Abstract
This study was planned to evaluate the effect of vitamin D administration on cytotoxicity due to fluoride exposure in vitro. NaF (IC50) and vitamin D (proliferative) were applied to human osteoblast (hFOB 1.19) cells. The major genes of apoptotic, autophagic, and necrotic pathways were determined by RT-PCR. 2-∆∆Ct formulation was used for expression analysis. In the NaF group, caspase 3, Bax, Bad, Bak, Bclx, Atg3, Atg5, Atg6, pG2, LC3-I, LC3-II, RIP1, and RIP3 genes were increased (2.6–15 times). It was observed that the expressions of these genes approached the control when vitamin D was given together with NaF. The Bcl2 gene increased significantly (sixfold) with the effect of NaF, and was down-regulated to some extent with additional vitamin D administration, but still more than in the control. As a result, it was determined that apoptotic, necrotic, and autophagic pathways were activated as the molecular basis of the damage in the bone tissue, which was most affected by fluorine, and these genes were down-regulated and approached the control group with the addition of vitamin D. It was concluded that this is an important data to explain the molecular basis of the protective and therapeutic effect of vitamin D against fluorine toxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fluorine is a trace element with very high electronegativity found in nature as different compounds. If it is taken for a long time and in high doses, it causes toxication called “fluorosis,” which causes damage to teeth, bones, and various tissues. Bone is significantly affected by fluoride toxicity [1,2,3,4]. Many studies have been conducted in many parts of the world and our country on fluorine toxicity and its complications [5,6,7].
Vitamin D has many important metabolic functions as a hormone-like molecule in steroid structure. There are studies investigating the importance of vitamin D in bone development and repair, normal calcium and phosphorus homeostasis, some cancer types, type 1 diabetes, and autoimmune diseases [8,9,10]. The active form of vitamin D, 1,25(OH)2D3 (cholecalciferol), binds to intracellular protein receptors and thus interacts with DNA in the nucleus of the target cell. It selectively either induces or inhibits gene transcription [11].
Apoptosis (programmed cell death) is rather essential for the normal functioning and survival of cellular organisms. High concentrations of fluorine induce osteoblast apoptosis [12,13,14].
Autophagy mechanism, which works as a quality control system, is a physiological phenomenon responsible for the destruction of long-lived proteins, dysfunctional organelles, cytosolic parts, and damaged macromolecules and pathogens. Autophagy enables cellular recycling after lysosomal degradation and helps the cell survive in various conditions such as starvation, growth factor deficiency, and oxidative stress. In conditions where apoptosis is insufficient due to cellular content, cell death occurs by autophagy [15, 16]. The role of fluorine application in autophagic cell death was investigated. In autophagy, the cell dies via the caspase-independent pathway, the non-apoptotic pathway [15, 17, 18].
Oxidative stress, which is based on various causes, plays a very important role in the formation of apoptosis [17]. It has been reported that organ damage caused by fluorine can be corrected with the help of antioxidants. For this purpose, there are studies in which vitamin application is recommended. It is suggested that the toxic effect of fluorine may increase in people who cannot be fed enough with antioxidants such as vitamin C and flavonoids [19, 20]. In addition, it has been reported that giving vitamin C, calcium, and vitamin D to young children may be beneficial in the prevention of fluorosis [21].
Based on some of the data obtained from 2 projects on this subject [22, 23] previously carried out in our laboratory, it was decided that further studies were needed and new studies should be conducted to reveal these mechanisms. This project was one of the studies planned for this purpose. The aim of this project is to investigate the mechanisms underlying the cytotoxicity that occurs due to NaF, especially its importance in the detection, treatment, and prognosis of possible complications leading to bone damage, and how it is affected by vitamin D treatment.
Material and Method
Cell Line
This study was performed using human osteoblast hFOB 1.19 (ATCC® CRL-11372™) cells.
Preparation of Sodium Fluoride (NaF) and Vitamin D Solutions
To determine the appropriate doses and dissolution media to be applied in the study, NaF [4, 24, 25] vitamin D [22, 26] doses were taken as a reference. Stock solutions were prepared. Concentrations of NaF were prepared in the cell line’s medium, with final concentrations of 50, 100, 250, 500, 1000, 2000, 5000, 7500, 10,000, and 20,000 µM. Vitamin D3 (cholecalciferol) stock solution in DMSO was prepared at 26,000 µM. Final concentrations (˂0.05% DMSO) of 1, 5, 10, 25, 50, and 100 μM were prepared in the cell’s medium.
Cell Culture
hFOB 1.19 cells: it was cultured and used in Ham’s F12 medium containing 10% FBS, 1% penicillin/streptomycin, and 2 mM L-glutamine at 37 °C, 5% CO2, and 95% humidity.
Cytotoxicity (MTT Cell Viability) Test
MTT test was performed to determine the cytotoxic effect of NaF on the hFOB 1.19 cell line and to determine the appropriate doses of vitamin D that would have a positive effect on hFOB 1.19 cells [14, 22, 27].
The study groups were determined as 4 groups: control, NaF, NaF + vitamin D, and only vitamin D (Table 1).
Total RNA Isolation and cDNA Synthesis
After determining the IC50 cytotoxic value of NaF and the proliferative concentration range in which vitamin D will reduce the toxicity of NaF, the expressions of the major genes belonging to different phenotypic mechanisms were determined to elucidate the molecular mechanisms underlying the cytotoxicity occurring at the determined values. For this purpose, total RNA and then DNA isolation was performed at the 24th hour in cells treated with NaF and vitamin D at determined concentrations.
Pure RNA was obtained by applying a 1-ml cold TRIzol protocol on the cell pellet [28]. To control the total RNAs, a run with 0.7% agarose gel electrophoresis was performed and the RNA image was observed. In the quantitative evaluation of total RNA (BioDrop, UK), both the amount and purity of the RNA were determined by measuring the absorbance in the nanodrop spectrophotometer. Then, to obtain cDNA, reverse transcription was performed with the Rotor-Gene Q (Qiagen, USA) device according to the commercial kit (WizbioWizScript cDNA Synthesis Kit, Korea) protocol. Obtained cDNAs were amplified with the help of primers (Sentegen, Turkey) designed specifically for the target gene region.
Real-Time PCR
Gene expression patterns of samples were determined in RT-qPCR using sSYBR Green Master mix (WizPure™ qPCR Master (SYBR). Apoptosis (Bcl-2, Bcl-Xl, Bax, Bad, Bim, Bak, caspase 3, 8, 9), expressions of major genes in autophagy (Atg 3, 5, 6, pG2 (SQSTM1), LC3-I, LC3-II), and necrotic (RIP1, RIP3) pathways were investigated. Glyceraldehyde phosphate dehydrogenase (GAPDH) was used as a control gene in expression analysis. The sample was read in 3 independent replicates; Ct (cycle threshold) was determined at the beginning of the logarithmic phase of the amplifications; 2-∆∆Ct formulation was used for expression analysis; the difference between the groups was compared with the increase–decrease coefficient of control gene expression [29].
Statistical Analysis
Descriptive statistics for the featured features, expressed as median, mean, standard deviation, minimum and maximum values, and whether there was a difference between the groups in terms of these characteristics, was determined with the appropriate test. SPSS (22.0) statistical package program was used for calculations.
Results
MTT Results
According to MTT results, NaF IC50 concentration was determined as 3200 µM in the hFOB 1.19 cell line. In these cells, it was observed that cell proliferation increased the most at vitamin D 10 µM concentration.
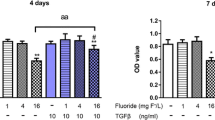
After 24 h of application of various concentrations of NaF and vitamin D concentration (10 µM) with the highest increase in cell proliferation, MTT % viability results are given below in the hFOB 1.19 cell line, assuming the control group as 100% viable (Table 2 and Fig. 1).
It was observed that cell viability changed positively after the application of vitamin D at NaF concentrations (100, 250, 500, 1000, 2000, 5000, 7500, and 10,000 μM) that increased cell proliferation.
In the hFOB 1.19 cell line, it was observed that the cell viability gradually decreased as the NaF level increased. In the groups treated with 10 µM vitamin D together with NaF, cell viability increased by 1–11% at all doses except the 250 µM dose compared to the NaF group.
Expression levels of target genes (Bcl-2, Bcl-Xl, Bax, Bad, Bim, Bak, caspase 3,8,9) in the mechanism of apoptosis are given below (Table 3, Fig. 2).
Expression results of target genes in the autophagy mechanism (Atg 3,5,6, pG2 (SQSTM1), LC3-I, LC3-II) are given below (Table 4, Fig. 3).
Expression results of necrotic pathway target genes (RIP1, RIP3) are given below (Table 5, Fig. 4).
Caspase 3, Bax, Bad, Bak, Bclx, Atg3, Atg5, Atg6, pG2, LC3-I, LC3-II, RIP1, and RIP3 genes were found to be up-regulated between 2.6 and 15 times in the groups given NaF. It was observed that the expression of these genes was not different from the control group, which was given only vitamin D. It was observed that the expressions of these genes approached the control with the administration of vitamin D together with NaF.
It was determined that the caspase 8 gene was up-regulated 2 times more in the groups given NaF and vitamin D separately, compared to the control. In the group given NaF + vitamin D together, it was down-regulated significantly compared to the control.
It was determined that the caspase 9 gene was not different in the NaF-treated group compared to the control group, and it was significantly down-regulated in the NaF + vitamin D-treated group.
It was found that Bcl2, an antiapoptotic gene, was significantly up-regulated (sixfold) in the NaF-treated group compared to the control group, and it was somewhat down-regulated in the NaF + vitamin D-treated group, but still more than in the control.
Discussion
NaF is used as a fluorine source in experimental studies on fluorosis caused by long-term exposure to the high concentration of fluoride [2,3,4].
As revealed in previous studies, NaF has a significant effect on cell proliferation depending on dose and time. In in vitro studies, it is known that NaF has a reducing effect on cell proliferation as the dose increases [14, 22, 23, 27, 30].
Regarding NaF cytotoxicity, there are many studies that examine the cytotoxic mechanisms and the mechanism of death one by one, but report that the cause of cytotoxicity has not been fully elucidated.
Studies are reporting that vitamin C, calcium, and vitamin D are applied in addition to substances with high antioxidant content to prevent organ damage caused by fluoride [14, 19,20,21,22,23, 27, 31].
In recent studies on the mechanism of cellular damage seen in fluorosis, it has been reported that different mechanisms are activated in various tissues. In studies investigating the effects of fluoride application in bone cells (MC3T3-E1, MG-63), it was revealed that NaF application decreases cell viability and increases apoptosis in cells depending on time and concentration [4, 12, 24]. A significant increase in osteoblast apoptosis was observed via caspase-3 and 9 [12, 24]. It has been found that NaF increases Bax expression in the renal tubules, suppresses Bcl-2, and thus induces apoptosis [32].
NaF stimulates apoptosis via the mitochondrial pathway by activating caspase-9, 3, Bak, Bax, Bcl-2, and Bcl-xL genes in many different cell lines [33,34,35] also increasing BAD expression [34]. Fluorine exposure caused apoptosis, DNA damage, and oxidative stress in lymphocytes and increased Bax expression [36]. Yang et al. [24] demonstrated that NaF application decreased cell viability in MC3T3-E1 osteoblast cells depending on time and concentration, and increased apoptosis even at low concentrations.
In osteoblast cells, Bim, caspase 9, 14, and (BCL2) BAX is increased, while caspase 3 is down-regulated and apoptosis occurs via the mitochondrial and death receptor pathways. Oxidative stress, apoptosis, and necrotic parameters increase together [37, 38].
Exposure to fluoride initiates autophagy. It has been reported that both transcript and protein levels of autophagic genes (Atg5, Atg7, Atg8/LC3, Beclin1) are significantly increased after fluoride exposure [39, 40].
Many studies have been conducted on the beneficial effects of antioxidants on fluorine-induced autophagy, apoptosis, and cytotoxicity [14, 18, 22, 23, 27, 31, 41].
Different treatments such as antiapoptotic molecular estrogen, calcitonin, different phosphonates, and vitamin D have been tried to stop apoptosis [4, 12,13,14, 22,23,24, 27, 30].
Vitamin D is important in the development and repair of bones, calcium and phosphorus homeostasis. It selectively stimulates or represses gene transcription. In the presence of parathormone (PTH), 1,25(OH)2D3 stimulates calcium mobilization from bone and intestinal absorption, increasing the amount of calcium and phosphate in the plasma [9,10,11].
Ekambaram and Chennai [41] showed that it protected against hypocalcemia in female rats treated with a combination of 500 ppm NaF and vitamin D for 60 days. In this study, which was planned to investigate the effects of vitamin D, which has antioxidant and protective properties, on the expression and translation of some apoptotic markers in the osteoblast (hFOB 1.19) cell line to which NaF was applied at IC50 ratio, it was reported that apoptotic caspase 3 and 8 enzymes increased and apoptotic M30 protein changed [22]. It has been shown that there is a relationship between apoptosis rate, intracellular ROS levels, Bax/Bcl-2 ratio, and protein expression (Caspase-3) [42].
Calcium, which is used as a preventive and therapeutic against fluorosis plus with vitamin D, can alleviate fluorosis when used alone, and Bax up-regulates caspase 12, 9, 7, and 3, while down-regulating Bcl-2 at both mRNA and protein expression levels [43].
Studies have been carried out in our laboratory to reveal the cytotoxic effect mechanism of NaF. It has been determined that there are significant changes in molecular pathways depending on time due to NaF. It was found that there was no significant increase in apoptotic and necrotic pathways except Atg3 at 3 and 12 h depending on the time. However, it was found that there was an increase in all cell death signaling pathways and increased genes over 24 h. It was concluded that cytotoxic mechanisms are activated by the addition of NaF and different mechanisms accelerate cellular death within 24 h [30].
In this study, the ability of vitamin D, which is an important molecule in many aspects, to inhibit flora-dependent cytotoxicity in osteoblast cells most affected by fluorine toxicity was investigated in a dose- and time-dependent manner. Thus, the possibilities of using vitamin D in the clinical treatment of NaF, which is known to be toxic and causes the death of cells and/or tissues in the organism, were evaluated.
In this study, all genes involved in apoptotic (caspase 3, Bax, Bad, Bak, Bclx), autophagic (Atg3, Atg5, Atg6, pG2, LC3-I, LC3-II), and necrotic (RIP1, RIP3) pathways, except for caspase 9, were investigated. It was found to be up-regulated between 2.6 and 15 times in the groups given NaF. It was observed that caspase 8 and 9 decreased significantly when vitamin and NaF were used together but did not change significantly with NaF. Bax/Bcl-2 ratio can act as a rheostat which determines cell susceptibility to apoptosis [21]. Lower levels of this ratio may lead to resistance of human cancer cells to apoptosis. Thus, Bax/Bcl-2 ratio can affect tumor progression and aggressiveness [44]. The Bax/Bcl-2 ratio is important because this rate increases mitochondrial apoptosis. In this study, the BAX/BCL-2 ratio was found to be significantly lower in the NaF + vitamin D group than in the NaF group alone.
Thus, it was determined that NaF causes damage to osteoblast cells through apoptotic, autophagic, and necrotic pathways. It was revealed that the administration of Vitamin D together with NaF decreased the expression of these genes. It was concluded that this is proof of the benefit of vitamin D in the prevention of NaF-induced bone cell damage.
As a result, it has been shown that apoptotic, necrotic, and autophagic pathways are activated as the molecular basis of the damage in the bone tissue, which is most affected by fluorine, and it has been shown that these pathways are inhibited by the application of vitamin D. It was concluded that this situation can be accepted as important evidence to explain the basis of the protective and therapeutic effect of vitamin D against fluorine toxicity.
Data Availability
The data that support the findings of this study are available from the corresponding author, [Semiha DEDE], upon reasonable request.
References
Agalakova NI, Gusev GP (2012) Molecular mechanisms of cytotoxicity and apoptosis induced by inorganic fluoride. ISRN Cell Biol 403835. https://doi.org/10.5402/2012/403835
Perumal E, Paul V, Govindarajan V, Panneerselvam L (2013) A brief review on experimental fluorosis. Toxicol Letter 233:236–251. https://doi.org/10.1016/j.toxlet.2013.09.005
Song GH, Gao JP, Chun FW et al (2014) Sodium fluoride induces apoptosis in the kidney of rats through caspase-mediated pathways and DNA damage. J Physiol Biochem 70(3):857–868. https://doi.org/10.1007/s13105-014-0354-z
Wei Y, Wu Y, Zeng B, Zhang H (2014) Effects of sodium fluoride treatment in vitro on cell proliferation, BMP-2 and BMP-3 expression in human osteosarcoma MG-63 cells. Biol Trace Elem Res 162(1–3):18–25. https://doi.org/10.1007/s12011-014-0148-8
Yur F, Dede S, Çiftçi-Yeğin S, Değer Y (2013) ACE activity in sheep with fluorosis. Van Vet J 24(1):25–27
Yur F, Mert N, Dede S et al (2013) Evaluation of serum lipoprotein and tissue antioxidant levels in sheep with fluorosis. Fluoride 46(2):90–96
Aydın N, Dede S, Tanrıtanır P (2014) The distribution of minerals in some tissues of sheep with fluorosis. Fluoride 47:43–48
Öngen B, Kabaroğlu C, Parıldar Z (2008) D vitamininin biyokimyasal ve laboratuvar değerlendirmesi. Türk Klin Biyokim Derg 61:23–31
Christakos S, DeLuca HF (2011) Minireview: Vitamin D: is there a role in extra skeletal health? Endocrinology 152:2930–2936. https://doi.org/10.1210/en.2011-0243
Tintino SR, Morais-Tintino CD, Campina FF et al (2016) Action of cholecalciferol and alpha tocopherol on Staphylococcus aureus efflux pumps. EXCLI J 15:315–322. https://doi.org/10.17179/excli2016-277
Ferrier DR (2014) Lippincott’s Illustrated Reviews: Biochemistry, 6th edn. Lippincott Williams & Wilkins, Baltimore
Yan X, Feng C, Chen Q et al (2009) Effects of sodium fluoride treatment in vitro on cell proliferation, apoptosis and caspase-3 and caspase-9 mRNA expression by neonatal rat osteoblasts. Arch Toxicol 83(5):451–458. https://doi.org/10.1007/s00204-008-0365-z
Liu L, Zhang Y, Gu H et al (2015) Fluorosis induces endoplasmic reticulum stress and apoptosis in osteoblasts in vivo. Biol Trace Elem Res 164(1):64–71. https://doi.org/10.1007/s12011-014-0192-4
Yüksek V, Dede S, Taspinar M (2017) The effects of vitamin D onto the expression of caspase enzymes in osteoblastic cell line treated with sodium fluoride (NaF). FEBS J 284:354
Öz-Arslan D, Korkmaz G, Gözüaçık D (2011) Otofaji: Bir hücresel stress yanıtı ve ölüm mekanizması. Acıbadem Üniv Sag Bil Derg 2(4):184–194
Karadağ A (2016) Otofaji: Programlı hücre ölümü. Ankara Sag Hiz Derg 15(2):19–26. https://doi.org/10.1501/Ashd_0000000117
Kannan K, Jain SK (2000) Oxidative and stress and apoptosis. Pathophysiology 7(3):153–163
Suzuki M, Bandoski C, Bartlett JD (2015) Fluoride induces oxidative damage and SIRT1/autophagy through ROS-mediated JNK signaling. Free Radic Biol Med 89:369–378. https://doi.org/10.1016/j.freeradbiomed.2015.08.015
Verma RJ, Sherlin DM (2001) Vitamin C ameliorates fluoride-induced embryotoxicity in pregnant rats. Human Exp Toxicol 20(12):619–623. https://doi.org/10.1191/096032701718890559
Susheela AK, Bhatnagar M (2002) Reversal of fluoride induced cell injury through elimination of fluoride and consumption of dietrich in essential nutrients and antioxidants. Mol Cell Biochem 234(235):335–340
Gupta SK, Gupta RC, Seth AK, Gupta A (1996) Reversal of fluorosis in children. Acta Paediatr Japonica 38(5):513–519
Yüksek V, Dede S, Taşpınar M, Çetin S (2017) The effects of vitamins A, D, E, and C on apoptosis and DNA damage in sodium fluoride-treated renal and osteoblast cell lines. Fluoride 50(3):300–313
Çetin S, Yur F, Taşpınar M, Dede S, Yüksek V (2017) The effects of lycopene application on sodium fluoride (NaF) applied renal cell line. Int J Second Metab 4(Special Issue 2):508–511. https://doi.org/10.21448/ijsm.377756
Yang S, Wang Z, Farquharson C et al (2011) Sodium fluoride induces apoptosis and alters Bcl-2 family protein expression in MC3T3-E1 osteoblastic cells. Biochem Biophys Res Commun 410(4):910–915
He H, Wang H, Jiao Y et al (2015) Effect of sodium fluoride on the proliferation and gene differential expression in human RPMI8226 cells. Biol Trace Elem Res 167:11–17. https://doi.org/10.1007/s12011-015-0271-1
Maj E, Filip-Psurska B, Świtalska M et al (2015) Vitamin D analogs potentiate the antitumor effect of ımatinibmesylate in a human a549 lung tumor model. Int J Mol Sci 16(11):27191–27207. https://doi.org/10.3390/ijms161126016
Yüksek V, Cetin S, Usta A, Komuroglu AU, Dede S (2017) Effect of some vitamins on antioxidant/prooxidant parameters in sodium fluoride (NaF)-treated cell line (hFOB 1.19). Turk J Vet Res 1(1):1–6
Chomczynski P, Mackey K (1995) Substitution of chloroform by bromo-chloropropane in the single-step method of RNA isolation. Analy Biochem 225:163–164
Bustin SA (2004) (ed.) A-Z of Quantitati and PCR. La Jolla, CA: International University Line; USA
Urut F, Dede S, Yuksek V et al (2021) In vitro evaluation of the apoptotic, autophagic, and necrotic molecular pathways of fluoride. Biol Trace Elem Res 199:3700–3706. https://doi.org/10.1007/s12011-020-02491-3
Oner AC, Dede S, Yur F, Oner A (2020) The effect of vitamin C and vitamin E on DNA damage, oxidative status and some biochemical parameters in rats with experimental fluorosis. Fluoride 53(1):154–163
Xu H, Jin XQ, Jing L, Li GS (2006) Effect of sodium fluoride on the expression of bcl-2 family and osteopontin in rat renal tubular cells. Biol Trace Elem Res 109(1):55–60
Deng H, Kuang P, Cui H et al (2017) Sodium fluoride induces apoptosis in mouse splenocytes by activating ROS-dependent NF-κBsignaling. Oncotarget 8(70):114428–114441. https://doi.org/10.18632/oncotarget.22826
Zhou BH, Tan PP, Jia LS et al (2018) PI3K/AKT signaling pathway involvement influoride-induced apoptosis in C2C12 cells. Chemosphere 199:297–302. https://doi.org/10.1016/j.chemosphere.2018.02.057
Aranda-Salomão PM, de Oliveira FA, Dos Santos DMS et al (2019) TiF4 and NaF varnishes induce low levels of apoptosis in murine and human fibroblasts through mitochondrial Bcl-2 family and death receptor signalling. Arch Oral Biol 97:245–252. https://doi.org/10.1016/j.archoralbio.2018.10.039
Wen P, Wei X, Liang G et al (2019) Long-term exposure to low level offlüorideinduces apoptosis via p53 pathway in lymphocytes of aluminum smelter workers. Environ Sci Pollut Res 26(3):2671–2680. https://doi.org/10.1007/s11356-018-3726-z
Panneerselvam L, Govindarajan V, Ameeramja J et al (2015) Single oral acuteflüorideexposure causes changes in cardiac expression of oxidant and antioxidant enzymes, apoptotic and necrotic markers in male rats. Biochimie 119:27–35. https://doi.org/10.1016/j.biochi.2015.10.002
Zhang YL, Luo Q, Deng Q et al (2015) Genes associated with sodiumfluoride-induced human osteoblast apoptosis. Int J Clin Exp Med 8(8):13171–13178
Zhang J, Zhu Y, Shi Y et al (2017) Fluoride-induced autophagy via the regulation of phosphorylation of mammalian targets of rapamycin in mice leydig cells. J Agri Food Chem 65(40):8966–8976. https://doi.org/10.1021/acs.jafc.7b03822
Pan Y, Li Z, Wang Y et al (2019) Sodium fluoride regulates the osteo/odontogenic differentiation of stem cells from apical papilla by modulating autophagy. J Cell Physiol. https://doi.org/10.1002/jcp.28269
Ekambaram P, Chennai VP (2003) Effect of vitamin D on chronic behavioral and dental toxicities of sodium fluoride in rats. Fluoride 36:189–197
Gu X, Wang Z, Gao J et al (2019) SIRT1 suppresses p53-dependent apoptosis by modulation of p21 in osteoblast-like MC3T3-E1 cells exposed tofluoride. Toxicol Vitro 57:28–38. https://doi.org/10.1016/j.tiv.2019.02.006
Wang J, Yang J, Cheng X et al (2019) Calcium alleviatesfluoride-induced bone damage by inhibiting endoplasmic reticulum stress and mitochondrial dysfunction. J Agri Food Chem 67(39):10832–10843. https://doi.org/10.1021/acs.jafc.9b04295
Khodapasand E, Jafarzadeh N, Farrokhi F et al (2015) Is Bax/Bcl-2 ratio considered as a prognostic marker with age and tumor location in colorectal cancer? Iran Biomed J 19(2):69–75. https://doi.org/10.6091/ibj.1366.2015
Acknowledgements
This research was carried out by Van Yuzuncu Yil University Scientific Research Projects Coordination Unit as project numbered TSA-2017-5949.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dede, S., Taşpinar, M., Yüksek, V. et al. The Effects of Vitamin D Application on NaF-Induced Cytotoxicity in Osteoblast Cells (hFOB 1.19). Biol Trace Elem Res 201, 698–705 (2023). https://doi.org/10.1007/s12011-022-03177-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03177-8