Abstract
Salidroside (Sal), a phenylpropanoid glycoside isolated from a popular traditional Chinese medicinal plant Rhodiola rosea L., possesses multiple pharmacological actions. This aim of this study is to investigate the effects of Sal against isoproterenol (ISO)-induced myocardial ischemia. Fifty male Sprague–Dawley rats were randomized equally to five groups: control group, ISO group, Sal (20 mg/kg; 40 mg/kg) treatments groups, and propranolol (Pro, 15 mg/kg) group. Rats were treated for 14 days and then given ISO (80 mg/kg) for 2 consecutive days by subcutaneous injection. In vitro, we used H9C2 cells to investigate the effects of Sal against hypoxia–reoxygenation. ST-segment elevation was measured after the last administration. Serum levels of creatine kinase (CK), lactate dehydrogenase (LDH), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), superoxide dismutase (SOD), and malondialdehyde (MDA); levels of NADPH oxidases 2 and 4 (Nox2 and Nox4), NF-κBP65, and AP1 in heart, and H9C2 cells were measured by Western blot. The hearts were excised for determining microscopic examination, SOD, and MDA measurements. Sal decreased the ST elevation induced by ISO, decreased serum levels of CK-MB, LDH, TNF-α, IL-6, SOD, and MDA. In addition, Sal increased SOD activity and decreased MDA content in myocardial tissue. Sal also decreased Nox2 and 4, NF-κBP65, P-NF-κBP65, and AP1 protein levels in the heart. The results support a further study of Sal as potential treatments for ischemic heart disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Acute myocardial ischemia (AMI) is the leading cause of morbidity and mortality in the Western world, and according to the World Health Organization, it will be the leading cause of death in the world [1, 2].
One common feature of AMI is the dysregulation of reactive oxygen species (ROS) production in the heart. Some of the most significant sources of ROS are NADPH oxidases (Noxs) [3]. Noxs are composed of one or two transmembrane proteins (such as Nox, Duox, and p22phox), and in some cases include regulatory subunits such as p47phox, NoxO1, p40phox, p67phox, or NoxOA1. Noxs are a major source of nonmitochondrial cellular ROS and a highly regulated dynamic complex containing both membrane and cytosolic proteins. ROS are the important intracellular second messenger and mediate the production of inflammation cytokines through the multiple signal pathways [4, 5].
Rhodiola rosea is a popular medicinal plant found in mountains at high altitudes and has long been used in traditional Tibetan medicine system as an adaptogen to enhance the body’s resistance to fatigue and to extend human life. Salidroside, a major active ingredient isolated from the plant R. rosea, has been used in the treatment of diabetes, hypertension, fatigue, and hypoxia [6]. While there is little evidence regarding the relevance of salidroside (Sal) on myocardial injury in vivo in vitro, in this study, we hypothesized that Sal would improve myocardial injury through regulation of Nox/NF-κB/AP1 pathway.
MATERIAL AND METHODS
Reagents
Sal (purity 99 %) was purchased from the National Institutes for Food and Drug Control (Beijing, China). Isoproterenol (ISO) was purchased from Sigma-Aldrich (St. Louis, MO, USA). The enzyme-linked immunosorbent assay (ELISA) kits for determination of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) were produced by Nanjing KeyGEN Biotech CO., LTD. (Nanjing, China). Antibody of NADPH oxidases 2 (Nox2), Nox4, NF-κBP65, p-NF-κBP65, and AP1 were purchased from Cell Signaling Technology (Danvers, USA).
Animal
Fifty male Wistar rats (200–250 g, 8 weeks old) were purchased from the Animal Experiment Center of China Pharmaceutical University. The animals were maintained in a temperature-controlled room at 20.1–23.11 °C and 40–50 % humidity, a 12-h light/dark cycle and free access to water.
Experimental Protocol
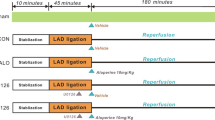
The rats were randomly assigned to five groups of 10 rats each. Two groups were given Sal (20, 40 mg/kg/day), and one was given propranolol (Pro, 15 mg/kg). A control group and an untreated model group were given distilled water. All treatments were oral. Rats were pretreated for 14 days and then intoxicated with ISO (80 mg/kg except for the control group) by subcutaneous injection on two consecutive days. Blood (5 ml) was collected from the abdominal aorta for serum enzyme assays. After treatment, hearts were excised, rinsed in ice-cold isotonic saline, blotted with filter paper, and homogenized in 0.05 M ice-cold phosphate buffer (pH 7.4) for biochemical assays and Western blot assay.
Determination of ST-Segment Elevation
Electrocardiograms (ECGs) recorded ST-segment elevation and heart rate at 20 min after the final injection of ISO or other drugs. ECGs were recorded under pentobarbital sodium anesthesia (30 mg/kg) using needle electrodes and a BL-420S Biological Function Experiment System purchased from Chengdu Thaimeng Technology Co. Ltd (Chengdu, China).
Determination of CK, LDH, TNF-α, IL-6, SOD, and MDA in the Serum
The levels of creatine kinase (CK), lactate dehydrogenase (LDH), superoxide dismutase (SOD), and malondialdehyde (MDA) were measured by a rate assay using an RT-9600 Semi-automatic Biochemical Analyzer (ShenZhenLeiDu life Science, LLC). TNF-α and IL-6 levels were measured by enzyme-linked immunosorbent assay (ELISA). All measurements were performed according to the kit manufacturers’ instructions.
SOD and MDA in Myocardial Tissue
Approximately 100 mg of myocardial tissue was removed from the apical part of the heart, immersed in ice-cold saline solution (1:9 W/V), and homogenized. The homogenate was centrifuged at 3000 rpm for 10 min at 4 °C, and the supernatant was used for the determining SOD and MDA levels according to the manufacturers’ instructions.
Histological Examination of Myocardium
The hearts were removed and fixed in 10 % formalin solution. The heart tissue was processed for sectioning and staining by standard histological methods. Sections (5 mm, Leica RM 2125, Germany) from the left ventricle were stained with hematoxylin and eosin (H&E) and examined by light microscopy (Nikon, Tokyo, Japan) at ×200 magnification.
Cell Culture
H9C2 cells, derived from rat embryonic ventricular cardiomyocytes, were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA); H9C2 cells were cultured in high glucose DMEM supplemented with 10 % fetal bovine serum and maintained in a humidified atmosphere consisting of 5 % CO2 and 95 % air at 37 °C.
Hypoxia–Reoxygenation Protocol
H9C2 cells were put into the anoxic tank (Thermo Fisher Scientific) filled with 94 % N2, 5 % CO2, and 1 % O2 for 6 h in culture medium deprived of glucose and serum. The hypoxia period was followed by reoxygenation by transferring cells to a normoxic incubator in 5 % CO2 and 95 % air for 1 h in normal medium. Sal at different concentrations (5, 10, 25 μM) was added at the time of reoxygenation.
Cell Viability Assay (MTT Assay)
H9C2 cell viability was measured by the 3-[4.5-dimethylthylthiazol-2-yl]-2.5 diphenyltetrazolium bromide (MTT) (Sigma, St. Louis, MO) method. The MTT cell viability OD value was detected.
Determination of TNF-α, IL-6, SOD, and MDA in Cell Supernatant
The levels of SOD and MDA in cell supernatant were measured by kits using an RT-9600 Semi-automatic Biochemical Analyzer (ShenZhenLeiDu life Science, LLC). TNF-α and IL-6 levels were measured by enzyme-linked immunosorbent assay (ELISA). All measurements were performed according to the kit manufacturers’ instructions.
Western Blot Analysis
Myocardial tissue and cells were homogenized in ice-cold RIPA buffer containing 0.1 % phenylmethylsulfonyl fluoride. The dissolved proteins were collected from the supernatant after centrifugation at 12,000g for 20 min. Protein concentrations were determined using Coomassie blue-based assay reagent. Protein extracts were separated by a SDS-polyacrylamide gel electrophoresis and then transferred onto a PVDF membrane. The membrane was blocked with 5 % skim milk in Tris buffer saline and then incubated at 4 °C overnight with respective primary antibodies for anti-Nox2 antibody (1:1000), anti-Nox4 antibody (1:1000), anti-NF-κBp65 (1:1000), anti-p-NF-κBp65 (1:1000), anti-AP1 (1:1000), and anti-GAPDH (inner control, 1:1000). After washing with Tris-buffered saline–tween 20 (TBST), the membranes were incubated with a horseradish peroxidase conjugated secondary antibody (1:12000) for 1.5 h at room temperature. The antibody-reactive bands were visualized by using enhanced chemiluminescence detection reagents and a gel imaging system (Tanon Science & Technology Co., Ltd., China).
Statistical Analysis
All data were normally distributed and are presented as mean ± SDs. In the case of single mean comparison, data were analyzed by a Student’s test. In the case of multiple mean comparisons, the data were analyzed by ANOVA and the Newman–Keuls post test, or two-way repeated measures ANOVA, followed by Bonferroni multiple comparison tests. p values less than 0.05 were regarded to reflect a significant difference.
RESULTS
Effect of Sal on ST-Segment Elevation
ST-segment elevation was reduced in the Sal groups compared with the ISO rats. Heart rates tended to stabilize and approximate the rate observed in the propranolol-treated group. Sal significantly decreased ST-segment elevation at doses of 20 and 40 mg/kg compared with the ISO group (Fig. 1).
Effect of Sal on SOD and MDA Levels in the Myocardium
Compared with the control group, SOD level in the ISO group was decreased. Sal increased SOD level compared with the untreated model group rats. Compared with the control group, the MDA levels in the ISO group increased. Sal decreased MDA level compared with the ISO group rats (Fig. 2).
Effects of Sal on CK-MB, LDH, SOD, MDA, TNF-α, and IL-6 Serum Levels
ISO increased injury marker enzymes, CK-MB, and LDH compared with the control rats. Pretreatment with Sal, the levels of CK-MB and LDH were decreased, compared with rats in the ISO group in a dose-dependent manner. Compared with the control group, serum levels of MDA, TNF-α, and IL-6 were increased, and SOD was decreased in the ISO group. Pretreatment with Sal decreased serum levels of MDA, TNF-α, and IL-6 levels and increased SOD, compared with the ISO group rats (Fig. 3).
Effect of Sal Myocardial on Histology
Control rat myocardium showed a normal myofibrillar structure with striations, branched appearance, and continuity with adjacent myofibrils. Tissue from the ISO rats given obvious myocardial cell swelling, degeneration, loss of transverse striations, and large numbers of infiltrating inflammatory cells. Tissues from rats pretreated with Sal showed a normal, well-preserved cardiac muscle cell histology (Fig. 4).
MTT Result
MTT results demonstrated that hypoxia–reoxygenation significantly inhibited H9C2 cell viability, which was alleviated by Sal. The cytoprotective effect of Sal dramatically inhibited hypoxia–reoxygenation-induced H9C2 cell viability loss (Fig. 5).
Determination of TNF-α, IL-6, SOD, and MDA in Cell Supernatant
Compared with the control group, levels of MDA, TNF-α, and IL-6 were increased and SOD was decreased in the hypoxia–reoxygenation group. Pretreatment with Sal decreased levels of MDA, TNF-α, and IL-6 levels and increased SOD, compared with the hypoxia–reoxygenation group (Fig. 6).
Effects of Sal on Nox/NF-κB/AP1 Pathway Proteins Expressions in the Heart and H9C2 Cells
The effect of Sal on Nox/NF-κB/AP1 pathway protein levels in the heart and H9C2 is shown in Fig. 7. Nox2, Nox4, and p-NF-κBp65 were increased in the heart and H9C2 cells, compared with that in control animals; administration of Sal was able to reverse the effects of ISO on Nox/NF-κB/AP1 pathway in the heart and H9C2 cells.
Effects of Sal on Nox/NF-κB/AP1 pathway in the heart and H9C2 cells. a control, b ISO, c ISO + Pro (15 mg/kg), d ISO + Sal (20 mg/kg), e ISO + Sal (40 mg/kg). All values given are the mean ± SDs. ## P < 0.01 vs. control group. **P < 0.01 vs. ISO vehicle group. e control, f hypoxia–reoxygenation, g hypoxia–reoxygenation + Sal (5 μM), h hypoxia–reoxygenation + Sal (10 μM), i hypoxia–reoxygenation + Sal (25 μM). All values given are the mean ± SDs. ##P < 0.01 vs. control group. **P < 0.01 vs. hypoxia–reoxygenation-vehicle group.
DISCUSSION
In this study, Sal reduced the ST-segment elevation induced by ISO; Sal also decreased CK-MB, LDH, SOD, MDA, TNF-α, and IL-6 levels in the serum. Sal also increased SOD level and decreased MDA level in the myocardium and reverses the effects of ISO on Nox/NF-κB/AP1 pathway. These results suggested that Sal had cardioprotective effects in myocardial ischemia that could be attributed to their anti-oxidative and anti-inflammatory properties.
ISO is a synthetic β-adrenergic agonist that can cause severe stress in the myocardium and necrosis of heart muscle cells. So, in the current study, we used ISO to build the acute myocardial ischemia contained in the study. In this study, the acute myocardial ischemia induced by ISO was confirmed by loss of integrity of myocardial membranes on histological examination, increased ST-segment elevation, and increased serum levels of CK-MB and LDH. Sal alleviated myocardial histological changes, reduced heart rate and ST-segment elevation, and decreased CK-MB and LDH.
Inflammation infiltration has been recognized as a major force in ischemia, and some evidence has shown that increased levels of inflammatory markers are related to ischemia [7]. The cytokines such as TNF-α and IL-6 are small proteins which regulate inflammation process. In this study, ISO and hypoxia–reoxygenation increased TNF-α and IL-6 levels. Sal decreased TNF-α and IL-6 levels, which suggested that their cardioprotective effects of Sal were associated with anti-inflammatory properties.
Mechanisms against inflammatory stresses are involved in anti-oxidant. The levels of SOD and MDA are associated with ischemic pathophysiology. SOD activity reflects the cellular capability of scavenging/quenching free radicals [8]. MDA level is often used as an indicator of lipid peroxidation. Increased serum MDA is an indicator of severe oxidative stress. One of the major causes of AMI is an imbalance between oxidants and anti-oxidant defense [9]. In this study, SOD activity was decreased and the level of MDA was increased in the heart tissue and H9C2 cells compared to controls. Sal increased SOD activity and decreased MDA level in the myocardium and H9C2 cells, which suggested that their cardioprotective effects of Sal were related to anti-oxidative properties.
Nox2 and Nox4 levels were significantly increased in the heart tissue by ISO and hypoxia–reoxygenation compared to controls. Sal decreased Nox2 and Nox4 levels in the heart and H9C2 cells. NF-κB and AP1 are two important transcriptional factors involved in regulating expression of pro-inflammatory mediators, including cytokines, chemokines, and adhesion molecules, and playing a critical role in mediating inflammatory responses. In this study, ISO and hypoxia–reoxygenation increased NF-κB and AP1 levels in the heart and H9C2 cells; Sal decreased NF-κB and AP1 levels in heart and H9C2 cells.
In conclusion, this study demonstrated that Sal had cardioprotective effects against acute ischemic myocardial injury induced by ISO in rats.
References
Lopez, A.D., C.D. Mathers, M. Ezzati, D.T. Jamison, and C.J.L. Murray. 2006. Global burden of disease and risk factors. Washington (DC): World Bank.
Yang, G., Y. Wang, Y. Zeng, G.F. Gao, X. Liang, M. Zhou, X. Wan, S. Yu, Y. Jiang, M. Naqhavi, T. Vos, H. Wang, A.D. Lopez, and C.J. Murray. 2013. Rapid health transition in China, 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet 381: 1987–2015.
Griendling, K.K., D. Sorescu, and M. Ushio-Fukai. 2000. NAD(P)H oxidase role in cardiovascular biology and disease. Circulation Research 86: 494–501.
Guha, M., W. Bai, J.L. Nadler, et al. 2000. Molecular mechanisms of tumor necrosis factor alpha gene expression in monocytic cells via hyperglycemia-induced oxidant stress-dependent and -independent pathways. Journal of Biological Chemistry 275: 17728e39.
Schreck, R., K. Albermann, and P.A. Baeuerle. 1992. Nuclear factor kappa B: an oxidative stress-responsive transcription factor of eukaryotic cells (a review). Free Radical Research Communications 17: 221e37.
Kucinskaite, A., V. Briedis, and A. Savickas. 2004. Experimental analysis of therapeutic properties of Rhodiola rosea L and its possible application in medicine. Medicina (Kaunas, Lithuania) 40: 614–619.
Willerson, J.T., and P.M. Ridker. 2004. Inflammation as a cardiovascular risk factor. Circulation 109: II2–10.
Zheng, W., L.Z. Huang, L. Zhao, B. Wang, H.B. Xu, G.Y. Wang, Z.L. Wang, and H. Zhou. 2008. Superoxide dismutase activity and malondialdehyde level in plasma and morphological evaluation of acute severe hemorrhagic shock in rats. American Journal of Emergency Medicine 26: 54–58.
Senthil, S., G. Chandramohan, and K.V. Pugalendi. 2007. Isomers (oleanolic and ursolic acids) differ in their protective effect against isoproterenol-induced myocardial ischemia in rats. International Journal of Cardiology 119: 131–133.
Acknowledgments
This work was supported by National twelve five major drug discovery project (2011ZX09102-002-01).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, L., Wei, T., Chang, X. et al. Effects of Salidroside on Myocardial Injury In Vivo In Vitro via Regulation of Nox/NF-κB/AP1 Pathway. Inflammation 38, 1589–1598 (2015). https://doi.org/10.1007/s10753-015-0134-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-015-0134-0