Abstract
Background
Sodium p-aminosalicylic acid (PAS-Na) was reported to exhibit anti-inflammatory effect in the nervous system. However, the mechanism by which PAS-Na exhibits anti-inflammatory effects on manganese (Mn)-stimulated BV2 microglia cells remains unclear. Thus, this study investigated the role of PAS-Na in Mn-stimulated BV2 microglial cells.
Methods
Microglia-like BV2 were treated with MnCl2 with or without the non-steroidal anti-inflammatory drug PAS-Na for 12 or 24 h to examine cell viability using MTT; for 24 or 48 h to examine levels of NLRP3, CASP1, IL-1β, and IL-18 mRNA using Real-Time quantitative PCR; for 48 h to examine levels of NLRP3 and CASP1 inflammasomes, measured by western blot analysis; and for 48 h to examine levels of inflammatory cytokines, measured by enzyme-linked immunosorbent assay.
Results
The MTT assay showed that PAS-Na produced significant neuroprotective effect by preventing Mn-induced inflammation in BV2 microglial cells. PAS-Na significantly concentration and time dependently inhibited Mn-induced production of NLRP3, CASP1, IL-1β, and IL-18.
Conclusion
Taken together, our results suggest that PAS-Na exerts anti-inflammatory effects in Mn-stimulated BV2 microglial cells via downregulation of NLRP3, CASP1, IL-1β, and I L-18. Furthermore, a high concentration and prolonged PAS-Na treatment appear necessary for its therapeutic efficacy. Taken together, we conclude that PAS-Na affords therapeutic efficacy in mitigating neurological conditions associated with neuroinflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Manganese (Mn) is an essential trace element that is widely distributed in the Earth’s crust and is crucial for multiple Mn-dependent enzyme and Mn metalloenzymes [1]. As such, Mn plays a key role in numerous biochemical reactions, including immune response, ATP generation, bone growth, digestion, and reproduction [2]. Additionally, Mn is an integral constituent of metalloenzymes, such as the Mn superoxide dismutase located within the mitochondria that facilitate the detoxification of superoxide free radicals [3]. Mn is required in human tissues and its concentrations regulated in several homeostatic mechanisms. The liver, kidney, pancreas, bone, and parts of the brain, including the basal ganglia and cerebellum, are particularly rich in Mn content [4]. Nutritional Mn deficiency has not been described in humans and has been attributed to its ubiquitous presence in the diet. In real-life scenarios, long-term exposure to elevated levels of Mn causes manganism, a neurodegenerative disorder characterized by Parkinson’s disease (PD)-like symptoms [5, 6]. Moreover, Mn is a risk factor for several neurodegenerative diseases including PD and Alzheimer’s disease (AD) [7]. Numerous investigations have indicated that Mn exposure induces classical activation in microglial cells in the central nervous system (CNS), leading to the production of pro-inflammatory cytokines [8, 9]. A plethora of pro-inflammatory factors cause neuronal damage [10]. However, the precise effect of Mn exposure on microglial cells has yet to be fully elucidated.
As the primary immune response cell in the CNS, microglia continuously monitor the microenvironment through pattern recognition receptors, including Toll-like receptors and Nod-like receptors. Once injury is sensed, microglia respond rapidly to stress, infection, and injury [11,12,13,14]. Mn has been shown to induce neurological injury, encompassing complex pathophysiological signaling mechanisms between neurons and glial cells [8, 15]. Glial cells are also an important target of Mn neurotoxicity, both for sequestration of the metal and for activation of inflammatory signaling pathways that trigger neuroinflammation by releasing multiple inflammatory cytokines [16, 17]. The accumulation of pro-inflammatory cytokines, such as IL-1β (interleukin 1 β) and IL-18 (interleukin 18), as well as NLRP3 (Nod-like receptor family, pyrin domain containing 3)-CASP1 (caspase-1) inflammasome activation has been invoked to damage hippocampal neuronal cells in the course of the pathogenesis of manganism [2, 10].
PAS-Na, a non-steroidal anti-inflammatory drug, has been used to treat tuberculosis [18]. The chemical structure of PAS-Na is comprised of carboxyl, hydroxyl, and amine groups, which provide promising chelating moieties for metals. An earlier study by Zheng et al. showed that in addition to its known anti-tuberculosis effect, PAS is also an efficient chelating agent [19]. In ensuing clinical trials, PAS-Na has shown efficacy in the treatment of Mn poisoning [20,21,22]. Moreover, animal studies have shown that PAS-Na has protective effect on spatial learning and memory abilities in rodent [23, 24]. Furthermore, PAS-Na has been reported to attenuate neurotoxicity in basal ganglia of rat [25, 26].
In spite of the many reports that have corroborated the clinical efficacy of PAS-Na in protecting the CNS by attenuating inflammatory response [24, 27], little is known about the relationship of PAS-Na and NLRP3-CASP1 inflammasome pathway. In addition, a previous study reported that NLRP3-CASP1 inflammasome-mediated neuroinflammation in microglia has specific relevance to manganism [2, 15]. Therefore, the present study investigated the neuroprotective effect of PAS-Na in Mn-stimulated BV2 microglial cells from the perspective of inflammatory responses.
Materials and Methods
Chemical Reagents
PAS-Na was purchased from Harbin Pharmaceutical Group (China). DMEM-F12 medium was purchased from HyClone (USA). 0.25% Trypsin (no EDTA) was purchased from Genotech (China). Fetal bovine serum (FBS) was purchased from Gibco (USA). JSH-23 (NF-κB inhibitor) was purchased from Selleck (USA). MnCl2·4H2O and dimethyl sulfoxide (DMSO) were purchased from Sigma (USA). BCA protein quantitative kit and Thiazolyl Blue Tetrazolium Bromide (MTT) were purchased from Beyotime (China). Eastep® Super total RNA extraction kit, GoScript™ Reverse Transcription Mix kit, and GoTaq® qPCR Master Mix kit were purchased from Promega (USA). The ELISA Kits were all purchased from Elabscience Company (China).
Cell Culture and Grouping
BV2 microglial cells were purchased from China Center for Type Culture Collection (CCTCC). BV2 microglial cells were placed at T25 flasks in DMEM-F12 medium supplemented with 10% FBS, penicillin G (100 units/mL), and streptomycin (100 mg/mL) and incubated at 37 °C in a humidified atmosphere containing 5% CO2 and passaged every 2–3 days to maintain growth.
To examine the effect of various concentrations of PAS-Na on Mn-induced cell viability, mRNA, and protein expression, BV2 microglial cells were randomly assigned into six groups in each group as follows: control, PAS-Na control, Mn-treated group (stimulated with 200 μmol/L MnCl2), and concentrations of 100, 200, and 400 μmol/L PAS-Na treatment groups (Mn + L, M, H groups). Cells cultured without any exposure and treatment were used as a control, and PAS-Na control group was treated with 400 μmol/L PAS-Na only.Different concentrations of PAS-Na treatment to Mn-induced Cell viability
Cell Viability Assay
BV2 microglial cells were inoculated into 96-well plate at a density of 6 × 103/well and then randomly assigned into different groups in different experiments. After 24 h of incubation, seeded cells were treated with various concentrations of MnCl2 with or without PAS-Na. After 24 h, 100 μl of MTT solution (5 mg/ml) was added to each well and kept in the incubator for 3 h at 37 °C. Then, 150 μl DMSO was added to dissolve the crystals, and cell viability was measured by taking absorbance at a wavelength of 490 nMin. The formula of the cell survival rate was as follows:
Real-Time Quantitative PCR
Real-Time Quantitative PCR (qPCR) was performed to investigate the effect of PAS-Na on NLRP3, CASP1, IL-1β, and IL-18 at mRNA level. 2 × 105/well BV2 microglial cells were seeded in 6-well plate and then randomly divided into six groups. After 24 h of incubation, 200 μmol/L MnCl2 was added to each well for 12 h prior to treatment with various concentrations of PAS-Na for 24 h or 48 h. Total RNA was isolated from cells according to the Eastep® Super total RNA extraction kit’s instructions. Total RNA (1 μg) was reverse transcribed using GoScript™ Reverse Transcription Mix kit. The optimal conditions for PCR amplification of cDNA were established using the manufacturer’s instructions. GAPDH was used as a housekeeping gene control, and untreated cells were used as a control to normalize the relative amounts of target gene expression.
qRT-PCR was performed in a total volume of 20 ul, consisting of 10 ul GoTaq® qPCR Master Mix, 0.8 μM of each primer, and diethylpyrocarbonate (DEPC)-treated water by using real-time fluorescence quantitative PCR instrument (Applied Biosystems, USA). The conditions for PCR cycles were as follows: stage 1, 95 °C 10 min; stage 2, 95 °C 15 s, 60 °C 1 min, 40 cycles; dissociation stage, 95 °C 15 s, 60 °C 15 s, 95 °C 15 s. Primer sequences (Sangon Biotech, China) were listed in Table 1. The cycle number at the linear amplification threshold (Ct) values for the endogenous control GAPDH and the target gene were recorded. Relative gene expression (target gene expression normalized to the expression of the endogenous control gene) was calculated using the comparative Ctmethod (2-∆∆Ct).
Western Blot Analysis
Proteins from BV2 microglial cells were extracted. Enhanced BCA protein assay kit (Beyotime, Shanghai, China) was applied to determine the concentrations of proteins, and double-distilled water was used to balance concentrations of samples before electrophoresis. Protein extracts were loaded onto a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) 10% gel for electrophoresis and then transferred to a polyvinylidene fluoride (PVDF) membrane (Merck Millipore, Darmstadt, Germany). After blockade with 5% BSA, membranes were incubated with primary antibodies (NLRP3, 1:1000, D4D8T, Cell Signaling Technology, USA; Cleaved CASP1, 1:1000, asp296, Cell Signaling Technology, USA; CASP1, 1:1000, ab1872, Abcam, USA; GAPDH, 1:2000, D16H11, Cell Signaling Technology, USA) overnight at 4 °C, respectively. The goat anti-rabbit secondary antibodies (1:5000, ab6721, Cell Signaling Technology, USA) were incubated at room temperature for 1 h. Finally, visualization of target proteins was achieved by detection of fluorescence produced by enhanced chemiluminescence (PierceTM ECL Western Blotting, Thermo).
Enzyme-Linked Immunosorbent Assay
The levels of IL-1β and IL-18 in BV2 microglial cells were determined using commercially available enzyme-linked immunosorbent assays (ELISA). To quantitatively the concentration of inflammatory cytokines, cells were incubated with 200 μmol/L MnCl2 acid for 12 h, then, different concentrations of PAS-Na treatment for 48 h. Next the cells were collected and analyzed for IL-1β and IL-18 content using an IL-1β ELISA kit and an IL-18 ELISA kit, respectively, according to the manufacturer’s protocol.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 8 and IBM SPSS Statistics 25 software. All data presented are representative of at least three independent experiments. The data are shown as the mean ± standard deviation (SD). Each experiment was repeated three times, and Student’s t test or one-way ANOVA were employed to quantify differences between two groups or among multiple groups, followed by LSD for post hoc comparisons. Statistical significance was set at P < 0.05.
Results
Establishment of BV2 Cell Inflammatory Injury Model Induced by Mn
In order to ensure the successful modeling of the inflammatory injury model, first, cell survival rate was assessed in BV2 microglial cells after Mn treatment. The results showed that the cell survival rate was significantly reduced in Mn treatment compared to the control group (P < 0.05, P < 0.01, Fig. 1). Therefore, we used cells cultured in 200 μmol/L MnCl2 for 12 h in the follow-up experiments.
Effect of MnCl2 on the Morphology of BV2 Microglial Cells
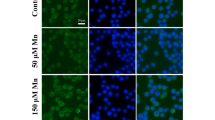
The morphology of control and MnCl2 treated is shown as Fig. 2. The morphology of control BV2 microglial cells showed small soma with fusiform, elliptical and synaptic. Mn-treated BV2 microglial cells had shorter branches which appeared to be resorbed into the cell body, while some cells became larger and amoeboid (Fig. 2). These data suggest that Mn exposure could impair immune system, and further indicate that our cells were a successful model of manganism.
Effect of MnCl2 on BV2 microglial cells morphology. The normal morphology of BV2 microglial cells cultured in normal medium for 12 h (left). Morphological changes of BV2 microglial cells were observed after exposure to 200 μmol/L MnCl2 for 12 h (right). Arrows indicate typical control and Mn-treated BV2 cells. Scale bar indicates 50 μm
Effect of PAS-Na on Cell Viability of BV2 Microglial Cells
To examine the effect of PAS-Na on cell viability, we examined the survival rate of PAS-Na-treated BV2 microglial cells. As shown in Fig. 3, the incubation of BV2 microglial cells with 0–1600 μmol/L PAS-Na for 24 h did not decrease cell viability within the range of concentrations used (P > 0.05). However, cellular viability was significantly decreased at concentrations of 3200 and 6400 μmol/L PAS-Na (P < 0.01). Therefore, 100(L), 200(M), and 400(H) μmol/L were utilized in the follow-up experiments.
PAS-Na Exerted Its Neuroprotection to BV2 Microglia Cells in a Concentration-Dependent Manner
Based on the results above, next, we investigated whether the neuroprotective effects of PAS-Na were concentration dependent. PAS-Na significantly increased cell viability of BV2 microglial cells with increasing concentration (Fig. 4). Thus, the results showed that PAS-Na might play a neuroprotective role in Mn-treated BV2 microglia cells in a concentration-dependent manner.
Effects of PAS-Na on cell viability of BV2 microglia cells exposed to Mn. BV2 microglial cells were stimulated with 200 μmol/L MnCl2 for 12 h prior to L, M, H-PAS treatment for 12 h. **, P < 0.01 compared to the control, ##, P < 0.01 compared to the Mn-treated group \( \overline{x}\pm \mathrm{SD} \) (μmol/L)
Effects of PAS-Na on Mn-Induced NLRP3, CASP1, IL-1β, and IL-18 mRNA Expression in BV2 Microglial Cells
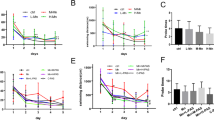
To assess temporal effect of PAS-Na treatment on Mn-induced inflammatory responses in microglia, NLRP3, CASP1, IL-1β, and IL-18 mRNA expression were detected by means of qPCR. As shown in Fig. 5, compared to control cells, mRNA levels of NLRP3, IL-1β, and IL-18 were significantly increased in cells treated with Mn (Fig. 4e, g, d, P < 0.05, P < 0.01). Furthermore, NLRP3 and IL-1β mRNA expression was significantly increased in PAS-Na-treated cells compared to that in Mn-treated group (Fig. 4a, c, P < 0.01). Interestingly, PAS-Na treatment for 48 h significantly decreased the levels of NLRP3 and IL-1β compared with the Mn-treated group (Fig. 4e, g, P < 0.05, P < 0.01). However, a significant decrease was observed in mRNA expression of CASP1 only (compared to Mn-treated group) (Fig. 4f, P < 0.05). Based on these results, we infer that the expression of NLRP3, CASP1, and IL-1β was significantly decreased, and IL-18 was significantly increased in BV2 microglial cells with prolonged PAS-Na incubation times.
Effects of PAS-Na on Mn-induced pro-inflammatory cytokines mRNA expression in BV2 microglial cells for 24 h (a, b, c, d) or 48 h (e, f, g, h). Each value indicates the mean ± SD and is representative of results obtained from three wells in all experiments. *, P < 0.05, **, P < 0.01, as compared with control group; #, P < 0.05, ##, P < 0.01, as compared with Mn-treated group (μmol/L)
Profiles of NLRP3, CASP1, IL-1β, and IL-18 Produced by Mn-Stimulated BV2 Cells After PAS Treatment
Protein Expression Levels of NLRP3 and CASP1 Inflammasomes
Mn exposure increased the expression of NLRP3 and CASP1 in the BV2 cells (Fig. 6b, c, P < 0.05, P < 0.01), compared to control cells. It is well established that CASP1 is cleaved into mature CASP1 to function. Thus, we determined protein expression of cleaved CASP1 and found its protein expression increased compared to control cells (Fig. 6d, P < 0.05). Furthermore, NLRP3 and cleaved CASP1 were significantly reduced after PAS treatment (Fig. 6b, d, P < 0.01), compared to the Mn-treated group. These results indicate that Mn activates the NLRP3 and CASP1 inflammasomes in BV2 cells. However, PAS-Na can effectively suppress induced inflammatory.
NLRP3/CASP1 expression in BV2 microglia cells. BV2 microglial cells were stimulated with 200 μmol/L MnCl2 for 12 h prior to L, M, H-PAS treatment for 48 h. Western blot was performed for NLRP3, CASP1, Cleaved CASP1, and GAPDH. Statistical analysis of relative target protein expression/internal control ratio. **, P < 0.01 compared to the control, ##, P < 0.01, compared to the Mn-treated group, \( \overline{x}\pm \mathrm{SD} \) (μmol/L)
Protein Expression Levels of IL-1β and IL-18 Inflammatory Cytokines
To measure the levels of IL-1β and IL-18 in the BV2 cells, we used the ELISA technique. Figure 7 presents the mean levels of the two cytokines, measured in repeated experiments, in cultured BV2 cells. The results showed a strong increase in IL-1β and IL-18 protein expression in the BV2 cells compared to the control at 12-h Mn stimulated (Fig. 7, P < 0.01). Importantly, PAS-Na treatment for 48 h significantly decreased the levels of IL-1β and IL-18 compared with the Mn-treated group (Fig. 7, P < 0.05, P < 0.01). Notably, the protein expression of IL-18 was reduced in dose-dependent manner.
IL-1β and IL-18 expression in BV2 microglia cells. BV2 microglial cells were stimulated with 200 μmol/L MnCl2 for 12 h prior to L, M, H-PAS treatment for 48 h. ELISA was performed for IL-1β and IL-18, **, P < 0.01 compared to the control, ##, P < 0.01 compared to the Mn-treated group, \( \overline{x}\pm \mathrm{SD} \) (μmol/L)
Discussion
Excessive brain Mn accumulation is known to cause an extrapyramidal disorder, including cognitive, memory, and motor deficits, as well as psychosis, an early effect in the course of the disease [28, 29]. In severe cases, manganism patients’ symptoms exhibit analogous neurological deficits to those of PD patients, such as tremor, bradykinesia, and gait difficulties, though the 2 diseases also have distinct features [30, 31]. Notably, inflammatory processes play an important role in neurodegenerative diseases and occur early in the pathogenesis of Mn neurotoxicity [13, 32]. As the primary source for pro-inflammatory cytokines, microglia are implicated as pivotal mediators of neuroinflammation and can induce or modulate a broad spectrum of cellular responses [14, 33]. Herein, we found that morphological changes after exposure to manganese are manifested as Mn poisoning, and the related mechanism of their changes were described below.
Previous studies have established the propensity of Mn to activate microglia, leading to neuroinflammation [34,35,36]. In the present study, we found that BV2 microglial cells showed increased release of pro-inflammatory IL-1β following Mn exposure. Li et al. observed that Mn caused enhancement in inflammatory cytokines IL-6, IL-1β, PGE2, and TNF-alpha levels in the hippocampus and thalamus [24]. The NLRP3 inflammasome, which comprises the NLRP3 scaffold, the PYCARD/ASC adaptor, and CASP1, has a critical role in the maturation and release of pro-inflammatory cytokines [37,38,39]. The inflammasome is formed and activated in response to infections, cellular damage, or metabolic disturbances and is involved in both host defense and sterile inflammation through proteolytically activating the highly pro-inflammatory cytokines, IL-1β, and IL-18 [40]. Here, we provide evidence that Mn exposure triggers the activation of NLRP3-CASP1 inflammasome pathway in microglia, corroborating in vivo observations by Wang et al [2]. Moreover, no significant increase was observed in the levels of IL-18 in BV2 microglial cells exposed to Mn in the present study. Collectively, these data suggest that microglia are activated by Mn both in vivo and in vitro, thus leading to the activation of the NLRP3-CASP1 inflammasome pathway, maturation, and release of pro-inflammatory cytokines (IL-1β and IL-18) and the ensuing innate immune response.
Microglia are the first line of response to brain injury or disease [41, 42]. Microglia play a critical homeostatic role in neuroinflammation, which is associated with various neurological diseases, including PD, AD, and Huntington disease [43,44,45]. Abnormal activation of microglia may induce neurotoxicity, and its excessive production of inflammatory mediators might aggravate blood-brain barrier disruption, facilitating CNS inflammatory responses [46,47,48]. Accordingly, controlling microglial activation might represent a potential strategy for the management of these diseases. Therapeutically, as a non-steroidal anti-inflammatory drug, PAS-Na has anti-inflammatory effects and has a proven efficacy against many different inflammation-associated disorders. Santos et al. found that in hippocampal pyramidal neurons, the Mn-induced increase in the level of PGE2 was reversed by PAS-Na [49]. Li et al. reported PAS-Na attenuated neuropathic pain through balancing pro-inflammatory and anti-inflammatory cytokine release in rat [24]. In addition, PAS-Na could mobilize and remove tissue Mn which is due to its chelating function [19]. In line with these reports, we ascertained that PAS-Na treatment impeded Mn-induced upregulation of IL-1β expression in BV2 microglial cells. Both the NLRP3 and CASP1 pathways were activated by Mn but were only partially repressed by PAS-Na treatment. Ram et al. found that novel NLRP3 inhibitor reduces nervous system injury by inhibiting NLRP3 and CASP1 pathways, consistent with our results [50]. Taken together, these data confirmed the anti-inflammatory functions of PAS-Na, providing evidences for its efficacy as a potential promising therapeutic for CNS disorders characterized by neuroinflammation.
The major function of inflammasomes is the generation of mature IL-1β and IL-18 [51]. NLRP3 is known as a critical mediator in neuroinflammation-mediated neurodegeneration [52]. We assessed whether the activation of the NLRP3 inflammasome was involved in Mn-induced release of IL-1β and IL-18 from microglia. Moreover, cleaved CASP1 triggers the maturation and secretion of potent pro-inflammatory mediators (IL-1β and IL-18) and culminating in the activation of the immune system and antimicrobial defense [53]. Using western blots, we showed that Mn exposure induced increased expression of NLRP3 and cleaved CASP1 in BV2 microglia. Mn-induced NLRP3-CASP1 inflammasome activation and the release of pro-inflammatory cytokines from microglia were shown to be blocked by treatment with PAS-Na. Herein, we found that PAS-Na Herein, we found that PAS-Na inhibited the Mn-induced increase in NLRP3 and cleaved CASP1 with concentration- and time-dependent, as well as the release of IL-1β and IL-18. Taken together, these results demonstrate that the NLRP3-CASP1 inflammasome pathway in BV2 microglial cells can be activated by Mn via non-canonical activation pathways. Additionally, the accumulation of pro-inflammatory cytokines, such as IL-1β and IL-18, may damage neuronal cells. Further, Mn exposure in the 48 h of PAS-Na treatment group showed that high-concentration of PAS-Na can reduce NLRP3, CASP, IL-1β, and IL-18 in different degree, while this was not the case in the other treatment groups. In other words, a high-concentration and prolonged PAS-Na treatment is necessary for its therapeutic efficacy. Notably, the combination of PAS-Na and Mn exposure increases NLRP3 and IL-1β in 24 h of treatment, which shown that PAS-Na might have some toxicity, and the magnitude of its toxicity needs further study. Meanwhile, additional work is required to further investigate the neurotoxic effect of IL-1β on neuronal cells, as well as the relationship between the abnormal activation of microglia and impairments in learning and memory in the hippocampal region. In addition, future research should be directed at the therapeutic efficacy of PAS-Na in attenuating in vivo Mn-induced neurological impairment.
Conclusion
The pathological mechanisms associated with Mn neurotoxicity are poorly understood, and evidence concerning the interrelationship between PAS-Na and neuroinflammation is lacking. Collectively, our novel data demonstrated that PAS-Na exerts an anti-inflammatory effect in Mn-stimulated BV2 microglial cells. Most specifically, the anti-inflammatory effect of PAS-Na on Mn-stimulated BV2 microglial cells is mediated by inhibition of the NLRP3-CASP1 inflammasome pathway, both in a concentration- and time-dependent manner. Therefore, PAS-Na may serve as a potential therapeutic agent for the treatment of neurodegenerative conditions with inherent neuroinflammation.
Data Availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- Mn:
-
manganese
- PD:
-
Parkinson’s disease
- AD:
-
Alzheimer’s disease
- CNS:
-
central nervous system
- IL1β:
-
interleukin 1 β
- IL18:
-
interleukin 18
- NLRP3:
-
NLR family, pyrin domain containing 3
- CASP1:
-
caspase-1
- PAS-Na:
-
sodium p-aminosalicylic acid
- qPCR:
-
quantitative real-time polymerase chain reaction
- ELISA:
-
enzyme-linked immunosorbent assay
References
Aschner JL, Aschner M (2005) Nutritional aspects of manganese homeostasis. Mol Asp Med 26(4-5):353–362. https://doi.org/10.1016/j.mam.2005.07.003
Wang D, Zhang J, Jiang W, Cao Z, Zhao F, Cai T, Aschner M, Luo W (2017) The role of NLRP3-CASP1 in inflammasome-mediated neuroinflammation and autophagy dysfunction in manganese-induced, hippocampal-dependent impairment of learning and memory ability. Autophagy 13(5):914–927. https://doi.org/10.1080/15548627.2017.1293766
Martinez-Finley EJ, Gavin CE, Aschner M, Gunter TE (2013) Manganese neurotoxicity and the role of reactive oxygen species. Free Radic Biol Med 62:65–75. https://doi.org/10.1016/j.freeradbiomed.2013.01.032
Tuschl K, Mills PB, Clayton PT (2013) Manganese and the brain. Int Rev Neurobiol 110:277–312. https://doi.org/10.1016/b978-0-12-410502-7.00013-2
Kim J, Pajarillo E, Rizor A, Son DS, Lee J, Aschner M, Lee E (2019) LRRK2 kinase plays a critical role in manganese-induced inflammation and apoptosis in microglia. PLoS One 14(1):e0210248. https://doi.org/10.1371/journal.pone.0210248
Langley MR, Ghaisas S, Ay M, Luo J, Palanisamy BN, Jin H, Anantharam V, Kanthasamy A, Kanthasamy AG (2018) Manganese exposure exacerbates progressive motor deficits and neurodegeneration in the MitoPark mouse model of Parkinson’s disease: relevance to gene and environment interactions in metal neurotoxicity. Neurotoxicology 64:240–255. https://doi.org/10.1016/j.neuro.2017.06.002
Ijomone OM, Aluko OM, Okoh COA, Martins AC Jr, Aschner M (2019) Role for calcium signaling in manganese neurotoxicity. J Trace Elem Med Biol 56:146–155. https://doi.org/10.1016/j.neuro.2017.06.002
Yin L, Dai Q, Jiang P, Zhu L, Dai H, Yao Z, Liu H, Ma X, Qu L, Jiang J (2018) Manganese exposure facilitates microglial JAK2-STAT3 signaling and consequent secretion of TNF-a and IL-1β to promote neuronal death. Neurotoxicology 64:195–203. https://doi.org/10.1016/j.neuro.2017.04.001
Crittenden PL, Filipov NM (2008) Manganese-induced potentiation of in vitro proinflammatory cytokine production by activated microglial cells is associated with persistent activation of p38 MAPK. Toxicol in Vitro 22(1):18–27. https://doi.org/10.1016/j.tiv.2007.07.004
Abe H, Ohishi T, Nakane F, Shiraki A, Tanaka T, Yoshida T, Shibutani M (2015) Exposure to MnCl2 . 4H2O during development induces activation of microglial and perivascular macrophage populations in the hippocampal dentate gyrus of rats. J Appl Toxicol 35(5):529–535. https://doi.org/10.1002/jat.3059
Colonna M, Butovsky O (2017) Microglia function in the central nervous system during health and neurodegeneration. Annu Rev Immunol 35:441–468. https://doi.org/10.1146/annurev-immunol-051116-052358
Kabba JA, Xu Y, Christian H, Ruan W, Chenai K, Xiang Y, Zhang L, Saavedra JM, Pang T (2018) Microglia: housekeeper of the central nervous system. Cell Mol Neurobiol 38(1):53–71. https://doi.org/10.1007/s10571-017-0504-2
Kirkley KS, Popichak KA, Afzali MF, Legare ME, Tjalkens RB (2017) Microglia amplify inflammatory activation of astrocytes in manganese neurotoxicity. J Neuroinflammation 14(1):99. https://doi.org/10.1186/s12974-017-0871-0
Kraft AD, Harry GJ (2011) Features of microglia and neuroinflammation relevant to environmental exposure and neurotoxicity. Int J Environ Res Public Health 8(7):2980–3018. https://doi.org/10.3390/ijerph8072980
Sarkar S, Rokad D, Malovic E, Luo J, Harischandra DS, Jin H, Anantharam V, Huang X, Lewis M, Kanthasamy A, Kanthasamy AG (2019) Manganese activates NLRP3 inflammasome signaling and propagates exosomal release of ASC in microglial cells. Sci Signal 12(563):eaat9900. https://doi.org/10.1126/scisignal.aat9900
Chen J, Su P, Luo W, Chen J (2018) Role of LRRK2 in manganese-induced neuroinflammation and microglial autophagy. Biochem Biophys Res Commun 498(1):171–177. https://doi.org/10.1016/j.bbrc.2018.02.007
Tjalkens RB, Popichak KA, Kirkley KA (2017) Inflammatory activation of microglia and astrocytes in manganese neurotoxicity. Adv Neurobiol 18:159–181
Parvez MM, Shin HJ, Jung JA, Shin JG (2017) Evaluation of para-aminosalicylic acid as a substrate of multiple solute carrier uptake transporters and possible drug interactions with nonsteroidal anti-inflammatory drugs in vitro. Antimicrob Agents Chemother 61(5). https://doi.org/10.1128/AAC.02392-16
Zheng W, Jiang YM, Zhang Y, Jiang W, Wang X, Cowan DM (2009) Chelation therapy of manganese intoxication with para-aminosalicylic acid (PAS) in Sprague–Dawley rats. NeuroToxicology 30(2):240–248. https://doi.org/10.1016/j.neuro.2008.12.007
Jiang YM, Mo XA, Du FQ, Fu X, Zhu XY, Gao HY, Xie JL, Liao FL, Pira E, Zheng W (2006) Effective treatment of manganese-induced occupational Parkinsonism with p-aminosalicylic acid: a case of 17-year follow-up study. J Occup Environ Med 48(6):644–649. https://doi.org/10.1097/01.jom.0000204114.01893.3e
Du QF, Mo XA, Jiang YM, Fu X, Gao HY, Liao FL (2008) A 17-year follow-up observation on a case of effective treatment of severe chronic manganese intoxication with p-aminosalicylic acid [in Chinese]. Chin Occup Med 35(5):398–399
Ky S, Deng H, Xie P, Hu W (1992) A report of two cases of chronic serious manganese poisoning treated with sodium para-aminosalicylic acid. Br J Ind Med 49(1):66–69. https://doi.org/10.1136/oem.49.1.66
Ky SQ, Hu WD, Jiang YM, Wei XM, Huang B, Hong XJ, Ge LH, Guo JX, Huang CP (1995) Sodium P-aminosalicylic acid of mechanism in treating chronic manganese poisoning [in Chinese]. J Guangxi Med Univ (04):477–483. https://doi.org/10.16190/j.cnki.45-1211/r.1995.04.001
Li SJ, Qin WX, Peng DJ, Yuan ZX, He SN, Luo YN, Aschner M, Jiang YM, Liang DY, Xie BY, Xu F (2018) Sodium P-aminosalicylic acid inhibits sub-chronic manganese-induced neuroinflammation in rats by modulating MAPK and COX-2. Neurotoxicology 64:219–229. https://doi.org/10.1016/j.neuro.2017.06.012
Li SJ, Li Y, Chen JW, Yuan ZX, Mo YH, Lu GD, Jiang YM, Ou CY, Wang F, Huang XW, Luo YN, Ou SY, Huang YN (2016) Sodium para-aminosalicylic acid protected primary cultured basal ganglia neurons of rat from manganese-induced oxidative impairment and changes of amino acid neurotransmitters. Biol Trace Elem Res 170(2):357–365. https://doi.org/10.1007/s12011-015-0472-7
Ou CY, Luo YN, He SN, Deng XF, Luo HL, Yuan ZX, Meng HY, Mo YH, Li SJ, Jiang YM (2016) Sodium P-aminosalicylic acid improved manganese-induced learning and memory dysfunction via restoring the ultrastructural alterations and γ-aminobutyric acid metabolism imbalance in the basal ganglia. Biol Trace Elem Res 176(1):143–153. https://doi.org/10.1007/s12011-016-0802-4
Peng DJ, Zhang YW, Li ZC, Li SJ, Cai M, Qin WX, Ou SY, Huang XW, Yuan ZX, Jiang YM (2019) Preventive impacts of PAS-Na on the slow growth and activated inflammatory responses in Mn-exposed rats. J Trace Elem Med Biol 54:134–141. https://doi.org/10.1016/j.jtemb.2019.04.013
Crossgrove J, Zheng W (2004) Manganese toxicity upon overexposure. NMR Biomed 17(8):544–553. https://doi.org/10.1002/nbm.931
Ward RJ, Crichton RR (2019) Manganese: its role in disease and health. Met Ions Life Sci 19. https://doi.org/10.1515/9783110527872-010
Dickerson RN (2001) Manganese intoxication and parenteral nutrition. Nutrition 17(7-8):689–693. https://doi.org/10.1016/s0899-9007(01)00546-9
Nizami S, Hall-Roberts H, Warrier S, Cowley SA, Di Daniel E (2014) Thalamic GABA predicts fine motor performance in manganese-exposed smelter workers. PLoS One 9(2):3515–3532. https://doi.org/10.1111/bph.14618
Wang H, Yang F, Xin R, Cui D, He J, Zhang S, Sun Y (2020) The gut microbiota attenuate neuroinflammation in manganese exposure by inhibiting cerebral NLRP3 inflammasome. Biomed Pharmacother 129:110449. https://doi.org/10.1016/j.biopha.2020.110449
Tang Y, Le W (2016) Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol 53(2):1181–1194. https://doi.org/10.1007/s12035-014-9070-5
Liu M, Cai T, Zhao F, Zheng G, Wang Q, Chen Y, Huang C, Luo W, Chen J (2009) Effect of microglia activation on dopaminergic neuronal injury induced by manganese, and its possible mechanism. Neurotox Res 16(1):42–49. https://doi.org/10.1007/s12640-009-9045-x
Filipov NM, Dodd CA (2012) Role of glial cells in manganese neurotoxicity. J Appl Toxicol 32(5):310–317. https://doi.org/10.1002/jat.1762
Zhao F, Cai T, Liu M, Zheng G, Luo W, Chen J (2009) Manganese induces dopaminergic neurodegeneration via microglial activation in a rat model of manganism. Toxicol Sci 107(1):156–164. https://doi.org/10.1093/toxsci/kfn213
Jo EK, Kim JK, Shin DM, Sasakawa C (2016) Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol 13(2):148–159. https://doi.org/10.1038/cmi.2015.95
Heneka MT, Kummer MP, Latz E (2014) Innate immune activation in neurodegenerative disease. Nat Rev Immunol 14(7):463–477. https://doi.org/10.1038/nri3705
Takahashi M (2019) Role of NLRP3 inflammasome in cardiac inflammation and remodeling after myocardial infarction. Biol Pharm Bull 42(4):518–523. https://doi.org/10.1248/bpb.b18-00369
Zhong Z, Sanchez-Lopez E, Karin M (2016) Autophagy, NLRP3 inflammasome and auto-inflammatory/immune diseases. Clin Exp Rheumatol 34(4 Suppl 98):12–16
Hui B, Zhang L, Zhou Q, Hui L (2018) Pristimerin inhibits LPS-triggered neurotoxicity in BV-2 microglia cells through modulating IRAK1/TRAF6/TAK1-mediated NF-kappaB and AP-1 signaling pathways in vitro. Neurotox Res 33(2):268–283. https://doi.org/10.1007/s12640-017-9837-3
Li L, Sun Q, Li Y, Yang Y, Yang Y, Chang T, Man M, Zheng L (2015) Overexpression of SIRT1 induced by resveratrol and inhibitor of miR-204 suppresses activation and proliferation of microglia. J Mol Neurosci 56(4):858–867. https://doi.org/10.1007/s12031-015-0526-5
Nizami S, Hall-Roberts H, Warrier S, Cowley SA, Di Daniel E (2019) Microglial inflammation and phagocytosis in Alzheimer’s disease: potential therapeutic targets. Br J Pharmacol 176(18):3515–3532. https://doi.org/10.1111/bph.14618
Peng Z, Luchtman DW, Wang X, Zhang Y, Song C (2019) Activation of microglia synergistically enhances neurodegeneration caused by MPP(+) in human SH-SY5Y cells. Eur J Pharmacol 850:64–74. https://doi.org/10.1016/j.ejphar.2019.01.024
Kumar A, Chaudhary T, Mishra J (2013) Minocycline modulates neuroprotective effect of hesperidin against quinolinic acid induced Huntington’s disease like symptoms in rats: behavioral, biochemical, cellular and histological evidences. Eur J Pharmacol 720(1-3):16–28. https://doi.org/10.1016/j.ejphar.2013.10.057
Voet S, Srinivasan S, Lamkanfi M, van Loo G (2019) Inflammasomes in neuroinflammatory and neurodegenerative diseases. EMBO Mol Med 11(6). https://doi.org/10.15252/emmm.201810248
Sarlus H, Heneka MT (2017) Microglia in Alzheimer’s disease. J Clin Invest 127(9):3240–3249. https://doi.org/10.1172/JCI90606
Chu F, Shi M, Zheng C, Shen D, Zhu J, Zheng X, Cui L (2018) The roles of macrophages and microglia in multiple sclerosis and experimental autoimmune encephalomyelitis. J Neuroimmunol 318:1–7. https://doi.org/10.1016/j.jneuroim.2018.02.015
Santos AP, Lucas RL, Andrade V, Mateus ML, Milatovic D, Aschner M, Batoreu MC (2012) Protective effects of ebselen (Ebs) and para-aminosalicylic acid (PAS) against manganese (Mn)-induced neurotoxicity. Toxicol Appl Pharmacol 258(3):394–402. https://doi.org/10.1016/j.taap.2011.12.003
Kuwar R, Rolfe A, Di L, Xu H, He L, Jiang Y, Zhang S, Sun D (2019) A novel small molecular NLRP3 inflammasome inhibitor alleviates neuroinflammatory response following traumatic brain injury. J Neuroinflammation 16(1):81. https://doi.org/10.1186/s12974-019-1471-y
Boucher D, Monteleone M, Coll RC, Chen KW, Ross CM, Teo JL, Gomez GA, Holley CL, Bierschenk D, Stacey KJ, Yap AS, Bezbradica JS, Schroder K (2018) Caspase-1 self-cleavage is an intrinsic mechanism to terminate inflammasome activity. J Exp Med 215(3):827–840. https://doi.org/10.1084/jem.20172222
Gustin A, Kirchmeyer M, Koncina E, Felten P, Losciuto S, Heurtaux T, Tardivel A, Heuschling P, Dostert C (2015) NLRP3 inflammasome is expressed and functional in mouse brain microglia but not in astrocytes. PLoS One 10(6):e0130624. https://doi.org/10.1371/journal.pone.0130624
Schroder K, Tschopp J (2010) The inflammasomes. Cell 140(6):821–832. https://doi.org/10.1016/j.cell.2010.01.040
Acknowledgments
Thanks to the fund support provided by grants from the National Natural Science Foundation of China (NSFC 81460505, 81973094). MA was supported in part by a grant from the National Institute of Environmental Health Science (NIEHS) R01ES10563.
Author information
Authors and Affiliations
Contributions
YF, DP, and YL carried out the studies and drafted the manuscript. LL and SO assisted with the establishment of the cell model. JL, LZ, and MA performed the statistical analysis. YF, DP, YL, LL, SO, JL, and LZ took part in the sample preparation and experimental analysis. YJ and MA designed the study. YJ led the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fang, Y., Peng, D., Liang, Y. et al. Sodium P-aminosalicylic Acid Inhibits Manganese-Induced Neuroinflammation in BV2 Microglial Cells via NLRP3-CASP1 Inflammasome Pathway. Biol Trace Elem Res 199, 3423–3432 (2021). https://doi.org/10.1007/s12011-020-02471-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02471-7