulled down by Bilo treatment, compared with those in MI group (Fig. 5B, C).
Bilo reduces infarct size and ameliorates cardiac fibrosis in MI mouse model. A H&E staining and Masson's trichrome staining were conducted to observe the morphological changes. B Cardiac infarct size and C fibrosis were quantified in Sham, Sham + Bilo, MI, and MI + Bilo groups. **p < 0.01; ***p < 0.001
Bilo Attenuates Myocardial Cell Apoptosis in MI Mouse Model
TUNEL assay in cardiac tissues further revealed that the TUNEL-positive cells were accumulated after the MI induction. However, Bilo treatment obviously reduced the TUNEL-positive cells compared to the MI group (Fig. 6A). Western blotting in cardiac tissues showed the protein level of Bcl-2 was decreased and the protein level of Bax was increased in MI mouse model. After the treatment of Bilo, the levels of apoptotic-related proteins were all partially reversed, suggesting the inhibitory effect of Bilo on myocardial cell apoptosis in MI mouse model (Fig. 6B).
Bilo Inactivates JNK/p38 MAPK Signaling in MI Mouse Model
The phosphorylation levels of JNK and p38 proteins were again examined in cardiac tissues. Western blotting presented higher levels of p-JNK and p-p38 in cardiac tissues from MI mouse model, compared with sham-operated group. Bilo treatment significantly lowered the levels of p-JNK and p-p38, compared with MI group, suggesting that Bilo exerted inhibitory role in apoptosis via inactivating JNK/p38 MAPK signaling in vivo (Fig. 7A).
Discussion
The anti-MI role of many TCMs has been widely reported. For example, mangiferin inhibit cardiomyocyte apoptosis by activating the silent information regulator 1 (Sirt1)/ forkhead box class O 3a (FoxO3a) signaling in MI [32]. Nootkatone abundantly found in grapefruit attenuates isoproterenol induced MI in rats [33]. Resveratrol also has been found to protect cardiomyocyte from ischemia–reperfusion injury [34]. In this study, we found Bilo from Ginkgo biloba leaves can reduce OGD induced cardiomyocytes apoptosis in H9c2 cells. Particularly, we also confirmed the protective effect of Bilo on MI-induced cardiac dysfunction in mice model and revealed the participation of JNK/p38 MAPK signaling pathways (Fig. 8).
Schematic of the cell and animal experiments. A Bilo suppressed OGD-induced H9c2 cell damage and apoptosis via inactivating JNK/p38 MAPK signaling pathways. B Bilo improved MI-induced cardiac dysfunction in mouse model by inhibiting myocardial cell apoptosis and myocardial fibrosis via the inactivation of JNK/p38 MAPK signaling pathways
As we know, cardiomyocytes apoptosis takes a critical part in the progression of MI [5]. The process of apoptosis is mainly modulated by pro-apoptotic proteins (BAX etc.) and anti-apoptotic proteins (Bcl-2 etc.) [35]. Previous studies have reported the inhibitory effect of Bilo on the apoptosis of various cell types such as hypoxia-treated neonatal rat cardiomyocytes and OGD-stimulated H9c2 cells [36, 37]. Similarly, an OGD model was performed to mimic the ischemic condition in H9c2 cardiomyocytes, The results of flow cytometry showed that the apoptosis rate was increased after the induction of OGD and then significantly reduced by Bilo. In particular, the level of anti-apoptotic protein Bcl-2 was downregulated by OGD stimulation, but partially upregulated by Bilo treatment. Inversely, OGD elevated the protein level of pro-apoptotic Bax, which was then obviously reduced by Bilo. We then found the viability of H9c2 cardiomyocytes was inhibited by OGD, compared with that in control group. While Bilo treatment promoted the suppressed viability of H9c2 cardiomyocytes induced by OGD treatment. In addition, OGD group showed a higher level of LDH release, which was significantly pulled down by Bilo treatment. All these results indicated the inhibitory effect of Bilo on OGD-induced cell damage and cell death in H9c2 cells.
Increasing studies highlight the important participation of the JNK/p38 MAPK signaling pathway in regulating cardiomyocytes apoptosis. For example, midazolam inhibits ischemia/reperfusion-induced cardiomyocyte apoptosis via the inactivation of the JNK/p38 MAPK signaling [38]. Asiatic acid exerts cardioprotective effects by limiting phosphorylation of p38-MAPK and JNK-MAPK [39]. Particularly, the regulatory mechanism of Bilo in JNK/ MAPK signaling pathways has been reported in cerebral ischemia and reperfusion injury and skeletal muscle ischemia/reperfusion injury [40, 41]. We thus hypothesized that the inhibitory effect of Bilo on OGD-induced cell apoptosis may exert through regulating JNK and MAPK signaling. By validating through western blotting, we for the first time confirmed that Bilo inhibited OGD-induced cell apoptosis via inactivating JNK/p38 MAPK signaling in H9c2 cells. These results were further confirmed by SB20358 (inhibitor of p38) and SP600125 (inhibitor of JNK), which also sharply decreased the apoptotic rate, as shown by flow cytometry.
During MI, the fibrotic region can be enlarged by myocardial fibroblast infiltration, leading to LV dilation and dysfunction [42, 43]. Consistent with previous studies, we found that the EF, FS, LVESP were decreased, and LVESD, LVEDD, LVEDP were obviously increased after the MI induction in mice. This result suggested the severity of cardiac damage, which was further evidenced by increased infarct size and infarct fibrosis in cardiac tissues of the MI mice. Interestingly, the Bilo-treated animal group presented significantly improved the cardiac dysfunction and markedly reduced infarct size and cardiac fibrosis compared with the model group. In addition, the results of TUNEL assays indicated that Bilo decreased the TUNEL-positive cells compared with mice in the MI group. Western blotting in cardiac tissues showed the protein level of Bcl-2 was increased and the protein level of Bax was decreased in MI mouse model after the treatment of Bilo, suggesting the inhibitory effect of Bilo on myocardial cell apoptosis in MI mouse model. More importantly, the phosphorylation levels of JNK and p38 proteins were significantly lowered by Bilo treatment compared with MI group, suggesting that Bilo alleviated MI development in mice via the inactivation of JNK/p38 MAPK signaling.
Evidence has showed that MAPK cascade contains three types of reversibly phosphorylated kinases (MAPK, MAPKK, and MAPKKK), which were all play an important role in various heart diseases [44]. In the future study, we will validate the upstream of JNK/p38 MAPK signaling and further confirm the underlying mechanisms of Bilo in MI. In addition to that, analytical methods for the determination of Bilo have been previously validated for accuracy, precession, limit of detection and quantification [45]. A previous study also revealed the accurate estimates of pharmacokinetic parameters of Bilo in rat plasma [46]. Thus, the analytical method validation for the determination of pharmacokinetics of Bilo as a potential cardioprotective drug is needed in the subsequent research.
Conclusion
For the first time, we demonstrated that Bilo had mitigating effects on MI development by inhibiting JNK/p38 MAPK signaling. Specifically, Bilo inhibited OGD-induced cardiomyocytes apoptosis in H9c2 cells and suppressed MI-induced cardiac fibrosis and apoptosis in mouse model. The findings of our study highlighted the anti-MI potential of Bilo, revealed the concrete mechanisms underlying its anti-MI action, and provided novel research orientation for improving the MI treatments.
References
Tsao, C. W., Aday, A. W., Almarzooq, Z. I., Alonso, A., Beaton, A. Z., Bittencourt, M. S., Boehme, A. K., Buxton, A. E., Carson, A. P., Commodore-Mensah, Y., Elkind, M. S. V., Evenson, K. R., Eze-Nliam, C., Ferguson, J. F., Generoso, G., Ho, J. E., Kalani, R., Khan, S. S., Kissela, B. M., Knutson, K. L.,… Martin, S. S. (2022) Heart Disease and Stroke Statistics-2022 Update: A report from the American Heart Association. Circulation, 145, e153–e639.
Jennings, R. B., Murry, C. E., Steenbergen, C., Jr., & Reimer, K. A. (1990). Development of cell injury in sustained acute ischemia. Circulation, 82, Ii2–12.
Talman, V., & Ruskoaho, H. (2016). Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration. Cell and Tissue Research, 365, 563–581.
Heusch, G., & Gersh, B. J. (2017). The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: A continual challenge. European Heart Journal, 38, 774–784.
Abbate, A., Bussani, R., Amin, M. S., Vetrovec, G. W., & Baldi, A. (2006). Acute myocardial infarction and heart failure: Role of apoptosis. International Journal of Biochemistry & Cell Biology, 38, 1834–1840.
Gottlieb, R. A., Burleson, K. O., Kloner, R. A., Babior, B. M., & Engler, R. L. (1994). Reperfusion injury induces apoptosis in rabbit cardiomyocytes. The Journal of Clinical Investigation, 94, 1621–1628.
Olivetti, G., Abbi, R., Quaini, F., Kajstura, J., Cheng, W., Nitahara, J. A., Quaini, E., Di Loreto, C., Beltrami, C. A., Krajewski, S., Reed, J. C., & Anversa, P. (1997). Apoptosis in the failing human heart. New England Journal of Medicine, 336, 1131–1141.
Teiger, E., Than, V. D., Richard, L., Wisnewsky, C., Tea, B. S., Gaboury, L., Tremblay, J., Schwartz, K., & Hamet, P. (1996). Apoptosis in pressure overload-induced heart hypertrophy in the rat. The Journal of Clinical Investigation, 97, 2891–2897.
Konstam, M. A., Kramer, D. G., Patel, A. R., Maron, M. S., & Udelson, J. E. (2011). Left ventricular remodeling in heart failure: Current concepts in clinical significance and assessment. JACC: Cardiovascular Imaging, 4, 98–108.
Chen, H., Moreno-Moral, A., Pesce, F., Devapragash, N., Mancini, M., Heng, E. L., Rotival, M., Srivastava, P. K., Harmston, N., Shkura, K., Rackham, O. J. L., Yu, W. P., Sun, X. M., Tee, N. G. Z., Tan, E. L. S., Barton, P. J. R., Felkin, L. E., Lara-Pezzi, E., Angelini, G., … Petretto, E. (2019) Author Correction: WWP2 regulates pathological cardiac fibrosis by modulating SMAD2 signaling. Nature Communications, 10, 4085.
Hayakawa, K., Takemura, G., Kanoh, M., Li, Y., Koda, M., Kawase, Y., Maruyama, R., Okada, H., Minatoguchi, S., Fujiwara, T., & Fujiwara, H. (2003). Inhibition of granulation tissue cell apoptosis during the subacute stage of myocardial infarction improves cardiac remodeling and dysfunction at the chronic stage. Circulation, 108, 104–109.
Leask, A. (2010). Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circulation Research, 106, 1675–1680.
Park, E. S., Kang, D. H., Yang, M. K., Kang, J. C., Jang, Y. C., Park, J. S., Kim, S. K., & Shin, H. S. (2014). Cordycepin, 3’-deoxyadenosine, prevents rat hearts from ischemia/reperfusion injury via activation of Akt/GSK-3β/p70S6K signaling pathway and HO-1 expression. Cardiovascular Toxicology, 14, 1–9.
Lu, S., Tian, Y., Luo, Y., Xu, X., Ge, W., Sun, G., & Sun, X. (2021). Iminostilbene, a novel small-molecule modulator of PKM2, suppresses macrophage inflammation in myocardial ischemia-reperfusion injury. Journal of Advanced Research, 29, 83–94.
Zhang, H. F., Huang, L. B., Zhong, Y. B., Zhou, Q. H., Wang, H. L., Zheng, G. Q., & Lin, Y. (2016). An overview of systematic reviews of Ginkgo biloba extracts for mild cognitive impairment and dementia. Frontiers in Aging Neuroscience, 8, 276.
Schwarzkopf, T. M., Koch, K. A., & Klein, J. (2013). Neurodegeneration after transient brain ischemia in aged mice: Beneficial effects of bilobalide. Brain Research, 1529, 178–187.
Sui, R. X., Miao, Q., Wang, J., Wang, Q., Song, L. J., Yu, J. W., Cao, L., Xiao, W., Xiao, B. G., & Ma, C. G. (2019). Protective and therapeutic role of Bilobalide in cuprizone-induced demyelination. International Immunopharmacology, 66, 69–81.
Goldie, M., & Dolan, S. (2013). Bilobalide, a unique constituent of Ginkgo biloba, inhibits inflammatory pain in rats. Behavioural Pharmacology, 24, 298–306.
Liu, J., Geng, Z., Zhang, Y., Alharbi, S., & Shi, Y. J. (2021). Sesquiterpenoid bilobalide inhibits gastric carcinoma cell growth and induces apoptosis both in vitro and in vivo models. Journal of Biochemical and Molecular Toxicology, 35, e22723.
Hao, Y., Wang, W., Wu, D., Liu, K., & Sun, Y. (2020). Bilobalide alleviates tumor necrosis factor-alpha-induced pancreatic beta-cell MIN6 apoptosis and dysfunction through upregulation of miR-153. Phytotherapy Research, 34, 409–417.
Zheng, Y., Wu, Z., Yi, F., Orange, M., Yao, M., Yang, B., Liu, J., & Zhu, H. (2018). By activating Akt/eNOS bilobalide B inhibits autophagy and promotes angiogenesis following focal cerebral ischemia reperfusion. Cellular Physiology and Biochemistry, 47, 604–616.
Chen, B., Cai, J., Song, L., Wang, X., & Chen, Z. (2005). Effects of Ginkgo biloba extract on cation currents in rat ventricular myocytes. Life Sciences, 76, 1111–1121.
Satoh, H.J.A.-F. (2003). Effects of Ginkgo biloba extract and bilobalide, a main constituent, on the ionic currents in guinea pig ventricular cardiomyocytes. Arzneimittel-Forschung, 53, 407–413.
Chen, J., Zeng, H., Chen, X., Su, C., & Lai, C. C. (2001). Induction of heme oxygenase-1 by Ginkgo biloba extract but not its terpenoids partially mediated its protective effect against lysophosphatidylcholine-induced damage. Pharmacological Research, 43, 63–69.
Wang, Z., Zhang, F., Liu, W., Sheng, N., Sun, H., & Zhang, J. (2021). Impaired tricarboxylic acid cycle flux and mitochondrial aerobic respiration during isoproterenol induced myocardial ischemia is rescued by bilobalide. Journal of Pharmaceutical Analysis, 11, 764–775.
Cao, A., & Li, X. J. (2019). Bilobalide protects H9c2 cell from oxygen-glucose-deprivation-caused damage through upregulation of miR-27a. Artificial Cells, Nanomedicine, and Biotechnology, 47, 2980–2988.
Xie, P., Guo, S., Fan, Y., Zhang, H., Gu, D., & Li, H. (2009). Atrogin-1/MAFbx enhances simulated ischemia/reperfusion-induced apoptosis in cardiomyocytes through degradation of MAPK phosphatase-1 and sustained JNK activation. Journal of Biological Chemistry, 284, 5488–5496.
Dhanasekaran, D. N., & Reddy, E. P. (2017). JNK-signaling: A multiplexing hub in programmed cell death. Genes & Cancer, 8, 682–694.
Cao, W., Xie, Y. H., Li, X. Q., Zhang, X. K., Chen, Y. T., Kang, R., Chen, X., Miao, S., & Wang, S. W. (2011). Burn-induced apoptosis of cardiomyocytes is survivin dependent and regulated by PI3K/Akt, p38 MAPK and ERK pathways. Basic Research in Cardiology, 106, 1207–1220.
Zuo, Y., Wang, Y., Hu, H., & Cui, W. (2016). Atorvastatin protects myocardium against ischemia-reperfusion injury through inhibiting miR-199a-5p. Cellular Physiology and Pharmacology, 39, 1021–1030.
Schwarzkopf, T., Koch, K., & Klein, J. (2013). Neurodegeneration after transient brain ischemia in aged mice: Beneficial effects of bilobalide. Brain Research, 1529, 178–187.
Chen, L., Li, S., Zhu, J., You, A., Huang, X., Yi, X., & Xue, M. (2021). Mangiferin prevents myocardial infarction-induced apoptosis and heart failure in mice by activating the Sirt1/FoxO3a pathway. Journal of Cellular and Molecular Medicine, 25, 2944–2955.
Meeran, M., Azimullah, S., Adeghate, E., & Ojha, S. (2021). Nootkatone attenuates myocardial oxidative damage, inflammation, and apoptosis in isoproterenol-induced myocardial infarction in rats. Phytomedicine, 84, 153405.
Zhang, X., Huang, L., Hua, L., Feng, H., & Shen, B. (2019). Resveratrol protects myocardial apoptosis induced by ischemia-reperfusion in rats with acute myocardial infarction via blocking P13K/Akt/e-NOS pathway. European Review for Medical and Pharmacological Sciences, 23, 1789–1796.
Xie, Z., Koyama, T., Suzuki, J., Fujii, Y., Togashi, H., Sawa, H., & Nagashima, K. (2001). Coronary reperfusion following ischemia: Different expression of bcl-2 and bax proteins, and cardiomyocyte apoptosis. Japanese Heart Journal, 42, 759–770.
Maerz, S., Liu, C. H., Guo, W., & Zhu, Y. Z. (2011). Anti-ischaemic effects of bilobalide on neonatal rat cardiomyocytes and the involvement of the platelet-activating factor receptor. Bioscience Reports, 31, 439–447.
Cao, A., & Li, X. (2019). Bilobalide protects H9c2 cell from oxygen-glucose-deprivation-caused damage through upregulation of miR-27a. Artificial Cells, Nanomedicine, and Biotechnology, 47, 2980–2988.
Zhou, W., & Cai, D. (2022). Midazolam suppresses ischemia/reperfusion-induced cardiomyocyte apoptosis by inhibiting the JNK/p38 MAPK signaling pathway. Canadian Journal of Physiology and Pharmacology, 100, 117–124.
Yi, C., Song, M., Sun, L., Si, L., Yu, D., Li, B., Lu, P., Wang, W., & Wang, X. (2022). Asiatic acid alleviates myocardial ischemia-reperfusion injury by inhibiting the ROS-mediated mitochondria-dependent apoptosis pathway. Oxidative Medicine and Cellular Longevity, 2022, 3267450.
Jiang, M., Li, J., Peng, Q., Liu, Y., Liu, W., Luo, C., Peng, J., Li, J., Yung, K., & Mo, Z. (2014). Neuroprotective effects of bilobalide on cerebral ischemia and reperfusion injury are associated with inhibition of pro-inflammatory mediator production and down-regulation of JNK1/2 and p38 MAPK activation. Journal of Neuroflammation, 11, 167.
Li, Y., Jiang, J., Tong, L., Gao, T., Bai, L., Xue, Q., Xing, J., Wang, Q., Lyu, H., Cai, M., & Sun, Z. (2020). Bilobalide protects against ischemia/reperfusion-induced oxidative stress and inflammatory responses via the MAPK/NF-휅B pathways in rats. BMC Musculoskeletal Disorders, 21, 449.
Shah, D. J., Kim, H. W., James, O., Parker, M., Wu, E., Bonow, R. O., Judd, R. M., & Kim, R. J. (2013). Prevalence of regional myocardial thinning and relationship with myocardial scarring in patients with coronary artery disease. JAMA, 309, 909–918.
Sun, M., Dawood, F., Wen, W. H., Chen, M., Dixon, I., Kirshenbaum, L. A., & Liu, P. P. (2004). Excessive tumor necrosis factor activation after infarction contributes to susceptibility of myocardial rupture and left ventricular dysfunction. Circulation, 110, 3221–3228.
Avkiran, M., & Marber, M. S. (2010). Feeling the stress: MAPKKK-MAPKK-MAPK signaling cascades in heart failure. Journal of Molecular and Cellular Cardiology, 48, 283–285.
Deng, F., & Zito, S. W. (2003). Development and validation of a gas chromatographic-mass spectrometric method for simultaneous identification and quantification of marker compounds including bilobalide, ginkgolides and flavonoids in Ginkgo biloba L. extract and pharmaceutical preparations. Journal of Chromatography A, 986, 121–127.
Wang, J., Ouyang, J., Liu, Y., Jia, X., You, S., He, X., & Di, X. (2014). Development of a sensitive LC-MS/MS method for the determination of bilobalide in rat plasma with special consideration of ex vivo bilobalide stability: Application to a preclinical pharmacokinetic study. Journal of Pharmaceutical and Biomedical Analysis, 95, 238–244.
Acknowledgements
We appreciate all participants who contributed to the study.
Funding
This work was supported by Henan medical science and technology research plan, joint construction project (No. LHGJ20200077) and Wuhan medical research project, youth project (No. WX20Q21).
Author information
Authors and Affiliations
Contributions
WS, ZC and MZ conceived and designed the experiments. FA, KC, JH, HF, XW, JM, XZ, WS, ZC and MZ carried out the experiments. FA, KC, WS, ZC and MZ analyzed the data. FA, KC, WS, ZC and MZ drafted the manuscript. All authors agreed to be accountable for all aspects of the work. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declared no competing interests in this study.
Ethical Approval
All procedures were approved by the Animal Protection and Utilization Committee of the Institute at Heart Center of Henan Provincial People's Hospital, Central China Fuwai Hospital, Central China Fuwai Hospital of Zhengzhou University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, W., Chen, Z., Zhang, M. et al. Bilobalide Prevents Apoptosis and Improves Cardiac Function in Myocardial Infarction. Mol Biotechnol 66, 442–453 (2024). https://doi.org/10.1007/s12033-023-00753-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-023-00753-8