Abstract
Fibrinogen (FB) is a soluble blood plasma protein and is a key molecule involved in coagulation. Oxidative modification of proteins, such as the formation of advanced oxidation protein products (AOPP), a heterogeneous family of protein compounds structurally modified and derived from oxidative stress, may be associated with the pathophysiology of a number of chronic inflammatory diseases. Therefore, the aim of this study was to determine whether the formation of this mediator of inflammation occurs from FB and whether its generation is associated with structural changes. Results of the present study suggest that the oxidation of FB may provoke the formation of AOPP, which in turn, may promote functional alterations in FB, thus causing changes in its structural domains and increasing its procoagulant activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Fibrinogen (FB) is a soluble blood plasma protein and is a key molecule involved in coagulation and hemostasis [1]. It is a dimeric glycoprotein with a molecular mass of 340 kDa that is synthesized primarily in the liver. After albumin and globulins, it represents the third most abundant protein in plasma, with an average concentration of 150–400 mg dL−1 and half-life of 3–5 days [2]. FB is composed of two pairs of three nonidentical chains termed Aα, Bβ, and γ. Together, the chains comprise a symmetrical molecule composed of one globular E region flanked on each side by globular D regions that are connected by three-stranded alpha-helical coiled coils [3, 4]. The E region, which is composed of all three chains, contains fibrinopeptides A and B [3, 5]. Cleavage of these peptides by thrombin exposes knobs A and B, thus resulting in the formation of fibrin monomers [6]. Specific sites in each of these chains are subject to oxidative modification [3]. Oxidative stress has been widely implicated in inflammatory processes related with diabetes mellitus, carcinogenesis, atherogenesis, and especially arterial and venous thrombosis. In this context, proteins are major targets for oxidants, and FB includes a large percentage of plasma proteins, which may be a target for oxidative posttranslational modifications [7].
Numerous mechanisms for the induction of protein modifications may lead to different types of these protein alterations. The detection of protein carbonyl groups is the most frequently used measure the level of protein modification [8]. However, advanced oxidation protein products (AOPP) are also markers of protein oxidation, as well as being mediators of inflammation [9]. AOPP are a heterogeneous family of compounds that are structurally modified and derived from oxidative stress, mainly from HOCl synthesized by myeloperoxidase (MPO), which is an enzyme broadly expressed in cells of the immune system. In some clinical conditions in which inflammation is involved, this enzyme can be constantly active, thus leading to an increased production of HOCl and the accumulation of AOPP in plasma. It has been demonstrated that the spectral characteristics of AOPP correspond to several chromophores, which include dityrosine, carbonyls, and pentosidine [10]. In this context, although albumin is considered the major target for the formation of AOPP, it is known that FB is also a key molecule in the reactivity of this product [11]. However, it has not been established yet whether FB is a source of AOPP formation. Since oxidative and inflammatory processes are implicated in the pathophysiology of a number of clinical conditions that involve the formation of AOPP, and since this biomarker can reflect these changes, it is important to evaluate the susceptibility of other proteins to form AOPP. Furthermore, it is important to investigate whether the formation of these products from FB may be associated with changes in the activity of this procoagulant protein. Therefore, the aim of this study was to determine whether AOPP is formed from FB, and if this generation is associated with structural changes and the appearance of FB fragments associated with band formation.
METHODS
Chemicals and Reagents
Purified human FB, chloramine-T, and citric acid (C6H8O7) were purchased from Sigma Chemical Co. (St. Louis, USA). Potassium iodide (KI) and sodium hydroxide (NaOH) were purchased from Vetec Chemistry (Rio de Janeiro, Brazil). FB solutions were prepared by diluting in 50 mM phosphate buffer (PBS). In this study, the term hypochlorous acid refers to the sum of both HOCl and OCl− species. The hypochlorite concentration was determined spectrophotometrically using a wavelength of 292 nm (ε = 350 M−1cm−1) after dilution with 10 mM NaOH. The assays were performed in 50 mM PBS (pH 7.4). All experiments were carried out in replicates (n = 5).
Treatment of Samples
FB was exposed to HOCl in order to produce FB-AOPP. The FB solution (300 mg dL−1) was incubated for 30 min with different concentrations of HOCl (1, 2, and 4 mM) at 37 °C since it has been reported that this incubation time is suitable for the formation of AOPP [12]. FB samples exposed to HOCl were dialyzed overnight against PBS in order to reduce the HOCl interference. We used 300 mg dL−1 FB because this is close to physiological concentrations of FB. FB incubated only with PBS (FB-PBS) was used as the control. We also tested a sample of FB incubated with NaOH and PBS (FB-NaOH) in order to demonstrate that the NaOH used for diluting HOCl does not interfere with the AOPP levels.
Determination of AOPP
First, a curve with different concentrations of HOCl (1, 2, and 4 mM) was obtained to check the concentration-dependent increase of AOPP. Next, we tested different concentrations of FB (30, 100, and 300 mg dL−1) in order to investigate the association between FB concentrations and AOPP levels. The AOPP concentrations were expressed in chloramines-T equivalents (μmol L−1) and were measured spectrophotometrically using the Cobas Mira® automated analyzer (Roche Diagnostics, Basel, Switzerland) by a method previously described [13].
Spectrophotometric Quantification of FB
In order to investigate the impact of FB exposure to HOCl on its concentration, FB was quantified using the UV-Visible Spectrophotometer UVmini-1240® (Shimadzu, Kyoto, Japan) at λ = 280 nm. The results were obtained from a calculation using the molar extinction coefficient ε = 1.6 mg mL−1 cm−1 [14]. PBS was used as the blank.
SDS-PAGE of FB-AOPP
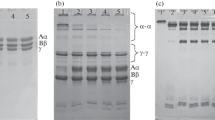
To determine if the oxidation of FB was associated with structural alterations in order to generate bands associated with fragments or aggregates of this protein, FB-AOPP was subjected to electrophoresis as described previously [15] on a 12 % polyacrylamide gel for 2.5 h at a constant current of 30 mA using the Mini-PROTEAN® Tetra Cell (Bio-Rad Laboratories, Hercules, CA, EUA). Gels were stained with Coomassie brilliant blue. FB-PBS was used as a control. The Aα, Bβ, and γ bands were quantified by scanning densitometry using DS-5000® (Loccus Biotechnology, Cotia, SP, Brazil). ImageJ software (National Institutes of Health, USA) was used to determine the concentration of the bands on the gel.
FB Activity Assays
To identify activity alterations of FB exposed to HOCl, we measured its activity through a detection method consisting of light scattering (λ = 660 nm) in the automated coagulation Ca-1500® (Sysmex, Kobe, Japan) using a Thrombin reagent (Sigma Chemical Co; St. Louis, MO, USA).
Statistical Analysis
All experiments were performed in replicates (n = 5). Data are expressed as mean ± standard error and were compared using one-way analysis of variance (ANOVA) followed by Tukey’s test for multiple comparisons. P < 0.05 was considered statistically significant. Statistical analyses were performed using the GraphPad Prism software version 4.00 for Windows (GraphPad Software, San Diego, CA, USA).
RESULTS
Exposure of FB to HOCl promoted the formation of AOPP in a concentration-dependent manner, as shown in Fig. 1. We also performed assays with different concentrations of FB (30, 100, and 300 mg dL−1), and the highest formation of AOPP occurred in FB at a concentration of 300 mg dL−1, as shown in Fig. 2. In addition, we observed lower concentrations of FB in the FB-AOPP group (Fig. 3). Based on this observation, FB-AOPP was subjected to SDS-PAGE so that we could observe the band formation with protein aggregates of higher molecular weight, as well as band alterations in the FB Aα, Bβ, and γ chains in relation to FB-PBS (Fig. 4). In addition, densitometric scanning of the gel showed that FB concentrations decreased in the Aα, Bβ, and γ bands (FB-PBS: Aα = 49.4 mg dL−1, Bβ = 29.8 mg dL−1, and γ = 31.7 mg dL−1; FB-NaOH: Aα = 42.3 mg dL−1, Bβ = 34.2 mg dL−1, and γ = 27.2 mg dL−1; FB-AOPP: Aα = 11.2 mg dL−1, Bβ = 15.4 mg dL−1, and γ = 16.5 mg dL−1) and the percentage of FB degradation in FB-AOPP in relation to FB-PBS was 32 %. As shown in Fig. 5, FB exposed to HOCl had increased activity when compared to FB-PBS, thus demonstrating that the formation of AOPP may lead to decreased clotting time.
Effects of FB oxidation on the electrophoretic pattern. Proteins bands on a 12 % SDS-PAGE gel were visualized by Coomassie Blue staining. The positions of FB-AOPP, FB-NaOH, and FB-PBS are indicated. Approximately 15 μL of sample was added to each lane. Molecular masses are shown on the left. A Protein aggregates of higher molecular mass. The intensity of the bands of the Aα, Bβ, and γ FB chains were quantified by densitometry. The experiment was repeated at least three times.
DISCUSSION
In the present study, we demonstrated that FB is a target of HOCl-induced damage and may be a source for the formation of AOPP. In this context, HOCl oxidation significantly increased FB-AOPP levels in a concentration-dependent manner. Although albumin is the major protein susceptible to the formation of AOPP [9], Selmeci et al. [11] demonstrated that plasma samples showed increased levels of AOPP in relation to serum samples, suggesting that FB could be involved in the reactivity of AOPP. Interestingly, this notion was supported by the observation that higher FB concentrations were associated with an enhanced molar ratio of AOPP to FB [11]. In this present study, we have demonstrated that incubating FB with different concentrations of HOCl induced an increase in AOPP formation in a concentration-dependent manner, thus suggesting that FB may be involved not only in the reactivity of AOPP, but also in the formation of these products.
AOPP is a heterogeneous family of structurally modified proteins that are derived from oxidative stress, specifically from HOCl synthesized by MPO [16]. HOCl produced by myeloperoxidase is likely the major oxidant from neutrophils, and may be an important contributor to inflammatory tissue injury. The plasma fibronectin oxidized by the myeloperoxidase system and by reagent HOCl resulted in the loss of tryptophan and cysteine [17]. FB treatment with HOCl leads to the preferential oxidation of specific methionine residues on the α, β, and γ chains. The oxidation of one or all of these residues is associated with reduced lateral aggregation of protofibrils, resulting in gels with smaller fibers and higher fiber density when compared to untreated fibrin gels [18]. Therefore, during pro-oxidant conditions associated with the formation of HOCl, FB may be a target of HOCl, thus triggering the formation of AOPP concomitantly with the formation of AOPP from albumin. In addition, AOPP formation from FB may promote functional and structural changes in this protein and may contribute to disorders in the coagulation process.
FB is a dimeric glycoprotein with a molecular mass of 340 kDa that is synthesized primarily in the liver [2]. It plays an essential role in blood coagulation and platelet aggregation and is also involved in inflammatory processes and atherogenesis. It is critical protein for clot formation, both in the fibrin network and in platelet aggregation, which are ultimately required for the generation of the hemostatic thrombus. Perturbations in these functions may influence the formation and properties of the fibrin network and promote pathological states, including thrombosis and thromboembolism [19, 20]. In this context, oxidative stress leads to covalent oxidative modifications of plasma proteins, including FB. It has been shown elsewhere that oxidized FB has an increased ability to form fibrin and that acetylation prevents the enhancement of clot formation [21]. We have also demonstrated that the FB exposure to HOCl promoted an increase in FB activity.
It has been reported that the treatment of FB with oxidizing reagents increased the concentration of the carbonyl proteins to approximately 20 times that of the levels observed in non-stressed cells. The formation of dityrosine, as well as the loss of tryptophan during the oxidation of FB [22], was also observed. In the present study, we demonstrated that FB treated with HOCl induced the formation of AOPP. We also observed changes in the concentration and activity of the FB. We detected a decrease in the FB concentration, which was in contrast to the increase in FB activity that we observed. Furthermore, we have also shown the presence of structural changes in FB incubated with HOCl, as well as alterations in the band content of the Aα, Bβ, and γ chains. We infer from this that the formation of high mass bands is likely a result of protein aggregation of FB. Moreover, based on the densitometry scanning analysis of the gels, a decrease in the FB concentration in the bands of the sample oxidized by HOCl was observed, thus suggesting that the change in the activity of this protein may be specifically related to changes in these structural domains of the molecule.
The main findings of this study was to demonstrate that the oxidation of FB may lead to the formation of AOPP in this in vitro model, and that this formation may promote functional alterations in FB, thus causing changes in their structural domains and increasing its activity. Finally, we suggest that the formation of FB-AOPP may contribute for the generation of thrombosis and that FB, as well as albumin, may be a source of AOPP formation. However, additional in vivo studies are required to confirm the role of FB-AOPP in the pathophysiology of thromboembolic diseases.
References
Liu, C.Y., H.L. Nossel, and K.L. Kaplan. 1979. The binding of thrombin by fibrin. The Journal of Biological Chemistry 254: 10421–10425.
Collen, D., G. Tygat, H. Claeys, and R. Piessens. 1972. Metabolism and distribution of fibrinogen. I. Fibrinogen turnover in physiological conditions in humans. British Journal of Haematology 22: 681–700.
Brow, J.H., N. Volkmann, G. Jun, A.H. Henschen-Edman, and C. Cohen. 2000. The crystal structure of modified bovine fibrinogen. Proceedings of National Academy of Sciences of the United States of America 97: 85–90.
Hall, C.E., and H.S. Slayter. 1959. The fibrinogen molecule: its size, shape, and mode of polymerization. The Journal of the Biophysical and Biochemical Cytology 5: 11–16.
Kollman, J.M., L. Pandi, M.R. Sawaya, M. Riley, and R.F. Doolittle. 2009. Crystal structure of human fibrinogen. Biochemistry 48: 3877–3886.
Budzynski, A.Z., S.A. Olexa, and B.V. Pandya. 1983. Fibrin polymerization sites in fibrinogen and fibrin fragments. Annals of the New York Academy of Sciences 408: 301–314.
Martinez, M., J.W. Weisel, and H. Ischiropoulos. Functional impact of oxidative posttranslational modifications on fibrinogen and fibrin clots. 2013. Free Radical Biology and Medicine 65: 411-418.
Dalle-Donne, I., R. Rossi, D. Giustarini, A. Milzani, and R. Colombo. 2003. Protein carbonyl groups as biomarkers of oxidative stress. Clinica Chimica Acta 329: 23–38.
Witko-Sarsat, V., M. Friedlander, C. Capeillère-Blandin, A.T. Nguyen-Khoa, A.T. Nguyen, J. Zingraff, P. Jungers, et al. 1996. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney International 49: 1304–1313.
Capeillere-Blandin, C., V. Gausson, B. Descamps-Latscha, and V. Witko-Sarsat. 2004. Biochemical and spectrophotometric significance of advanced oxidized protein products. Biochimica et Biophysica Acta 1689: 91–102.
Selmeci, L., M. Székely, P. Soós, L. Seres, N. Klinga, and A. Geiger. 2006. Human blood plasma advanced oxidation protein products (AOPP) correlates with fibrinogen levels. Free Radical Research 40: 952–958.
Bochi, G.V., V.D. Torbitz, L.P. Cargnin, M.B. Sangoi, R.C. Santos, P. Gomes, et al. 2012. Fructose-1,6-bisphosphate and N-acetylcysteine attenuate the formation of advanced oxidation protein products, a new class of inflammatory mediators, in vitro. Inflammation 35: 1786–1792.
Hanasand, M., R. Omdal, K.B. Norheim, L.G. Gøransson, C. Brede, and G. Jonsson. 2013. Improved detection of advanced oxidation protein products in plasma. Clinica Chimica Acta 413: 901–906.
Carr, Jr., and J. Hermans. 1978. Size and density of fibrin fibers from turbidity. Macromolecules 11: 46–50.
Laemmli, U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685.
Witko-Sarsat, V., V. Gausson, A.T. Nguyen, M. Touam, T. Drüeke, F. Santangelo, et al. 2003. AOPP-induced activation of human neutrophil and monocyte oxidative metabolism: a potential target for N-acetylcysteine treatment in dialysis patients. Kidney International 64: 82–91.
Margret, C.M., and C.C. Winterbourn. 1991. Oxidative damage to fibrinonectin I. Effects of the Neutrophil myeloperoxidase system and HOCl. The Archieves of Biochemistry and Biophysics 15: 53–59.
Weigandt, K.M., N. White, D. Chung, E. Ellingson, Y. Wang, and X. Fu. 2012. Fibrin clot structure and mechanics associated with specific oxidation of methionine residues in fibrinogen. Biophysical Journal 103: 2399–2407.
Kannel, W.B., P.A. Wolf, W.P. Castelli, and R.B. D’Agostino. 1987. Fibrinogen and risk of cardiovascular disease. The Framingham Study. JAMA 258: 1183–1186.
Stec, J.J., H. Silbershatz, G.H. Tofler, T.H. Matheney, P. Sutherland, I. Lipinska, et al. 2000. Association of fibrinogen with cardiovascular risk factors and cardiovascular disease in the Framingham Offspring Population. Circulation 102: 1634–1638.
Upchurch Jr., G.R., N. Ramdev, M.T. Walsh, and J. Loscalzo. 1998. Prothrombotic consequences of the oxidation of fibrinogen and their inhibition by aspirin. Journal of Thrombosis and Thrombolysis 5: 9–14.
Belisario, M.A., C. Di Domenico, A. Pelagalli, R. Della Morte, and N. Staiano. 1997. Metal-ion catalyzed oxidation affects fibrinogen activity on platelet aggregation and adhesion. Biochimie 79: 449–455.
Acknowledgments
This study was supported by scholarships from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil).
Conflict of Interest
There are no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Torbitz, V.D., Bochi, G.V., de Carvalho, J.A.M. et al. In Vitro Oxidation of Fibrinogen Promotes Functional Alterations and Formation of Advanced Oxidation Protein Products, an Inflammation Mediator. Inflammation 38, 1201–1206 (2015). https://doi.org/10.1007/s10753-014-0085-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-014-0085-x