Abstract
Objective
To investigate whether high serum homocysteine (Hcy) levels is associated with the risk of developing Alzheimer’s disease (AD) by performing a meta-analysis based on updated published data.
Methods
We conducted a comprehensive research using Medline (Pubmed), Scopus, Web of Science and EMBASE databases to identify all prospective studies published any time to July 7, 2020 evaluating the association between elevated Hcy levels and AD risk.
Results
From an initial screening of 269 published papers, 9 prospective investigations conducted on a total of 7474 subjects with mean follow-up of 9.5 years (range: 3.7–10) were included in the meta-analysis. Eight seventy-five of these subjects converted to AD. Hcy was significantly higher in these individuals (HRadjusted:1.48, 95% CI:1.23–1.76, I2=65.6%, p<0.0001) compared with who did not convert to AD. There was a significant publication bias (Egger’s test, t=6.39, p=0.0003) and this was overcome by the trim and fill method, which allowed to calculate a bias-corrected imputed risk estimate of HRadjusted:1.20, 95% CI:1.01–1.44, Q value=41.92.
Conclusions
The present meta-analysis found that having higher Hcy increases the risk of AD in the elderly and this finding is consistent with the widely suggested role of this non-proteinogenic α-amino acid in AD neurodegeneration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mounting epidemiological and clinical evidences have demonstrated a considerable overlap between Vascular dementia (VaD) and Alzheimer’s disease (AD) (1, 2). The emerging scenario highlights that cardiovascular disease (CVD), atherosclerosis, and cerebral microvasculature abnormalities mutually interact promoting neurodegeneration since the earliest stage of AD, and influencing the disease progression (3, 4). In support of this view, several studies have demonstrated the presence of an association between cardiometabolic risk factors and development AD, besides VAD (5–9).
In this regard, hyperhomocysteinemia (H-Hcy), which represents a well-established cardiovascular risk factor (10), represents an emblematic example in this frame. The first solid demonstration showing that increased H-Hcy is an independent risk factor for the development of AD, and more in general dementia, was presented in 2002 (11). Since then, several epidemiological studies have been consistent with this finding, (12, 13), suggesting Hcy as a potential target for both non- and pharmacological treatments (14). Unfortunately, the causality of H-Hcy in AD has not yet been definitely confirmed, although experimental evidence clearly suggests its implication in pathogenic mechanism of the neurodegenerative disease (15, 16). One of the most intriguing hypotheses linking H-Hcy and AD onset, is inspired by the role of Hcy in the metabolism of methionine, and the importance of the latter in phosphatidylcholine synthesis. Indeed, Hcy is a product of methionine catabolism, but it can also be recycled back to the essential amino acid by the vitamin-B12 dependent methionine synthase, as well as via a folate-independent pathway (17, 18). Owing the crucial role of these two vitamins in methionine synthesis, a deficiency of either of them can result in H-Hcy and low bioavailability of methionine (19). In turn, a decrease in methionine may cause a lower synthesis of phosphatidylcholine (methionine is a precursor of this phospholipid), which serves as important carrier for docosahexaenoic acid (DHA) through the blood-brain barrier. Importantly, DHA is the most abundant fatty acid in the brain and its deficiency is associated with AD (20).
The clinical relevance of the topic, prompted us to provide an updated systematic review and meta-analysis based on published prospective studies evaluating the role of H-Hcy and the risk of AD.
Methods
Search strategy
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline (Supplementary file 1) (21). Data were obtained searching MEDLINE, Scopus and Web of Science and EMBASE for all prospective studies in English language and without age restrictions, published any time to July 7, 2020 evaluating the association between H-Hcy and the risk of AD in the elderly. The risk of AD due to H-Hcy was chosen as the primary outcome of the study.
Study selection
The selection of studies to be included in our analysis was independently conducted by 2 authors (MZ, GZ) in a blinded fashion. Any discrepancies in study selection was resolved consulting a third author (CC). The following MeSH terms were used for the search: “Homocysteine” OR “Hyperhomocisteinemia” AND “Alzheimer’s disease” OR “Dementia”. Moreover, we searched the bibliographies of target studies for additional references. Case reports, review articles, abstracts, editorials/letters, and case series with less than 10 participants were excluded. Data extraction was independently conducted by 2 authors (AT, MZ). Any disagreements were resolved by consensus after discussion. Studies were included in the present analysis if they were prospective investigations or prospective nested case-control studies assessing the relationship between H-Hcy and AD and the results expressed as hazard ratio (HR) and relative 95% confidence interval (CI).
Data extraction
For each investigation included into the final analysis, the following items were extracted: year of publication, country, sample size, male gender, mean follow-up duration, diagnostic criteria for AD, method used for the assessment of blood Hcy concentration and covariates used in the multivariate analyses of each manuscript. The quality of included studies was graded using the Newcastle-Ottawa quality assessment scale (22).
Statistical analysis
Continues variables were expressed as mean ± standard deviation (SD) or range while categorical variables were presented as numbers and relative percentages. From each study, the adjusted hazard ratio (aHR) and 95% confidence interval (CI) for the higher versus the lower Hcy category comparison was pooled using a random-effects model, while a traditional forest plot was adopted to visually evaluate the results. Statistical heterogeneity between groups was measured using the Higgins I2 statistic. Specifically, a I2=0 indicated no heterogeneity while we considered low, moderate, and high degrees of heterogeneity the values of I2 as <25%, 25–75% and above 75%, respectively. Moreover, tau-squared (τ2) was also calculated to see the extent of variation among the effects observed in different studies. To evaluate potential bias, both the Egger’s test and funnel plots were computed. In case of significant Egger’s test, the Begg’s rank correlation test was also carried out and the trim-fill method was used to re-calculate the pooled risk estimates. A p-value < 0.05 was considered statistically significant. All meta-analyses were conducted using Comprehensive Meta-Analysis software, version 3 (Biostat, USA).
Results
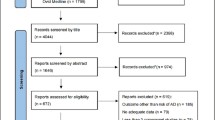
A total of 269 articles were retrieved after excluding duplicates. The initial screening excluded 186 articles because they did not meet inclusion criteria, leaving 83 articles to assess for eligibility. After evaluation of the full-text articles, 74 were excluded and 9 prospective investigations met the inclusion criteria (Figure 1) (11, 23–29).
Overall, 7474 community-dwelling adults (mean age 71 years, 53% male), with a mean follow-up of 9.5 years were analysed (Table 1). Eight seventy-five of these subjects (11.7%) converted to AD.
The diagnostic criteria used in the studies were: the National Institute of Neurological and Communicative Disorders and Stroke (NINCDS) (11,25), the National Institute of Neurological and Communicative Disorders and Stroke of the United States and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) (23, 24, 26, 27), the revised Diagnostic and Statistical Manual of Mental Disorders criteria (DSM-III-R) (29, 30), DSM IV (28) and the National Institute of Neurological Disorders and Stroke (NINDS) Association Internationale pour la Recherche et l’Enseignement en Neurosciences (AIREN) criteria (NINDS-AIREN) (23).
The different confounders considered for the estimation of Hazard Risk in each analysis are shown in Table 2. Age (n=9 studies), sex (n=8), Apo E4 (n=7), education (n=9) and body mass index (n=7) were the most considered covariates; surprisingly, the most important determinants of Hcy, folate and vitamin B12, in less than 50% of the investigations. The studies included into the meta-analysis resulted of moderate-high quality according to the NOS.
The pooled analysis, based on a random effect model, revealed that subjects with higher vs. lower levels of blood Hcy had an increased risk of AD (Figure 2, HRadjusted:1.48, 95% CI:1.23–1.76, p<0.0001), with a moderate heterogeneity in effects size between the studies (I2=65.6%).
However, as displayed by the Funnel Plot (Figure 3), there was a significant publication bias confirmed also by the Egger’s test (t=6.39, p=0.0003). To overcome this limitation, the trim and fill method calculated a bias-corrected imputed risk estimate of HRadjusted:1.20, 95% CI:1.01–1.44, Q value=41.92.
Discussion
The result of the present meta-analysis confirms that higher concentration of blood Hcy increases the risk of developing AD in older individuals. The clinical value of Hcy is beyond its mere use as static biomarker; indeed, this cysteine homologue represents a well-known modifiable risk factor, especially in the field of cardiovascular prevention, as well as a potential therapeutic target.
H-Hcy has been found to be related with cognitive decline, global and regional brain atrophy (including hippocampus volumes), white matter damage, formation and/or accumulation of the major AD-neuropathological hallmarks, neurofibrillary tangles and neuritic plaques (31). Notably, some authors found that a nutritional model of B vitamin deficiency with Hcy cycle alteration could lead to increased amyloid β (Aβ) deposition, due to over-expression in presenilin 1 and β-secretase 1 activity (32). Similarly, Li and co-workers reported a dietary approach that leads to an increase in Hcy levels resulting in a typical AD phenotype where Aβ and tau neuropathology were accompanied by memory deficit (33). More recently, it has been shown that supraphysiological concentrations of Hcy (>0.5 µM) caused a decrease in synaptic proteins in AD animal model, with the concomitant increase in oxidative stress and excitatory transmission hyperactivity, which are all considered to be neurotoxic effects (34). Furthermore, it has been reported that H-Hcy plays a causal role in stroke (35), a frequent co-existing pathology and potent risk factor of AD (36, 37), and has deleterious effects on the cerebral vasculature, including blood brain barrier disruption (38), a well-recognized early event in AD pathogenesis (39).
A meta-analysis on studies published until June 2018 showed increase of 1 µmol/L in Hcy in the blood is linearly associated with a 15% increase in the relative risk of AD (40). Our work adds to those performed to Zhou et al, since we have considered around one thousand and six hundred more patients. Moreover, the cited authors performed a dose-response meta-analysis on the risk all-cause dementia (AD and vascular dementia), while our study aimed to confirm whether patients of general population with H-Hcy, were at higher risk of AD. Indeed, evaluating the risk of AD in terms of fixed increase of blood Hcy, as every 5 µmol/L results directly correlated with the baseline values. Conversely, it could be more useful for clinicians to establish a direct relationship between H-Hcy and AD in the evaluation of patients with dementia. Furthermore, whether the risk between H-Hcy and AD follow a linear or exponential growth, has not yet been defined.
H-Hcy remains a major and yet underrecognized risk factor for cognitive impairment and dementia in daily clinical practice (41). This is mostly due to the contrasting results of the clinical trials that failed to show a clear beneficial effect of Hcy-lowering B vitamins (B-6, B-12 and folic acid) supplementation on cognitive decline. However, some studies found that baseline Hcy levels could be predictive of the response with beneficial effects of B vitamins administration only in subjects with high baseline Hcy (42, 43). The effectiveness of B vitamins supplementation could depend on other endogen and exogen factors; therefore, it could be helpful to identify subgroups that are likely to benefit of such supplementation in clinical trials. Of particular relevance to this context, two studies reported a beneficial effect of B vitamins supplementation on brain atrophy and cognitive decline only on those subjects which had high baseline levels of plasma omega (ω)-3 fatty acids (FA) (44, 45). Interestingly, the recent findings of Jerenlen et al. clearly suggest that B vitamins, Hcy and ω-3 FA influence each other. In fact, FAs supplementation seems to be effective on cognitive and clinical outcomes performance only on those AD patients with low baseline levels of Hcy (46).
Our findings confirm that the assessment of Hcy level in serum is a promising tool for the evaluation of AD risk in general population. However, it is undeniable that any case of h-Hcy should be adequately interpreted in a multidimensional evaluation because it could be expression of an underlying causal condition (e.g. chronic renal failure, alcohol consumption, smoke, use of some medications) or a consequence of cognitive decline itself (e.g. malnutrition in demented patients). Our analysis has some limitations. Firstly, being based on observational studies, the possibility of remaining residual confounding items, due either to unmeasured or underestimated risk factors in the reviewed studies cannot be excluded, representing a potential source of biases. At the same manner, we cannot exclude that patients enrolled in the reviewed cohort might be treated with vitamin B supplementation. However, potential bias resulted mitigated by the fact that some of the reviewed studies demonstrated that H-Hcy remained associated with AD, after adjustment for vitamin B levels (24, 30). However, the relative long follow-up period of the studies considered, our findings are less prone to be biased due to potential reversed causalities over the time. Finally, the lack of standardized cut-offs for H-Hcy represents another important limitation in our findings and analysis.
Conclusions
Our meta-analysis found that H-Hcy increases the risk of AD in the elderly and this finding is consistent with the potential role of Hcy in promoting neurodegeneration. Although interventional studies analysing B vitamins supplementation in terms of prevention of Hcy-related cognitive decline have shown scant results, promotion of healthy lifestyle, screening of high-risk subjects and earlier therapeutic approaches, before neurological damages have occurred, could get better results.
Key points
-
1
]Homocysteine might play an important role in Alzheimer’s disease-related neurodegeneration
-
2
]In elderly, higher blood levels of homocysteine are associated with a greater risk of developing Alzheimer’s disease
Data availability statement: Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
Ravaglia G, Forti P, Montesi F, Lucicesare A, Pisacane N, Rietti E, et al. Mild cognitive impairment: epidemiology and dementia risk in an elderly Italian population. J Am Geriatr Soc. 2008;56(1):51–8.
Villeneuve S, Jagust WJ. Imaging Vascular Disease and Amyloid in the Aging Brain: Implications for Treatment. J Prev Alzheimer’s Dis. 2015;2(1):64–70.
de la Torre JC. Is Alzheimer’s disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol. 2004;3(3):184–90.
Iturria-Medina Y, Sotero RC, Toussaint PJ, Mateos-Pérez JM, Evans AC. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat Commun. 2016;7(1):11934.
Cervellati C, Trentini A, Romani A, Bellini T, Bosi C, Ortolani B, et al. Serum paraoxonase and arylesterase activities of paraoxonase-1 (PON-1), mild cognitive impairment, and 2-year conversion to dementia: A pilot study. J Neurochem. 2015;135(2):395–401.
Hottman DA, Chernick D, Cheng S, Wang Z, Li L. HDL and cognition in neurodegenerative disorders. Neurobiol Dis. 2014;72 Pt A:22–36.
Craft S. The role of metabolic disorders in Alzheimer disease and vascular dementia: two roads converged. Arch Neurol. 2009;66(3):300–5.
Wood WG, Li L, Müller WE, Eckert GP. Cholesterol as a causative factor in Alzheimer’s disease: a debatable hypothesis. J Neurochem. 2014;129(4):559–72.
van Eersel MEA, Joosten H, Gansevoort RT, Slaets JPJ, Izaks GJ. Treatable Vascular Risk and Cognitive Performance in Persons Aged 35 Years or Older: Longitudinal Study of Six Years. J Prev Alzheimer’s Dis. 2019;6(1):42–9.
Chrysant SG, Chrysant GS. The current status of homocysteine as a risk factor for cardiovascular disease: a mini review. Expert Rev Cardiovasc Ther. 2018;16(8):559–65.
Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D’Agostino RB, et al. Plasma Homocysteine as a Risk Factor for Dementia and Alzheimer’s Disease. N Engl J Med. 2002;346(7):476–83.
Ma F, Wu T, Zhao J, Ji L, Song A, Zhang M, et al. Plasma Homocysteine and Serum Folate and Vitamin B12 Levels in Mild Cognitive Impairment and Alzheimer’s Disease: A Case-Control Study. Nutrients. 2017;9(7):725.
Handing EP, Small BJ, Reynolds SL, Kumar NB. Impact of Dietary Factors and Inflammation on Cognition among Older Adults. J Prev Alzheimer’s Dis. 2015;2(4):220–6.
Elias MF, Sullivan LM, D’Agostino RB, Elias PK, Jacques PF, Selhub J, et al. Homocysteine and Cognitive Performance in the Framingham Offspring Study: Age Is Important. Am J Epidemiol. 2005;162(7):644–53.
McCully KS. Chemical pathology of homocysteine. IV. Excitotoxicity, oxidative stress, endothelial dysfunction, and inflammation. Ann Clin Lab Sci. 2009;39(3):219–32.
Bonetti F, Brombo G, Zuliani G. The relationship between hyperhomocysteinemia and neurodegeneration. Neurodegener Dis Manag. 2016;6(2):133–45.
Selhub J, Miller JW. The pathogenesis of homocysteinemia: interruption of the coordinate regulation by S-adenosylmethionine of the remethylation and transsulfuration of homocysteine. Am J Clin Nutr. 1992;55(1):131–8.
Froese DS, Fowler B, Baumgartner MR. Vitamin B 12, folate, and the methionine remethylation cycle-biochemistry, pathways, and regulation. J Inherit Metab Dis. 2019;42(4):673–85.
Yamada K, Kawata T, Wada M, Isshiki T, Onoda J, Kawanishi T, et al. Extremely Low Activity of Methionine Synthase in Vitamin B-12–Deficient Rats May Be Related to Effects on Coenzyme Stabilization Rather than to Changes in Coenzyme Induction. J Nutr. 2000;130(8):1894–900.
Sugasini D, Yalagala PCR, Goggin A, Tai LM, Subbaiah P V. Enrichment of brain docosahexaenoic acid (DHA) is highly dependent upon the molecular carrier of dietary DHA: lysophosphatidylcholine is more efficient than either phosphatidylcholine or triacylglycerol. J Nutr Biochem. 2019;74:108231.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Hartling L, Milne A, Hamm MP, Vandermeer B, Ansari M, Tsertsvadze A, et al. Testing the Newcastle Ottawa Scale showed low reliability between individual reviewers. J Clin Epidemiol. 2013;66(9):982–93.
Chen S, Honda T, Ohara T, Hata J, Hirakawa Y, Yoshida D, et al. Serum homocysteine and risk of dementia in Japan. J Neurol Neurosurg Psychiatry. 2020;91(5):540–6.
Ravaglia G, Forti P, Maioli F, Chiappelli M, Montesi F, Tumini E, et al. Blood inflammatory markers and risk of dementia: The Conselice Study of Brain Aging. Neurobiol Aging. 2007;28(12):1810–20.
Luchsinger JA, Tang M-X, Shea S, Miller J, Green R, Mayeux R. Plasma homocysteine levels and risk of Alzheimer disease. Neurology. 2004 Jun 8;62(11):1972–6.
Ravaglia G, Forti P, Maioli F, Martelli M, Servadei L, Brunetti N, et al. Homocysteine and folate as risk factors for dementia and Alzheimer disease. Am J Clin Nutr. 2005;82(3):636–43.
Hooshmand B, Solomon A, Kareholt I, Leiviska J, Rusanen M, Ahtiluoto S, et al. Homocysteine and holotranscobalamin and the risk of Alzheimer disease: A longitudinal study. Neurology. 2010;75(16):1408–14.
Miwa K, Tanaka M, Okazaki S, Yagita Y, Sakaguchi M, Mochizuki H, et al. Increased Total Homocysteine Levels Predict the Risk of Incident Dementia Independent of Cerebral Small-Vessel Diseases and Vascular Risk Factors. J Alzheimer’s Dis. 2015;49(2):503–13.
Zylberstein DE, Lissner L, Björkelund C, Mehlig K, Thelle DS, Gustafson D, et al. Midlife homocysteine and late-life dementia in women. A prospective population study. Neurobiol Aging. 2011;32(3):380–6.
Kivipelto M, Annerbo S, Hultdin J, Bäckman L, Viitanen M, Fratiglioni L, et al. Homocysteine and holo-transcobalamin and the risk of dementia and Alzheimers disease: a prospective study. Eur J Neurol. 2009;16(7):808–13.
Smith AD, Smith SM, de Jager CA, Whitbread P, Johnston C, Agacinski G, et al. Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: a randomized controlled trial. PLoS One. 2010;5(9):e12244.
Fuso A, Nicolia V, Cavallaro RA, Ricceri L, D’Anselmi F, Coluccia P, et al. B-vitamin deprivation induces hyperhomocysteinemia and brain S-adenosylhomocysteine, depletes brain S-adenosylmethionine, and enhances PS1 and BACE expression and amyloid-β deposition in mice. Mol Cell Neurosci. 2008;37(4):731–46
Li J-G, Chu J, Barrero C, Merali S, Praticò D. Homocysteine exacerbates β-amyloid pathology, tau pathology, and cognitive deficit in a mouse model of Alzheimer disease with plaques and tangles. Ann Neurol. 2014;75(6):851–63.
Montecinos-Oliva C, Arrázola MS, Jara C, Tapia-Rojas C, Inestrosa NC. Hormetic-Like Effects of L-Homocysteine on Synaptic Structure, Function, and Aβ Aggregation. Pharmaceuticals. 2020;13(2):24.
Gu SX, Sonkar VK, Katare PB, Kumar R, Kruger WD, Arning E, et al. Memantine Protects From Exacerbation of Ischemic Stroke and Blood Brain Barrier Disruption in Mild But Not Severe Hyperhomocysteinemia. J Am Heart Assoc. 2020;9(4):e013368.
de la Torre JC. Alzheimer Disease as a Vascular Disorder. Stroke. 2002;33(4):1152–62.
Cervellati C, Wood PL, Romani A, Valacchi G, Squerzanti M, Sanz JM, et al. Oxidative challenge in Alzheimer’s disease: state of knowledge and future needs. J Investig Med. 2016;64(1):21–32.
Beard RS, Reynolds JJ, Bearden SE. Hyperhomocysteinemia increases permeability of the blood-brain barrier by NMDA receptor-dependent regulation of adherens and tight junctions. Blood. 2011;118(7):2007–14.
Cervellati C, Trentini A, Pecorelli A, Valacchi G. Inflammation in Neurological Disorders: The Thin Boundary Between Brain and Periphery. Antioxid Redox Signal. 2020;33(3):191–210.
Zhou F, Chen S. Hyperhomocysteinemia and risk of incident cognitive outcomes: An updated dose-response meta-analysis of prospective cohort studies. Ageing Res Rev. 2019;51:55–66.
Price BR, Wilcock DM, Weekman EM. Hyperhomocysteinemia as a Risk Factor for Vascular Contributions to Cognitive Impairment and Dementia. Front Aging Neurosci. 2018;10:350.
Douaud G, Refsum H, de Jager CA, Jacoby R, Nichols TE, Smith SM, et al. Preventing Alzheimer’s disease-related gray matter atrophy by B-vitamin treatment. Proc Natl Acad Sci U S A. 2013;110(23):9523–8.
Jager CA, Oulhaj A, Jacoby R, Refsum H, Smith AD. Cognitive and clinical outcomes of homocysteine-lowering B-vitamin treatment in mild cognitive impairment: a randomized controlled trial. Int J Geriatr Psychiatry. 2012;27(6):592–600.
Jernerén F, Elshorbagy AK, Oulhaj A, Smith SM, Refsum H, Smith AD. Brain atrophy in cognitively impaired elderly: the importance of long-chain ω-3 fatty acids and B vitamin status in a randomized controlled trial. Am J Clin Nutr. 2015;102(1):215–21.
Oulhaj A, Jernerén F, Refsum H, Smith AD, de Jager CA. Omega-3 Fatty Acid Status Enhances the Prevention of Cognitive Decline by B Vitamins in Mild Cognitive Impairment. J Alzheimer’s Dis. 2016;50(2):547–57.
Jernerén F, Cederholm T, Refsum H, Smith AD, Turner C, Palmblad J, et al. Homocysteine Status Modifies the Treatment Effect of Omega-3 Fatty Acids on Cognition in a Randomized Clinical Trial in Mild to Moderate Alzheimer’s Disease: The OmegAD Study. J Alzheimers Dis. 2019;69(1):189–97.
Funding
Funding information: The research reported did not receive any specific grant from funding agencies in the public, commercial, or not- for- profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest: All authors declare that they have no conflicts of interest.
Approval by ethical committee: Not necessary (systematic review)
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Zuin, M., Cervellati, C., Brombo, G. et al. Elevated Blood Homocysteine and Risk of Alzheimer’s Dementia: An Updated Systematic Review and Meta-Analysis Based on Prospective Studies. J Prev Alzheimers Dis 8, 329–334 (2021). https://doi.org/10.14283/jpad.2021.7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.14283/jpad.2021.7