Abstract
The role of heavy metals on human health has often been discussed regarding disease pathogenesis and risk factors. Alzheimer’s disease (AD), a prevalent neurodegenerative disease, is no exception. We conducted a comprehensive examination to assess the concentrations of lead (Pb), cadmium (Cd), arsenic (As), and mercury (Hg) in biological samples of AD patients in comparison with a group of individuals without AD, with the objective of our study being to perform a systematic review on this topic. We performed this systematic review by searching various databases/search engines, including Web of Science, Scopus, PubMed, and Google Scholar, until December 7, 2022. Relevant studies were scrutinized for eligibility. Meta-analysis was performed on studies with sufficient data using STATA software. Twenty-seven out of 3349 studies met pre-defined eligibility criteria and were included in our systematic review. For the meta-analysis, 22 studies with 3346 participants (1291 AD patients and 2055 healthy controls) had sufficient data for inclusion in the analysis. Using a random-effects model, we found that pooled data indicated patients with AD had significantly higher levels of Cd in their biological samples compared to controls (Hedges’ g, 0.83; 95% CI, 0.11, 1.54; p = 0.023). However, other heavy metals were not significantly different in circulatory samples of AD patients compared to healthy controls (p > 0.05). This systematic review and meta-analysis indicated that Cd concentrations in AD patients were significantly higher than in healthy controls. Other included heavy metals may not be directly associated with increased risk of AD.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is recognized as a global priority by the World Health Organization (WHO). AD is a prevalent neurodegenerative condition that results in cognitive impairment and memory loss, making it the leading cause of dementia [1, 2]. More than 46 million people are diagnosed with dementia worldwide, with approximately 70% of cases involving AD [3,4,5].
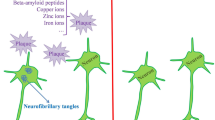
Although several pathological mechanisms have been proposed for AD, two hallmark pathologies involve amyloid-beta peptide (Aβ) formation and tau protein aggregation, causing extracellular plaque deposition and neurofibrillary tangle formations in the brain, respectively [3]. Despite the high prevalence of AD, there is no established treatment [1]. Therefore, characterizing risk factors and prevention is prudent.
The risk of AD is mainly attributed to genetic and environmental factors [5]. Due to the ongoing expansion of industry in developing countries, which results in the emission of toxic airborne chemicals, heavy metal exposures are more commonly reported. Heavy metals are contributors and risk factors in many diseases [5]. For instance, cadmium (Cd) and lead (Pb) are notable for their association with neurotoxicity [5, 6]. Electronic wastes, inexpensive jewelry, and toys are common sources of Cd [6].
In contrast, lead sources include lead-based paints and solders, bullets, ceramics, industrial emissions, contaminated food, water, soil, and alternative health supplements [7]. Arsenic (As) is associated with peripheral neuropathy and oxidative DNA damage [6]. According to the WHO, human exposure to As is primarily due to groundwater consumption and crop irrigation from said water source [6]. Mercury (Hg) exposure in humans can occur via natural and anthropogenic sources such as volcanic eruption, gold mining activities, chemical wastes, and consumption of contaminated seafood and induce AD by Aβ formation via oxidative stress [8, 9]. These toxic metals cross the blood–brain barrier (BBB), with evidence suggesting a link between Aβ formation, tau accumulation, and oxidative stress, leading to apoptosis in neural cells [5]. These metals traverse the BBB with passive diffusion and facilitated transport processes. Pb can easily cross the BBB due to its structural similarity to calcium, allowing it to mimic calcium-dependent transport systems [10] as it enters the tissue using aquaporin channels and sodium-coupled nutrient transporters [11]. Hg can pass through the BBB as the organic and inorganic forms, with the organic methylmercury being more lipophilic and readily crossing the barrier [12]. Lastly, Cd disrupts the integrity of the BBB and enters the brain through divalent metal transporters and calcium channels [13].
A previous meta-analysis study on a similar matter has shown that the level of Pb is significantly lower in AD patients, which cannot be justified due to the toxic effect of Pb on the neurological system [4]. Additionally, it has been stated that Cd and Hg are significantly higher in AD patients compared to controls, however, more related studies require analyses with subgrouping to better contextualize these results [4]. The method used to measure toxic metals and the countries where these studies were conducted can increase heterogeneity and influence the results.
Identifying environmental risk factors of AD may advance and inform a more comprehensive understanding of the disease pathology, which may impact treatment and prevention. Consequently, due to the limited literature reviews on this subject matter, our objective was to conduct a systematic review and meta-analysis to evaluate the concentrations of the four relevant toxic heavy metals (Hg, Pb, Cd, As) in biological samples of AD patients compared to healthy controls.
Methods
Study Design and Literature Search
This systematic review and meta-analysis have been carried out according to PRISMA guidelines. Three independent individuals (AK, RF, and SK) performed an extensive database search until December 7, 2022, utilizing both keywords and MESH terms. There was no date limit, and all studies were included from the earliest time available. We conducted searches in four databases/search engines, namely Web of Science, Scopus, PubMed, and Google Scholar, using MESH terms and keywords. The searches were performed on the title or abstract of the studies. The search strategies employed in all four databases are detailed in Table 1. The records of different databases were exported and managed using Mendeley software. We also added our systematic review to PROSPERO registration with ID CRD558007.
Eligibility Criteria
Inclusion Criteria
Studies that investigated human blood or cerebrospinal fluid (CSF) concentrations for at least one of the heavy metals of interest were included. Studies with two groups of AD patients and controls were considered. Since studies are mainly performed in adults due to AD, we did not consider an age limit for the systematic review and meta-analysis. No limitations on language and time of studies were imposed, and articles in English language or at least one English abstract were included. The references of relevant studies were also assessed for additional related documents. Studies that reported mean and standard deviation for the concentration of the metals in two groups of AD patients and healthy controls were included. The results of relevant articles with insufficient data (did not have any numerical form of report for the concentrations of metals that can be analyzed) for meta-analysis were only reported in our systematic review section. In all the studies, the control groups were age- and gender-matched with the AD group. Further, controls were healthy and had not been diagnosed with AD or another neurodegenerative disease.
Exclusion Criteria
Review studies, books, letters to the editor, and experimental studies were not included. Irrelevant studies that did not report outcomes of interest were also excluded in the different stages of document screening. First, the total records of the databases were pooled and the duplicates were deleted. Next, the studies that did not meet our inclusion criteria were excluded after a review of their titles and abstracts. The remaining studies that were deemed relevant underwent a full-text examination.
A third author (AK) resolved the discrepancies between the reviewers regarding article inclusion.
Data Extraction
Information from related studies is obtained and presented in Table 2. An electronic data abstraction form was used for recording study characteristics, such as the name of the first author, year, country, type of study, diagnostic criteria for AD, sample size, mean age of participants, biological sex, assessment method for different metals in circulatory samples, type of body fluid, and the main results of the study. For studies in the full text that have yet to be found, we included them if the data were enough for analysis and inclusion in the systematic review by abstract. If full text was unavailable, we emailed the authors and did our best to find any relevant data’s full text. Therefore, we have included more studies than other similar studies, which can make our findings more precise.
Quality Assessment
A pre-defined checklist was used to appraise the quality of the included studies. Each study was evaluated by two reviewers using the Joanna Briggs Institute (JBI) critical assessment tool. The discrepancy was rechecked by a third researcher (SN). The JBI tool consists of questions that must be answered with “yes” or “no.” If the answer is “yes,” it earns one point, while a “no” or unclear answer earns zero points [14]. No studies that met our research criteria were excluded from the quality evaluation stage.
Meta-Analysis
The meta-analysis was conducted using STATA software (V.17). The I-squared (I2) and chi-square–based Q-test assessed heterogeneity in the included studies. If a considerable heterogeneity (I2 statistic more than 70% and p-value of Q-test < 0.1) was observed, we analyzed the pooled estimates with a random-effects model. The publication bias was examined using Egger’s regression and Begg’s rank correlation tests. The statistical analysis was conducted using Hedge’s g to calculate the effect sizes for the primary outcome measures. Hedge’s g was chosen to provide an unbiased estimate of the effect size, accounting for potential differences in sample sizes between conditions.
Effect size estimates and their 95% confidence intervals are reported to indicate the magnitude of treatment effects.
Most studies used μg/dl as the unit; the data for other units has been converted to μg/dl. Most studies reported data with mean and standard deviation (SD). Some used the median ± interquartile range, which has been converted into mean and SD. No study was excluded in this section unless the data were incomplete.
Subgroup analysis has been done to reduce possible heterogeneity and increase our knowledge about the findings. We used the human development index (HDI), body fluids, and measurement method for subgrouping, as these results were available in most studies. Studies that did not mention this information were titled as not mentioned (NM) in the analysis.
To investigate the effects of age and gender on our outcomes, we employed a meta-regression analysis.
Results
Study Characteristics
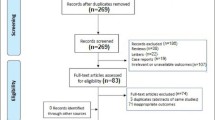
Various databases/search engines yielded 7399 studies. Three thousand eight hundred fifty-four articles were deleted as duplicates using Mendeley software. Afterward, we examined the remaining research papers and eliminated 3286 because they were either duplicates, reviews, letters, or books, or did not match our inclusion criteria. Subsequently, we assessed the complete content of the remaining studies and removed 232 of them. Ultimately, 27 studies met the study inclusion criteria for the quality assessment and systematic review (Fig. 1).
The included studies measured at least one of the four heavy metals of interest (Pb, As, Cd, Hg) in patients with AD and healthy controls’ serum, plasma, or CSF. Among 27 studies, 22 had sufficient data to be analyzed and were included in the meta-analysis. The publication dates for the included studies span from 1991 to 2022. The concentrations of the heavy metals were assessed using different methods, primarily using inductively coupled plasma (ICP) [15,16,17,18,19,20,21] or atomic absorption spectroscopy (AAS) [22,23,24,25,26]. Most studies used NINCDS-ADRDA (National Institute of Neurological and Communicative Disorders-Alzheimer’s Disease and Related Disorders Association) criteria for diagnosis of AD [15,16,17,18,19,20,21,22,23,24,25,26]. The results are shown in Table 2.
Systematic Review and Meta-analysis
Cadmium (Cd)
Eighteen studies measured Cd concentrations in different body fluids, including CSF and blood, in AD patients and controls [15,16,17, 19, 23, 24, 26,27,28,29,30,31,32,33,34,35,36,37].
Three studies measured CSF concentrations of Cd [15, 27, 34]. Two reported non-significant results [15, 34], while the other reported lower Cd concentrations of CSF in AD patients compared to the controls [27].
Six studies measured Cd in whole blood [24, 28, 31, 33, 35, 36]. Four reported non-significant results [28, 31, 33, 36], while two reported higher Cd concentrations in the whole blood of AD patients compared to controls [24, 35].
Nine studies measured serum Cd concentrations [16, 17, 23, 26, 29,30,31,32,33]. Five studies reported non-significant results [17, 29,30,31,32], while four others reported higher Cd concentrations in the serum of AD patients [16, 23, 26, 33].
Four studies measured the plasma concentrations of Cd [15, 19, 27, 37]. Three reported a non-significant difference in plasma concentrations of Cd in AD patients compared to controls [15, 19, 37], and one reported higher concentrations in patients with AD [27].
Also, for the meta-analysis, the random effect model pooled Cd concentrations in 618 AD patient samples compared to 819 healthy controls (12 studies). The results showed that Cd concentrations in AD patients were significantly higher than in controls (Hedges’ g, 0.80; 95% CI, 0.16, 1.45; p = 0.014) (Fig. 2). The pooled studies were heterogeneous (I2 = 97.04%, Q = 112.48, p < 0.001).
a, b Point and pooled estimates of Hedge’s g effect size, along with 95% confidence intervals for the concentration of Cd in patients with AD when compared to healthy controls in the subgroups of different body fluids (plasma, serum, whole blood) and assessment methods (AAS, ICP). Additionally, heterogeneity indices and the p-value for Cochran’s Q-test of heterogeneity are provided (Cd, cadmium; AD, Alzheimer's disease; ICP, individually coupled plasma; AAS, atomic absorption spectrometry; HDI, human development index; K, number of studies)
The Egger’s regression test suggested the presence of a publication bias (z = 3.69, p = 0.0002).
The results of subgroup analysis based on different samples showed that pooled concentrations of Cd in serum were significantly higher in AD patients than in controls (Hedges’ g, 1.22; 95% CI, 0.01, 2.42; p = 0.047). Additionally, the subgroup analysis based on the assessment method showed that Cd concentrations had significant differences in the ICP method (Hedges’ g, 0.50; 95% CI, 0.16, 0.83; p = 0.004) but not in the AAS method. Results in both developing and developed countries in human development index (HDI) subgrouping showed significantly higher Cd concentrations in AD patients compared to controls (respectively; Hedges’ g, 2.25; 95% CI, 0.54, 3.96; p = 0.010; Hedges’ g, 0.26; 95% CI, 0.07, 0.44; p = 0.006).
The results of meta-regression analyses revealed that continuous factors such as age (β = 0.25, p = 0.01) were positively related to Cd concentrations, but gender (female) (β = − 0.20, p = 0.002) was negatively associated with Cd concentrations.
Arsenic (As)
Seven studies measured As concentrations in various body fluids in AD patients versus healthy controls [16, 20, 27, 30, 32, 35, 37]. Two studies measured As concentrations in the plasma [27, 37]. Four studies measured As in the serum of AD patients and controls [16, 20, 30, 32].
All the studies found no statistically significant differences in As concentrations in AD patients compared to controls. However, one study measured As serum concentrations and found higher concentrations in AD patients than in healthy controls [16]. The study that measured As concentrations in whole blood reported that As was undetected [35].
As for the meta-analysis, five studies compared blood As concentrationss of 180 AD patients with 175 controls. This analysis showed a non-significant difference in As concentrations in AD patients’ blood compared to healthy controls (Hedges’ g, 0.77; 95% CI, − 0.57, 2.11; p = 0.260) (Fig. 3). Heterogeneity was found in the pooled studies (I2 = 97.02%, Q = 56.55, p < 0.001). The Begg’s rank correlation test suggested no publication bias (z = 1.71, p = 0.0864). Furthermore, meta-regression analyses explained that age (β = 0.02, p = 0.40) and gender (female) (β = 0.006, p = 0.76) had no statistically significant impact on As concentrations.
a, b Point and pooled estimates of Hedge’s g effect size, along with 95% confidence intervals for the concentration of As in patients with AD when compared to healthy controls in the subgroups of different body fluids (plasma, serum, whole blood) and assessment method (ICP). Additionally, heterogeneity indices and the p-value for Cochran’s Q-test of heterogeneity are provided (As, arsenic; AD, Alzheimer's disease; ICP, individually coupled plasma; HDI, human development index; K, number of studies)
Lead (Pb)
Twenty-two studies measured Pb concentrations in different body fluids, including CSF and blood, in AD patients and controls [15,16,17,18,19, 21, 23,24,25,26,27, 29,30,31,32,33,34,35,36,37,38,39].
Four studies measured Pb concentrations in CSF [14, 30, 34, 38]. Two reported significantly lower Pb concentrations in AD patients [15, 34] and two reported a non-significant difference compared to controls [34, 38].
Six studies measured Pb concentrations in whole blood [21, 24, 31, 33, 35, 36]. Two documents reported lower Pb concentrations [21, 31], two reported no significant difference [33, 36], one reported higher Pb concentrations [24], and one reported Pb as undetectable in AD patients’ whole blood compared to controls [35].
Twelve studies measured Pb serum concentrations [16,17,18, 23, 25, 26, 29,30,31,32,33, 39]. Eight studies reported non-significant differences in serum Pb concentrations in AD patients and controls [17, 18, 29,30,31,32,33, 39] and four reported higher Pb serum concentrations in AD patients [16, 23, 25, 26].
Five studies measured Pb plasma concentrationsof AD patients and controls [15, 19, 27, 37, 38]. Four reported that the Pb plasma concentrations were not significantly different between AD patients and controls [15, 19, 27, 37] and one reported higher Pb plasma concentrations [38].
In the meta-analysis, this section included 1042 AD patient samples and 1657 control samples (16 studies). The findings obtained from the random effect model revealed there was no noticeable variation in the Pb concentrations between individuals with AD and those without the disease (Hedges’ g, 0.38; 95% CI, − 0.09, 0.85; p = 0.110) (Fig. 4). The pooled studies were heterogeneous (I2 = 96.78, Q = 152.01, p < 0.001). There was publication bias based on Egger’s regression test results (z = 3.05, p = 0.0023). Subgroup analysis indicated a higher concentration of Pb in the AAS method (Hedges’ g, 2.03; 95% CI, 0.63, 3.42; p = 0.004) and developing countries (Hedges’ g, 1.83; 95% CI, 0.9, 2.76; p < 0.001) in AD patients compared to healthy controls.
a, b Point and pooled estimates of Hedge’s g effect size, along with 95% confidence intervals for the concentration of Pb in patients with AD when compared to healthy controls in the subgroups of different body fluids (plasma, serum, whole blood) and assessment methods (AAS, ICP). Additionally, heterogeneity indices and the p-value for Cochran’s Q-test of heterogeneity are provided (Pb, lead; AD, Alzheimer's disease; ICP, individually coupled plasma; AAS, atomic absorption spectrometry; HDI, human development index; K, number of studies; NM, not mentioned)
Furthermore, meta-regression analyses explained that age (β = − 0.02, p = 0.82) and gender (female) (β = − 0.004, p = 0.98) had no statistically significant impact on Pb concentrations.
Mercury (Hg)
Eighteen studies measured Hg concentrations in the body fluids of AD patients and healthy controls [15, 16, 21,22,23, 27, 29,30,31,32,33,34,35,36,37,38, 40, 41].
Three studies measured CSF mercury concentrations and reported non-significant results [15, 27, 34].
Among seven studies that measured Hg whole blood concentrations [21, 22, 31, 33, 35, 36, 40], one reported lower [33] and two reported higher [21, 31] Hg concentrations [22, 35] in AD patients compared to controls. Other studies reported non-significant results [21, 31, 36, 40].
Seven studies measured serum Hg concentrations [16, 23, 29,30,31,32,33]. One reported lower [30] and three reported higher serum Hg concentrations in AD patients [1, 32, 33]. On the other hand, three studies indicated no significant change in serum Hg concnetrations between individuals with AD and those without the disease [16, 23, 33].
Among three studies that measured plasma Hg concentrations [15, 37, 38], two reported a non-significant result [37, 38], while others reported higher Hg concentrations in AD patients [15].
Regarding the meta-analysis, among 714 AD patient samples and 1046 control samples (13 studies), there was no significant difference between these two groups (Hedges’ g, 0.35; 95% CI, − 0.65, 1.35; p = 0.492) (Fig. 5). The pooled studies were heterogeneous (I2 = 99.03, Q = 569.78, p < 0.001).
a, b Point and pooled estimates of Hedge’s g effect size, along with 95% confidence intervals for the concentration of Hg in patients with AD when compared to healthy controls in the subgroups of different body fluids (plasma, serum, whole blood) and assessment methods (AAS, ICP). Additionally, heterogeneity indices and the p-value for Cochran’s Q-test of heterogeneity are provided (Hg, mercury; AD, Alzheimer's disease; ICP, individually coupled plasma; AAS, atomic absorption spectrometry; NM, not mentioned; HDI, human development index; K, number of studies)
The Egger’s regression test did not reveal any evidence of publication bias (z = 1.81, p = 0.0697). Results in subgroup analysis indicated higher Hg concentrations in AD patients in developing countries compared to healthy controls (Hedges’ g, 1.84; 95% CI, 0.78, 2.91; p = 0.001). Furthermore, meta-regression analyses explained that age (β = 0.06, p = 0.17) and gender (female) (β = − 0.02, p = 0.21) had no statistically significant impact on Hg concentrations.
Discussion
Heavy metals have a well-documented history as risk factors for myriad diseases, including various cancers, autoimmune diseases, and neurocognitive disorders [42,43,44,45]. Data on four commonly implicated toxic heavy metals were gathered.
Our analysis indicated that there is a significant increase in Cd levels in the blood of individuals with AD compared to healthy controls. Furthermore, the subgroup analysis indicated a significant elevation in of serum Cd concentrations when employing the ICP method. However, Cd concentrations measured using the ICP method showed tighter confidence intervals than those measured using AAS. Additionally, we added a subgroup analysis based on the Human Development Index (HDI) sorted by the International Monetary Fund (IMF) and the United Nations (UN) [46]. Our HDI subgrouping indicated that developing and developed countries have statistically higher circulatory Cd concentrations in AD patients than in healthy controls. This finding requires further investigation. One hypothesis is that higher Cd concentrations might be related to poor regulation and increased exposure to industrial Cd in developing countries [47]. Cd has garnered increasing attention due to exposure through tobacco smoking and food products. Tobacco can cause the accumulation of considerable Cd in smokers, while polluted soil and water can lead to Cd accumulation in plants and crops [48]. The exact mechanism of Cd pathogenicity in inducing AD has not been clearly illustrated; however, evidence suggests Aβ formation as a primary pathological contributor to AD [49]. An increase in the level of these toxic metals is capable of leading to AD pathogenicity. The Cd excretion rate is relatively low and can increase the BBB’s permeability [50]. Cd can also interfere with neurotransmitters [50], which are capable of increasing dopamine and 5-hydroxytryptamine (5-HT) in specific brain regions, as shown in animal models [51].
Arsenic is associated with carcinogenic effects [52]. Although the pathogenicity hypothesis of As in AD is not robust, with the exact mechanism unestablished, some studies propose cell proliferation and DNA-methylated oxidative stress. Altered DNA repair may be closely associated with these effects [52]. It has also been proposed that As can invoke brain inflammatory responses and cause tau and Aß formation [53]. It triggers the generation of reactive oxygen species (ROS) and impairs mitochondrial function in brain tissue. This fact leads to the activation of apoptotic pathways, causing neuronal cell death and brain tissue degeneration [54]. The outcomes of our study suggest there is no significant alteration in As concentrations among individuals with AD compared to healthy individuals, as observed in the subgroups. Due to the limited number of studies, this metal subgrouping has relatively larger confidence intervals and none of the subgrouping was significant.
Lead is a historically well-known toxic metal that can cause brain white matter damage and cell death [55]. Exposure can affect multiple organ systems, namely the cardiovascular, renal, and reproductive systems [56]. Studies showed that Pb can enter BBB by calcium channels and has an approximate half-life of two years. After entering the brain tissue, Pb can suppress neurotransmitter release and cause neural death by oxidative stress pathways [57]. Pb can substitute for calcium in various cellular processes, leading to the impairment of tight junctions and increased permeability of the BBB [58]. This phenomenon allows for the enhanced entry of Aβ peptides into the brain, which play a crucial role in AD pathogenesis [59]. In addition, some studies have suggestedt an increase in Pb concentrations can lead to DNA methylation and neurodegenerative diseases, including AD [56]. Although our overall analysis indicated that circulatory Pb concentrations are not significantly different between AD patients and controls, residents of developing countries—specifically India, Turkey, and Iran—tend to demonstrate higher Pb concentrations in their AD patients. This phenomenon has not been well studied, however, we hypothesize that this may be related to more common industrial exposures and weaker regulatory measures in said countries [7]. Further, using the AAS method, Pb concentrations were significantly higher in AD patients than in healthy individuals with a wide confidence interval. Developing countries again indicated that the Pb concentrations are higher in AD patients than in controls. Though, as previously discussed, there is limited evidence requiring further investigation regarding this association [60].
No significant change was observed in the circulating Hg concentrations among individuals with AD except in two studies performed in Iraq, the only developing country measuring Hg concentrations that showed slightly higher concentrations in AD patients compared to controls. Despite this, Hg has been proposed to influence AD pathogenesis. Major Hg sources include anthropogenic sources, fish consumption, nature exposure, and vaccines [61, 62]. In addition, experimental studies have shown that in some concentrations, Hg may be associated with increased oxidative stress pathways, the creation of neurofibrillary tangles, glutathione consumption, and Aß formation [61]. Hg, particularly in its organic form (methylmercury), can cross the BBB and accumulate in the brain. This metal impairs mitochondrial function and disrupts synaptic transmission, which can lead to neuronal dysfunction and even death [12]. Similar to the other metals, the subgroup analysis of Hg concentrations showed that the ICP method again demonstrated a smaller confidence interval and non-significant differences verus the AAS method. The developing countries demonstrated a significant increase in Hg concentrations in AD patients compared to controls. In body fluid subgrouping, there was no significant difference between AD and controls Hg concentrations in any of the measured fluids.
Another noteworthy result of this meta-analysis pertains to the HDI subgroup analysis. Our results indicated that pooled data for developing countries had wider confidence intervals with all four toxic heavy metals of interest than developed countries’ relatively narrow confidence intervals. This conclusion may be influenced by stricter protocols and advanced equipment indeveloped countries in measuring blood heavy metal concentrations versus developing countries.
Previous studies on this review had limitations, and therefore, our study willfurther contextualize this subject matterr. Previous reviews included less related data, and the results, as a study published previously, included 14 studies on toxic metals while we included 27 studies. Also, a similar previous study did not include As in the toxic metals. We also used new subgrouping methods, such as HDI, which had not been discussed previously.
Limitations
There are several limitations in our systematic review and meta-analysis. The results should be interpreted cautiously, considering the variation in outcomes with different methods and high heterogeneity. Future prospective studies are warranted to evaluate circulatory toxic heavy metals concentrations (measuring intended metals with ICP and AAS methods to obtain more reliable results) and their potential pathogenic roles in AD patients. Another limitation of our study is the possible bias that the diagnosis of AD in different studies may cause. There is no other way to improve this bias; however, we recommend that AD diagnoses be predicated upon up-to-date, evidence-based clinical criteria.
Conclusion
Our findings demonstrated a potential relationship between higher overall Cd concentrations, higher Hg concentrations in developing countries, and Pb concentrations with AD pathogenicity. Conversely, the circulatory concentrations of As were similar in AD patients and healthy controls. These findings may be used for larger-scale prospective studies to assess heavy metal concentrations and their physiological roles and implications in AD patients. It is essential to establish safety thresholds and interpretations of the heavy metal concentrations in various body fluids and continuously reduce or mitigate toxic heavy metal sources as AD prevalence rises. Our results demonstrate that developing countries exhibit higher correlations between the heavy metals of interest and AD occurrence. Therefore, early comprehensive screenings may be effective interventions as secondary prevention measures that can help identify risk factors, aid in source control, and inform more timely diagnosis and management.
Data Availability
No datasets were generated or analysed during the current study.
References
Scheltens P, Blennow K, Breteler MM, De Strooper B, Frisoni GB, Salloway S, Van der Flier WM (2016) Alzheimer’s disease. Lancet (London, England) 388:505–517
Ghazal TM, Issa G (2022) Alzheimer disease detection empowered with transfer learning. Comput Mater Contin 70:5005–5019
Qiu C, Kivipelto M, Von Strauss E (2009) Epidemiology of Alzheimer’s disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin Neurosci 11(2):111–28
Xu L, Zhang W, Liu X, Zhang C, Wang P, Zhao X (2018) Circulatory levels of toxic metals (aluminum, cadmium, mercury, lead) in patients with Alzheimer’s disease: a quantitative meta-analysis and systematic review. J Alzheimer’s Dis 62:361–372
Bakulski KM, Seo YA, Hickman RC, Brandt D, Vadari HS, Hu H, Park SK (2020) Heavy metals exposure and Alzheimer’s disease and related dementias. J Alzheimer’s Dis 76:1215–1242
World Health Organization (2023) Exposure to lead: a major public health concern. Preventing disease through healthy environments, 3rd edn. World Health Organization
Obeng-Gyasi E (2019) Sources of lead exposure in various countries. Rev Environ Health 34:25–34
Klotter J (2007) Alzheimer’s disease & mercury. Townsend Lett: Examiner Alternative Med 287:36–8
Hightower JM, Moore D (2003) Mercury levels in high-end consumers of fish. Environ Health Perspect 111:604–608
Lidsky TI, Schneider JS (2003) Lead neurotoxicity in children: basic mechanisms and clinical correlates. Brain 126:5–19
Rao MV, Sharma PSN (2001) Protective effect of vitamin E against mercuric chloride reproductive toxicity in male mice. Reprod Toxicol 15:705–712
Clarkson TW, Magos L (2006) The toxicology of mercury and its chemical compounds. Crit Rev Toxicol 36:609–662
Brzóska MM, Moniuszko-Jakoniuk J (2001) Interactions between cadmium and zinc in the organism. Food Chem Toxicol 39:967–980
JBI’s critical appraisal tools available at: https://jbi.global/critical-appraisal-tools. Accessed: december 2021.
Gerhardsson L, Lundh T, Minthon L, Londos E (2008) Metal concentrations in plasma and cerebrospinal fluid in patients with Alzheimer’s disease. Dement Geriatr Cogn Disord 25:508–515. https://doi.org/10.1159/000129365
Lavanya RD, Reddy BS, Abdul Sattar S, Rao ADP (2021) Trace element imbalances in blood serum of Alzheimer’s disease patients. Spectrosc Lett 54:458–471. https://doi.org/10.1080/00387010.2021.1941124
González-Domínguez R, García-Barrera T, Gómez-Ariza JL (2014) Characterization of metal profiles in serum during the progression of Alzheimer’s disease. Metallomics 6:292–300
Hare DJ, Faux NG, Roberts BR, Volitakis I, Martins RN, Bush AI (2016) Lead and manganese levels in serum and erythrocytes in Alzheimer’s disease and mild cognitive impairment: results from the Australian Imaging, Biomarkers and Lifestyle Flagship Study of Ageing. Metallomics 8:628–632
Guan C, Dang R, Cui Y, Liu L, Chen X, Wang X, Zhu J, Li D, Li J, Wang D (2017) Characterization of plasma metal profiles in Alzheimer’s disease using multivariate statistical analysis. PloS One 12(7):e0178271
Baum L, Chan IHS, Cheung SK-K, Goggins WB, Mok V, Lam L, Leung V, Hui E, Ng C, Woo J (2010) Serum zinc is decreased in Alzheimer’s disease and serum arsenic correlates positively with cognitive ability. Biometals 23:173–179
Giacoppo S, Galuppo M, Calabrò RS, D’Aleo G, Marra A, Sessa E, Bua DG, Potortì AG, Dugo G, Bramanti P, Mazzon E (2014) Heavy metals and neurodegenerative diseases: an observational study. Biol Trace Element Res 161:151–60
Hock C, Drasch G, Golombowski S, Müller-Spahn F, Willershausen-Zönnchen B, Schwarz P, Hock U, Growdon JH, Nitsch RM (1998) Increased blood mercury levels in patients with Alzheimer’s disease. J Neural Transm 105:59–68
Lafta H, AlMayaly I (2021) Potential effect of several heavy metals with Alzheimer’s disease infection. Ann Rom Soc Cell Biol 25:3476–3484
Omotosho IO, Ngwube MR, Abdumalik JO (2020) Oxidative stress, trace and toxic metal levels in Alzheimer’s disease in sub-Sahara Africa. Preprint (Version 1) available at Research Square. https://doi.org/10.21203/rs.3.rs-38308/v1
BabakFathabadi MD, Mohammad Dehghanifiroozabadi MD, Fathabadi B, Dehghanifiroozabadi M, Aaseth J, Sharifzadeh G, Nakhaee S, Rajabpour-Sanati A, Amirabadizadeh A, Mehrpour O et al (2018) Comparison of blood lead levels in patients with Alzheimer’s Disease and healthy people. Am J Alzheimer’s Dis Other Dementias 33:541–547
Arslan A, Tüzün FA, Tamer S, Demir H, Aycan A, Demir C, Tasin M, Gönüllü E (2016) Change of antioxidant enzyme activities, some metals and lipid peroxidation in Alzheimer’s disease. Acta Medica Mediterr 32:1643–1649. https://doi.org/10.19193/0393-6384_2016_5_144
Basun H, Forssell LG, Wetterberg L, Winblad B (1991) Metals and trace elements in plasma and cerebrospinal fluid in normal aging and Alzheimer’s disease. J Neural Transm Park Dis Dement Sect 3:231–258
Basun H, Lind B, Nordberg M, Nordström M, Björkstén K, Winblad B (1994) Cadmium in blood in Alzheimer’s disease and non-demented subjects: results from a population-based study. Biometals 7:130–134
Alimonti A, Ristori G, Giubilei F, Stazi MA, Pino A, Visconti A, Brescianini S, Monti MS, Forte G, Stanzione P, Bocca B (2007) Serum chemical elements and oxidative status in Alzheimer’s disease, Parkinson disease and multiple sclerosis. Neurotoxicolog 28(3):450–6
Paglia G, Miedico O, Cristofano A, Vitale M, Angiolillo A, Chiaravalle AE, Corso G, Di Costanzo A (2016) Distinctive pattern of serum elements during the progression of Alzheimer’s disease. Sci Rep 6. https://doi.org/10.1038/srep22769.
Lee JY, Kim JH, Choi DW, Lee DW, Park JH, Yoon HJ, Pyo HS, Kwon HJ, Park KS (2012) The association of heavy metal of blood and serum in the Alzheimer’s diseases. Toxicol Res 28:93–8
Park J-H, Seo SW, Kim C, Kim SH, Kim GH, Kim ST, Jeon S, Lee JM, Oh SJ, Kim JS et al (2014) Effects of cerebrovascular disease and amyloid beta burden on cognition in subjects with subcortical vascular cognitive impairment. Neurobiol Aging 35:254–260. https://doi.org/10.1016/j.neurobiolaging.2013.06.026
Bocca B, Forte G, Petrucci F, Pino A, Marchione F, Bomboi G, Senofonte O, Giubilei F, Alimonti A (2005) Monitoring of chemical elements and oxidative damage in patients affected by Alzheimer’s disease. Ann Ist Super Sanita [Internet] 41:197–203
Gerhardsson L, Lundh T, Londos E, Minthon L (2011) Cerebrospinal fluid/plasma quotients of essential and non-essential metals in patients with Alzheimer’s disease. J Neural Transm 118:957–962. https://doi.org/10.1007/s00702-011-0605-x
Yadav J, Verma AK, Ahmad MK, Garg RK, Shiuli Mahdi AA, Srivastava S (2021) Metals toxicity and its correlation with the gene expression in Alzheimer’s disease. Mol Biol Rep 48:3245–3252. https://doi.org/10.1007/s11033-021-06386-x
Yang Y-W, Liou S-H, Hsueh Y-M, Lyu W-S, Liu C-S, Liu H-J, Chung M-C, Hung P-H, Chung C-J (2018) Risk of Alzheimer’s disease with metal concentrations in whole blood and urine: a case–control study using propensity score matching. Toxicol Appl Pharmacol 356:8–14. https://doi.org/10.1016/j.taap.2018.07.015
Lin Y-K, Liang C-S, Tsai C-K, Tsai C-L, Lee J-T, Sung Y-F, Chou C-H, Shang H-S, Yang B-H, Lin G-Y, et al (2022) A metallomic approach to assess associations of plasma metal levels with amnestic mild cognitive impairment and Alzheimer’s disease: an exploratory study. J Clin Med 11. https://doi.org/10.3390/jcm11133655.
Zhang S, Liu N, LI Q, Gao M, Cui D, Zhou L (2010) Relationship between plasma heavy metal concentrations and Alzheimer’s disease and vascular dementia. Int J Cerebrovasc Dis 18(8):585–589
Luo X-N, Zhong X-M, Hou L (2012) Content changes of metal elements concentrations in serum and CSF in patients with Alzheimer disease and dementia with Lewy body. J Clin Neurol 25:413–415
Fung YK, Beatty M, Durham T, Meade A, Rack E, Blotcky A, Claassen J (1995) Mercury determination in Alzheimer’s patients. FASEB J 9:A384
Cho J, Noh J, Cho S, Choi JE, Kim C (2017) [P3–534]: Blood mercury level and risks of amnestic mild cognitive impairment and Alzheimer’s disease: a case-control study. Alzheimer’s Dement 13:P1182–P1182
Cojocaru M, Chicoş B (2014) The role of heavy metals in autoimmunity. Rom J Intern Med Rev Roum Med Interne 52:189–191
Vella V, Malaguarnera R, Lappano R, Maggiolini M, Belfiore A (2017) Recent views of heavy metals as possible risk factors and potential preventive and therapeutic agents in prostate cancer. Mol Cell Endocrinol 457:57–72
Sá I, Semedo M, Cunha ME (2016) Kidney cancer. Heavy metals as a risk factor. Porto Biomed J. 1:25–28
Karri V, Schuhmacher M, Kumar V (2016) Heavy metals (Pb, Cd, As and MeHg) as risk factors for cognitive dysfunction: a general review of metal mixture mechanism in brain. Environ Toxicol Pharmacol 48:203–213
World Economic and Financial Surveys World Economic Outlook Database—WEO Groups and Aggregates Information [Internet]. IMF.org. Int. Monet. Fund. [cited 2022 Jun 2]. Available from: https://www.imf.org/external/pubs/ft/weo/2022/01/weodata/groups.htm.
Wang M, Chen Z, Song W, Hong D, Huang L, Li Y (2021) A review on cadmium exposure in the population and intervention strategies against cadmium toxicity. Bull Environ Contam Toxicol 106:65–74
Jain RB (2017) Factors affecting the variability in the observed levels of cadmium in blood and urine among former and current smokers aged 20–64 and≥ 65years. Environ Sci Pollut Res 24:8837–8851
Yano K, Hirosawa N, Sakamoto Y, Katayama H, Moriguchi T (2003) 500 Aggregations of amyloid beta-proteins in the presence of metal ions. Toxicol Lett 144:s134
Wang B, Du Y (2013) Cadmium and its neurotoxic effects. Oxid Med Cell Longev 1:12
Antonio MT, Benito MJ, Leret ML, Corpas I (1998) Gestational administration of cadmium alters the neurotransmitter levels in newborn rat brains. J Appl Toxicol 18(2):83–8
Hughes MF (2002) Arsenic toxicity and potential mechanisms of action. Toxicol Lett 133:1–16
Gong G, O’Bryant SE (2010) The arsenic exposure hypothesis for Alzheimer disease. Alzheimer Dis Assoc Disord 24:311–316
Vázquez Cervantes GI, González Esquivel DF, Ramírez Ortega D, Blanco Ayala T, Ramos Chávez LA, López-López HE, Salazar A, Flores I, Pineda B, Gómez-Manzo S (2023) Mechanisms associated with cognitive and behavioral impairment induced by arsenic exposure. Cells 12:2537
Khalil N, Morrow LA, Needleman H, Talbott EO, Wilson JW, Cauley JA (2009) Association of cumulative lead and neurocognitive function in an occupational cohort. Neuropsychology 23:10
Bihaqi SW (2019) Early life exposure to lead (Pb) and changes in DNA methylation: relevance to Alzheimer’s disease. Rev Environ Health 34:187–195
Reuben A (2018) Childhood lead exposure and adult neurodegenerative disease. J Alzheimers Dis 64:17–42. https://doi.org/10.3233/JAD-180267.Cited:in: (PMID: 29865081)
Zheng W, Aschner M, Ghersi-Egea J-F (2003) Brain barrier systems: a new frontier in metal neurotoxicological research. Toxicol Appl Pharmacol 192:1–11
Jomova K, Valko M (2011) Advances in metal-induced oxidative stress and human disease. Toxicology 283:65e87
Ericson B, Landrigan P, Taylor MP, Frostad J, Caravanos J, Keith J, Fuller R (2016) The global burden of lead toxicity attributable to informal used lead-acid battery sites. Ann Glob Heal 82:686–699
Mutter J, Naumann J, Sadaghiani C, Schneider R, Walach H (2004) Alzheimer disease: mercury as pathogenetic factor and apolipoprotein E as a moderator. Neuroendocrinol Lett 25:331–339
Sundseth K, Pacyna JM, Pacyna EG, Pirrone N, Thorne RJ (2017) Global sources and pathways of mercury in the context of human health. Int J Environ Res Public Health 14:105
Author information
Authors and Affiliations
Contributions
AK, SN, OM, AN, RF, and SK participated in the creation, planning, and writing of the manuscript. AK, SN, AA, RF, and SK were responsible for collecting and analyzing the data, as well as providing input for its interpretation. They played roles in gathering and examining the information, and all authors (AK, SN, OM, RF, AN, SK, VV, and AA) contributed to the drafting and critical review of the manuscript, ensuring its intellectual integrity. The final version of the manuscript was reviewed and approved by all authors.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kooshki, A., Farmani, R., Mehrpour, O. et al. Alzheimer’s Disease and Circulatory Imbalance of Toxic Heavy Metals: A Systematic Review and Meta-analysis of Clinical Studies. Biol Trace Elem Res (2024). https://doi.org/10.1007/s12011-024-04326-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12011-024-04326-x