Abstract
Sepsis is a severe clinical condition that is a result of the cellular and biochemical response to infection. The present study evaluated the therapeutic potential of tryptophan against lipopolysaccharide (LPS)-induced acute lung injury (ALI) in rats. Rats were grouped into sham, control (ALI), and ALI + 1, 25, and 50 mg/kg body weight l-tryptophan. Supplementation with 1, 25, and 50 mg/kg l-tryptophan reduced the total protein content by 4.9%, 33.4%, and 64.5%; the levels of neutrophils (12.5%, 31.8%, and 65.1%), lymphocytes (15.1%, 41.7%, and 63.3%), total cells (12.6%, 42.4%, and 65.7%); lipid peroxidation (9.4%, 28.4%, and 68.7%); myeloperoxidase levels (12.1%, 33.4%, and 68.2%); migration inhibitory factor (12.7%, 39.5%, and 68.2%), interleukin (IL)-8 (5.5%, 46.8%, and 78.5%), tumor necrosis factor (TNF)-α (10.8%, 39.8%, and 72.2%), respectively. Supplementation with 1, 25, and 50 mg/kg l-tryptophan reduced mRNA expression of TNF-α (4.5%, 21.8%, and 41.8%), IL-1β (5.2%, 17.9%, and 46.2%); and the protein expression of TNF-α (2.8%, 15.2%, and 35.7%) and IL-1β (5.2%, 15.6%, and 28.6%), respectively. It also reduced glutathione (to near normal levels), neutrophilic infiltration and edema, and the wet/dry ratio of lung tissue. It significantly increased catalase, superoxide dismutase, glutathione peroxidase levels, as well as the partial pressure of oxygen (PaO2) by 21.9%, 52.8%, and 87.4%, respectively. Altogether, our results suggest that supplementation with l-tryptophan has a strong protective effect against LPS-induced ALI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sepsis is a severe clinical condition that is a result of the cellular and biochemical response to infection (Zhai et al. 2018). Baracchi et al. (2011) reported a higher rate of sepsis-induced mortality in individuals in intensive care. Sepsis causes dysfunction in lungs and other organ (Kim and Hong 2016). In particular, patients with tachypnea and hypoxia experience acute lung injury (ALI) (Raghavendran and Napolitano 2011). ALI leads to acute respiratory distress syndrome (ARDS), resulting in higher morbidity and mortality rates (Matthay and Ware 2012; Yao et al. 2013). Matute-Bello et al. (2008) reported that poor lung performance, edema, protein-rich fluid accumulation in airspaces, pulmonary neutrophil infiltration, and accelerated alveolar-capillary membrane permeability are major symptoms of ALI. Kojicic et al. (2012) reported that increased health-care resources and prolonged hospitalization are required for patients with ARDS/ALI. Bice et al. (2013) reported that therapy becomes overly costly when ARDS/ALI patients require mechanical ventilation. At present, there is no permanent therapeutic approach for ALI; sepsis also increases other associated death rates (Favarin et al. 2013). Effective drugs are thus required for the treatment of ALI.
Tryptophan is a key amino acid in the synthesis of proteins in animals. It acts as a precursor for melatonin, serotonin, and vitamin B3 (Slominski et al. 2002). Tryptophan is involved in the anchoring of cell membrane proteins (Palme and Nagy 2008). Bitzer-Quintero et al. (2010) reported the antioxidant potential of tryptophan following endotoxic shock in experimental rats. Hu et al. (2017) reported the protective effects of melatonin against septic injury and bacterial infections; Xu et al. (2019) reported the therapeutic efficacy of melatonin against microbial sepsis via neutrophilic antibacterial activity. Wang et al. (2016) reported that 5-methoxytryptophan also exhibits anti-inflammatory activity against systemic inflammation. In this study, we evaluated the therapeutic efficacy of tryptophan against lipopolysaccharide (LPS)-induced ALI in rats.
Materials and methods
Rats and housing conditions
Male albino Wistar rats were purchased from the Animal House of the Fourth Medical Centre of the Chinese PLA General Hospital (Beijing, China). The rats (170–200 g) were maintained in cages with standard atmospheric conditions and 12 h light/dark periods. The relative humidity was maintained at 60 ± 5% and the temperature was kept at 25 ± 0.5 °C.
Experimental design
Experimental ALI was induced in rats according to previously described method (Zhang et al., 2018). Rats were grouped into sham, control (ALI), ALI + 1, 25 and 50 mg/kg body weight l-tryptophan (Sigma-Aldrich, T0254). Each group contained six rats. Rats were grouped into sham, control (ALI), and ALI + 1, 25, and 50 mg/kg body weight l-tryptophan (Sigma-Aldrich, T0254). We conducted preliminary study with various concentrations of l-tryptophan (1–100 mg/kg). However, we observed the optimum and significant effect of l-tryptophan up to 50 mg/kg. Thus, we selected 25 mg/kg and 50 mg/kg in this study. Each group contained six rats. l-Tryptophan was dissolved in water and administered orally for 28 consecutive days. The dose volume was adjusted to 1 mL, and an equal volume of water was given to the control and sham rats. At the end of treatment, blood and bronchoalveolar lavage fluid (BALF) were collected for further analyses.
Determination of biochemical markers
Total protein levels in BALF were measured using the bicinchoninic acid (BCA) method as previously described (Hua et al. 2017). Protein levels in the supernatant of BALF were measured using a biochemical analyzer (Bio-Rad Laboratories, Hercules, CA, USA). Neutrophil, lymphocyte, and total cell levels in BALF were estimated according to Hua et al. (2017); 100 cells per slide were counted. The lipid peroxidation level was measured as malondialdehyde (MDA) content in lung tissue as previously described (Samarghandian et al. 2014). Catalase, glutathione peroxidase (Gpx), superoxide dismutase (SOD), and reduced glutathione (GSH) in fresh lung tissue were determined according to previously described methods (Weydert and Cullen 2010; Baydas et al. 2002). Myeloperoxidase (MPO) activity levels in lung tissue homogenate were measured as described by Pulli et al. (2013). Serum levels of tumor necrosis factor (TNF)-α, migration inhibitory factor (MIF), and interleukin (IL)-8 were determined as previously described (Talebi-Garakani and Safarzade 2013). The partial pressure of oxygen (PaO2) in blood samples was determined according to Hua et al. (2017). Briefly, 2 mL blood was collected from the aorta abdominalis to measure PaO2 in an automatic blood gas analyzer (Bio-Rad Laboratories).
Histopathological analyses
Histopathological evaluation of lung tissue was performed as previously described (Talebi-Garakani and Safarzade 2013). The right lower pulmonary lobe was removed, immersed in neutral-buffered formalin (10%), and embedded in paraffin. Tissues were sliced, sectioned (4 µm), stained with hematoxylin and eosin, and examined for histopathological changes under a light microscope (Olympus BX53, Tokyo, Japan).
Determination of wet/dry weight ratio
The wet/dry weight ratio of lung tissue was measured as previously described (Huang et al. 2017). Briefly, wet weight was determined through excision of right upper pulmonary lobes, and dry weight was determined by placing these lobes in an oven at 70 °C for 48 h.
RT-PCR
Total RNA was extracted from lung tissue and converted into cDNA using reverse transcriptase III (ab63979, Abcam, Cambridge, UK). Expression of TNF-α and IL-1β was analyzed using specific primers (TNF-α: forward, 5′-TAT GGC TCA GGG TCC AAC TC-3′ and reverse, 5′-CTC CCT TTG CAG AAC TCA GG-3′; IL-1β: forward, 5′-GAC CTT CCA GGA TGAGGA CA-3′ and reverse, 5′-AGG CCA CAG GTA TTT TGTCG-3′). Relative mRNA expression was calculated using the 2–ΔΔCt method (Muthuraman et al. 2014).
Immunohistochemistry
Lung tissues were sectioned, fixed, and permeabilized in 0.1% Triton X-100 for 10 min. Then the cells were incubated with bovine serum albumin for 60 min, washed repeatedly, and incubated with primary antibodies against TNF-α (ab6671, Abcam) and IL-1β (ab9722, Abcam) for 12 h, followed by incubation with FITC-conjugated secondary antibody for 60 min.
Statistical analyses
The values are presented as the mean ± standard deviation. Experimental results were analyzed using analysis of variance (ANOVA), and Tukey’s post hoc test was carried out to compare the results. The difference was taken as significant at P < 0.05.
Results
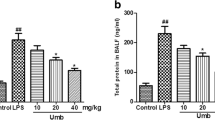
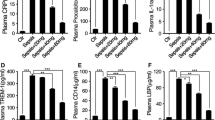
In this study, we evaluated the effects of tryptophan in LPS-induced ALI in rats. The total protein level in BALF was drastically increased by 321.1% in the control group. However, supplementation with L-tryptophan reduced the total protein content by 4.9%, 33.4%, and 64.5% at 1, 25, and 50 mg/kg, respectively (P < 0.05, Fig. 1). In the control group, neutrophils, lymphocytes, and total cells were substantially increased by 468.5%, 307.2%, and 354.9%, respectively (P < 0.05, Fig. 2). However, supplementation reduced them by 12.5%, 31.8%, and 65.1%; 15.1%, 41.7%, and 63.3%; and 12.6%, 42.4%, and 65.7%, respectively (P < 0.05, Fig. 2). MDA content was increased by 521.7% in the control group, and supplementation reduced these to 9.4%, 28.4%, and 68.7% (Fig. 3, P < 0.05). Catalase, SOD, Gpx, and GSH were lower in control rats than in sham rats, and supplementation significantly increased these antioxidant markers to near normal levels (Table 1, P < 0.05). MPO activity was substantially increased (609.8%) in the control group, and supplementation reduced levels by 12.1%, 33.4%, and 68.2% (Fig. 4, P < 0.05). In control rats, MIF, IL-8, and TNF-α level were increased by 441.7%, 717.3%, and 532.9%, respectively. However, l-tryptophan reduced these by 12.7%, 39.5%, and 68.2%; 5.5%, 46.8%, and 78.5%; and 10.8%, 39.8%, and 72.2%, respectively (Fig. 5, P < 0.05).
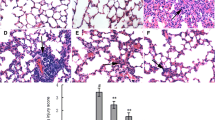
PaO2 was reduced by 506.6% in the control group, and supplementation increased it by 21.9%, 52.8%, and 87.4% (Fig. 6, P < 0.05). Neutrophilic infiltration and edema are the predominant proinflammatory changes following ALI. However, supplementation resulted in significant recovery of these (Fig. 7). The wet/dry ratio of fresh lung tissue was significantly increased in control rats; this ratio was reduced following supplementation with l-tryptophan (Fig. 8, P < 0.05).
The mRNA expression of TNF-α and IL-1β was increased 120% and 90%, respectively, in the control group. However, l-tryptophan reduced these by 4.5%, 21.8%, and 41.8% and 5.2%, 17.9%, and 46.2%, respectively (P < 0.05, Fig. 9a). Immunohistochemical analyses showed reduced TNF-α and IL-1β expression in a dose-dependent manner (Fig. 9b). Protein expression of TNF-α and IL-1β was increased by 110% and 92% in the control group, respectively, and supplementation reduced these by 2.8%, 15.2%, and 35.7% and 5.2%, 15.6%, and 28.6% (P < 0.05, Fig. 9c).
Discussion
The present study evaluated the effects of tryptophan in sepsis-induced ALI in rats. Poor lung performance, edema, protein-rich fluid accumulation in airspaces, pulmonary neutrophil infiltration, and accelerated alveolar-capillary membrane permeability are major symptoms of ALI (Matute-Bello et al. 2008). Destruction of the pulmonary endothelium and alveolar epithelium is the primary cause of lung dysfunction (Herrero et al. 2018). Cytokines play a major role in inflammation, and understanding the effects of L-tryptophan on the production of inflammatory cytokines in sepsis-induced ALI is important. Chen et al., (2018) have reported that the heme oxygenase-1 reduces sepsis-induced endoplasmic reticulum stress and ALI. Activated neutrophils and macrophages release increased levels of ILs and TNF-α in ALI (Fuller et al. 2015), which leads to the accumulation of neutrophils in lung tissue (Carney et al. 1999). Supplementation of 25 and 50 mg/kg l-tryptophan reduced TNF-α levels, confirming the protective effects of l-tryptophan against sepsis and inflammation.
IL-8 plays a key role in endothelial activation, capillary leaks, and ischemia–reperfusion injury (Zhang 2008) and MIF levels are increased in sepsis-induced ALI (Gao et al. 2007). Supplementation of 25 and 50 mg/kg l-tryptophan reduced IL-8 and MIF levels, further confirming the protective effects of l-tryptophan against sepsis and inflammation. Wang et al. (2016) reported the anti-inflammatory activity of 5-methoxytryptophan against systemic inflammation. Evaluation of antioxidant and lipid peroxidation status will improve our understanding of the mechanism of l-tryptophan against sepsis. The active role in ALI-induced pathology has been researched extensively (Li et al. 2015). Reduced levels of antioxidants have been reported in sepsis-induced ALI (Zolali et al. 2014). Supplementation of 25 and 50 mg/kg l-tryptophan increased catalase, SOD, Gpx, and GSH, but reduced lipid peroxidation, confirming the antioxidant effects of l-tryptophan against sepsis and inflammation. Nayak and Buttar (2016) recently demonstrated the strong antioxidant potential of l-tryptophan in human cancer cells.
Determination of protein levels could be used as a marker to evaluate lung permeability. Supplementation of l-tryptophan reduced protein content, indicating a reduction in pulmonary permeability. It also reduced alveolar distortion, pulmonary edema, and neutrophil infiltration in sepsis conditions. The wet/dry ratio of fresh lung tissue indicates the level of pulmonary edema. Supplementation reduced the wet/dry ratio to a near normal ratio, confirming the protective effects of l-tryptophan against sepsis-induced ALI. Altogether, our results suggest that supplementation with l-tryptophan is effective against LPS-induced ALI.
Limitations of the study
In the current study, no toxic effects of l-tryptophan were detected and no mortality occurred in all l-tryptophan-treated groups. However, further studies are required to determine whether repeated long-term administration of l-tryptophan produces toxic effects.
Availability of data and materials
The corresponding author will provide all the experimental data on valid request.
Abbreviations
- ALI:
-
Acute lung injury
- MPO:
-
Myeloperoxidase
- TNF-α:
-
Tumor necrosis factor alpha
- MIF:
-
Migration inhibitory factor
- IL-8:
-
Interleukin-8
- PaO2:
-
Partial pressure of oxygen
- ARDS:
-
Acute respiratory distress syndrome
- BALF:
-
Bronchoalveolar lavage fluid
- MDA:
-
Malondialdehyde
- ANOVA:
-
Analysis of variance
- GSH:
-
Reduced glutathione
- SOD:
-
Superoxide dismutase
References
Baracchi F, Ingiosi AM, Raymond RM, Opp MR (2011) Sepsis-induced alterations in sleep of rats. Am J Physiol Regul Integr Comp Physiol 301:R1467–R1478
Baydas G, Gursu MF, Yilmaz S, Canpolat S, Yasar A, Cikim G, Canatan H (2002) Daily rhythm of glutathione peroxidase activity, lipid peroxidation and glutathione levels in tissues of pinealectomized rats. Neurosci Lett 323:195–198
Bice T, Cox CE, Carson SS (2013) Cost and health care utilization in ARDS–different from other critical illness? Semin Respir Crit Care Med 34:529–536
Bitzer-Quintero OK, Dávalos-Marín AJ, Ortiz GG, Meza AR, Torres-Mendoza BM, Robles RG, Huerta VC, Beas-Zárate C (2010) Antioxidant activity of tryptophan in rats under experimental endotoxic shock. Biomed Pharmacother 64:77–81
Carney DE, Lutz CJ, Picone A (1999) Soluble tumor necrosis factor receptor prevents post-pump syndrome. J Surg Res 83:113–121
Chen X, Wang Y, Xie X, Chen H, Zhu Q, Ge Z, Wei H, Deng J, Xia Z, Lian Q (2018) Heme oxygenase-1 reduces sepsis-induced endoplasmic reticulum stress and acute lung injury. Mediators Inflamm 2018:9413876
Favarin DC, de Oliveira JR, de Oliveira CJ, Rogerio Ade P (2013) Potential effects of medicinal plants and secondary metabolites on acute lung injury. Biomed Res Int 2013:576479
Fuller BM, Mohr NM, Graetz TJ, Lynch IP, Dettmer M, Cullison K, Coney T, Gogineni S, Gregory R (2015) The impact of cardiac dysfunction on acute respiratory distress syndrome and mortality in mechanically ventilated patients with severe sepsis and septic shock: an observational study. J Crit Care 30:65–70
Gao L, Flores C, Fan-Ma S (2007) Macrophage migration inhibitory factor in acute lung injury: expression, biomarker, and associations. Transl Res 150(1):18–29
Herrero R, Sanchez G, Lorente JA (2018) New insights into the mechanisms of pulmonary edema in acute lung injury. Ann Transl Med 6(2):32
Hu W, Deng C, Ma Z (2017) Utilizing melatonin to combat bacterial infections and septic injury. Br J Pharmacol 174(9):754–768
Hua S, Liu X, Lv S, Wang Z (2017) Protective effects of cucurbitacin b on acute lung injury induced by sepsis in rats. Med Sci Monit 23:1355–1362
Huang C, Pan L, Lin F, Dai H, Fu R (2017) Monoclonal antibody against Toll-like receptor 4 attenuates ventilator-induced lung injury in rats by inhibiting MyD88- and NF-κB-dependent signalling. Int J Mol Med 39:693–700
Kim WY, Hong SB (2016) Sepsis and acute respiratory distress syndrome: recent update. Tuberc Respir Dis (Seoul) 79(2):53–57
Kojicic M, Li G, Hanson AC (2012) Risk factors for the development of acute lung injury in patients with infectious pneumonia. Crit Care 16(2):R46
Li S, Tan HY, Wang N (2015) The role of oxidative stress and antioxidants in liver diseases. Int J Mol Sci 16(11):26087–26124
Matthay MA, Ware LB, Zimmerman GA (2012) The acute respiratory distress syndrome. J Clin Invest 122:2731–2740
Matute-Bello G, Frevert CW, Martin TR (2008) Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 295(3):L379–L399
Muthuraman P, Ravikumar S, Muthuviveganandavel V, Kim J (2014) Effect of cortisol on calpains in the C2C12 and 3T3-L1 cells. Appl Biochem Biotechnol 172:3153–3162
Nayak BN, Buttar HS (2016) Evaluation of the antioxidant properties of tryptophan and its metabolites in in vitro assay. J Complement Integr Med 13(2):129–136
Palme K, Nagy F (2008) A new gene for auxin synthesis. Cell 133:31–32
Pulli B, Ali M, Forghani R (2013) Measuring myeloperoxidase activity in biological samples. PLoS ONE 8(7):e67976
Raghavendran K, Napolitano LM (2011) Definition of ALI/ARDS. Crit Care Clin 27(3):429–437
Samarghandian S, Afshari R, Sadati A (2014) Evaluation of lung and bronchoalveolar lavage fluid oxidative stress indices for assessing the preventing effects of safranal on respiratory distress in diabetic rats. Sci World J 2014:251378
Slominski A, Semak I, Pisarchik A, Sweatman T, Szczesniewski A, Wortsman J (2002) Conversion of l-tryptophan to serotonin and melatonin in human melanoma cells. FEBS Lett 511:102–106
Talebi-Garakani E, Safarzade A (2013) Resistance training decreases serum inflammatory markers in diabetic rats. Endocrine 43:564–570
Wang YF, Hsu YJ, Wu HF, Lee GL, Yang YS, Wu JY, Yet SF, Wu KK, Kuo CC (2016) Endothelium-derived 5-methoxytryptophan is a circulating anti-inflammatory molecule that blocks systemic inflammation. Circ Res 119:222–236
Weydert CJ, Cullen JJ (2010) Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat Protoc 5:51–66
Xu L, Zhang W, Kwak M, Zhang L, Lee PCW, Jin JO (2019) Protective effect of melatonin against polymicrobial sepsis is mediated by the anti-bacterial effect of neutrophils. Front Immunol 10:1371
Yao S, Mao T, Fang W, Xu M, Chen W (2013) Incidence and risk factors for acute lung injury after open thoracotomy for thoracic diseases. J Thorac Dis 5(4):455–460
Zhai X, Yang Z, Zheng G, Yu T, Wang P, Liu X, Ling Q, Jiang L, Tang W (2018) Lactate as a potential biomarker of sepsis in a rat cecal ligation and puncture model. Mediators Inflamm 2018:8352727
Zhang C (2008) The role of inflammatory cytokines in endothelial dysfunction. Basic Res Cardiol 103(5):398–406
Zhang Y, He H, Zhang B, Chen Q, Yao S, Gui P (2018) Amelioration of lipopolysaccharide-induced acute lung injury in rats by Na-H exchanger-1 inhibitor amiloride is associated with reversal of ERK mitogen-activated protein kinase. Biomed Res Int 2018:3560234
Zolali E, Hamishehkar H, Maleki-Dizaji N (2014) Selenium effect on oxidative stress factors in septic rats. Adv Pharm Bull 4(3):289–293
Funding
None.
Author information
Authors and Affiliations
Contributions
SL, FS, and WZ conducted experiments and collected data. XU, XZ, and YY carried out data interpretation, review of literature, and manuscript drafting.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All animal experiments were approved by the ethical committee of the Fourth Medical Centre of the Chinese PLA General Hospital, Beijing, China; State Key Laboratory of Kidney Disease, The Chinese PLA General Hospital, Beijing, China, 100048.
Additional information
Handling editor: J. Broos.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, S., She, F., Zhang, W. et al. Tryptophan decreases the intensity of lipopolysaccharide-induced acute lung injury in a rat model. Amino Acids 52, 1139–1147 (2020). https://doi.org/10.1007/s00726-020-02878-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-020-02878-5