Abstract
Sepsis is related to systemic inflammation and oxidative stress, the primary causes of death in intensive care units. Severe functional abnormalities in numerous organs can arise due to sepsis, with acute lung damage being the most common and significant morbidity. Spirulina, blue-green algae with high protein, vitamins, phycocyanin, and antioxidant content, shows anti-inflammatory properties by decreasing the release of cytokines. In addition, zinc (Zn) and selenium (Se) act as an antioxidant by inhibiting the oxidation of macromolecules, as well as the inhibition of the inflammatory response. The current study aimed to examine the combined properties of Zn, Se, and phycocyanin oligopeptides (ZnSePO) against lipopolysaccharide-D-galactosamine (LPS-GalN)-induced septic lung injury through survival rate, inflammatory, and histopathological changes in Balb/c mice. A total of 30 mice were allocated into three groups: normal control, LPS-GalN (100 ng of LPS plus 8 mg of D-galactosamine), LPS-GalN + ZnSePO (ZnPic, 52.5 µg/mL; SeMet, 0.02 µg/mL; and phycocyanin oligopeptide (PO), 2.00 mg/mL; at 1 h before the injection of LPS-GalN). Lung tissue from mice revealed noticeable inflammatory reactions and typical interstitial fibrosis after the LPS-GalN challenge. LPS-GalN-induced increased mortality rate and levels of IL-1, IL-6, IL-10, TGF-β, TNF-α, and NF-κB in lung tissue. Moreover, treatment of septic mice LPS-GalN + ZnSePO reduced mortality rates and inflammatory responses. ZnSePO considerably influenced tissue cytokine levels, contributing to its capacity to minimize acute lung injury (ALI) and pulmonary inflammation and prevent pulmonary edema formation in LPS-GalN-injected mice. In conclusion, ZnSePO treatment enhanced the survival rate of endotoxemia mice via improving inflammation and oxidative stress, indicating a possible therapeutic effect for patients with septic infections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sepsis is a significant health problem worldwide, caused by an uncontrolled inflammatory response to infection, which can cause multi-organ failures and even death [1]. The incidence of sepsis is associated with wounds, bacteria, or bacterial toxins such as lipopolysaccharide (LPS), an essential element of the Gram-negative bacterial outer membrane. Previous data have presented that low-dose LPS application in mice can contribute to the onset of systemic inflammation and apoptosis [2, 3]. LPS stimulates the transcription factor nuclear factor kappa B (NF-κB), which after translocation to the nucleus, stimulates the production of cytokines and chemokines, including tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β). TNF-α and IL-1β, which can cause systemic problems by directing leukocytes to the inflamed area, have an essential role in the early stages of inflammation and defense. TNF-α overproduction can harm the body and cause septic shock [4]. Increased cytokine levels cause rapid onset of systemic inflammatory response syndrome (SIRS) and dose-related mortality [5].
Spirulina platensis (Arthrospira platensis) is a microalga rich in vitamins (vitamins E and C), proteins, minerals, phytopigments, phenolic acids, betacarotene, gamma-linoleic acid, phycocyanins, and chlorophyll. These substances have antimicrobial, antioxidant, anticancer, anti-inflammatory, hypolipidemic, hypoglycemic, antiplatelet, hepatoprotective, and antihypertensive properties [6]. It has been demonstrated that spirulina exhibits anti-inflammatory possessions, mainly by decreasing the release of cytokines [7]. C-Phycocyanin (PO), a pigment protein with many biological activities like antioxidant and anti-inflammatory activities, immunomodulation, antiplatelet, and hepatoprotective, is highly dominant in spirulina. It is gaining popularity due to its bioactive properties in reported studies [8]. PO is used as a crude material to prepare nutraceutical products [9]. Even anticancer bioactivity has been reported [10]. Recent research has found that the PO has considerable antioxidant effects, suggesting that it might be used to treat oxidative stress-related disorders [11]. So far, in vivo experimental findings regarding PO’s immunomodulatory and antioxidant effects are insufficient, and more in vivo studies are needed before human studies [12].
Zinc (Zn) is a crucial trace element because of its function in energy metabolism and antioxidant qualities in the body, particularly in the cellular immune system. Zinc is required for optimal growth and function in neutrophils, natural killer cells, and macrophages [13]. Studies have shown that a Zn deficiency negatively impacts chemotaxis and immune system functions [14]. Zinc is vital for the proper structure and function of many biological enzymes, such as lactate dehydrogenase, superoxide dismutase, and carbonic anhydrase [15]. One of the critical properties of zinc is that it modulates the production of inflammatory cytokines. Studies have reported that Zn deficiency can increase inflammation, organ injury, and mortality and that short-term zinc supplementation can improve these effects [16].
Selenium (Se) is a vital trace element needed by humans and animals. It is a critical co-factor in the antioxidant enzyme system and is found in at least 25 selenoproteins [17]. Se deficiency can cause several pathological reactions, such as apoptosis, autophagy, and necrosis [18]. Furthermore, inflammation is a significant pathological response to a Se deficiency. As a result of Se deficiency, pro-inflammatory factors such as TNF-α and NF-κB are also released, worsening inflammatory lesions [19,20,21]. Selenomethionine, the selenium analog of methionine, has been a reactive oxygen species scavenger. Selenomethionine has been shown to reduce oxidative stress, accelerate cell viability and growth, and heal tissue and organ damage [22].
Various forms of Zn, Se, and PO, alone or together, have been used as supplements to investigate their metabolic effects in animals and humans [12, 23]. For example, it has also been reported that spirulina is more effective when enriched with a few essential elements, such as selenium [24]. However, there are no detailed studies investigating the effect of the combination of zinc picolinate (ZincPic), selenomethionine (SeMet), and PO on survival, inflammation, and antioxidant status in mice injected with lipopolysaccharide-D-galactosamine (LPS-GalN). Therefore, we hypothesized that ZincPic, SeMet, and PO combinations improve survival, lipid peroxidation, antioxidant enzyme (SOD) and glutathione peroxidase (GPx), and lung IL-1β, IL-6, IL-10, NF-κB, TGF-β, and TNF-α levels in mice injected with LPS-GalN.
Materials and Methods
Animals
A total of 30 Balb/c mice (8 weeks, weighing 20–25 g) were from the Firat University (FUDAM). The mice were housed in agreement with the laboratory animal use and care procedures at the Laboratory Animal Research Center of Firat University. Mice were fed in a controlled room (22 ± 2 °C, 50 ± 5 relative humidity, 12-h light and dark). The study was permitted by the University Animal Ethics Committee and done according to the standard ethical guidelines described in the European Economic Community rules (EEC, 1986).
Study Design
Except for control mice, animals were injected with an i.p. injection of LPS plus D-galactosamine (Sigma, St. Louis, MO). The LPS-galactosamine mixture was freshly prepared at 500 µL (consisting of 100 ng of LPS plus 8 mg of D-galactosamine, Sigma, St. Louis, MO) and administered to each mouse (i.p.). Control animals were treated with 0.5 mL normal saline (NS), i.e., an aqueous solution of 0.9% NaCl instead of ZnSePO [25]. Thirty BALB/c male mice, all genetically identical and of the same age, were allocated into 3 groups (n = 10): (1) control, mice injected (i.p.) with normal saline; (2) LPS-GalN, mice were injected (i.p.) with a single dose of LPS combined with D-galactosamine; (3) LPS-GalN + ZnSePO, mice were given LPS-GalN and ZnSePO (Zn picolinate, 52.5 µg/mL; selenomethionine, 0.02 µg/mL; phycocyanin oligopeptide, 2.00 mg/mL solution) at 1 h before the injection of LPS-GalN. All supplemental products were obtained by Nutrition 21 (NY, USA). After then, the mice’s survival was tracked for 24 h. Standard diet and water were provided ad libitum. The standard rodent diet was used in this study (Table 1).
Malondialdehyde (MDA) Levels and Activities of SOD and GPx Enzymes
According to the manufacturer’s procedures, the activities of SOD and GPx in the lung tissues were detected using commercially available kits (Cayman Chemical, Ann Arbor, MI, USA). As earlier defined, MDA levels were determined in the lungs [25]. Tissue samples were analyzed for MDA using high-performance liquid chromatography (HPLC, Shimadzu, Kyoto, Japan). Specifically, an HPLC system equipped with the LC solution Software (Shimadzu, Kyoto, Japan), a UV Detector (SPD-20A), and a column (Inertsil ODS-3, 250 × 46 mm, 5 mm) were used. Samples (0.3 g) were homogenized in a mixture of 0.5 mL of HClO4 (0.5 M), 2.5 mL distilled water, and 2[6]-di-tert-butyl-p-cresol (BHT) for precipitating proteins. Then, the samples were centrifuged at 4500 rpm for 5 min, and supernatants were injected into the HPLC system. The mobile phase was 30 mM KH2PO4-methanol (82.5 + 17.5, v/v %, pH 3.6), and the flow rate was 1 mL min−1. The injection volume was 30 μL, and chromatograms were scanned at 250 nm.
Western Blot Analyses
Lung tissue IL-1β, IL-6, IL-10, NF-κB, TGF-β, and TNF-α levels were determined by western blot analysis [25]. After electrophoresis with SDS-PAGE, the separated proteins from the gel were transferred onto a nitrocellulose membrane Antibodies (Abcam, Cambridge, UK) sensitive to IL-1β (ab282021), IL-6 (ab208113), IL-10 (ab9969), NF-κB (ab16502), TGF-β (ab31013), and TNF-α (ab6671) proteins (containing 0.05% Tween®-20) were diluted 1:1000 in PBS buffer. The loaded proteins were checked with β-actin against monoclonal mouse antibodies (A5316; Sigma). The obtained bands were analyzed densitometrically with the Image J image analysis system (National Institute of Health, Bethesda, MD, USA).
Quantitative Real-Time qPCR Analyses
Total RNA was extracted from the mouse lung using a GeneJET RNA extraction kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s guidelines, and the quality and concentration of the RNA were verified by Thermo Qubit 4.0 equipment from Invitrogen™ by Life Technologies (Thermo Fisher Scientific, Waltham, MA, USA). Complementary DNA (cDNA) was generated from 1 μg of total RNA in a 20-μL reaction volume using an RT2 HT First Strand Kit (Catalog no. 330411, Qiagen, Germany). Real-time quantitative reverse transcription PCR was performed on cDNA aliquots with an RT2 SYBR® Green ROX FAST Mastermix (Catalog no. 330620, Qiagen) to quantitatively assess the gene expressions on Rotor‐Gene Q (Qiagen). Glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) was used as the internal control. The primers used for the amplification are as follows: gene ID 16,176, forward sequence TGGACCTTCCAGGATGAGGACA, reverse sequence GTTCATCTCGGAGCCTGTAGTG; gene ID 16,193, forward sequence TACCACTTCACAAGTCGGAGGC, reverse sequence CTGCAAGTGCATCATCGTTGTTC; gene ID 21,803, forward sequence TGATACGCCTGAGTGGCTGTCT, reverse sequence CACAAGAGCAGTGAGCGCTGAA; gene ID 21,926, forward sequence GGTGCCTATGTCTCAGCCTCTT, reverse sequence GCCATAGAACTGATGAGAGGGAG; gene ID 14,433, forward sequence CATCACTGCCACCCAGAAGACTG, reverse sequence ATGCCAGTGAGCTTCCCGTTCAG. Each PCR was made in triplicate, and the mean Ct value was used for statistical analysis. mRNA expressions were standardized using the GAPDH expression levels and then normalized to the control group.
Histopathological Analyses
Tissue samples from the lungs of all the mice in each group were taken for pathological examination once they were dead. Another piece was taken for additional analysis, and 3-m slices were cut, deparaffinized, dried, and stained with hematoxylin and eosin (H&E) after being fixed in 10% formalin solution and processed for histopathologic evaluation.
Statistical Analysis
Data were analyzed using the SPSS software (IBM, SPPS Version 21, or/and GraphPad Prism version 8.0) for Windows. The Shapiro–Wilk test evaluated whether the variables were normally distributed. Continuous data were analyzed by parametric analysis of variance (ANOVA) test for normally distributed variables (Shapiro–Wilk test result p ≥ 0.05). ANOVA was performed, and the Tukey test was used for post hoc comparisons among the groups. The daily survival rates of mice in various treatment groups were analyzed using the Kaplan–Meier approach and the log-rank chi-square test. Log-rank survival analysis was performed to compare survival between different groups. All p values were two-tailed, and values < 0.05 were considered to indicate statistical significance.
Results
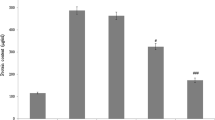
The survival rate of the mice was monitored for 24 h. As shown in Fig. 1, mice treated only saline (control) all survived. Mice injected only with LPS-GalN suffered subsequent death in 5.36 h, and the mortality rate was 100%. However, we reported the mortality rate of pretreatment with ZnSePO on LPS-GalN-induced lung damage significantly decreased. In contrast to the consistently fatal treatment outcome found in LPS-GalN mice, who had a median survival period of 5.36 h, 60% of mice treated with ZnSePO survived, with an average survival duration of 24 h (log-rank = 11.72, p = 0.0006; Fig. 1). The lung MDA levels were much higher in LPS-GalN-exposed mice than in healthy control group mice. The activities of SOD and glutathione peroxidase (GPx) were substantially lower, representing severe oxidative stress in tissue (p < 0.0001, Fig. 2). When compared to LPS-GalN-exposed mice, lung MDA levels were lower (p < 0.001), and SOD and glutathione peroxidase (GPx) activities were greater (p < 0.0001) in the ZnSePO treatment group (Fig. 2).
The effects of LPS-GalN + ZnSePO administration on lung malondialdehyde (MDA, panel A) levels, superoxide dismutase (SOD, panel B), and glutathione peroxidase (GPx, panel C) activities in a mouse septic shock model. Differences between groups were demonstrated using one-way analysis of variance (ANOVA) and the Tukey test as a post hoc test
Western blot examination of lung tissue cytokine levels in LPS-GalN-injected mice revealed significant results compared with control mice not injected with LPS-GalN (p < 0.0001 for all; Fig. 3). In mice exposed to LPS-GalN, transcription factor NF-κB and inflammatory cytokine levels (IL-1β, IL-6, IL-10, TGF-β, and TNF-α) were dramatically elevated at death (p < 0.0001 for all, Fig. 3), consistent with a cytokine storm and severe systemic inflammation. IL-1β, IL-6, and IL-10 were noticeably lower in LPS-GalN + ZnSePO-treated mice compared to LPS-GalN-injected mice alone (p < 0.0001 for all; Fig. 3). In addition, western blot results indicated that the NF-κB, TGF-β, and TNF-α levels were significantly decreased in lungs treated with ZnSePO compared with the LPS-GalN group (p < 0.0001 for all; Fig. 3). RT-qPCR was performed to investigate the effects of ZnSePO administration on inflammatory cytokines and NF-κB signaling pathway activation in lung tissue, and the results indicated that the mRNA expressions of IL-1β, IL-6, TGF-β, and TNF-α were increased in the LPS-GalN group compared with the control (p < 0.0001 for all; Fig. 4). However, the results indicated that the expressions of IL-1β (p < 0.0001), IL-6 (p = 0.0035), TGF-β (p < 0.0001), and TNF-α (p = 0.0008) were decreased in the ZnSePO group compared with LPS-GalN (Fig. 4).
The effects of LPS-GalN + ZnSePO administration on interleukin 1β (IL-1β, panel A), interleukin-6 (IL-6, panel B), interleukin-10 (IL-10, panel C), nuclear factor kappa B (NF-κB, panel D), transformer growth factor-beta (TGF-β, panel E), tumor necrosis factor-alpha (TNF-α, panel F) protein levels, and bands (panel G). Differences between groups were demonstrated using one-way analysis of variance (ANOVA) and the Tukey test as a post hoc test
Effects of LPS-GalN + ZnSePO administration on interleukin 1β (IL-1β, panel A), IL-6 (panel B), transforming growth factor-beta (TGF-β, panel C), and tumor necrosis factor-alpha (TNF-α, panel D) mRNA protein expression levels in a mouse septic shock model. Differences between groups were demonstrated using one-way analysis of variance (ANOVA) and the Tukey test as a post hoc test
Histological alterations associated with severe acute ALI were seen in H/E-stained lung tissues from LPS-GalN-injected mice, including alveolar hemorrhages, thickening of the alveolar wall, edemas/congestion, and leukocyte infiltration (Fig. 5). The lung tissues of the mice that were not administrated with LPS-GalN did not show any such histological alterations. ZnSePO significantly reduced LPS-GalN-induced ALI. The ZnSePO treatment dramatically decreased alveolar wall thickness, a marker of pulmonary edema (Fig. 5). In LPS-GalN-injected mice, ZnSePO seemed to inhibit the development of pulmonary edema. ZnSePO administration reduced the LPS-GalN-related mortality rate by reducing the systemic inflammatory response and improving lung injury.
Discussion
This study examined the properties of combining Zn, Se, and PO on septic lethality and sepsis-induced inflammation using a mouse model of mice injected with LPS-GalN. A combination of Zn, Se, and PO pretreatment contributed 60% to the survival rates of septic mice by reducing pro-inflammatory and anti-inflammatory cytokines in septic mice.
Combining current methodologies can help researchers better understand certain elements of sepsis development and generate new and creative treatment options [26]. Previous studies show that the synergistic impact of a broad range of antioxidants is more beneficial than utilizing a single antioxidant [27]. It is known that antioxidants derived from natural sources have better bioavailability and, as a result, a stronger protective efficiency than antioxidants derived from synthetic sources [28]. Several vital elements, such as selenium, were supplemented with spirulina in some studies. Hassan et al. showed that the combined impact of spirulina enhanced with Zn and Se in rabbits has resulted in better growth than alone [29]. The beneficial effects of spirulina, selenium, and zinc supplementations had already been described, also evidenced in our experiment. Castel et al. demonstrated that Se (88%) rats had better survival than Se + spirulina (50%) rats. They also stated that spirulina reduces the positive results of sepsis and that more research is needed on this subject [30]. Contrary to this study, our data revealed the valuable properties of spirulina in combination with zinc and selenium in septic mice. According to our results, the ZnSePO application not only prolonged the survival time but also increased the survival rate to 60%. High nitrogenous substances in spirulina biomass, especially free amino acids and phenolic compounds, have been reported to provide antimicrobial or bacteriostatic properties and better growth and survival [31]. Ganatra et al. reported that juvenile mice treated with zinc supplementation showed significantly improved survival (50%) in sepsis secondary to polymicrobial peritonitis [16].
We observed that lung MDA levels were considerably enhanced, whereas the activities of SOD and GPx were lowered, indicating increased lipid peroxidation associated with LPS-GalN-related lung damage and severe oxidative stress. ZnSePO decreased the levels of pro-inflammatory cytokines in the lungs, decreased MDA levels, and increased SOD and GPx activities. Similar to our results, Hassan et al. (2021) reported that the Zn, Se, and spirulina combination showed an antioxidant effect in rabbits under heat stress and was linked with lower thiobarbituric acid reactive substances (TBARS), higher total antioxidant capacity (T-AOC), glutathione peroxidase enzyme (GPx), catalase (CAT), and SOD activities. PO is a potent antioxidant due to the high content of antioxidant phenols or flavonoids. In various animal models, spirulina has exhibited antioxidant capacity [32, 33]. In septic mice, Abdel-Daim et al. observed that spirulina decreased pro-inflammatory cytokines in serum and improved antioxidant status (MDA, SOD, CAT, GSH, and GSH-Px) in the liver, kidney, and brain tissues [34]. Ou et al. reported that mice treated with PO showed decreased MDA levels and increased serum total antioxidant capacity [35]. In cellular models, PO showed antioxidant activity regulating the activities of SOD, CAT, and GSH-Px [31]. In addition, Castel et al. reported that the Se + spirulina-supplemented group showed 2.5 times higher GSH-Px mRNA content than the Se-increased group [30]. On the other hand, Se or zinc alone or in combinations would be expected to decrease MDA concentrations since they are involved in antioxidant defense systems [36, 37]. Previous studies have shown that Se increases enzymatic antioxidant activity and reduces lipid peroxidation (MDA) in animal studies and in vitro. It has also been reported that selenium has antioxidant properties because it is an essential component of GSH-Px [29]. Antioxidant effects of Zn are well recognized and have been proven in several researches [38]. Prasad et al. showed that Zn increases oxidative activity and improves animal health [37]. Alissa et al. showed that plasma TBAR concentration decreased in association with the decrease in plasma lipid peroxides of zinc [39]. Interestingly, Nasirian et al. showed that oral supplementation of Spirulina platensis (20 and 30 mg/kg body weight) increased plasma levels of zinc, selenium, iron, and copper required for synthesizing antioxidant enzymes while decreasing the plasma concentration of MDA, TNF-α, and IL-6 [33].
Our findings showed that ZnSePO improved LPS-GalN-induced sepsis, protected LPS-induced tissue damage by reducing levels of inflammatory cytokines, and increased septic shock survival rates. Oxidative stress is closely related to the inflammatory response. Oxidative stress caused by LPS-GalN can increase the expression of NF-κB and TNF-α [19]. An increase in pro-inflammatory cytokines (TNF-α and IL-6) and anti-inflammatory cytokines (IL-10 and TGF-β) is associated with the worse progression of sepsis [40]. Previous studies have reported that spirulina has immunomodulatory, anti-inflammatory, antioxidant, and antimicrobial activity in animals [41, 42]. In animal models of experimental arthritis and colitis, the antioxidant activity of PO was associated with anti-inflammatory effects. Phycocyanin exerts its anti-inflammatory and antioxidant effects by inhibiting NF-κB [31]. Activation of the NF-kB pathway mainly increases inflammation by promoting pro-inflammatory factors’ expression [43]. In our study, a decrease in NF-κB and other inflammatory cytokine levels (IL-1β, IL-6, TNF-α, IL-10, and TGF-β) was observed in mice treated with ZnSePO. Many animal studies have examined the effects of prophylactic zinc supplementation on anti-inflammatory effects. Similar to our study, zinc supplementation before sepsis induction showed beneficial effects such as better survival, lower pro-inflammatory cytokine (IL-1β, IL-6, IL-2) concentrations, lower bacterial load, or better pulmonary function compared to the control group [16, 44]. Al-Rasheed et al. reported a decrease in inflammatory cytokines such as IL-1β, IL-6, and TNF-α in rats treated with zinc [15]. According to Visalakshy et al., plasma zinc levels are strongly linked to sepsis-related death [13]. Utomo et al. showed that zinc treatment improved sepsis status via regulating cytokines, resulting in lower levels of pro-inflammatory cytokines (TNF-α and IL-6) and higher levels of anti-inflammatory cytokines (IL-10 and TGF-β) [40]. It has been shown that 92% of septic patients in critical care units are selenium deficient, suggesting that selenium deficiency may play a part in sepsis development [30]. Selenium has been reported to reduce LPS-induced inflammatory cytokines (TNF-a, IL-1, IL-6, IFN-y), inhibit oxidative stress formation, and alleviate myocardial tissue damage [45]. Lack of selenium is linked to worse clinical results, a rise in nosocomial infections, and even death. The advantages of selenium treatment in septic patients are still being contested, and more research is needed to comprehend their effects fully [46, 47]. The studies mentioned above have demonstrated the antioxidant and anti-inflammatory properties of zinc, selenium, and spirulina. In our study, oxidative stress markers and inflammatory cytokines decreased with the synergistic effect of zinc, selenium, and spirulina.
In this study, ZnSePO has an anti-inflammatory role in inflammation reasoned by LPS-GalN and has tissue-protective effects in sepsis induced by LPS-GalN. The levels of IL-1β, IL-10, IL-6, NF-κB, and TNF-α, along with TGF-β, in the lungs of mice exposed to LPS-GalN were significantly reduced after treatment with ZnSePO. TGF-β is also implicated in acute respiratory distress syndrome (ARDS)-related lung tissue improvement and fibrosis [48]. We demonstrated that ZnSePO treatment suppressed TGF-β in the lungs, reducing pulmonary inflammation and ARDS-related tissue damage in mice. This study was consistent with other studies showing that spirulina, Zn, and selenium would regulate the production of inflammatory cytokines in inflammatory conditions. Mahmoud et al. showed that spirulina ameliorates gastric mucosal injury by improving antioxidant and cytoprotective protection and attenuating oxidative stress and inflammation in albino mice. Also, it has been demonstrated that spirulina enhances the enzymatic antioxidant system (GSH, GPx, SOD, and CAT) and alleviation of the tissue levels of lipid peroxidation marker (MDA) and inflammatory mediators (TNF-α) [49]. Many studies have shown that Se has protective effects on inflammatory damage of various tissues and organs. Qu et al. used selenomethionine to ameliorate LPS-induced inflammation and tissue damage by suppressing the NF-κB signaling pathway in broiler liver tissue [22]. Luo et al. revealed that Se supplementation could preserve against modifies in the liver by regulating the mRNA expression levels of inflammatory factors and exert a considerable protective effect against oxidative stress by increasing the activities of antioxidant enzymes [50]. Wang J et al. showed that Se supplementation improves tissue damage in the small intestine by increasing GPx activity and regulating the NF-κB pathway [51]. Wang X et al. reported that selenium supplementation decreased pro-inflammatory cytokine expression and oxidative stress and showed protective effects on LPS-induced myocardial damage [52]. Knoell et al. reported a significant effect of zinc supplementation on inflammation, organ damage, and mortality in a polymicrobial sepsis model [53].
The limitation of this study should be acknowledged in a way that treatments did not contain Zn, Se, and PO or their combination such as Zn + PO or Se + PO groups, which would give an idea of the properties of their combination before evaluating the combination of these organic minerals and PO. It would also be interesting to see a possible effect(s) of the combination of Zn + PO or Se + PO. Study results from a combination of organic Zn and Se and PO in animal models have been scarce in the literature. Hassan et al. [23] reported that supplemental zinc and/or selenium-enriched spirulina or their combination improved nutrient digestibility, plasma biochemicals, and antioxidant status of growing rabbits.
Conclusion
Our findings reported that a combination of ZnSePO treatment efficiently prevented LPS-GalN-induced death in mice. ZnSePO also inhibited lipid peroxidation and inflammation markers, including IL-1β, IL-6, IL-10, NF-κB, TGF-β, and TNF-α. The combination also increased the SOD and GPx activities. Although the pretreatment of ZnSePO showed a promising protective activity by protecting the lung tissues, further studies involving molecular mechanisms with a longer treatment period and investigating the effects of these two elements together with PO separately are needed to evaluate the post-treatment effects.
References
P’erez-Hernandez EG, Delgado-Coello B, Luna-Reyes I, Mas-Oliva J (2021) New insights into lipopolysaccharide inactivation mechanisms in sepsis. Biomed Pharmacother 141:111890. https://doi.org/10.1016/j.biopha.2021.111890
Seemann S, Zohles F, Lupp A (2017) Comprehensive comparison of three different animal models for systemic inflammation. J Biomed Sci 24(1):60. https://doi.org/10.1186/s12929-017-0370-8
Azambuja JH, Mancuso RI, Della Via FI, Torello CO, Saad STO (2022) Protective effect of green tea and epigallocatechin-3-gallate in a LPS-induced systemic inflammation model. J Nutr Biochem 101:108920. https://doi.org/10.1016/j.jnutbio.2021.108920
Keller M, Manzocchi E, Rentsch D, Lugarà R, Giller K (2021) Antioxidant and inflammatory gene expression profiles of bovine peripheral blood mononuclear cells in response to Arthrospira platensis before and after LPS challenge. Antioxidants (Basel) 10(5):814. https://doi.org/10.3390/antiox10050814
Recknagel P, Gonnert FA, Halilbasic E, Gajda M, Jbeily N, Lupp A, Rubio I, Claus RA, Kortgen A, Trauner M, Singer M, Bauer M (2013) Mechanisms and functional consequences of liver failure substantially differ between endotoxaemia and faecal peritonitis in rats. Liver Int 33(2):283–293. https://doi.org/10.1111/liv.12012
Hajati H, Zaghari M, Oliveira HC (2020) Arthrospira (Spirulina) Platensis can be considered as a probiotic alternative to reduce heat stress in laying Japanese quails. Braz J Poult Sci 22:1–8. https://doi.org/10.1590/1806-9061-2018-0977
Pham TX, Lee Y, Bae M, Hu S, Kang H (2019) Spirulina supplementation in a mouse model of diet-induced liver fibrosis reduced the pro-inflammatory response of splenocytes. Br J Nutr 121:748–755. https://doi.org/10.1017/S0007114519000126
Grover P, Bhatnagar A, Kumari N, Bhatt AN, Nishad DK, Purkayastha J (2021) C-Phycocyanin-a novel protein from Spirulina platensis- in vivo toxicity, antioxidant and immunomodulatory studies. Saudi J Biol Sci 28(3):1853–1859. https://doi.org/10.1016/j.sjbs.2020.12.037
Silveira ST, Burkert JF, Costa JAV, Burkert CAV, Kalil SJ (2007) Optimization of phycocyanin extraction from Spirulina platensis using factorial design. Bioresour Technol 98(8):1629–1634. https://doi.org/10.1016/j.biortech.2006.05.050
Wang H, Liu Y, Gao X, Carter CL, Liu ZR (2007) The recombinant beta subunit of C-phycocyanin inhibits cell proliferation and induces apoptosis. Cancer Lett 247(1):150–158. https://doi.org/10.1016/j.canlet.2006.04.002
Bannu SM, Lomada D, Gulla S, Chandrasekhar T, Reddanna P, Reddy MC (2019) Potential therapeutic applications of C-phycocyanin. Curr Drug Metab 20(12):967–976. https://doi.org/10.2174/1389200220666191127110857
Hamedifard Z, Farrokhian A, Reiner Ž, Bahmani F, Asemi Z, Ghotbi M, Taghizadeh M (2020) The effects of combined magnesium and zinc supplementation on metabolic status in patients with type 2 diabetes mellitus and coronary heart disease. Lipids Health Dis 19(1):112. https://doi.org/10.1186/s12944-020-01298-4
Visalakshy J, Surendran S, Pillai MPG, Rajendran A, Sherif AA (2017) Could plasma zinc be a predictor for mortality and severity in sepsis syndrome? Int J Res Med Sci 5(9):3929–3934. https://doi.org/10.18203/2320-6012.ijrms20173956
Ibs KH, Rink L (2003) Zinc-altered immune function. J Nutr 133:1452S-S1456. https://doi.org/10.1093/jn/133.5.1452S
Al-Rasheed NM, Attia HA, Mohamed RA, Al-Rasheed NM, Al-Amin MA (2013) Preventive effects of selenium yeast, chromium picolinate, zinc sulfate and their combination on oxidative stress, inflammation, impaired angiogenesis and atherogenesis in myocardial infarction in rats. J Pharm Pharm Sci 16:848–867. https://doi.org/10.18433/j34c7n
Ganatra HA, Varisco BM, Harmon K, Lahni P, Opoka A, Wong HR (2017) Zinc supplementation leads to immune modulation and improved survival in a juvenile model of murine sepsis. Innate Immun 23(1):67–76. https://doi.org/10.1177/1753425916677073
Li M, Zhang Y, Li S (2020) Effects of selenium deficiency on testis development and autophagy in chicks. Ital J Anim Sci 19(1):753–761. https://doi.org/10.1080/1828051X.2020.1786739
Cao C, Li X, Qin L, Luo J, Zhang M, Ou Z, Wang K (2018) High selenium yeast mitigates aluminum-induced cerebral inflammation by increasing oxidative stress and blocking NO production. Biometals 31(5):835–843. https://doi.org/10.1007/s10534-018-0128-0
Liu J, Wang S, Zhang Q, Li X, Xu S (2020) Selenomethionine alleviates LPS-induced chicken myocardial inflammation by regulating the miR-128-3p-p38 MAPK axis and oxidative stress. Metallomics 12(1):54–64. https://doi.org/10.1039/c9mt00216b
Fan R, Yao H, Cao C, Zhao X, Khalid A, Zhao J, Zhang Z, Xu S (2017) Gene silencing of selenoprotein K induces inflammatory response and activates heat shock proteins expression in chicken myoblasts. Biol Trace Elem Res 180(1):135–145. https://doi.org/10.1007/s12011-017-0979-1
Cao C, Luo J, Li X, Zhang M, Zhang H, Zhang J, Wang K (2018) Selenium-rich yeast protects against aluminum-induced renal inflammation and ionic disturbances. Biol Trace Elem Res 186(2):467–473. https://doi.org/10.1007/s12011-018-1324-z
Qu J, Wang W, Zhang Q, Li S (2020) Inhibition of lipopolysaccharide-induced inflammation of chicken liver tissue by selenomethionine via TLR4-NF-κB-NLRP3 signaling pathway. Biol Trace Elem Res 195(1):205–214. https://doi.org/10.1007/s12011-019-01841-0
Thoen RU, Barther NN, Schemitt E, Bona S, Fernandes S, Coral G, Marroni NP, Tovo C, Guedes RP, Porawski M (2019) Zinc supplementation reduces diet-induced obesity and improves insulin sensitivity in rats. Appl Physiol Nutr Metab 44(6):580–586. https://doi.org/10.1139/apnm-2018-0519
Chen T, Wong YS (2008) In vitro antioxidant and antiproliferative activities of selenium-containing phycocyanin from selenium-enriched Spirulina platensis. J Agric Food Chem 56(12):4352–4358. https://doi.org/10.1021/jf073399k
Uckun FM, Carlson J, Orhan C, Powell J, Pizzimenti NM, Hv W, Ozercan IH, Mand V, Sahin K (2020) Rejuveinix shows a favorable clinical safety profile in human subjects and exhibits potent preclinical protective activity in the lipopolysaccharide-galactosamine mouse model of acute respiratory distress syndrome and multi-organ failure. Front Pharmacol 11:594321. https://doi.org/10.3389/fphar.2020.594321
Korneev KV (2019) Mouse models of sepsis and septic shock. Mol Biol (Mosk) 53(5):799–814. https://doi.org/10.1134/S0026898419050100
Sahin K, Orhan C, Kucuk O, Tuzcu M, Sahin N, Ozercan IH, Sylla S, Ojalvo SP, Komorowski JR (2022) Effects of magnesium picolinate, zinc picolinate, and selenomethionine co-supplementation on reproductive hormones, and glucose and lipid metabolism-related protein expressions in male rats fed a high-fat diet. Food Chem: Mole Sci 4:100081. https://doi.org/10.1016/j.fochms.2022.100081
Benedetti S, Benvenuti F, Pagliarani S, Francogli S, Scoglio S, Canestrari F (2004) Antioxidant properties of a novel phycocyanin extract from the blue-green alga Aphanizomenon flos-aquae. Life Sci 75(19):2353–2362. https://doi.org/10.1016/j.lfs.2004.06.004
Hassan F, Mobarez S, Mohamed M, Attia Y, Mekawy A, Mahrose K (2021) Zinc and/or selenium enriched spirulina as antioxidants in growing rabbit diets to alleviate the deleterious impacts of heat stress during summer season. Animals (Basel) 11(3):756. https://doi.org/10.3390/ani11030756
Castel T, Theron M, Pichavant-Rafini K, Guernec A, Joublin-Delavat A, Gueguen B, Leon K (2021) Can selenium-enriched spirulina supplementation ameliorate sepsis outcomes in selenium-deficient animals? Physiol Rep 9(14):e14933. https://doi.org/10.14814/phy2.14933
Finamore A, Palmery M, Bensehaila S, Peluso I (2017) Antioxidant, immunomodulating, and microbial-modulating activities of the sustainable and ecofriendly spirulina. Oxid Med Cell Longev 2017:3247528. https://doi.org/10.1155/2017/3247528
Gargouri M, Soussi A, Akrouti A, Magné C, El Feki A (2018) Ameliorative effects of Spirulina platensis against lead-induced nephrotoxicity in newborn rats: modulation of oxidative stress and histopathological changes. EXCLI J 17:215–232. https://doi.org/10.17179/excli2017-1016
Nasirian F, Dadkhah M, Moradi-kor N, Obeidavi Z (2018) Effects of Spirulina platensis microalgae on antioxidant and anti-inflammatory factors in diabetic rats. Diabetes Metabo Syndr Obes 11:375–380. https://doi.org/10.2147/DMSO.S172104
Abdel-Daim M, El-Bialy BE, Rahman HGA, Radi AM, Hefny HA, Hassan AM (2016) Antagonistic effects of Spirulina platensis against sub-acute deltamethrin toxicity in mice: biochemical and histopathological studies. Biomed Pharmacother 77:79–85. https://doi.org/10.1016/j.biopha.2015.12.003
Ou Y, Lin L, Yang X, Pan Q, Cheng X (2013) Antidiabetic potential of phycocyanin: effects on KKAy mice. Pharm Biol 51:539–544. https://doi.org/10.3109/13880209.2012.747545
Labunskyy VM, Hatfield DL, Vadim N, Gladyshe VN (2014) Selenoproteins: molecular pathways and physiological roles. Physiol Rev 94:739–777. https://doi.org/10.1152/physrev.00039.2013
Prasad AS, Bao B (2019) Molecular mechanisms of zinc as a pro-antioxidant mediator: clinical therapeutic implications. Antioxidants 8:164. https://doi.org/10.3390/antiox8060164
Powell SR (2000) The antioxidant properties of zinc. J Nutr 130(5S):1447S-S1454. https://doi.org/10.1093/jn/130.5.1447S
Alissa EM, Bahijri SM, Lamb DJ, Gordon A, Ferns GAA (2004) The effects of coadministration of dietary copper and zinc supplements on atherosclerosis, antioxidant enzymes and indices of lipid peroxidation in the cholesterol-fed rabbit. Int J Exp Pathol 85(5):265–275. https://doi.org/10.1111/j.0959-9673.2004.00392.x
Utomo MT, Sudarmo SM, Sudiana K (2020) Zinc supplementation in cytokine regulation during LPS-induced sepsis in rodent. J Int Dent Med Res 13(1):46–50
Michalak I, Mahrose KM (2020) Seaweeds, intact and processed, as a valuable component of poultry feed. J Mar Sci Eng 8(8):620. https://doi.org/10.3390/jmse8080620
Gabr GA, El-Sayed SM, Hikal MS (2020) Antioxidant activities of phycocyanin: a bioactive compound from Spirulina platensis. J Pharm Res Int 32(2):73–85. https://doi.org/10.9734/JPRI/2020/v32i230407
Hu X, Chi Q, Liu Q, Wang D, Zhang Y, Li S (2019) Atmospheric H2S triggers immune damage by activating the TLR-7/MyD88/NF-kB pathway and NLRP3 inflammasome in broiler thymus. Chemosphere 237:124427. https://doi.org/10.1016/j.chemosphere.2019.124427
Wessels I, Cousins RJ (2015) Zinc dyshomeostasis during polymicrobial sepsis in mice involves zinc transporter Zip14 and can be overcome by zinc supplementation. Am J Physiol Gastrointest Liver Physiol 309:G768–G778. https://doi.org/10.1152/ajpgi.00179.2015
Shalihat A, Hasanah AN, Mutakin LR, Budiman A, Gozali D (2021) The role of selenium in cell survival and its correlation with protective effects against cardiovascular disease: a literature review. Biomed Pharmacother 134:111125. https://doi.org/10.1016/j.biopha.2020.111125
Huang TS, Shyu YC, Chen HY, Lin LM, Lo CY, Yuan SS, Chen PJ (2013) Effect of parenteral selenium supplementation in critically ill patients: a systematic review and metaanalysis. PLoS ONE 8(1):e54431. https://doi.org/10.1371/journal.pone.0054431
Landucci F, Mancinelli P, De Gaudio AR (2014) Selenium supplementation in critically ill patients: a systematic review and meta-analysis. J Crit Care 29(1):150–156. https://doi.org/10.1016/j.jcrc.2013.08.017
Nithiananthan S, Crawford A, Knock JC, Lambert DW, Whawell SA (2017) Physiological fluid flow moderates fibroblast responses to TGF-β1. J Cell Biochem 118:878–890. https://doi.org/10.1002/jcb.25767
Mahmoud YI, Abd El-Ghffar EA (2019) Spirulina ameliorates aspirin-induced gastric ulcer in albino mice by alleviating oxidative stress and inflammation. Biomed Pharmacother 109:314–321. https://doi.org/10.1016/j.biopha.2018.10.118
Luo J, Li X, Li X, He Y, Zhang M, Cao C, Wang K (2018) Selenium-rich yeast protects against aluminum-induced peroxidation of lipide and inflammation in mice liver. Biometals 31(6):1051–1059. https://doi.org/10.1007/s10534-018-0150-2
Wang J, Liu Z, He X, Lian S, Liang J, Yu D, Sun D, Wu R (2018) Selenium deficiency induces duodenal villi cell apoptosis via an oxidative stress-induced mitochondrial apoptosis pathway and an inflammatory signaling-induced death receptor pathway. Metallomics 10:1390–1400. https://doi.org/10.1039/c8mt00142a
Wang X, Yang B, Cao HL, Wang RY, Lu ZY, Chi RF, Li B (2021) Selenium supplementation protects against lipopolysaccharide-induced heart injury via sting pathway in mice. Biol Trace Elem Res 199(5):1885–1892. https://doi.org/10.1007/s12011-020-02295-5
Knoell DL, Julian MW, Bao S, Besecker B, Macre JE, Leikauf GD, DiSilvestro RA, Crouser ED (2009) Zinc deficiency increases organ damage and mortality in a murine model of polymicrobial sepsis. Crit Care Med 37(4):1380–1388. https://doi.org/10.1097/CCM.0b013e31819cefe4
Funding
This work was supported by KOSGEB (Elazig, Turkey) and the Turkish Academy of Sciences (KS).
Author information
Authors and Affiliations
Contributions
K.S. designed the study; P.O., B.E., and C.O. performed the experiment; P.O. drafted the study; and K.S. edited the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oner, P., Er, B., Orhan, C. et al. Combination of Phycocyanin, Zinc, and Selenium Improves Survival Rate and Inflammation in the Lipopolysaccharide-Galactosamine Mouse Model. Biol Trace Elem Res 201, 1377–1387 (2023). https://doi.org/10.1007/s12011-022-03433-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03433-x