Abstract
Manganese (Mn), an essential trace metal for protein synthesis and particularly neurotransmitter metabolism, preferentially accumulates in basal ganglia. However, excessive Mn accumulation may cause neurotoxicity referred to as manganism. Sodium para-aminosalicylic acid (PAS-Na) has been used to treat manganism with unclear molecular mechanisms. Thus, we aim to explore whether PAS-Na can inhibit Mn-induced neuronal injury in basal ganglia in vitro. We exposed basal ganglia neurons with 50 μM manganese chloride (MnCl2) for 24 h and then replaced with 50, 150, and 450 μM PAS-Na treatment for another 24 h. MnCl2 significantly decreased cell viability but increased leakage rate of lactate dehydrogenase and DNA damage (as shown by increasing percentage of DNA tail and Olive tail moment). Mechanically, Mn reduced glutathione peroxidase and catalase activity and interrupted amino acid neurotransmitter balance. However, PAS-Na treatment reversed the aforementioned Mn-induced toxic effects. Taken together, these results showed that PAS-Na could protect basal ganglia neurons from Mn-induced neurotoxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Manganese (Mn) is an essential trace metal that maintains normal physiological and biochemical functions in all tissues [1]. It plays a critical role in maintaining functions of some enzymes and amino acids, e.g., superoxide dismutase, glutamine synthetase, arginine, and pyruvate carboxylase. However, excessive Mn accumulation in the central nervous system can cause manganism [2], a neurodegenerative disorder with symptoms similar to those of Parkinson’s disease [3]. Chronic excessive Mn exposure occurs in occupational workers including Mn ferroalloy factory workers, vehicle maintenance workers, battery manufacturers, and welders [4, 5]. More importantly, environmental Mn pollution has become a public concern [6].
Manganism patients display all sorts of defects including mental, cognitive, and behavioral impediments and motor dysfunctions that were associated with different degrees of basal ganglia injuries [7]. Magnetic resonance imaging examination on Mn-exposed workers showed a high T1-weighted density in globus pallidus, suggesting that Mn accumulated in γ-aminobutyric acid (GABA) enriched areas [8, 9]. However, the exact mechanisms of Mn neurotoxicity are yet unclear. Previous studies mainly focused on alterations of iron homeostasis [10], inflammatory reactions [11], disruption of mitochondrial metabolism, and induction of oxidative stress [12]. Moreover, several recent studies showed that Mn-induced neurodegeneration diseases may be associated with disturbance of neurotransmitter metabolism [13, 14]. GABA and glycine (Gly) are the major inhibitory neurotransmitters in the basal ganglia–thalamus–cortex loop [15, 16], whereas glutamate (Glu) is the most abundant excitatory neurotransmitter. GABA, which is synthesized from glutamine (Gln) by glutamic acid decarboxylase and then released from synapse through GABA transporter, binds to GABA receptor and results in neuron hyperpolarization and unresponsiveness [17]. Mn-induced neurotoxicities were found to be associated with an interruption of Gln/Glu-GABA cycle between astrocytes and neurons [18, 19].
Sodium para-aminosalicylate (PAS-Na), a non-steroidal anti-inflammatory drug, is clinically approved for tuberculosis treatment. In 1992, we first applied PAS-Na as a clinically effective drug in treatment of two cases of manganism patients [20, 21]. Accumulative results found that PAS-Na or p-aminosalicylic acid (PAS) could improve Mn-induced oxidative stress [22], impairment of mitochondrial membrane potentials [23], disruption of dopamine system [24], cell apoptosis, and inflammatory effects [25, 26]. Our recent studies found that PAS-Na may also have an antagonistic effect on Mn-induced neurotransmitter imbalance [13]. In the present study, we aim to further explore the protective mechanism of PAS-Na on Mn-induced oxidative stress, DNA damage, and neurotransmitter alterations in basal ganglia neurons in vitro.
Material and Methods
Primary Rat Basal Ganglia Neuron Culture
Primary culture of basal ganglia neurons was obtained from brain of newborn Sprague–Dawley rat (provided by the Experimental Animal Center of Guangxi Medical University [SCXKG2009-0003]), and all experimental procedures were conducted in conformity with Institutional Guidelines For The Care And Use of Laboratory Animals in Animal Committee of Guangxi Medical University, Nanning, China. Briefly, newborn rats were decapitated and the basal ganglia were obtained by digestion with 0.25 % Trypsin-EDTA (Gibco BRL Co., Ltd, Grand Island, USA) for 8 min. Then, the cells were plated at a density of 1 × 106 cells/ml in 6-, 12-, or 96-well plates precoated with poly-l-lysine (Sigma-Aldrich, San Francisco, U.S.), and maintained in DMEM/F12 with growth supplement B27 and 10 % (v/v) heat-inactivated fetal bovine serum (FBS), penicillin G (10,000 U/ml), and streptomycin (10 mg/ml; Solarbio Technology Co., Ltd., Beijing, China). The cells were maintained in a humidified incubator (5 % CO2, 95 % air at 37 °C) and replenished with fresh medium two times a week. On the third and fifth day, 10 μmol/l cytarabine (Sigma-Aldrich, San Francisco, USA) was added to remove non-neuron cells. We have validated the basal ganglia neurons according to the procedures as reported by our lab previously [25]. Neurons were specifically labeled by mouse anti-rat neuro-filament monoclonal antibody and stained by both DAB and hematoxylin. The neurons showed dark brown cytoplasm, slender neurites and brown spindle, and oval or vertebral cell bodies, while the non-neurons were only labeled by hematoxylin with purple blue nucleus. Under these culture conditions, more than 90 % of the cells are neurons.
Neuron Treatments with Mn and PAS-Na
On the eighth day, the validated basal ganglia neurons were randomly divided into six groups: control, Mn treatment (50 μM Mn), PAS-Na intervention (50 μM Mn plus 50, 150, or 450 μM PAS-Na), and PAS-Na control (450 μM PAS-Na) group. In the intervention groups, the neurons were treated by 50 μM manganese chloride (MnCl2, Sigma-Aldrich) for 24 h, replaced with PAS-Na (50, 150, or 450 μM, respectively) in fresh medium for another 24 h.
Assessment of Cell Viability by MTT
After washed with phosphate-buffered saline (PBS) buffer, neurons cultured in 96-well plates were supplemented with 20 μl 5 mg/ml 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) for 4 h at 37 °C. Purple formazan crystals produced by viable cells were then dissolved by addition of 150 μl DMSO. The absorbance was measured at a wavelength of 490 nm with an enzyme-linked immunosorbent assay (Multiskan FC, Thermo Scientific, USA). Cell viability = (optical density (OD) of the indicated group/OD of the control group) × 100 %.
Assessment of Leakage Rate of LDH
Basal ganglia neurons were cultured in 24-well plates. After the indicated treatment, the culture medium was collected and centrifuged at 1000 rpm for 5 min. Meanwhile, the basal ganglia neurons were harvested by trypsinization and disrupted by ultrasonication, and then the supernatant was collected after centrifugation at 2500 rpm for 5 min. the lactate dehydrogenase (LDH) activities in culture medium and cells were assayed for at a wavelength of 340 nm by an enzyme-linked immunosorbent assay. The leakage rate of LDH = [OD of the culture medium / (OD of the culture medium + OD of the cells)] × 100 %.
Assessments of GSH-Px and CAT Activity
Basal ganglia neurons were plated at a density of 1 × 106 cells/ml in 12-well plates. Following the treatment, the basal ganglia neurons were harvested by trypsinization and disrupted by ultrasonication. The supernatants were collected after centrifugation at 2500 rpm for 5 min and then assayed for catalase (CAT) and glutathione peroxidase (GSH-Px) activity by an enzyme-linked immumosorbent assay, and total protein concentration was determined by BCA assay, according to the previous study [27]. Results were expressed as U/mg protein.
Determination of DNA Damage by Using SCGE
DNA damage was detected by single-cell gel eletrophoresis (SCGE) as described in a previous study [25] with modifications. Following the indicated treatment, the basal ganglia neurons cultured in six-well plates were harvested by trypsinization and washed two times with PBS and then centrifuged at 1000 rpm for 5 min. Approximately 2 × 104 cells were mixed with 0.7 % low melting point agarose (Sigma-Aldrich) and pipetted immediately on a frosted glass microscope slide that were precoated with a layer of 0.7 % normal melting point agarose. The frosted glasses with agarose were leaved at 4 °C for 5 min and then immersed in lysis buffer [2.5 M NaCl, 100 mM EDTA, 10 mM Tris–HCl, 5 %(v/v) DMSO, and 2 %(v/v) Triton X-100; pH 10] at 4 °C for 2 h. The slides were washed with 0.01 M PBS for three times, before placing in unwinding liquid (300 mM NaOH) at 4 °C for 30 min. After the slides were equilibrated in fresh denaturation buffer (300 mM NaOH and 1 mM Na2-EDTA) for 40 min, electrophoresis was performed at 25 V for 30 min at an ambient temperature of 4 °C. The slides were then washed three times with neutralization buffer (400 mM Tris–HCl, pH 7.5, 5 min), before staining with 20 μl ethidium bromide (20 μg/ml) and observing under a fluorescence microscope (Olympus Optical, Tokyo, Japan) connected to a CCD video camera. At least 100 cells in each sample were analyzed by software CASP (casp-1.2.2, CASP lab, Wroclaw, Poland). The tail DNA percentage and Olive tail moment were used as the indicators of the extent of DNA damage.
Assessments of Intracellular and Extracellular Amino Acid Levels in Basal Ganglia Neurons
After the indicated treatment of neurons on 12-well plates, the culture medium was first collected and centrifuged at 14,000 rpm for 5 min, and the supernatants were stored at −80 °C for HPLC analysis. The cells were washed two times with PBS and then lysed in hypotonic lysis buffer. After centrifugation at 14,000 rpm for 5 min, the supernatants were kept at −80 °C for HPLC analysis. Apart from determination of total protein concentration, supernatant samples were prepared for HPLC analysis by using 0.4 mol/l perchlorate to precipitate and a derivatization reagent to derive, and eluted in the chromatographic condition according to a previously published method [13] with minor modifications. The derivatization reagent (0.027 g O-phthalaldehyde; Sigma-Aldrich) was dissolved in 0.5 ml carbinol and then added with 20 μl 2-mercaptoethanol and 4.5 ml sodium tetraborate buffer solution (pH = 9.6) and stored in a refrigerator at 4 °C. Twenty microliters of 2-mercaptoethanol was then added freshly into the derivatization reagent. The mobile phase “A” was 0.05 mol/l phosphate buffer solution mixed with 0.8 % (v/v) THF (pH = 5.8). The mobile phase “B” was carbinol mixed with 25 % (v/v) acetonitrile. The gradient elution was as follows: 0∼6 min B%, 25∼25 %; 6.01∼8 min B%, 25∼42 %; 8.01∼11 min B%, 42∼50 %; 11.01∼18 min B%, 50∼75 %; 18.01∼19 min B%, 75∼95 %; 19.01∼21 min B%, 95∼95 %; and 21.01 min, finished. The detection wavelength was λex = 340 nm and λem = 455 nm. The flow rate was 1 ml/min, the injection volume was 20 μl, and column temperature was 30 °C. The entire chromatography process took 25 min. Results were expressed as the content of amino acid μmol/mg or nmol/mg protein.
Statistical Analysis
Statistical analysis was performed by SPSS 16.0 for Windows (SPSS, Inc, Chicago, USA). One-way analysis of variance (ANOVA) was used to compare the means among the groups, and Tukey’s test was used in the post hoc multiple comparisons. Kruskal level–Wallis H test was used to analyze multiple comparisons, and Nemenyi test was used to analyze paired comparison in the data of DNA damage. The data were presented as means ± SE, except for the data of DNA damage using median. Results were considered statistically significant at P value <0.05.
Results
The Effects of Mn or PAS-Na on Basal Ganglia Neurons
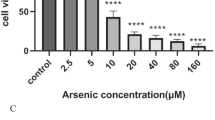
In our preliminary study, the primary basal ganglia neurons were exposed to 25–200 μΜ (25, 50, 100, 200 μΜ) Mn for 24 h, and the cell survival rates were about 92.4, 77.3, 56.5, and 45.5 %, respectively (Fig. 1a), compared with those of untreated cells. The result showed that Mn decreased the survival of basal ganglia neurons in a dose–response manner (r = −0.59, P < 0.05). The aim of this study was to investigate the changes of neurons exposed to low-concentration Mn; therefore, 50 μΜ of Mn was chosen. On the other hand, primary basal ganglia neurons were exposed to 5–5000 μΜ (5, 50, 500, and 5000 μΜ) PAS-Na for 24 h, and the cell survival rates were about 100 % at all doses except 5000 μΜ (86.1 %, Fig. 1b). Therefore, we chose 50–450 μM of PAS-Na to treat neurons.
The effects of Mn or PAS-Na on basal ganglia neurons. To determine the effects of Mn or PAS-Na on the basal ganglia, the cell viability was measured as indicated. Cell viability was displayed as percentage of control group. Error bars represent standard deviation (n = 6 per group). *P < 0.05, significant as compared to the control group; # P < 0.05, significant as compared to the 25 μM Mn group; △ P < 0.05, significant as compared to the 50 μM Mn group;◇ P < 0.05, significant as compared to the 100 μM Mn group
PAS-Na Protected Mn-Induced Cytotoxicity
In order to determine the cytotoxicity of Mn in basal ganglia neurons, the cell viability and LDH leakage rate were measured (Fig. 2). After 24-h Mn (50 μM) treatment and another 24- h recovery in fresh medium, the cell viability was significantly decreased to 71 % as compared to the control group (P < 0.05, Fig. 2a). Mn exposure also increased the leakage rate of LDH (184 % of control) indicative of impaired integrity of cell membranes (Fig. 2b). It is noteworthy that PAS-Na was not toxic to basal ganglia neurons (Fig. 2). In contrast, a high dose of PAS-Na (450 μM) treatment rescued the Mn-induced reduction in cell viability to 92 % of control, respectively (Fig. 2a). Furthermore, treatment with 50 or 150 μM PAS-Na for 24 h significantly prevented Mn-induced LDH leakage rate to 132 and 143 % of control, respectively, but 450 μM PAS-Na failed (Fig. 2b). These results collectively suggested that PAS-Na treatment may decrease Mn-induced cytotoxicity in basal ganglia neurons.
PAS-Na protected Mn-induced cytotoxicity in basal ganglia neurons. To determine the effects of PAS-Na on Mn cytotoxicity in the basal ganglia, the cell viability (a) and leakage rate of LDH (b) were measured as indicated. Cell viability was displayed as percentage of control group. Error bars represent standard deviation (n = 6 per group). *P < 0.05, significant as compared to the control group; # P < 0.05, significant as compared to the Mn 50 μM group
PAS-Na Attenuated Mn-Induced Reduction of Anti-oxidant Enzyme Activity
To investigate whether Mn induced oxidative stress in the basal ganglia neurons, activities of key anti-oxidant enzymes glutathione peroxidase (GSH-Px) and catalase (CAT) were measured. After 24-h Mn exposure, GSH-Px and CAT activity in the basal ganglia were decreased as compared to the control group by 40 and 15 %, respectively (Fig. 3). But, 450 μM PAS-Na alone did not cause any effects on anti-oxidant enzyme activity in the basal ganglia (Fig. 3). In contrast, higher doses of PAS-Na treatment (150 and 450 μM, but not 50 μM) for 24 h significantly increased CAT activity in a dose-dependent manner in the Mn-pretreated neurons. PAS-Na treatment (150 μM) robustly increased GSH-Px activity. Therefore, the results showed that PAS-Na could inhibit oxidative stress caused by Mn.
PAS-Na Rescued Mn-Caused DNA Damage
Single-cell gel (SCGE), also nicknamed as Comet assay, is a useful approach for assessing DNA damage. SCGE was thus used to determine whether low level of Mn (50 μM) exposure caused DNA damage in basal ganglia neurons. Compared to the nuclei of the control and PAS-Na alone group with circular fluorescent cores and no observable tails (Fig. 4a, f), the damaged nuclei found in the Mn toxic group (50 μM) showed evident comet tails after the fluorescent heads (Fig. 4b∼e). Specifically, after Mn exposure for 24 h, the percentage of tail DNA and Olive tail moment were significantly increased as compared to those of the untreated control group (P < 0.05, Table 1). In contrast, treatment with PAS-Na for 24 h dose-dependently decreased both tail DNA and Olive tail moment in the Mn-pretreated neurons. Taken together, these results suggested that PAS-Na may play an important role in rescuing the DNA damage caused by Mn in the basal ganglia neurons.
PAS-Na treatments protected the Mn-induced DNA damages in basal ganglia neurons. Basal ganglia neurons were pretreated with 50 μM MnCl2 for 24 h followed by the treatment of 50, 150, or 450 μM PAS-Na for 24 h. Nuclei head and comet tails were shown by representative pictures from each groups: a control (circular fluorescent nuclei with no tails as indicated by arrows), b Mn (comet head with long tail, as indicated by arrows), c Mn + 50 μM PAS-Na, d Mn + 150 μM PAS-Na, e Mn + 450 μM PAS-Na group, and f PAS-Na 450 μM alone. Scale bar = 20 μm. One hundred cells were randomly selected and measured in each group. After low-level Mn treatment, the percentage of tail DNA and Olive tail moment significantly increased, while PAS-Na treatment decreased both
PAS-Na Reverted Mn-Altered Amino Acid Neurotransmitter Metabolism
In order to investigate the effects of Mn on amino acid neurotransmitter metabolism in basal ganglia neurons, both intracellular and extracellular concentrations of Glu, Gln, Gly, and GABA were measured. Mn exposure for 24 h caused a robust increase in intracellular and extracellular Gly (to the levels of ∼129 %; ∼125 % of control, respectively) and intracellular GABA (123 % of control), but not extracellular GABA in the basal ganglia neurons. But, extracellular Glu levels were decreased in Mn (50 μM) toxic group as compared to those in the control group (by 15 %; Fig. 5). The increasing doses of PAS-Na treatment for 24 h consistently caused a significant reduction of intracellular and extracellular Gly levels which were increased by Mn treatment (by 17∼27 and 15∼23 %, respectively). We also observed the protective effects of PAS-Na on the Mn-induced changes in Glu and GABA, but the effects were dependent on specific dose. For example, 50 μM PAS-Na treatment induced a robust decrease of intracellular GABA levels (by 21.7 %; Fig. 5), 150 μM PAS-Na treatment restored the decrease of extracellular Glu levels to ∼108 % of control, and the other treatment dose of PAS-Na had no effects. These results collectively indicated that Mn interrupts the balance of amino acid neurotransmitter and that PAS-Na may recover these changes.
PAS-Na reversed Mn-induced disruption of amino acid neurotransmitters. To investigate the effects of Mn on amino acid neurotransmitter metabolism in basal ganglia neurons and the effect of PAS-Na, the intracellular (a) and extracellular (b) Glu, Gln, Gly, and GABA concentrations were measured in the basal ganglia neurons (n = 6 per group)
Discussion
Mn is an essential trace metal for protein synthesis and various enzymatic reactions, particularly for the synthesis and metabolism of neurotransmitters [28]. Mn exists in all tissues but preferentially accumulates in the brain. However, excessive Mn accumulation in the basal ganglia may cause manganism [5]. Herein, we used an in vitro model to test the effects of Mn in basal ganglia neurons of rats. The results showed that Mn exposure significantly decreased viability of basal ganglia neurons (Figs. 1a and 2a), which is consistent with previous studies that Mn reduced viability of hippocampus neurons and AF5 cells in a dose–response manner [25, 29]. Moreover, our study indicated that Mn increased the leakage rate of LDH (184 % of control; Fig. 2b) in the basal ganglia neurons, suggesting impaired integrity of cell membranes. This result is also in line with a previous study that Mn increased the leakage rate of LDH in PC12 cells [30].
Oxidative stress, as a major factor contributing to the vulnerability and progressive degeneration of dopaminergic cells, has been proposed to be one of the major mechanisms of Mn-induced neurotoxicity [12]. Excessive reactive oxygen species may damage cellular contents such as nuclear acids, lipids, and proteins. The peroxidation of lipids by reactive oxygen species, for example, plays an important role in cell injury [31]. Mn exposure elevated malondialdehyde levels, a by-product of free radical attack on lipids [12]. These papers suggested that Mn exposure elevated reactive oxygen species by suppressing the anti-oxidant system of central nervous system. GSH-Px and CAT are important anti-oxidative enzymes to neutralize reactive oxygen species. GSH-Px protects the structural and functional integrity of the cell membrane by catalyzing the reduction reaction of glutathione on H2O2, whereas CAT decomposes hydroxyl radicals to resist the peroxidative damages by reactive oxygen species. Therefore, we tested the cellular GSH-Px and CAT activities to confirm oxidative damage induced by Mn. Our results showed that Mn reduced the antioxidant capacity of basal ganglia neurons by decreasing the activity of GSH-Px and CAT (by 15 and 40 %, respectively, Fig. 3), suggesting that CAT is more sensitive to Mn. These may be because that CAT possesses an iron(III) protoporphyrin and shares the same transporter with Mn so that CAT could be easily affected by Mn exposure [32, 33]. But, GSH-Px is a selenium-containing enzyme. These results were consistent with another cell study that showed a reduction of GSH-Px and CAT activity in Mn-exposed PC12 cells [34]. Additionally, Mn exposure caused a marked decrease in GSH-Px activity, immunohistochemical expression, and protein levels of GSH-Px in the striatum of aged rats [35]. More importantly, epidemiological investigations have found that in occupational Mn exposure workers, GSH-Px and CAT activities are significantly decreased [36].
Previous studies indicated that DNA damage plays an important role in neurodegenerative disease processes such as Alzheimer’s disease and Parkinson’s disease [10, 37]. Earlier in vitro studies reported that Mn increased the length of comet tail and percentage of comet-like nuclei in PC12 cells [34] and in SH-SY5Y cells [38]. Our previous studies also showed that Mn exposure induced DNA damage in hippocampus neurons by increasing percentage of tail DNA and Olive tail moment [25, 34]. Herein, we used SCGE to measure DNA damage and found that 50 μM Mn exposure for 24 h increased the percentage of comet tail and Olive tail moment of basal ganglia neurons.
A large number of studies found that excessive Mn exposure might induce neurotransmission changes, especially the contents of dopamine (DA). For example, Blecharz-Klin et al. found that depression of cognitive ability was evoked by deregulation of dopaminergic, serotonergic, and noradrenergic neurotransmission and their metabolites in the cortex, striatum, and hippocampus [39]. This was confirmed by studies of other labs that Mn exposure markedly impaired DA, expression of dopamine receptors, and transporter in the brain [40, 41]. However, low cumulative Mn exposure has significant effects on striatal GABA but not on DA [42]. And, there are many studies that linked Mn neurotoxicology with the disruption of Gln/Glu-GABA cycle. Glu is the most abundant excitatory neurotransmitter, whereas GABA is the most prevalent inhibitory neurotransmitter [15, 16]. On one hand, magnetic resonance imaging of Mn-exposed workers suggested that there was a negative correlation between Glu levels and Mn levels in erythrocyte [4, 8]. An in vitro study supported this finding by showing that excessive Mn exposure reduced the Glu levels in the primary cultured cortex neurons [19]. Cano et al. detected the levels and distributions of excitatory neurotransmitter receptors in the cortex, hypothalamus, basal ganglia, and cerebellum in Mn-exposed rats (8 weeks) by using autoradiography and found that the content of Glu and Glu receptor in the above regions was decreased [43]. On the other hand, some other studies showed that Mn reduced Gln levels [44] but increased Gly levels [27] in the globus pallidus. Consistently, our present results showed that Mn decreased extracellular Glu levels but increased intracellular GABA and Gly levels in basal ganglia neurons (Fig. 5), resulting in the imbalance of excitatory (Glu) and inhibitory neurotransmitter (GABA, Gly). In addition, Mn affected extracellular neurotransmitters in a similar pattern. These results indicated that Mn exposure might disrupt amino acid neurotransmitter metabolism in basal ganglia neurons in vitro.
PAS has been first demonstrated to have beneficial effects of excreting Mn in animal models [37], and PAS (100 and 200 mg/kg/day) increased Mn excretion in urine and dung [10]. PAS-Na, as a salt product of PAS, was then clinically applied in the treatment of manganism with a good prognosis [20, 21]. These findings were subsequently supported by following mechanical studies that PAS or PAS-Na relieved Mn-induced inflammatory effects, oxidative stress, and interruption of choline acetyltransferase system [24, 26, 45, 46]. However, the mechanism of how PAS or PAS-Na reversed Mn-induced oxidative stress is still unclear. There are two main explanations on this effect. PAS has been considered as a chelating agent to mobilize and remove tissue Mn2+, whereas it would be converted to Mn3+ and produced a lot of ROS [10]. The other hypothesis is that PAS may be an anti-oxidant and would enhance defense of the cell [47]. On the one hand, our present results showed that the PAS-Na reversed Mn-induced damages in cell viability, DNA damage, and oxidative stress in basal ganglia neurons, which is consistent with our previous in vivo rat study [25]. On other hand, our present study also showed that PAS-Na restored the decreased Glu and Gln levels caused by Mn exposure, and increased Gly and GABA. These presented that PAS-Na has antagonistic effects on Mn toxicity on amino acid neurotransmitter in basal ganglia neurons in vitro. These results are consistent with our previous study that PAS-Na (200 mg/kg) treatment for 6 weeks or PAS-Na (200 mg/kg) preventive restored Gly, and GABA levels of the Mn-exposed rats to normal levels [13]. However, we also found that PAS-Na may increase the Gln levels of basal ganglia neurons. However, this study is solely based on primary culture of basal ganglia neurons alone. Future co-culture studies of neurons and astrocytes may better explain whether PAS-Na has cytotoxicity on neurons.
Although PAS or PAS-Na has been demonstrated to have beneficial therapeutic effects on manganism, their applications were limited by the following reasons. First, PAS was notorious for its side effects of gastrointestinal intolerance which caused vomiting, frequent nausea, and abdominal discomfort [48]. Second, our study also found that a high dose (5000 μΜ) of PAS-Na has a certain toxic effect on the primary basal ganglia neurons. Last but not least, the mechanism on how PAS-Na exerts interventional effects on manganism is yet unclear and controversial [10]. However, further studies of PAS or PAS-Na may help find an appropriate specific drug with a wide therapeutic window for manganism.
In conclusion, a low level of Mn (50 μM) induced cytotoxicity in basal ganglia neurons, as evidenced by reduced cell viability, impaired membrane integrity, increased oxidative stress and DNA damages, and disrupted amino acid neurotransmitter (Glu/Gln-GABA) balance. However, PAS-Na could protect ganglia neurons by reversing the aforementioned Mn-induced toxic effects. The exact molecular mechanisms of PAS-Na-mediated protective effects from Mn neurotoxicity need to be further investigated.
References
Takeda A (2003) Manganese action in brain function. Brain Res Brain Res Rev 41:79–87
Gonzalez-Cuyar, L.F., G. Nelson, S.R. Criswell, et al. (2013) Quantitative neuropathology associated with chronic manganese exposure in South African mine workers. Neurotoxicology
Alaimo A, Gorojod RM, Beauquis J et al (2014) Deregulation of mitochondria-shaping proteins Opa-1 and Drp-1 in manganese-induced apoptosis. PLoS One 9:e91848
Jiang Y, Zheng W, Long L et al (2007) Brain magnetic resonance imaging and manganese concentrations in red blood cells of smelting workers: search for biomarkers of manganese exposure. Neurotoxicology 28:126–35
Criswell SR, Perlmutter JS, Huang JL et al (2012) Basal ganglia intensity indices and diffusion weighted imaging in manganese-exposed welders. Occup Environ Med 69:437–43
Maffeo E, Montuschi A, Stura G et al (2014) Chronic acquired hepatocerebral degeneration, pallidal T1 MRI hyperintensity and manganese in a series of cirrhotic patients. Neurol Sci 35:523–30
Pal PK, Samii A, Calne DB (1999) Manganese neurotoxicity: a review of clinical features, imaging and pathology. Neurotoxicology 20:227–38
Dydak U, Jiang YM, Long LL et al (2011) In vivo measurement of brain GABA concentrations by magnetic resonance spectroscopy in smelters occupationally exposed to manganese. Environ Health Perspect 119:219–24
Chang Y, Woo ST, Kim Y et al (2010) Pallidal index measured with three-dimensional T1-weighted gradient echo sequence is a good predictor of manganese exposure in welders. J Magn Reson Imaging 31:1020–6
Zheng W, Jiang YM, Zhang Y et al (2009) Chelation therapy of manganese intoxication with para-aminosalicylic acid (PAS) in Sprague–Dawley rats. Neurotoxicology 30:240–8
Mokgobu, M.I., M.C. Cholo, R. Anderson, et al. (2014) Oxidative induction of pro-inflammatory cytokine formation by human monocyte-derived macrophages following exposure to manganese in vitro. J Immunotoxicol
Liu XF, Zhang LM, Guan HN et al (2013) Effects of oxidative stress on apoptosis in manganese-induced testicular toxicity in cocks. Food Chem Toxicol 60:168–76
Ou CY, Huang ML, Jiang YM et al (2011) Effect of sodium para-aminosalicylic on concentrations of amino acid neurotransmitters in basal ganglia of manganese-exposed rats. Zhonghua Yu Fang Yi Xue Za Zhi 45:422–5
Long Z, Li XR, Xu J et al (2014) Thalamic GABA predicts fine motor performance in manganese-exposed smelter workers. PLoS One 9:e88220
Winer JA, Larue DT, Pollak GD (1995) GABA and glycine in the central auditory system of the mustache bat: structural substrates for inhibitory neuronal organization. J Comp Neurol 355:317–53
Song, A., X. Gao, D. Li, et al.(2002) Immunohistochemistry study on GABAergic neurons of subareas of basal ganglia in unilateral rat model of Parkinson's disease. Chinese Journal of Neuroanatomy: 232-234 + 291.
Albrecht J, Sidoryk-Wegrzynowicz M, Zielinska M et al (2010) Roles of glutamine in neurotransmission. Neuron Glia Biol 6:263–76
Fan X, Luo G, Yang D et al (2010) Critical role of lysosome and its associated protein cathepsin D in manganese-induced toxicity in cultured midbrain astrocyte. Neurochem Int 56:291–300
Zwingmann C, Leibfritz D, Hazell AS (2003) Energy metabolism in astrocytes and neurons treated with manganese: relation among cell-specific energy failure, glucose metabolism, and intercellular trafficking using multinuclear NMR-spectroscopic analysis. J Cereb Blood Flow Metab 23:756–71
Ky SQ, Deng HS, Xie PY et al (1992) A report of two cases of chronic serious manganese poisoning treated with sodium para-aminosalicylic acid. Br J Ind Med 49:66–9
Jiang YM, Mo XA, Du FQ et al (2006) Effective treatment of manganese-induced occupational Parkinsonism with p-aminosalicylic acid: a case of 17-year follow-up study. J Occup Environ Med 48:644–9
Yoon H, Kim DS, Lee GH et al (2009) Protective effects of sodium para-amino salicylate on manganese-induced neuronal death: the involvement of reactive oxygen species. J Pharm Pharmacol 61:1563–9
Crawford S, Davis K, Saddler C et al (2011) The ability of PAS, acetylsalicylic acid and calcium disodium EDTA to protect against the toxic effects of manganese on mitochondrial respiration in gill of Crassostrea virginica. In Vivo (Brooklyn) 33:7–14
Nelson M, Huggins T, Licorish R et al (2010) Effects of p-aminosalicylic acid on the neurotoxicity of manganese on the dopaminergic innervation of the cilia of the lateral cells of the gill of the bivalve mollusc, Crassostrea virginica. Comp Biochem Physiol C Toxicol Pharmacol 151:264–70
Wang F, Wang C, Jiang Y et al (2014) Protective role of sodium para-amino salicylic acid against manganese-induced hippocampal neurons damage. Environ Toxicol Pharmacol 37:1071–8
Li, S.J., H.Y. Meng, X.F. Deng, et al. (2014) Protective effects of sodium p-aminosalicylic acid on learning and memory via increasing the number of basal forebrain choline acetyltransferase neurons in manganese-exposed rats. Hum Exp Toxicol
Santos D, Batoreu MC, Almeida I et al (2012) Manganese alters rat brain amino acids levels. Biol Trace Elem Res 150:337–41
Takeda A, Sotogaku N, Oku N (2003) Influence of manganese on the release of neurotransmitters in rat striatum. Brain Res 965:279–82
Crooks DR, Welch N, Smith DR (2007) Low-level manganese exposure alters glutamate metabolism in GABAergic AF5 cells. Neurotoxicology 28:548–54
Alinovi R, Vettori MV, Mutti A et al (1996) Dopamine (DA) metabolism in PC12 cells exposed to manganese (Mn) at different oxidation states. Neurotoxicology 17:743–50
Chang CY, Shen CY, Kang CK et al (2014) Taurine protects HK-2 cells from oxidized LDL-induced cytotoxicity via the ROS-mediated mitochondrial and p53-related apoptotic pathways. Toxicol Appl Pharmacol 279:351–63
Zachara BA (1992) Mammalian selenoproteins. J Trace Elem Electrolytes Health Dis 6:137–51
Bravo J, Mate MJ, Schneider T et al (1999) Structure of catalase HPII from Escherichia coli at 1.9 A resolution. Proteins 34:155–66
Chen, J., Y. Chen, W. Luo, et al. (2002) Toxicity effects of manganese on PC12 cells. Journal of Hygiene Research: 223–225
Deng, Y., C. Jiao, C. Mi, et al. (2014) Melatonin inhibits manganese-induced motor dysfunction and neuronal loss in mice: involvement of oxidative stress and dopaminergic neurodegeneration. Mol Neurobiol
Liu, L., X. Chen, G. Luo, et al. (2005) Study on the effects of occupational manganese exposure on lipid peroxidation in welders. Prev Med Trib: 385–386
Tandon SK, Singh J (1975) Removal of manganese by chelating agents from brain and liver of manganese treated rats: as in vitro and an in vivo study. Toxicology 5:237–41
Stephenson AP, Schneider JA, Nelson BC et al (2013) Manganese-induced oxidative DNA damage in neuronal SH-SY5Y cells: attenuation of thymine base lesions by glutathione and N-acetylcysteine. Toxicol Lett 218:299–307
Blecharz-Klin K, Piechal A, Joniec-Maciejak I et al (2012) Effect of intranasal manganese administration on neurotransmission and spatial learning in rats. Toxicol Appl Pharmacol 265:1–9
Guilarte TR, Burton NC, McGlothan JL et al (2008) Impairment of nigrostriatal dopamine neurotransmission by manganese is mediated by pre-synaptic mechanism(s): implications to manganese-induced parkinsonism. J Neurochem 107:1236–47
Roth JA, Li Z, Sridhar S et al (2013) The effect of manganese on dopamine toxicity and dopamine transporter (DAT) in control and DAT transfected HEK cells. Neurotoxicology 35:121–8
Gwiazda RH, Lee D, Sheridan J et al (2002) Low cumulative manganese exposure affects striatal GABA but not dopamine. Neurotoxicology 23:69–76
Cano G, Suarez-Roca H, Bonilla E (1997) Alterations of excitatory amino acid receptors in the brain of manganese-treated mice. Mol Chem Neuropathol 30:41–52
Zwingmann C, Leibfritz D, Hazell AS (2007) Nmr spectroscopic analysis of regional brain energy metabolism in manganese neurotoxicity. Glia 55:1610–7
Santos AP, Lucas RL, Andrade V et al (2012) Protective effects of ebselen (Ebs) and para-aminosalicylic acid (PAS) against manganese (Mn)-induced neurotoxicity. Toxicol Appl Pharmacol 258:394–402
Santos D, Batoreu MC, Aschner M et al (2013) Comparison between 5-aminosalicylic acid (5-ASA) and para-aminosalicylic acid (4-PAS) as potential protectors against Mn-induced neurotoxicity. Biol Trace Elem Res 152:113–6
Yoon H, Lee GH, Kim DS et al (2011) The effects of 3, 4 or 5 amino salicylic acids on manganese-induced neuronal death: ER stress and mitochondrial complexes. Toxicol In Vitro 25:1259–68
Sy SK, de Kock L, Diacon AH et al (2015) N-Acetyltransferase genotypes and the pharmacokinetics and tolerability of para-aminosalicylic acid in patients with drug-resistant pulmonary tuberculosis. Antimicrob Agents Chemother 59:4129–38
Conflict of interest
The authors declare that there are no conflicts of interest.
Grant support
This study was supported by grants from the National Natural Science Foundation of China (NSFC 81072320, 81460505, 30760210) and Guangxi Natural Science Foundation (2014GXNSFAA 118232).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, SJ., Li, Y., Chen, JW. et al. Sodium Para-aminosalicylic Acid Protected Primary Cultured Basal Ganglia Neurons of Rat from Manganese-Induced Oxidative Impairment and Changes of Amino Acid Neurotransmitters. Biol Trace Elem Res 170, 357–365 (2016). https://doi.org/10.1007/s12011-015-0472-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-015-0472-7