Abstract
Advanced oxidation protein products (AOPPs) are novel biomarkers of oxidative damage to proteins and a novel class of inflammatory mediators. AOPPs can promote oxidative stress (OS) and inflammation and thus participate in many pathophysiological disease processes. Atherosclerosis is a chronic inflammatory disease of blood vessels that is characterized by low-density lipoprotein infiltration into the endothelial intima and the formation of atherosclerotic plaques. Inflammation and OS are established risk factors for the formation of atherosclerosis. Accumulated studies show that AOPPs can accelerate the progression of atherosclerosis through OS and inflammation. Additionally, AOPPs can accelerate the formation of atherosclerotic plaques by inhibiting high-density lipoprotein receptor scavenger receptor class B type I-mediated high-density lipoprotein cholesterol reverse transport, leading to metabolic disturbances. Some studies have suggested that plasma AOPPs levels are independently positively correlated with blood pressure and are also independent risk factors for cardiovascular disease. AOPPs can trigger oxidative bursts of neutrophils, monocytes and phagocytic cells, increase the generation of reactive oxygen species and promote the secretion of cytokines to accelerate endothelial cell injury. Detecting the levels and inhibiting the formation of AOPPs may provide a novel approach to monitor the progress and improve the prognosis of atherosclerosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atherosclerosis is one of the most common complications in many diseases, such as chronic renal failure (CRF) and diabetes, the major cause of heart attack, stroke and myocardial infarction and the most common cause of death worldwide [1–3]. Therefore, the development of novel therapeutic strategies that specifically target atherosclerosis is desired. The etiologies of cardiovascular disease (CVD) are multifactorial, and no feasible therapeutic recommendations exist because the diseases underlying the interactions are too complex to be well understood [1].
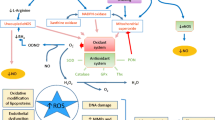
Atherosclerosis is a chronic inflammatory disease. Oxidative stress, inflammatory mediators, oxidative lipids and protein modifications play complex roles in the initiation and progression of atherosclerosis [4]. Inflammation-induced oxidative modifications contribute to a variety of important clinical manifestations of atherosclerosis, such as endothelial dysfunction and plaque disruption. The classic risk factors for the development of atherosclerosis are at least partially response to endothelial dysfunction, high blood pressure, cell adhesion and proliferation, and lipid metabolism disorder [5]. These risk factors all have a strong association with AOPPs (Fig. 1). In this review, we show that AOPPs may play an important role in the development of atherosclerosis. Understanding the mechanisms underlying this role will help establish new preventive and therapeutic strategies.
Schematic diagram of the roles and mechanisms of AOPPs in atherosclerosis. AOPPs is involved in the formation of As, which is closely related to inflammation, OS, modifying lipoproteins and RCT. OS and inflammation can stimulate the productions of AOPPs in blood circulation. The increasing AOPPs further aggravate OS and inflammation. In turn, this positive feedback loop can amplify or maintain OS and inflammation. AOPPs induce the activations of macrophages, monocytes and mast cells and increase the levels of inflammatory cytokines (IL-6, IL-1, MCP-1 and SDF-1α). AOPPs also induce the productions of ROS, CRP and oxygen free radicals (O2−, H2O2, OH-), which result in endothelial dysfunction and lipoprotein infiltration. AOPPs promote the modification of lipoprotein and increase the abnormal compositions of lipoprotein in circulation. Additionally, AOPPs inhibit RCT by combining with SR-BI and downregulating the expressions of ABCA1 and ABCG1 in macrophages, which result in the accumulation of cholesterol, format the foam cells and promote the development of As. As Atherosclerosis, AOPPs advanced oxidation protein products, OS oxidative stress, IL-1 interleukin-1, MCP-1 monocyte chemotactic protein 1, SDF-1α stromal cell-derived factor-α, ROS reactive oxygen species, CPR C-reactive protein, O2– superoxide, H 2 O 2 peroxide ions, OH- hydroxyl radical, SR-BI scavenger receptor class B type I, ABCA1 ATP-binding cassette transporter A1, ABCG1 ATP-binding cassette transporter G1, RCT reverse cholesterol transport
AOPPs Formation and Characteristics
The identification of AOPPs as novel OS biomarkers was first reported in the plasma of chronic uremic patients by Witko-Sarsat et al. [6]. AOPPs can be formed in many conditions, including oxidant–antioxidant imbalance, glycol oxidation processes and coexisting inflammation [7]; however, most AOPPs result from myeloperoxidase-derived OS [8–11]. AOPPs are derived from oxidation-modified albumin, which is the primary origin of AOPPs. Albumin is a multifunctional protein that plays key roles in maintaining appropriate oncotic pressure and transporting fatty and amino acids, metal ions, cholesterol, bilirubin, hormones and various other ligands and drugs [12]. These functions are related to the albumin structure, which may be destroyed by OS to form AOPPs. Free radicals can also impair albumin functions, especially its transport ability [13]. Human serum albumin (HAS) is the major and predominant antioxidant in plasma and can exert important antioxidant activities against oxidative damage. The ratio of the oxidized form of albumin to the normal form can serve as a useful marker to evaluate the systemic redox state, which can be used to assess the progression and therapeutic efficacy of diseases [14].
AOPPs are also derived from fibrinogen (FB) [6, 8, 15, 16]. Selmeci et al. [9] demonstrated that plasma samples had increased AOPPs levels compared to serum samples, suggesting that FB could be involved in the reactivity of AOPPs. Torbitz et al. showed that hypochlorous acid (HOCl)-induced oxidative damage of FB might induce AOPPs formation and significantly increased the FB-AOPPs levels in a concentration-dependent manner. FB-AOPPs formation may promote the change of FB function and structure, as well as increasing its coagulation activity [17]. Studies have shown that hypercholesterolemia can spontaneously produce AOPPs, suggesting that hyperlipidemia may enhance the in vivo process of AOPPs formation via increasing oxidative stress. The occurrence of OS in hyperlipidemia has been shown to result in an imbalance between the oxidant and antioxidant systems that is accompanied by increased oxidized low-density lipoprotein (ox-LDL) [18]. Furthermore, studies have shown that collagen modified by HOCl increased collagen-AOPPs levels in a dose-dependent manner and these products induced the generation of AOPPs, nitric oxide (NO) and O2-induced apoptosis and decreased neutrophil viability [19]. In previous study, it was reported that AOPPs might be generated via the Fenton reaction. Because the Fenton reaction is an important source of reactive oxygen species (ROS), especially during the inflammatory process, and the hydroxyl radical (OH–) formed by this reaction is the most harmful free radical, this reaction may represent an alternative pathway for AOPPs generation [20].
Although the exact AOPPs structure is not completely clear, AOPPs are a group of dityrosine-, pentosidine- and carbonyl-containing protein products that are generated by the reaction of plasma proteins with HOCl and chloramines during OS [7, 21]. Dityrosine, which is a sign of protein injury in the terminal phase, is also a sign of AOPPs [22, 23]. AOPPs can be simply and rapidly detected using a semiautomated microplate-based spectrophotometric technique at 340 nm [24]. The AOPPs concentration is largely overestimated in plasma samples due to lipid interference. Precipitating triglycerides prior to the analysis can overcome this problem and allow the AOPPs concentration to accurately reflect OS [25, 26].
AOPPs can be divided into two types based on their molecular weights (low molecular weight AOPPs and high molecular weight AOPPs). The low molecular weight AOPPs contain the monomeric form of albumin, whereas the high molecular weight AOPPs account for the majority of AOPPs due to the propensity of albumin to form aggregates via disulfide bridges and/or dityrosine cross-linking [8, 11]. Under reducing or denaturing conditions, polymers of the high molecular weight AOPPs are dissociated into the low molecular weight AOPPs [6]. The key molecule responsible for human plasma AOPPs reactivity is post-translationally modified FB [9]. Oxidized plasma proteins, especially albumin, carry AOPPs. The formation of AOPPs is irreversible, and they cannot be easily hydrolyzed by proteolytic enzymes or reduced by antioxidants such as vitamin C and glutathione [27]. AOPPs are mostly eliminated by the liver and spleen, and AOPPs stored at −20 and −80 °C for 6 months to maintain their stability [7, 28, 29].
In vivo, AOPPs may be produced by the oxidation of albumin by HOCL formed through the myeloperoxidase (MPO)—catalyzing reaction in circulating neutrophils. In vitro, AOPPs are capable of inducing respiratory bursts from neutrophils and monocytes to induce systemic inflammation and increase the circulation of TNF-α, C-reactive protein (CPR) and other inflammatory mediators. Similar to advanced glycosylation end products, AOPPs also bind to the same receptor and have their own particular biological proprieties. AOPPs that can enhance OS and inflammation have been identified as a novel class of inflammatory mediators and novel biomarkers of oxidative damage to proteins in many studies [7].
The Roles of AOPPs in Atherosclerosis
AOPPs participate in many pathophysiological disease processes. Many pathological conditions are linked to the accumulation of AOPPs, including osteoarthritis [30], uremia, hypertriglyceridemia [29], coronary artery disease (CAD) and atherosclerosis [7, 9]. The AOPPs levels gradually increase with the severity of the disease and can be used to monitor the disease progress and prognosis. Therefore, inhibiting AOPPs formation may be a potential effective therapeutic strategy in many diseases [6].
As the main pattern underlying CVD, the characteristics of atherosclerosis include chronic inflammatory disease of the blood vessels [31]. Inflammation and OS are established risk factors for atherosclerosis. Numerous studies found that the occurrence and progression of atherosclerosis were closely associated with high plasma AOPPs levels, which could be used as an early marker of atherosclerosis and an independent strong predictor of the risk of atherosclerotic cardiovascular events and CAD [32–36]. The plasma AOPPs levels are also closely related to the carotid intima-media thickness in patients with carotid atherosclerosis plaques. AOPPs not only reflect the severity of OS, but also act as a mediator of inflammation [35].
Lipid metabolism also plays an important role in atherogenesis and contributes to atherosclerotic plaque formation in vivo. Mo et al.’s [37] experimental study showed that AOPPs increased lipid accumulation and exacerbated atherosclerosis by downregulating ATP-binding cassette transporter A1 (ABCA1) and ATP-binding cassette transporter G1 (ABCG1) expression in apoE-KO mice fed an atherogenic diet. AOPPs promoted the increase in lipid accumulation in the aortic sinus plaques and livers in the apoE-KO mice. AOPPs also exacerbated the atherosclerotic lesions in the aortic sinus.
AOPPs participate in the formation of atherosclerosis through a variety of mechanisms [38]. First, AOPPs can lead to the production of ROS and oxidative bursts in neutrophils and monocytes. Second, AOPPs can induce the synthesis of inflammatory cytokines and stimulate the expression of chemokines, including SDF-1α and MCP-1, to promote cellular adhesion. Finally, AOPPs can contribute to the progression of atherosclerosis by intensifying metabolic disturbances [39, 40]. These mechanisms are important for atherosclerosis progression (Fig. 1).
Mechanisms by Which AOPPs Promotes Atherosclerosis Development
Oxidative Stress as a Bridge Between AOPPs and Atherosclerosis
OS primarily results from an imbalance between the excessive generation of oxidant compounds and insufficient antioxidant prevention mechanisms and is closely associated with the formation of a large number of free radicals that may impair tissues and cells [39]. Free radicals are highly reactive and can bind to proteins, lipoproteins, nucleic acids and enzymes in a short time. The oxidative metabolism of AOPPs may result in the generation of oxygen free radicals, such as superoxide (O2–), peroxide ions (H2O2) and OH–. In the endothelium, the combination of free radicals with NO can reduce the vasodilation ability and account for the occurrence of coronary ischemia [41].
Many lines of evidence indicate that OS is related to atherosclerosis. For example, OS promotes platelet activation with the release of serotonin. The platelet poor plasma (PPP) serotonin levels and PPP/whole blood (WB) serotonin ratio were significantly correlated with the levels of OS in coronary heart disease patients, indicating that OS promoted serotonin production and contributed to vascular inflammation and atherosclerosis development [42].
Overwhelming OS consumes endogenous redox buffer systems, which not only triggers immune responses, but also leads to endothelial and smooth muscle cell dysfunctions that are critical for atherosclerosis progression [1]. The first sign of atherosclerosis may be endothelial dysfunction, which is an early event in CVD in type 2 diabetes patients and can occur before the occurrence of albuminuria. An increased plasma AOPPs level is an independent risk factor for impaired endothelial vasodilation and increased plasma soluble intercellular adhesion molecule-1 (ICAM-1) in type 2 diabetes patients and therefore may be an early marker of vasculopathy in individuals with early stage diabetes [41, 43]. OS plays a predominant role in the pathogenesis of atherosclerosis by impairing endothelial functions and reducing endothelial activity. AOPPs are reliable markers of oxidative injury originating from oxidative and carbonyl stress and have functions in the enhancement of systematic inflammatory activity [41]. AOPPs levels are closely related to endothelial function [44]. AOPPs can induce endothelial dysfunction by generating oxygen free radicals, decreasing the NO level and reducing the activity of endothelial cells by inducing ROS production. ROS can also prompt the oxidation of albumin into AOPPs, and antioxidants have good protective effects on OS injury and the reduction in cell viability induced by AOPPs [39, 45].
Intravenous injection of animals with AOPPs can increase the plasma TNF-α concentration and promote endothelial cell proliferation and macrophage infiltration into the smooth muscle. Thus, AOPPs are associated with the proliferation of vascular smooth muscle cells (VSMCs), which can accelerate the formation of atherosclerosis [18]. AOPPs can also induce the expression of ICAM-1 and other cytokines to accelerate endothelial cell injury. A high plasma AOPPs level is an independent risk factor for endothelial dysfunction and the strongest risk factor for impairment of endothelial vasodilation and therefore may be an early marker of vasculopathy in atherosclerosis [43].
Presented data indicate that AOPP plays a significant role in many disorders with chronic background, because of they reflect intensification of OS and the degree of pathological changes connected with OS [46]. In peritoneal dialysis (PD) patients, the AOPPs levels increase to more than 50 % of the baseline value. Thus, a prooxidant status defined by continuous increase in AOPPs levels would thus reflect a cardiovascular-prone event status. Probably, the prooxidant status will most likely maintain permanent endothelial dysfunction and promote new cardiovascular events. Therefore, AOPPs are significantly associated with past and future CVDs. Indeed, these patients had a 4.7-fold increased risk of suffering from subsequent CVD [27]. Aside from PD patients, atherosclerosis and calcification in the cardiovascular system are the most frequent causes of morbidity and mortality in hemodialysis (HD) patients with end-stage renal disease, with almost half of deaths ascribed to atherosclerosis [47, 48]. The accumulation of AOPPs in renal disease and CAD patients represents an excellent novel marker of OS. Thus, the role of OS in the development of CVD might be of great importance [49].
AOPPs constitute a novel molecular basis for the deleterious activity of oxidants [34]. AOPPs levels, which are reliable markers to estimate the degree of and variation in oxidant-mediated protein damage, are more sensitive than ox-LDL for the evaluation of OS [32, 50]. Under normal conditions, low levels of ROS as byproducts of electron transport chain reactions are primarily generated in the mitochondria [51]. The small amount of ROS in the human body can remove external pathogens and tumor cells and plays an important role in the body’s defense system. In vivo, OS can induce the accumulation of ROS, which contributes to the pathogenesis of many chronic diseases by changing protein structures and functions and causing oxidative damage to endothelial cells [52]. Additionally, the permeability of the blood vessel wall increases, allowing plasma lipoprotein infiltration into the endothelial gap and uptake by macrophages to form foam cells. Studies have shown that ROS production by human umbilical vein endothelial cells, polymorphonuclear neutrophils and monocytes in vitro is induced by AOPPs-BSA [39]. Moreover, AOPPs-BSA decreases endothelial cell viability via OS induction.
Importantly, AOPPs are real mediators of the pro-inflammatory effect of OS and can stimulate the NADPH oxidase respiratory burst, MPO production in monocytes, neutrophils and phagocytic cells, and cell inflammation; the intensity of the reaction is positively related to the oxidative degree of the AOPPs [34, 53, 54].
AOPPs are closely related with neopterin, CRP and monocyte-derived cytokines such as tumor necrosis factor α (TNF-α) and its antagonist and IL-1 and its receptor antagonist [10]. Neopterin, which is a byproduct of the guanosine triphosphate pathway, activates T lymphocytes to produce IFN-γ, which is the major stimulating factor for neopterin formation and can be indirectly induced by TNF-α; interestingly, AOPPs can induce TNF-α secretion. Elevated neopterin levels may be a significant predictor of coronary events and mortality [55, 56]. Neopterin is a useful marker for monocyte activation and OS and has emerged as an important independent and predictive marker for cardiovascular risk assessment [57, 58]. The increase in the neopterin concentration is significantly related to the lowering of plasma levels of vitamins C, E and B and several other antioxidant compounds [1, 59]. Neopterin not only associates with the systemic inflammatory process in atherosclerosis, but also might be important for the inflammatory process within the plaque and plaque instability [58, 60]. Furthermore, neopterin can promote cellular adhesion molecules and tissue factor expression to induce an atherosclerotic phenotype in human coronary endothelial cells [61].
Superoxide dismutase (SOD), which is a scavenger of superoxide anions, and l-arginine, which is a precursor of NO, ameliorate the impairment of endothelium-dependent relaxation to decrease NO production/release and increase oxygen free radical production by exogenous AOPPs-BSA in rat aortic rings. Several studies have demonstrated that AOPPs accumulation coexists with decreased antioxidant enzyme levels, such as glutathione peroxidase and SOD, and that an abnormal decrease in these plasma antioxidant enzymes may further aggravate the detrimental effects of potential AOPPs-mediated ROS production [39].
AOPPs is Involved in Atherosclerosis by Increasing Inflammation
Both circulating inflammation and local inflammation in the blood vessel play key roles in the initiation and progression of atherosclerosis [62]. The incidence of cardiovascular events in CRF patients is tightly related to the degree of inflammation [63]. AOPPs were elevated in the plasma of CRF patients and increased with the deterioration of renal functions; thus, AOPPs were proposed to be novel markers of OS in CRF. Atherosclerosis is associated with vascular calcification (VC). Inflammatory cytokines, such as the TNF and IL-6 superfamilies, and the inflammation-related transcription factor NF-kB play important roles in the VC process, indicating that inflammation accelerates VC progression and promotes the development of atherosclerosis [64]. Chronic inflammation can impact endogenous antioxidant capacities through the continuous production of high ROS levels. IFN-γ is the most important trigger for the formation and release of ROS. ROS can lead to the depletion of antioxidants such as vitamins C and E and glutathione, thereby contributing to OS development. Enhanced OS markers and low plasma antioxidant levels are common patterns in atherosclerosis patients.
The production of pro-inflammatory mediators by immune competent cells in the lesions, such as macrophages, T lymphocytes, endothelial cells, smooth muscle cells and mast cells, is required to maintain a chronic inflammatory state [65]. Considerable evidence indicates that mast cells and their products play key roles in inflammation and atherosclerosis. Activated mast cells release a broad spectrum of pro-inflammatory cytokines, growth factors, vasoactive substances and proteolytic enzymes that have detrimental effects on their immediate surroundings in the vessel wall. These products increase lipid uptake, provoke matrix degradation, enhance apoptosis and recruit inflammatory cells, which actively contribute to atherosclerosis and plaque formation [66]. Activated macrophages also play a critical role in atherosclerotic plaque formation, fibrous cap disruption and thrombus formation. Neopterin is a marker of macrophage activation. As mentioned above, a significant correlation was reported between AOPPs and neopterin, demonstrating that AOPPs are closely linked to macrophage activation [1].
AOPPs are not only exquisite markers of phagocyte-derived OS and the molecular basis for the deleterious activity of oxidants, but also active inflammatory mediators because they can trigger oxidative bursts in neutrophils and monocytes and promote the synthesis of inflammatory cytokines [21, 54, 67], such as IL-6, TNF-α and IL-1, which exert important effects in the process of atherosclerosis. IL-1 can induce a large portion of the local and systemic inflammatory response manifestations in atherosclerosis. IL-1 is a chemoattractant for a variety of cells; it improves the adhesion of blood cells to vascular endothelial cells and increases the blood procoagulant activity. TNF-α induces apoptosis, leading to the production of ROS, O2 and NO; it is also an important factor in atherosclerotic plaque destabilization, which leads to the development of acute coronary syndrome. IL-6 plays a role in the development of atherosclerosis. The main biological effect of IL-6 is participation in the inflammatory response. IL-6 affects the synthesis of acute phase proteins by hepatocytes more than other inflammatory cytokines. Additionally, IL-6 promotes the exacerbation of chronic inflammatory processes and the transformation of acute inflammation into chronic inflammation. Thus, inflammatory cytokines play a role during every stage of the development of atherosclerotic lesions in the arterial wall. [68].
In addition to monocytes and neutrophils, AOPPs can decrease the activity of vascular endothelial cells in vitro by inducing the respiratory bursts of endothelial cells and phagocytic cells and ROS generation. These effects can be inhibited by the NADPH oxidase inhibitor in a manner that is dependent on the AOPPs dose. In uremia patients, AOPPs are involved in monocyte-mediated inflammatory disorders [47, 53]. O2 arises during the respiratory burst and can induce AOPPs generation under the action of the chlorine oxidant. The inflammatory reaction is amplified in this vicious cycle, which can be seen as positive feedback of AOPPs [67, 69, 70].
The adhesion of monocytes and T lymphocytes to the damaged endothelium and their migration into the tunica media and the accumulation of monocytes/macrophages in the lesion are key factors in the pathogenesis of atherosclerosis [1, 71]. MCP-1 and SDF-1α are effective chemokines. MCP-1 can stimulate the migration of monocytes through the four arterial endothelial monolayers. MCP-1 has been detected in atherosclerotic lesions and is expressed in a variety of cells, including endothelial cells, monocytes and smooth muscle cells [72, 73]. AOPPs show remarkable potential for the stimulation of MCP-1 mRNA expression and MCP-1 protein secretion by p38 MAPK. Disposal of VSMCs with AOPPs results in a prominent increase in MCP-1 expression in a time- and dose-dependent manner [34]. SDF-1α is a highly effective chemokine of lymphocytes and monocytes, with chemotactic activity that is ten times stronger than MCP-1. AOPPs-BSA can promote SDF-1α mRNA expression in ECV304 cells in a dose-dependent manner [74]. AOPPs may also accelerate the progression of atherosclerosis by promoting the expression of a variety of inflammatory cytokines, which in turn stimulate SDF-1α expression. Several studies showed that the delayed clearance of AOPPs from serum was an independent risk factor for CAD in uremia [75].
More recent work on MPOs has clearly shown that MPOs of neutrophils are responsible for generating chlorine oxidant and AOPPs. In HD patients, a 50 % increase in AOPPs production was dependent on MPO activity [13]. MPO participates in the inflammatory process associated with atherosclerosis and can be detected in the atherosclerotic plaques of the aorta but not in normal aortae [53, 76]. AOPPs have dual identities as the cause of systemic inflammation and the result of OS; AOPPs are not only a bridge between inflammation and OS, but also a bridge between monocytes and polymorphonuclear phagocytes, which are key effector cells in inflammation and OS.
AOPPs Promote the Development of Atherosclerosis by Modifying Lipoproteins
In addition to albumin, many other types of proteins participate in the pathogenesis of immune dysfunctions and accelerate atherosclerosis; these proteins are modified by AOPPs. One of the most important proteins is the LDL. Based on the “oxidative modification hypothesis” of atherosclerosis, increasing LDL oxidation is an early event in atherosclerosis [77]. LDL is easily denatured by oxidative modification [3]. LDL oxidation is promoted by a variety of free radicals, which results in structural and functional modifications of the molecule [5].
The deposition of ox-LDL on the vessel wall can lead to the formation of foam cells, contribute to the formation of an oxidative environment, stimulate ROS formation, promote endothelial dysfunction and induce the infiltration of mononuclear cells to the vascular wall, thereby leading to the proliferation of smooth muscle cells [36] and contributing to the pathogenesis of vascular dysfunctions in the development of early atherosclerosis [1, 78]. AOPPs have been implicated in atherosclerosis primarily through LDL, which is extremely sensitive to AOPPs modification. AOPPs-LDL was found deposited on atherosclerotic vessel walls and plaques in CRF patients; the process involved the rapid development and formation of atherosclerotic lesions by increasing inflammation in circulation and activating endothelial cells [76, 78, 79]. Additionally, studies report that AOPPs can promote the formation of atherosclerotic plaques in animal models with high cholesterol [80], which is direct evidence that AOPPs may be involved in atherosclerotic lesions. Circulating ox-LDL was also detected in CVD patients [81]. The presence of ox-LDL has been implicated in the progression to advanced atherosclerotic lesions, which are characterized by instability and a propensity for plaque rupture with resultant generation of an occlusive intravascular thrombus [5, 27]. However, although ox-LDL was present in the vessels, the amount was small [82]. The elevation of ox-LDL in the plasma is predictive of endothelial dysfunction and coronary heart disease [81–83].
AOPPs Promote the Development of Atherosclerosis by Inhibited Reverse Cholesterol Transport
Reverse cholesterol transport (RCT) has been proposed as the major mechanism by which HDL reduces the incidence of atherosclerosis. Moreover, a variety of other mechanisms have been ascribed to HDL, including anti-inflammatory activity and antioxidant activity, modification of coagulation parameters, alteration of platelet functions, interaction with the metabolism of triglyceride-rich lipoproteins and improvement in endothelial functions [5, 6]. Recent data have elucidated the role of HDL as a natural antioxidant. HDL possesses significant antioxidant activity that is primarily mediated via inhibiting LDL oxidation with a subsequent reduction in cellular uptake by the monocyte macrophage system [5, 23]. HDL is closely associated with antioxidant enzymes such as paraoxonase-1 [3]. Paraoxonase-1 is located on the surface of HDL particles and is a calcium-dependent enzyme that contributes to the antioxidant and anti-inflammatory roles of HDL; paraoxonase-1 can protect both HDL and LDL from oxidation [84]. Preclinical and large-scale epidemiological studies have found that low HDL-C level is a robust predictor of lipid peroxidation. The plasma HDL-C level has been inversely correlated with the risk of CVD [85].
AOPPs accelerate the progression of atherosclerosis by increasing the plasma lipid levels and impairing the macrophage cholesterol efflux. In vitro generated AOPPs–albumin has a high binding affinity for the HDL receptor SR-BI. SR-BI can recognize a broad range of oxidized and modified proteins that play protective roles against CVD and act as the primary pathway for the disposal of HDL-borne cholesterol ester (CE) and triglycerides. A molar concentration of AOPPs–albumin could block the binding of HDL with SR-BI and effectively inhibit SR-BI-mediated CE uptake in vitro and in vivo. Administration of AOPPs–albumin may significantly interfere with the clearance of HDL-CE mediated by SR-BI in mice [49]. Macrophages participate in the RCT process. Stimulation of the macrophage cholesterol efflux by enhancing ABCA1 and ABCG1 expression is predicted to inhibit foam cell formation and consequently reduce atherosclerosis [86]. AOPPs accelerate the progression of atherosclerosis by increasing the plasma lipid levels and impairing the macrophage cholesterol efflux [37]. In CRF patients, depressing the clearance of HDL-C may contribute to the abnormal composition of HDL and the increased cardiovascular risk [8, 49]. AOPPs directly impair HDL metabolism, which may play a potential key role in the development of CVD.
Anti-AOPPs Strategies and Atherosclerosis
Atherosclerosis contributes significantly to morbidity and mortality worldwide. AOPPs play an important role in many of the pathological conditions of atherosclerosis; thus, inhibiting AOPPs formation may provide a novel approach to improve the prognosis. To date, studies have found many ways to act on AOPPs and affect the disease prognosis. AOPPs can induce myocardial cell death and contribute to myocardial injury through the activation of Nox2/Rac1/superoxide-dependent TRAF3IP2/JNK and TRAF3-interacting protein 2 knockdown, which can blunt AOPPs-induced apoptosis. TRAF3IP2 may represent a potential therapeutic target for CAD [11]. AOPPs can induce MCP-1 mRNA and protein overproduction by activating the ROS/NF-κB pathway, but this pathway can be inhibited by SOD (ROS inhibitor) and sesquiterpene lactone, which inhibit IκBα degradation [87]. Arctiin can decrease the epithelial–mesenchymal transition of mouse podocytes by alleviating the endoplasmic reticulum stress caused by AOPPs [88]. Garlic significantly reduced AOPPs levels in the brain tissue of rats administered radiofrequency electromagnetic radiation [89]. The persimmon flavone effectively inhibited the AOPPs-induced proliferation of vascular adventitial fibroblasts [90]. Metformin and lifestyle modifications both effectively reduced the adverse effects of AOPPs and OS alone [91].
Because there are many risk factors for CVDs in individuals, it is necessary to take effective treatment measures against these risks. N-acetyl cysteine is capable of inhibiting the formation of HOCl-induced AOPPs and alleviating OS in a concentration-dependent manner. The synergistic effect promoted by the association of these drugs is more effective than the use of the drug alone [92, 93]. AOPPs-BSA markedly inhibits the endothelium-dependent relaxation response to acetylcholine in a dose-dependent manner. Captopril can completely reverse the inhibitory effect of AOPPs-BSA, thereby treating hypertension and inhibiting the progression of atherosclerosis by scavenging oxygen free radicals. Captopril also protected the endothelium against the functional damage induced by AOPPs-BSA in the rat aorta by increasing the bioavailability of NO [39, 94]. Olmesartan resulted in a significant reduction in intracellular ROS production. Furthermore, olmesartan reduced the cytotoxicity and vascular endothelial growth factor (VEGF) secretion induced by AOPPs or H2O2. A low antioxidant intake (particularly of vitamins) is suggested to be associated with a reduced risk of CVD. Vitamin E is an antioxidant that can alleviate OS and may be able to reduce atherosclerotic lesions. Several clinical trials found that vitamin E supplementation led to a reduction in the risk of fatal and nonfatal acute myocardial infarction [1, 95, 96].
Matrine is a type of herb, which are used to treat inflammatory diseases. Matrine inhibited vascular cellular adhesion molecule 1 (VCAM-1) and ICAM-1 expression in TNF-α-stimulated human umbilical smooth muscle cells (HASMCs) via the suppression of ROS production and NF-κB and MAPK pathway activation. As a consequence, matrine may have a potential therapeutic use for the prevention of the advancement of atherosclerotic lesions [97]. Additionally, other natural plants (especially plants with factors with medicinal properties such as stereocalpin A and sulforaphane) may have therapeutic potential. Stereocalpin A prevented the induction of adhesion molecule expression in a concentration-dependent manner after inflammatory cytokine stimulation [98]. Sulforaphane was also shown to inhibit the expression of TNF-α-induced adhesion molecules in VSMCs [99]. Astaxanthin inhibits LDL oxidation and increases the high-density lipoprotein (HDL) cholesterol and adiponectin levels; astaxanthin could exert preventive actions against CVD via its potential ability to improve oxidative stress, inflammation, lipid metabolism and glucose metabolism [100].
Some potential novel anti-atherosclerotic drug preparations with anti-cytokine mechanisms have emerged, such as canakinumab (a human monoclonal antihuman IL-1β antibody), anakinra (an IL-1 receptor antagonist), tocilizumab (an IL-6 inhibitor), etanercept (a TNF-α inhibitor) and darapladib (a direct inhibitor of lipoprotein-associated phospholipase A2). Classical anti-inflammatory drugs, such as colchicine and methotrexate, are the most promising agents for atherosclerosis medication under study [101]. Lipid-lowering drugs, such as statins, have been used for atherosclerosis prevention. These drugs prevent the increase in the thickness of the intimal–medial layer of the carotid arteries, which is a surrogate marker of atherosclerosis [102]. Their antiatherosclerotic effects are caused not only by their lipid-lowering activity but also by their anti-inflammatory, antioxidant, anti-platelet and immunomodulation properties [103, 104]. Lipid-lowering drugs from other groups are also effective as anti-atherosclerotic agents.
In summary, atherosclerosis is a multifactorial disease. The prevention of AOPPs formation has been demonstrated to be an efficient method for the prevention of atherosclerosis and can also influence the treatment efficacy. Additionally, other measures can prevent and slow down the progression of atherosclerosis.
Perspectives
This review summarizes the role of AOPPs in atherosclerosis with a particular focus on inflammation and OS, which play critical roles through expediting the initiation and progression of atherosclerosis. OS and inflammation can stimulate the production of AOPPs in circulation. The increase in AOPPs further aggravates OS and inflammation. In turn, this positive feedback loop could amplify or maintain OS and inflammation. AOPPs are not only exquisite markers of OS and the molecular basis for the deleterious activity of oxidants but also active inflammatory mediators. AOPPs are involved in the development of atherosclerosis through enhancing OS and inflammation, inducing endothelial dysfunction, increasing the production of inflammatory cytokines, chemokines and free radicals, stimulating VSMCs proliferation, disordering lipoprotein metabolism and promoting cell adhesion, including monocytes and macrophages, all of which contribute to the formation of atherosclerosis. AOPPs are independent and powerful predictors of the atherosclerosis risk. Interfering with AOPPs formation may be an efficient method for atherosclerosis treatment.
Abbreviations
- AOPPs:
-
Advanced oxidation protein products
- OS:
-
Oxidative stress
- LDL:
-
Low-density lipoprotein
- HDL:
-
High-density lipoprotein
- SR-BI:
-
Scavenger receptor class B type I
- HDL-C:
-
High-density lipoprotein cholesterol
- CVD:
-
Cardiovascular disease
- CRF:
-
Chronic renal failure
- HAS:
-
Human serum albumin
- FB:
-
Fibrinogen
- HOCL:
-
Hypochlorous acid
- NO:
-
Nitric oxide
- ROS:
-
Reactive oxygen species
- OH:
-
Hydroxyl radical
- MPO:
-
Myeloperoxidase
- CPR:
-
C-reactive protein
- CAD:
-
Coronary artery disease
- ABCA1:
-
ATP-binding cassette transporter A1
- ABCG1:
-
ATP-binding cassette transporter G1
- O2−:
-
Superoxide
- H2O2 :
-
Peroxide ions
- PPP:
-
Platelet poor plasma
- WB:
-
Whole blood
- ICAM-1:
-
Intercellular adhesion molecule-1
- VSMCs:
-
Vascular smooth muscle cells
- PD:
-
Peritoneal dialysis
- HD:
-
Hemodialysis
- SOD:
-
Superoxide dismutase
- VC:
-
Vascular calcification
- RCT:
-
Reverse cholesterol transport
- VEGF:
-
Vascular endothelial growth factor
- VCAM-1:
-
Vascular cellular adhesion molecule 1
- HASMCs:
-
Human umbilical smooth muscle cells
References
Mangge, H., Becker, K., Fuchs, D., & Gostner, J. M. (2014). Antioxidants, inflammation and cardiovascular disease. World Journal of Cardiology, 6, 462–477.
Hansson, G. K. (2005). Inflammation, atherosclerosis, and coronary artery disease. New England Journal of Medicine, 352, 1685–1695.
Vasdev, S., Gill, V., & Singal, P. (2007). Role of advanced glycation end products in hypertension and atherosclerosis: Therapeutic implications. Cell Biochemistry and Biophysics, 49, 48–63.
Jamkhande, P. G., Chandak, P. G., Dhawale, S. C., Barde, S. R., Tidke, P. S., & Sakhare, R. S. (2014). Therapeutic approaches to drug targets in atherosclerosis. Saudi Pharmaceutical Journal, 22, 179–190.
Bandeali, S., & Farmer, J. (2012). High-density lipoprotein and atherosclerosis: The role of antioxidant activity. Current Atherosclerosis Reports, 14, 101–107.
Witko-Sarsat, V., Friedlander, M., Capeillere-Blandin, C., Nguyen-Khoa, T., Nguyen, A. T., Zingraff, J., et al. (1996). Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney International, 49, 1304–1313.
Piwowar, A. (2010). Advanced oxidation protein products. Part I. Mechanism of the formation, characteristics and property. Polski Merkuriusz Lekarski, 28, 166–169.
Selmeci, L. (2011). Advanced oxidation protein products (AOPP): Novel uremic toxins, or components of the non-enzymatic antioxidant system of the plasma proteome? Free Radical Research, 45, 1115–1123.
Selmeci, L., Szekely, M., Soos, P., Seres, L., Klinga, N., Geiger, A., & Acsady, G. (2006). Human blood plasma advanced oxidation protein products (AOPP) correlates with fibrinogen levels. Free Radical Research, 40, 952–958.
Witko-Sarsat, V., Friedlander, M., Nguyen, K. T., Capeillere-Blandin, C., Nguyen, A. T., Canteloup, S., et al. (1998). Advanced oxidation protein products as novel mediators of inflammation and monocyte activation in chronic renal failure. The Journal of Immunology, 161, 2524–2532.
Valente, A. J., Yoshida, T., Clark, R. A., Delafontaine, P., Siebenlist, U., & Chandrasekar, B. (2013). Advanced oxidation protein products induce cardiomyocyte death via Nox2/Rac1/superoxide-dependent TRAF3IP2/JNK signaling. Free Radical Biology and Medicine, 60, 125–135.
Halliwell, B. (1988). Albumin—An important extracellular antioxidant? Biochemical Pharmacology, 37, 569–571.
Sciskalska, M., Zalewska, M., Grzelak, A., & Milnerowicz, H. (2014). The influence of the occupational exposure to heavy metals and tobacco smoke on the selected oxidative stress markers in smelters. Biological Trace Element Research, 159, 59–68.
Anraku, M., Chuang, V. T., Maruyama, T., & Otagiri, M. (2013). Redox properties of serum albumin. Biochimica et Biophysica Acta, 1830, 5465–5472.
Himmelfarb, J., & McMonagle, E. (2001). Albumin is the major plasma protein target of oxidant stress in uremia. Kidney International, 60, 358–363.
Liu, B., Hou, X., Zhou, Q., Tian, J., Zhu, P., Xu, J., et al. (2011). Detection of advanced oxidation protein products in patients with chronic kidney disease by a novel monoclonal antibody. Free Radical Research, 45, 662–671.
Torbitz, V. D., Bochi, G. V., de Carvalho, J. A., de Almeida, V. R., Da, S. J., & Moresco, R. N. (2015). In vitro oxidation of fibrinogen promotes functional alterations and formation of advanced oxidation protein products, an inflammation mediator. Inflammation, 38, 1201–1206.
Liu, S. X., Hou, F. F., Guo, Z. J., Nagai, R., Zhang, W. R., Liu, Z. Q., et al. (2006). Advanced oxidation protein products accelerate atherosclerosis through promoting oxidative stress and inflammation. Arteriosclerosis, Thrombosis, and Vascular Biology, 26, 1156–1162.
Bochi, G. V., Torbitz, V. D., de Campos, L. P., Sangoi, M. B., Fernandes, N. F., Gomes, P., et al. (2016). In vitro oxidation of collagen promotes the formation of advanced oxidation protein products and the activation of human neutrophils. Inflammation, 39, 916–927.
Bochi, G. V., Torbitz, V. D., Cargnin, L. P., de Carvalho, J. A., Gomes, P., & Moresco, R. N. (2014). An alternative pathway through the Fenton reaction for the formation of advanced oxidation protein products, a new class of inflammatory mediators. Inflammation, 37, 512–521.
Capeillere-Blandin, C., Gausson, V., Descamps-Latscha, B., & Witko-Sarsat, V. (2004). Biochemical and spectrophotometric significance of advanced oxidized protein products. Biochimica et Biophysica Acta, 1689, 91–102.
Heinecke, J. W., Li, W., Daehnke, H. R., & Goldstein, J. A. (1993). Dityrosine, a specific marker of oxidation, is synthesized by the myeloperoxidase-hydrogen peroxide system of human neutrophils and macrophages. Journal of Biological Chemistry, 268, 4069–4077.
Ohkubo, Y., Kishikawa, H., Araki, E., Miyata, T., Isami, S., Motoyoshi, S., et al. (1995). Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: A randomized prospective 6-year study. Diabetes Research and Clinical Practice, 28, 103–117.
Witko, V., Nguyen, A. T., & Descamps-Latscha, B. (1992). Microtiter plate assay for phagocyte-derived taurine-chloramines. Journal of Clinical Laboratory Analysis, 6, 47–53.
Anderstam, B., Ann-Christin, B. H., Valli, A., Stenvinkel, P., Lindholm, B., & Suliman, M. E. (2008). Modification of the oxidative stress biomarker AOPP assay: Application in uremic samples. Clinica Chimica Acta, 393, 114–118.
Valli, A., Suliman, M. E., Meert, N., Vanholder, R., Lindholm, B., Stenvinkel, P., et al. (2007). Overestimation of advanced oxidation protein products in uremic plasma due to presence of triglycerides and other endogenous factors. Clinica Chimica Acta, 379, 87–94.
Witko-Sarsat, V., & Descamps-Latscha, B. (1997). Advanced oxidation protein products: Novel uraemic toxins and pro-inflammatory mediators in chronic renal failure? Nephrology, Dialysis, Transplantation, 12, 1310–1312.
Iwao, Y., Anraku, M., Hiraike, M., Kawai, K., Nakajou, K., Kai, T., et al. (2006). The structural and pharmacokinetic properties of oxidized human serum albumin, advanced oxidation protein products (AOPP). Drug Metabolism and Pharmacokinetics, 21, 140–146.
Matteucci, E., Biasci, E., & Giampietro, O. (2001). Advanced oxidation protein products in plasma: Stability during storage and correlation with other clinical characteristics. Acta Diabetologica, 38, 187–189.
Kadowaki, D., Anraku, M., Sakaya, M., Hirata, S., Maruyama, T., & Otagiri, M. (2015). Olmesartan protects endothelial cells against oxidative stress-mediated cellular injury. Clinical and Experimental Nephrology, 19, 1007–1014.
Suzuki, M., Minami, A., Nakanishi, A., Kobayashi, K., Matsuda, S., Ogura, Y., & Kitagishi, Y. (2014). Atherosclerosis and tumor suppressor molecules (Review). International Journal of Molecular Medicine, 34, 934–940.
Komosinska-Vassev, K., Olczyk, P., Winsz-Szczotka, K., Kuznik-Trocha, K., Klimek, K., & Olczyk, K. (2012). Age- and gender-related alteration in plasma advanced oxidation protein products (AOPP) and glycosaminoglycan (GAG) concentrations in physiological ageing. Clinical Chemistry and Laboratory Medicine, 50, 557–563.
Yang, X. B., Hou, F. F., Wu, Q., Zhou, H., Liu, Z. R., Yang, Y., & Zhang, X. (2005). Increased levels of advanced oxidation protein products are associated with atherosclerosis in chronic kidney disease. Zhonghua Nei Ke Za Zhi, 44, 342–346.
Peng, K. F., Wu, X. F., Zhao, H. W., & Sun, Y. (2006). Advanced oxidation protein products induce monocyte chemoattractant protein-1 expression via p38 mitogen-activated protein kinase activation in rat vascular smooth muscle cells. Chinese Medical Journal, 119, 1088–1093.
Descamps-Latscha, B., Witko-Sarsat, V., Nguyen-Khoa, T., Nguyen, A. T., Gausson, V., Mothu, N., et al. (2005). Advanced oxidation protein products as risk factors for atherosclerotic cardiovascular events in nondiabetic predialysis patients. American Journal of Kidney Diseases, 45, 39–47.
Kaneda, H., Taguchi, J., Ogasawara, K., Aizawa, T., & Ohno, M. (2002). Increased level of advanced oxidation protein products in patients with coronary artery disease. Atherosclerosis, 162, 221–225.
Mo, Z. C., Xiao, J., Tang, S. L., Ouyang, X. P., He, P. P., Lv, Y. C., et al. (2014). Advanced oxidation protein products exacerbates lipid accumulation and atherosclerosis through downregulation of ATP-binding cassette transporter A1 and G1 expression in apolipoprotein E knockout mice. Circulation Journal, 78, 2760–2770.
Guo, Z. J., Hou, F. F., Liu, S. X., Zhang, W. R., Zhou, Z. M., & Liu, Z. Q. (2004). Proteins modified with glycation or oxidation products accelerate atherosclerosis in experimental hypercholesterolemic rabbits. Beijing Da Xue Xue Bao, 36, 127–130.
Chen, S., Liu, L., Sun, X., Liu, Y., & Song, T. (2005). Captopril restores endothelium-dependent relaxation induced by advanced oxidation protein products in rat aorta. Journal of Cardiovascular Pharmacology, 46, 803–809.
Piwowar, A. (2010). Advanced oxidation protein products. Part II. The significance of oxidation protein products in the pathomechanism of diabetes and its complications. Polski Merkuriusz Lekarski, 28, 227–230.
Skvarilova, M., Bulava, A., Stejskal, D., Adamovska, S., & Bartek, J. (2005). Increased level of advanced oxidation products (AOPP) as a marker of oxidative stress in patients with acute coronary syndrome. Biomedical papers of the Medical Faculty of the University Palacký, Olomouc, Czechoslovakia Republic, 149, 83–87.
Sugiura, T., Dohi, Y., Yamashita, S., Hirowatari, Y., Fujii, S., & Ohte, N. (2016). Serotonin in peripheral blood reflects oxidative stress and plays a crucial role in atherosclerosis: Novel insights toward holistic anti-atherothrombotic strategy. Atherosclerosis, 246, 157–160.
Liang, M., Wang, J., Xie, C., Yang, Y., Tian, J. W., Xue, Y. M., & Hou, F. F. (2014). Increased plasma advanced oxidation protein products is an early marker of endothelial dysfunction in type 2 diabetes patients without albuminuria. Journal of Diabetes, 6, 417–426.
Kocak, H., Gumuslu, S., Sahin, E., Ceken, K., Gocmen, Y. A., Yakupoglu, G., et al. (2009). Advanced oxidative protein products are independently associated with endothelial function in peritoneal dialysis patients. Nephrology (Carlton), 14, 273–280.
Yuan, F., Liu, S. X., & Tian, J. W. (2004). Advanced oxidation protein products induce reactive oxygen species production in endothelial cells. Di Yi Jun Yi Da Xue Xue Bao, 24, 1350–1352.
Piwowar, A. (2014). Biochemical and clinical aspects of advanced oxidation protein products in kidney diseases and metabolic disturbances. Postepy Higieny i Medycyny Doswiadczalnej (Online), 68, 179–190.
Descamps-Latscha, B., & Witko-Sarsat, V. (2003). Oxidative stress in chronic renal failure and hemodialysis. Nephrologie, 24, 377–379.
Krasniak, A., Drozdz, M., Pasowicz, M., Chmiel, G., Kowalczyk-Michalek, M., Szumilak, D., et al. (2007). Influence of microinflammation and oxidative stress on atherosclerosis progression and calcifications in cardiovascular system of hemodialyzed patients during two years follow-up. Przeglad Lekarski, 64, 140–147.
Marsche, G., Frank, S., Hrzenjak, A., Holzer, M., Dirnberger, S., Wadsack, C., et al. (2009). Plasma-advanced oxidation protein products are potent high-density lipoprotein receptor antagonists in vivo. Circulation Research, 104, 750–757.
Zadrazil, J., Horak, P., Strebl, P., Krejci, K., Kajabova, M., Schneiderka, P., et al. (2012). In vivo oxidized low-density lipoprotein (ox-LDL) aopp and tas after kidney transplantation: A prospective, randomized one year study comparing cyclosporine A and tacrolimus based regiments. Biomedical papers of the Medical Faculty of the University Palacky, Olomouc, Czechoslovakia Republic, 156, 14–20.
Le Bras, M., Clement, M. V., Pervaiz, S., & Brenner, C. (2005). Reactive oxygen species and the mitochondrial signaling pathway of cell death. Histology and Histopathology, 20, 205–219.
Sun, N., Yang, L., Li, Y., Cheng, Y., Zhang, Z., & Cai, D. (2013). Advanced oxidation protein products inhibit proliferation and differentiation of rat osteoblasts through oxidative stress. Nan Fang Yi Ke Da Xue Xue Bao, 33, 356–359.
Witko-Sarsat, V., Gausson, V., & Descamps-Latscha, B. (2003). Are advanced oxidation protein products potential uremic toxins? Kidney International, 84, S11–S14.
Witko-Sarsat, V., Gausson, V., Nguyen, A. T., Touam, M., Drueke, T., Santangelo, F., & Descamps-Latscha, B. (2003). AOPP-induced activation of human neutrophil and monocyte oxidative metabolism: A potential target for N-acetylcysteine treatment in dialysis patients. Kidney International, 64, 82–91.
Werner-Felmayer, G., Werner, E. R., Fuchs, D., Hausen, A., Reibnegger, G., & Wachter, H. (1989). Tumour necrosis factor-alpha and lipopolysaccharide enhance interferon-induced tryptophan degradation and pteridine synthesis in human cells. Biological Chemistry Hoppe-Seyler, 370, 1063–1069.
Sugioka, K., Naruko, T., Matsumura, Y., Shirai, N., Hozumi, T., Yoshiyama, M., & Ueda, M. (2010). Neopterin and atherosclerotic plaque instability in coronary and carotid arteries. Journal of Atherosclerosis and Thrombosis, 17, 1115–1121.
Wirleitner, B., Reider, D., Ebner, S., Bock, G., Widner, B., Jaeger, M., et al. (2002). Monocyte-derived dendritic cells release neopterin. Journal of Leukocyte Biology, 72, 1148–1153.
Fuchs, D., Avanzas, P., Arroyo-Espliguero, R., Jenny, M., Consuegra-Sanchez, L., & Kaski, J. C. (2009). The role of neopterin in atherogenesis and cardiovascular risk assessment. Current Medicinal Chemistry, 16, 4644–4653.
Murr, C., Winklhofer-Roob, B. M., Schroecksnadel, K., Maritschnegg, M., Mangge, H., Bohm, B. O., et al. (2009). Inverse association between serum concentrations of neopterin and antioxidants in patients with and without angiographic coronary artery disease. Atherosclerosis, 202, 543–549.
Adachi, T., Naruko, T., Itoh, A., Komatsu, R., Abe, Y., Shirai, N., et al. (2007). Neopterin is associated with plaque inflammation and destabilisation in human coronary atherosclerotic lesions. Heart, 93, 1537–1541.
Cirillo, P., Pacileo, M., de Rosa, S., Calabro, P., Gargiulo, A., Angri, V., et al. (2006). Neopterin induces pro-atherothrombotic phenotype in human coronary endothelial cells. Journal of Thrombosis and Haemostasis, 4, 2248–2255.
Libby, P., Ridker, P. M., & Maseri, A. (2002). Inflammation and atherosclerosis. Circulation, 105, 1135–1143.
Matuszkiewicz-Rowinska, J. (2002). The association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Polskie Archiwum Medycyny Wewnetrznej, 108, 901–905.
Henaut, L., Sanchez-Nino, M. D., Aldamiz-Echevarria, C. G., Sanz, A. B., & Ortiz, A. (2016). Targeting local vascular and systemic consequences of inflammation on vascular and cardiac valve calcification. Expert Opinion on Therapeutic Targets, 20, 89–105.
Frostegard, J. (2013). Immunity, atherosclerosis and cardiovascular disease. BMC Medicine, 11, 117.
Conti, P., & Shaik-Dasthagirisaeb, Y. (2015). Atherosclerosis: A chronic inflammatory disease mediated by mast cells. Central European Journal of Immunology, 40, 380–386.
Descamps-Latscha, B., & Witko-Sarsat, V. (2001). Importance of oxidatively modified proteins in chronic renal failure. Kidney International, 78, S108–S113.
Kirichenko, T. V., Sobenin, I. A., Nikolic, D., Rizzo, M., & Orekhov, A. N. (2015). Anti-cytokine therapy for prevention of atherosclerosis. Phytomedicine. doi:10.1016/j.phymed.2015.12.002.
Krzystek-Korpacka, M., Neubauer, K., Berdowska, I., Boehm, D., Zielinski, B., Petryszyn, P., et al. (2008). Enhanced formation of advanced oxidation protein products in IBD. Inflammatory Bowel Diseases, 14, 794–802.
Tesarova, P., Kalousova, M., Trnkova, B., Soukupova, J., Argalasova, S., Mestek, O., et al. (2007). Carbonyl and oxidative stress in patients with breast cancer—Is there a relation to the stage of the disease? Neoplasma, 54, 219–224.
Fenyo, I. M., & Gafencu, A. V. (2013). The involvement of the monocytes/macrophages in chronic inflammation associated with atherosclerosis. Immunobiology, 218, 1376–1384.
Rolin, J., & Maghazachi, A. A. (2014). Implications of chemokines, chemokine receptors, and inflammatory lipids in atherosclerosis. Journal of Leukocyte Biology, 95, 575–585.
Zernecke, A., & Weber, C. (2014). Chemokines in atherosclerosis: Proceedings resumed. Arteriosclerosis, Thrombosis, and Vascular Biology, 34, 742–750.
Shi, C. H., Jiang, Y. N., Shan, L. J., Lu, Y., Zhang, Y., & Gao, Y. G. (2013). Advanced oxidation protein products promote expression of stromal-cell derived factor-1alpha of ECV304 cells through ERK signal pathway. Zhongguo Ying Yong Sheng Li Xue Za Zhi, 29, 142–146.
Kalousova, M., Zima, T., Tesar, V., & Lachmanova, J. (2002). Advanced glycation end products and advanced oxidation protein products in hemodialyzed patients. Blood Purification, 20, 531–536.
Hazell, L. J., Arnold, L., Flowers, D., Waeg, G., Malle, E., & Stocker, R. (1996). Presence of hypochlorite-modified proteins in human atherosclerotic lesions. Journal of Clinical Investigation, 97, 1535–1544.
Chisolm, G. M., & Steinberg, D. (2000). The oxidative modification hypothesis of atherogenesis: An overview. Free Radical Biology and Medicine, 28, 1815–1826.
Yu, L., Cecil, J., Peng, S. B., Schrementi, J., Kovacevic, S., Paul, D., et al. (2006). Identification and expression of novel isoforms of human stromal cell-derived factor 1. Gene, 374, 174–179.
Juarez, J., Bendall, L., & Bradstock, K. (2004). Chemokines and their receptors as therapeutic targets: The role of the SDF-1/CXCR4 axis. Current Pharmaceutical Design, 10, 1245–1259.
Ma, Q., Jones, D., Borghesani, P. R., Segal, R. A., Nagasawa, T., Kishimoto, T., et al. (1998). Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proceedings of the National Academy of Sciences USA, 95, 9448–9453.
Holvoet, P., Mertens, A., Verhamme, P., Bogaerts, K., Beyens, G., Verhaeghe, R., et al. (2001). Circulating oxidized LDL is a useful marker for identifying patients with coronary artery disease. Arteriosclerosis, Thrombosis, and Vascular Biology, 21, 844–848.
Fraley, A. E., & Tsimikas, S. (2006). Clinical applications of circulating oxidized low-density lipoprotein biomarkers in cardiovascular disease. Current Opinion in Lipidology, 17, 502–509.
Holvoet, P., Vanhaecke, J., Janssens, S., Van de Werf, F., & Collen, D. (1998). Oxidized LDL and malondialdehyde-modified LDL in patients with acute coronary syndromes and stable coronary artery disease. Circulation, 98, 1487–1494.
Ferretti, G., Bacchetti, T., Masciangelo, S., & Bicchiega, V. (2010). HDL-paraoxonase and membrane lipid peroxidation: A comparison between healthy and obese subjects. Obesity (Silver Spring), 18, 1079–1084.
Zelzer, S., Fuchs, N., Almer, G., Raggam, R. B., Pruller, F., Truschnig-Wilders, M., et al. (2011). High density lipoprotein cholesterol level is a robust predictor of lipid peroxidation irrespective of gender, age, obesity, and inflammatory or metabolic biomarkers. Clinica Chimica Acta, 412, 1345–1349.
Jun, H. J., Hoang, M. H., Lee, J. W., Yaoyao, J., Lee, J. H., Lee, D. H., et al. (2012). Iristectorigenin B isolated from Belamcanda chinensis is a liver X receptor modulator that increases ABCA1 and ABCG1 expression in macrophage RAW 264.7 cells. Biotechnology Letters, 34, 2213–2221.
Wang, J. C., Zhao, Y., Chen, S. J., Long, J., Jia, Q. Q., Zhai, J. D., et al. (2013). AOPPs induce MCP-1 expression by increasing ROS-mediated activation of the NF-kappaB pathway in rat mesangial cells: Inhibition by sesquiterpene lactones. Cellular Physiology and Biochemistry, 32, 1867–1877.
Zhang, J., Guo, T. T., Yang, L., Du, Q. S., Hua, J., Liu, R. Z., & Tang, X. (2012). Effect of arctiin on mouse podocyte epithelial-mesenchymal transition induced by advanced oxidation protein products. Nan Fang Yi Ke Da Xue Xue Bao, 32, 379–382.
Avci, B., Akar, A., Bilgici, B., & Tuncel, O. K. (2012). Oxidative stress induced by 1.8 GHz radio frequency electromagnetic radiation and effects of garlic extract in rats. International Journal of Radiation Biology, 88, 799–805.
Camps, J., Marsillach, J., & Joven, J. (2009). The paraoxonases: Role in human diseases and methodological difficulties in measurement. Critical Reviews in Clinical Laboratory Sciences, 46, 83–106.
Esteghamati, A., Eskandari, D., Mirmiranpour, H., Noshad, S., Mousavizadeh, M., Hedayati, M., & Nakhjavani, M. (2013). Effects of metformin on markers of oxidative stress and antioxidant reserve in patients with newly diagnosed type 2 diabetes: A randomized clinical trial. Clinical Nutrition, 32, 179–185.
Bochi, G. V., Torbitz, V. D., Cargnin, L. P., Sangoi, M. B., Santos, R. C., Gomes, P., & Moresco, R. N. (2012). Fructose-1,6-bisphosphate and N-acetylcysteine attenuate the formation of advanced oxidation protein products, a new class of inflammatory mediators, in vitro. Inflammation, 35, 1786–1792.
Lazarova, M., Stejskal, D., Lacnak, B., Vaclavik, J., Adamovska, S., Ochmanova, R., et al. (2004). The antioxidant acetylcysteine reduces oxidative stress by decreasing level of AOPPs. Biomedical papers of the Medical Faculty of the University Palacky, Olomouc, Czechoslovakia Republic 148, 131–133.
Bordignon, M., Da Dalt, L., Marinelli, L., & Gabai, G. (2013). Advanced oxidation protein products are generated by bovine neutrophils and inhibit free radical production in vitro. The Veterinary Journal, 199, 162–168.
Rimm, E. B., Stampfer, M. J., Ascherio, A., Giovannucci, E., Colditz, G. A., & Willett, W. C. (1993). Vitamin E consumption and the risk of coronary heart disease in men. New England Journal of Medicine, 328, 1450–1456.
Stampfer, M. J., Hennekens, C. H., Manson, J. E., Colditz, G. A., Rosner, B., & Willett, W. C. (1993). Vitamin E consumption and the risk of coronary disease in women. New England Journal of Medicine, 328, 1444–1449.
Liu, J., Zhang, L., Ren, Y., Gao, Y., Kang, L., & Lu, S. (2016). Matrine inhibits the expression of adhesion molecules in activated vascular smooth muscle cells. Molecular Medicine Reports, 13, 2313–2319.
Byeon, H. E., Park, B. K., Yim, J. H., Lee, H. K., Moon, E. Y., Rhee, D. K., & Pyo, S. (2012). Stereocalpin A inhibits the expression of adhesion molecules in activated vascular smooth muscle cells. International Immunopharmacology, 12, 315–325.
Kim, J. Y., Park, H. J., Um, S. H., Sohn, E. H., Kim, B. O., Moon, E. Y., et al. (2012). Sulforaphane suppresses vascular adhesion molecule-1 expression in TNF-alpha-stimulated mouse vascular smooth muscle cells: Involvement of the MAPK, NF-kappaB and AP-1 signaling pathways. Vascular Pharmacology, 56, 131–141.
Kishimoto, Y., Yoshida, H., & Kondo, K. (2016). Potential anti-atherosclerotic properties of astaxanthin. Marine Drugs, 14, 345.
Owens, C. D. (2012). Statins and other agents for vascular inflammation. Journal of Vascular Surgery, 56, 1799–1806.
Huang, Y., Li, W., Dong, L., Li, R., & Wu, Y. (2013). Effect of statin therapy on the progression of common carotid artery intima-media thickness: An updated systematic review and meta-analysis of randomized controlled trials. Journal of Atherosclerosis and Thrombosis, 20, 108–121.
Profumo, E., Buttari, B., Saso, L., & Rigano, R. (2014). Pleiotropic effects of statins in atherosclerotic disease: Focus on the antioxidant activity of atorvastatin. Current Topics in Medicinal Chemistry, 14, 2542–2551.
Violi, F., Carnevale, R., Pastori, D., & Pignatelli, P. (2014). Antioxidant and antiplatelet effects of atorvastatin by Nox2 inhibition. Trends in Cardiovascular Medicine, 24, 142–148.
Acknowledgments
This work was supported by financial support from the Natural Sciences Foundation of Hunan Province (2016JJ6133), the National Natural Sciences Foundation of China (81100211), the Science and Technology Plan Projects of Hengyang City (Nos. 2013KJ04 and 2014KS34), the Construct Program of the Key Discipline in Hunan Province (Basic Medicine Sciences in University of South China) and the Zhengxiang Scholar (Xiangyang Tang) Program of the University of South China.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest for this article.
Additional information
Hanxiao Ou and Zhuping Huang have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ou, H., Huang, Z., Mo, Z. et al. The Characteristics and Roles of Advanced Oxidation Protein Products in Atherosclerosis. Cardiovasc Toxicol 17, 1–12 (2017). https://doi.org/10.1007/s12012-016-9377-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-016-9377-8