Abstract
Excessive intake of manganese (Mn) may cause neurotoxicity. Sodium para-aminosalicylic acid (PAS-Na) has been used successfully in the treatment of Mn-induced neurotoxicity. The γ-aminobutyric acid (GABA) is related with learning and memory abilities. However, the mechanism of PAS-Na on improving Mn-induced behavioral deficits is unclear. The current study was aimed to investigate the effects of PAS-Na on Mn-induced behavioral deficits and the involvement of ultrastructural alterations and γ-aminobutyric acid (GABA) metabolism in the basal ganglia of rats. Sprague-Dawley rats received daily intraperitoneally injections of 15 mg/kg MnCl2.4H2O, 5d/week for 4 weeks, followed by a daily back subcutaneously (sc.) dose of PAS-Na (100 and 200 mg/kg), 5 days/week for another 3 or 6 weeks. Mn exposure for 4 weeks and then ceased Mn exposure for 3 or 6 weeks impaired spatial learning and memory abilities, and these effects were long-lasting. Moreover, Mn exposure caused ultrastructural alterations in the basal ganglia expressed as swollen neuronal with increasing the electron density in the protrusions structure and fuzzed the interval of neuropil, together with swollen, focal hyperplasia, and hypertrophy of astrocytes. Additionally, the results also indicated that Mn exposure increased Glu/GABA values as by feedback loops controlling GAT-1, GABAA mRNA and GABAA protein expression through decreasing GABA transporter 1(GAT-1) and GABA A receptor (GABAA) mRNA expression, and increasing GABAA protein expression in the basal ganglia. But Mn exposure had no effects on GAT-1 protein expression. PAS-Na treatment for 3 or 6 weeks effectively restored the above-mentioned adverse effects induced by Mn. In conclusion, these findings suggest the involvement of GABA metabolism and ultrastructural alterations of basal ganglia in PAS-Na’s protective effects on the spatial learning and memory abilities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Manganese (Mn), an essential trace metal element, plays a crucial role in maintaining normal physiological functions, especially the neurotransmitters homeostasis [13]. However, excessive Mn exposure may cause a progressive neurological damage with extrapyramidal motor disorder called manganism. Manganism is characterized by variety of psychiatric, cognitive, and motor disturbances resembling to those of Parkinson’s disease [15]. More seriously, chronic excessive Mn exposure not only occurs in occupational workers but also happens in environmental Mn pollutions which has obtained public concern [3].
After penetrating the blood-brain barrier, Mn is distributed to different brain regions, especially the basal ganglia. Mn-induced the effects on basal ganglia have been concerned as it relates to the movement abnormalities [11]. Mn has been shown to interfere with several neurotransmission systems, especially the dopaminergic (DAergic) system [10]. The deregulation of DA signaling has been concerned as a major focus of manganism. However, it has recently clear that the alterations in the biology of glutamate (Glu)-glutamine (Gln)/GABA cycle were involved in the etiology of Mn neurotoxicity [1, 12]. Experimental studies also showed that the primary brain target of Mn is the γ-aminobutyric acid (GABA) enrichment area, such as basal ganglia [7]. Autopsy studies showed that nerve cell loss in the globus pallidus with astrocytosis, but no significant changes in the substantia nigra [26, 41]. The major inhibitory neurotransmitter, GABA, involves in projecting neuronal signals in the basal ganglia and thalamic regions to coordinate the performance of movement [22]. Moreover, the increasing ratio of the Glu and GABA may cause the learning and memory impairment [8]. The emerging evidence suggests that Mn-induced memory ability deficits may be related with the interruption of Glu-Gln/GABA cycle which may also induce unltrastructural alterations and influences each other. However, it is yet unclear how Mn interrupts the Glu-Gln/GABA cycle, especially through GABA transporters.
Many drugs have been used to treat Mn-induced neurotoxicity, including levodopa and ethylene diamine tetraacetic acid [28, 34]. However, the treatment of these drugs showed a limited clinical efficacy [23]. Para-aminosalicylic acid (PAS) and its salt sodium para-aminosalicylic acid (PAS-Na) have been used successfully in the treatment of Mn-induced neurotoxicity [15, 18]. So we hypothesized that the successful effect of PAS-Na on manganism might possibly be related to GABA. Thus, the present study was conducted to investigate the effects of Mn exposure on spatial learning and memory dysfunction, unltrastructural of the basal ganglia, and the GABA system. We also explore whether PAS-Na has protective effects on the above-mentioned changes.
Materials and Methods
Reagents
Manganese chloride tetrahydrate (MnCl2·4H2O), guarantee reagent (GR), were purchased from Tianjin Bodi Chemical Co. Ltd., China; PAS-Na was bought from Liaoning Beiqi Pharmaceutical Co. Ltd., China. All reagents were of analytical grade, the best available pharmaceutical grade or HPLC grade.
Experimental Animals
A total of 120 male Sprague-Dawley (SD) rats (specific pathogen-free, weighing 180.2 ± 14.7 g) were bought from the Experimental Animal Centre of Guangxi Medical University, Nanning, China [SCXK2009-0003]. The animals were housed at a climate-controlled animal room (temperature 24 ± 1 °C, humidity 55 ± 10 %, and 12 h/12 h light/dark cycle), and acclimated for 1 week before the experimentation. Food and water were available ad libitum. All experimental procedures were conducted according to the National Institutes of Health Guide for Care and Use of Laboratory Animals and approved by committee of the care and use of laboratory animals in Guangxi Medical University, Nanning, China.
Experimental Design and Treatments
There were 15 rats in each group. The exposure and treatment lasted 7 weeks or 10 weeks according groups. To induce learning and memory impairment, rats in the Mn-only treatment (Mn) group received intraperitoneal (i.p.) injection of 15 mg/kg MnCl2.4H2O, once a day, 5 days a week for 4 weeks; they were then ceased Mn exposure and received daily back subcutaneous (s.c.) injection with saline, 5 days per week for another 3 or 6 weeks.
Rats in PAS-Na treatment (designed as Mn + 100 PAS-Na and Mn + 200 PAS-Na) groups received the same daily i.p. injections of 15 mg/kg MnCl2.4H2O as those in the Mn group. Following 4 weeks of Mn exposure, exposure ceased and the rats were received daily back s.c. injection of 100 mg/kg (Mn + 100 PAS-Na group) or 200 mg/kg (Mn + 200 PAS-Na group) PAS-Na, 5 days per week for 3 or 6 weeks. Rats in the normal control (Control) group received i.p. and back s.c. injection of physiological saline at the same volume equivalent to the Mn group throughout the experiment.
Morris Water Maze Test (MWM)
MWM test was carried out within 24 h after last injection for six consecutive days as described in the previous study [19, 20]. The MWM consisted of a flat black galvanized metal tank (2.1 m in diameter and 0.6 m deep) equipped with a platform 2 cm below the surface of the water in the center of the third quadrant of the pool (temperature 26 ± 1 °C). A camera was mounted above the maze pool and connected to a computer which was equipped with the MWM analysis software (Huaibei Zhenghua biological equipment Co., China) to record the swimming track. On the training days, the rats were placed on the escape platform for 15 s to familiarize themselves with the task. The training trials were carried out four times per day each rat in a different quadrant for five consecutive days. The trail was terminated once the rats reach the escape platform. If the rats failed to find the escape platform within 90 s, the rats were guided to the platform and placed for 15 s, and the escape latency was recorded as 90 s. On the sixth day, spatial probe trial was performed without platform. The cumulative times spent in the original platform location were recorded during a period of 120 s. The spatial probe trial was used to measure the memory ability. All tests were performed in the same time period from 10 AM to 3 PM.
Transmission Electron Microscopy (TEM)
Rat were anaesthetized with chloral hydrate (i.p. 300 mg/kg) within 24 h after the last injection, and perfused transcardially with 150 ml 0.9 % NaCl, followed by 250 ml fixative solution (contains 2 % paraformaldehyde and 2.5 % glutaraldehyde in 0.1 M sodium phosphate buffer, pH = 7.2–7.4) at room temperature. After perfusion, the basal ganglia was rapidly dissected and immersed in the same fixative solution at 4 °C for overnight. The basal ganglia was sliced into 1-mm-thick coronal slices and immersed in the fresh fixative (1 % glutaraldehyde in 0.1 M PB) overnight at 4 °C, and then postfixed with 1 % osmium tetroxide and 0.01 % postassium dichromate in the same fixed solution overnight at 4 °C. The tissues were dehydrated in a graded aqueous solutions of acetone from 50 to 90 % (each for 10 min), and then 100 % acetone (three times, 10 min/time). After dehydration, the tissues were infiltrated in epoxy resin and pure acetone mixture (v:v = 1:1) for 2 h at room temperature. Each slice was placed on an Aclar film (Honeywell International Inc., Morristown, New Jersey, USA), covered with a capsule containing pure epoxy resin for 48 h at 60 °C. Slices in blocks were then coded. All further analyses were carried out with the investigator blinded to the experimental status of the tissue. The sections of the selected areas were cut and collected in a 200-mesh copper grid, counter stained with with uranyl acetate and lead citrate and examined by using transmission electron microscope (TEM H-500H-7650, Hitachi, Tokyo, Japan) on morphological change of neuron, astrocyte, and neuropil.
Determination of Glu and GABA Ratio
The basal ganglia tissues were homogenized with ten volumes of ice cold homogenization buffer and centrifuged at 3000g for 15 min. Apart from determination of total protein concentration, supernatant samples were prepared for high performance liquid chromatography (HPLC) analysis. The samples were precipitated by using 0.4 mol/L perchlorate and derived by using derivatization reagent according to the previous study [19, 20] with minor modifications. The mobile phase “A”: 0.5 mol/L phosphate buffer solution was mixed with 0.8 % (v:v) THF (pH = 5.8). The mobile phase “B”: carbinol was mixed with 25 % (v:v) acetonitrile. The gradient elution was as follows: 0~6 min B%: 25~25 %, 6.01~8 min B%: 25~42 %, 8.01~11 min B%: 42~42 %, 11.01~14 min B%: 42~50 %, 14.01~18 min B%: 75 %, 18.01 min: finished. The detection wavelength: λex = 340 nm, λem = 455 nm. The flow rate: 1 ml/min, the injection volume: 20 μl, column temperature was 30 °C. The entire chromatography process took 20 min. Ratio of Glu and GABA (Glu/GABA values) = Glu levels/GABA levels.
Real-Time Polymerase Chain Reaction (RT-PCR) Analysis
Total RNA was extracted from the basal ganglia samples by using Trizol (Tiangen Biochemical Technology co., China). The reverse transcription of total RNA was carried out by using iScript II Reverse Transcription Supermix (Bio-Rad Laboratories, Mississauga, Canada). The primers were synthesized by Shanghai Invitrogen Co., China and the sequence was listed as follows: 5′-GGCTTGACTTCTTTCGGGTTCTA-3′ (forward primer) and 5′-GGCTTGACTTCTTTCGGGTTCTA-3′ (reverse primer) for GABAA; 5′-CTCTCCCCTCTGGGCTATCC-3′ (forward primer) and 5′-GAATTCACGGCGATTGCG-3′ (reverse primer) for GAT-1 and 5′-GTTCAACGGCACAGTCAAGG-3′ (forward primer) and 5′-CGCCAGTAGACTCCACGACA-3′ (reverse primer) for GAPDH.
Western Blotting
Proteins (30 μg) were separated in 12% SDS-PAGE gels and transferred onto PVDF membranes. After blocking nonspecific sites in T-TBS buffer (containing 5 % nonfat dry milk) at room temperature for 40 min, the membranes were incubated overnight at 4 °C with the following primary antibodies: anti-GABAA(1:800, Abcam ab48341, USA, anti-GAT-1(1:800, Abcam ab426), anti-GAPDH(1:5000, Boster, China), respectively. And then, the membranes were incubated with HRP-conjugated goat anti-rabbit IgG (1:7500, Boster, China) at room temperature for 1 h. After detection with the enhanced chemiluminescence system (Bio-RadLaboratories-segrate, Milan, Italy), the results were analyzed using Image J software (NIH, Bethesda, MD).
Statistical Analysis
The data was presented as mean ± standard deviation. All statistical analyses were performed by SPSS software version 16 for windows. The repeated measures ANOVA with independent variances time and different treatment was used to analyze the escape latency and swimming distance, and Tukey’s post hoc test was also used for analyzing between-group differences among multiple sets of data. One-way ANOVA and Dunnett’s multiple comparison post hoc tests were used for the data of other indexes analysis. TEM analysis was descriptive only and aimed to identify the possible difference among the different treatment at the ultrastructural level. Results were considered statistically significant at p value <0.05.
Results
PAS-Na Treatment Improved Mn-Induced Memory and Learning Impairment
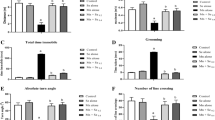
In order to assess the spatial learning and memory ability, MWM test was performed. The training test was used for assessing learning abilities. Mn exposure for 4 weeks and ceased Mn exposure for 3 weeks increased the escaping latency and swimming distance on the fifth day (p < 0.05, Fig. 1a, b). These neurotoxic effects were long-lasting, because ceased Mn exposure for 6 weeks did not improve the Mn neurotoxicity but deteriorated learning abilities as early as the fourth day (p < 0.05 or 0.01, Fig. 1c, d). In contrast, treatment with 200 mg/kg PAS-Na for 3 weeks significantly decreased the escaping latency and swimming distance on the fifth day (p < 0.05, Fig. 1a, b). The effects were more obviously that both 100 and 200 mg/kg treatment with PAS-Na for 6 weeks restored Mn-induced neurotoxicity via decreasing escaping latency and swimming distance (p < 0.05 or 0.01, Fig. 1c, d).
PAS-Na protected Mn-induced learning impairment via restored the increase of escape latency and swimming distance in rats. The average escape latency and swimming distance in the 7-week period (a, b), in the 10-week period (c, d). The experimental procedures were conducted as in Material and Methods. Rats were given four times per day and data represent as mean ± SD. n = 10 per group. * P or ** P < 0.05 or 0.01: significant as compared to control at the same periods; # P or ## P < 0.05 or 0.01: significant as compared to Mn-exposed group at the same periods
The spatial probe trial was used for accessing the memory abilities. Mn exposure for 4 weeks and ceased Mn exposure for 3 or 6 weeks increased the times of first cross (p < 0.01, Table 1) Mn exposure and ceased Mn exposure for 3 weeks decreased the ratio of platform quadrant time and distance (p < 0.05, Table 1). Treatment with 100 mg/kg PAS-Na for 3 weeks restored Mn-induced above-mentioned changes (p < 0.05 or 0.01, Table 1). Both 100 and 200 mg/kg PAS-Na treatment for 6 weeks significantly restored Mn-induced increasing of the first cross time.
PAS-Na Treatment Restored Mn-Induced Changes in Neurons, Astrocytes, and Neuropil Ultrastructural in the Basal Ganglia
The neuronal ultrastructural in the control group did not show any pathological changes (Fig. 2a). The nucleolus was clear and contained integrated nuclear membrane; the chromatin was well distributed and contained abundant rough endoplasmic reticulum and mitochondria. After Mn exposure for 4 weeks and ceased Mn exposure for 3 (Fig. 2b) or 6 (Fig. 2c) weeks, the neuronal ultrastructure was obviously abnormal as exhibited by the shrinkage and swelling nucleus, collapsed nucleolus, intense chromatin condensation, nuclear membrane disruption, swollen and degranulated rough endoplasmic reticulum and less cytoplasmic organelles. In contrast, PAS-Na treatment for 3 (Fig. 2d, e) or 6 weeks (Fig. 2f, g) reduced the above-mentioned impairment, especially 200 mg/kg PAS-Na treatment for 3 (e) and 6 (g) weeks.
PAS-Na restored the changes of neuronal ultrastructural in the basal ganglia induced by Mn (scale bars represent 125 nm, magnification ×10,000). a Normal neuronal ultrastructural in the basal ganglia. The nucleolus was obvious (black arrow) and contained integrated nuclear membrane (black cross-arrow); the chromatin was well distributed and contained abundant rough endoplasmic reticulum (white arrow-heads) and mitochondria (black arrow-heads). b, c Neuronal ultrastructural in basal ganglia of Mn group of 7- and 10-week period, respectively. The neuronal ultrastructure was obviously abnormal as exhibited by the shrinkage (b) and swelling nucleus (c), nucleolus collapsed (white cross-arrows), intense chromatin condensation (black arrow-heads), an apparent loss of nuclear membrane integrity (black arrows), swollen and degranulated rough endoplasmic reticulum (white arrow-heads), swollen mitochondria (black arrow-heads) and presence of lysosomes (white arrow). d, e Mn + 100 PAS-Na and Mn + 200 PAS-Na (treatment for 3 weeks). f, g Mn + 100 PAS-Na and Mn + 200 PAS-Na (treatment for 6 weeks). PAS-Na treatment restored Mn-induced above-mentioned changes in neuronal ultrastructure, especially 200 mg/kg PAS-Na treatment for 3 (e) and 6 (g) weeks
Signs of normal neuropil showed clearly visible synaptic cleft and presynaptic vesicles (Fig. 3a). Mn exposure for 4 weeks and ceased Mn exposure for 3 (Fig. 3b) or 6 (Fig. 3c) weeks increased the electron density in the protrusions structure and fuzzed interval of neuropil. PAS-Na treatment for 3 or 6 weeks reduced the impairment (Fig. 3d–g).
PAS-Na restored the ultrastructural changes of neuropil in the basal ganglia induced by Mn (scale bars represent 125 nm, magnification ×10,000). a The normal neuropil. The normal neuropil showed clearly visible synaptic cleft and presynaptic vesicles. b Neuropil in the Mn group of 7-week period. c Neuropil in the Mn group of 10-week period. Mn exposure increased the electron density in the protrusions structure and fuzzed interval of neuropil. d, e Neuropil in Mn + 100 PAS-Na and Mn + 200 PAS-Na group of 7-week period. f, g Neuropil in Mn + 100 PAS-Na and Mn + 200 PAS-Na group of 10-week period. PAS-Na treatment for 3 or 6 weeks reduced the impairment (Fig. 3d–g)
The astrocytic ultrastructural in the control groups showed a dense and homogeneous cytoplasm and well-distributed chromatin and integrated nuclear membrane (Fig. 4a). After Mn exposure for 4 weeks and ceased Mn exposure for 3 (Fig. 4b) or 6 (Fig. 4c) weeks, the astrocytic ultrastructural was obviously abnormal as visualized by apparent loss of nuclear membrane integrity, focal hyperplasia, and hypertrophy accompanied with increasing cell volume and cytoplasm. The cytoplasm contained more free ribosomes, mitochondria, and lysosomes than those of the control. Additionally, higher electronic density, irregular nucleus, chromatin condensation were also been found in the Mn-treated astrocytes (Fig. 4b, c). However, PAS-Na treatment for 3 or 6 weeks improved Mn-induced above-mentioned changes in astrocytes (Fig. 4d–g), especially 200 mg/kg PAS-Na treatment for 3 (Fig. 4e) and 6 (Fig. 4g) weeks.
PAS-Na revert the changes of astrocytic ultrastructural in the basal ganglia induced by Mn (scale bars represent 125 nm, magnification ×10,000). a The normal astrocytic ultrastructural. The normal astrocytic ultrastructural showed integrated nuclear membrane (black arrow), and the chromatin was well distributed. b, c The Mn group in 7- and 10-week period, respectively. The astrocytic ultrastructural of the Mn groups were obviously abnormal as visualized by an apparent focal hyperplasia (b), loss of nuclear membrane integrity (black arrows), accompanied with intense chromatin condensation (black arrow-heads). d, e Mn + 100 PAS-Na and Mn + 200 PAS-Na groups in 7-week period. f, g Mn + 100 PAS-Na and Mn + 200 PAS-Na groups in 10-week period. PAS-Na treatment for 3 or 6 weeks improved above-mentioned changes of astrocytic ultrastructural induced by Mn, especially 200 mg/kg PAS-Na treatment for 3 (e) and 6 (g) weeks
PAS-Na Treatment Restored Mn-Induced the Increase of Glu/GABA Values
Mn exposure for 4 weeks and ceased Mn exposure for 3 weeks increased Glu/GABA values (Fig. 5a, p < 0.01). However, Mn exposure for 4 weeks and ceased Mn exposure for 6 weeks did not alter the Glu/GABA values (Fig. 5b, p > 0.05). In contrast, treatment with 200 mg/kg PAS-Na for 3 weeks restored the alteration of Glu/GABA values induced by Mn (Fig. 5a, p < 0.01).
PAS-Na Treatment for 6 Weeks Reverted the Decrease of GAT-1 mRNA Expression but No GABAA mRNA Expression Induced by Mn
Mn exposure for 4 weeks and ceased Mn exposure for 3 weeks did not alter the GABAA and GAT-1 mRNA expression (Fig. 6a, p > 0.05). However, Mn exposure for 4 weeks and ceased Mn exposure for 6 weeks decreased GABAA and GAT-1 mRNA expression to 61.5 and 57.1 % of the control, respectively (Fig. 6b, p < 0.05). Treatment with PAS-Na for 6 weeks reverted the GAT-1 mRNA expression levels (95.6 and 96.3 % of the control, respectively, Fig. 6b, p < 0.05), but no effects on the GABAA mRNA expression (Fig. 6b, p > 0.05).
Mn Exposure Increased GABAA Protein Expression but No Effects on GAT-1
Mn exposure for 4 weeks and ceased Mn exposure for 3 weeks increased the GABAA protein expression to ~190 % levels of the control (Fig. 7a, p < 0.05), but Mn exposure for 4 weeks and ceased Mn exposure for 6 weeks did not alter the GABAA protein expression (Fig. 7b, p > 0.05). And treatment with PAS-Na for 3 weeks had not effects on Mn-induced the alteration of GABAA protein expression (Fig. 7a, p > 0.05). We did not find Mn has no significant effects on GAT-1 protein expression (Fig. 7, p > 0.05).
Discussions
A large body of evidences which were confirmed by meta-analyses [27, 32] showed that prolonged occupational Mn exposure even at a low level may cause motor deficits. Even after the Mn exposure ends, these damages may persist and progress worse [14]. More seriously, studies on children have shown that both neuromotor and cognitive abnormalities [16, 33] were associated with excessive Mn exposure in airborne particles [25] and deposited dust [24]. Morris water maze (MWM) test has been universally used to assess the spatial learning and memory ability. Hence, we used MWM to test the spatial learning and memory ability of the experimental animals. Our results showed that Mn exposure for 4 weeks and ceased Mn exposure for 3 weeks significantly damaged the spatial learning and memory ability indicated as increase of the escaping latency and swimming distance in the training test (Fig. 1a, b) and the first cross times in the spatial probe trial on the rats (Table 1). The neurotoxic effects were long-lasting, because 6-week period of no Mn exposure did not improve the Mn-induced neurotoxicity but deteriorated learning abilities as early as the fourth day (Fig. 1c, d).
Ultrastructural changes in the brain have been confirmed to be a common brain pathological response in the neurodegenerative diseases, including Alzheimer’s disease, PD, and manganism [29, 39]. The earliest study showed that Mn exposure induced ultrastructural changes in caudate nucleus such as swollen rough endoplasmatic reticulum [4]. It was corroborated by recently study which reported that Mn exposure produced ultrastructural changes in the caudate nucleus of mice expressed as neuronal and glial edema, myelin disarrangement, and swollen mitochondria. Our previous study showed that low levels of Mn exposure decreased the dendritic branching of primary cultured hippocampus neurons [40]. More importantly, Mn-induced injuries in the basal ganglia were related with Mn-induced the cognitive deficits and neuropsychological [11]. The above-mentioned evidences suggest that excessive Mn exposure can alter the basal ganglia unlrastructural and destroy memory ability [26, 31, 41]. The present study found that Mn-exposure for 4 weeks and ceased Mn exposure for 3 or 6 weeks induced ultrastructural alteration in the basal ganglia exhibited as neuronal shrinkage and swollen, nucleolus collapsed, irregular nucleus with higher electron density, intense chromatin condensation, increased the electron density in the protrusions structure and fuzzed interval of neuropil, swollen, focal hyperplasia, and hypertrophy in astrocytes (Fig. 7). Unfortunately, we have not shown the correlation between the behavior deficit and the ultrastructural alterations in the basal ganglia induced by Mn. But the involvement of injuries of the basal ganglia in Mn-induced cognitive deficits is confirmed in the present study.
Historically, studies on the effects of Mn have concerned with the effects on DAergic system damage because of its relation with movement abnormalities. However, emerging studies provide significant evidences of Mn effects on GABA-ergic system which is more primary affected by Mn than the DAergic systerm [10, 12]. Moreover, it is well known that GABA and Glu are involved in the regulation of movement performance, and the alterations of GABA and Glu in the basal ganglia are associated with movement deficits [22, 36]. But the effects of Mn exposure on these two key neurotransmitter involved remain controversial [12, 19–21, 37, 44]. Although the studies on the alteration of Mn-induced GABAergic system were in contradiction, the GABA level changes in basal ganglia are well recognized to be related with Mn-induced neurotoxicity [9, 35]. Additionally, Mn also altered GAT-1, GABAA and GABAB protein expression [2, 5]. DAergic nuclei and GABAergic nuclei may reciprocally affect each other [2]. The present results showed that Mn exposure for 4 weeks and ceased Mn exposure for 3 weeks increased the ratio of Glu and GABA, while after ceased Mn exposure for longer times (6 weeks) restored these changes. Moreover, t Mn exposure for 4 weeks and ceased Mn exposure for 3 weeks increased GABAA protein expression, but no effects on the GABAA mRNA, GAT-1 mRNA and protein expression. However, Mn exposure for 4 weeks and ceased Mn exposure for 6 weeks decreased both GAT-1 and GABAA mRNA expression levels, but no effects on the protein expression. Unfortunately, we did not observe similar changes on Mn-induced alteration of GABAA and GAT-1 mRNA in the protein level. The reason of why the results of mRNA and protein expression were not consistent may be due to that we used different samples to determine the mRNA and protein expression which was similar to the results of the previous studies [2].
PAS-Na, an anti-berculosis drug, has been firstly confirmed to be efficient on promoting Mn excrete [38]. More importantly, our labs found that PAS-Na has clinical effects on manganism treatment with good long-lasting prognosis [15, 18]. However, how PAS-Na makes a therapeutic effect in the treatment of manganism is unclear. Among various therapeutic mechanisms of PAS-Na treatment on Mn-induced neurotoxicology, Glu excitotoxicity has also been considered [6, 17, 19, 20, 42, 43]. The present study found that PAS-Na restored Mn-induced spatial learning and memory ability impairment, and higher and more prolonged PAS-Na treatments were more effects. We also found that PAS-Na treatment reduced Mn-induced long-lasting ultrastructural alterations in neuron, astrocytes, and neuropil of the basal ganglia. Furthermore, our study showed that PAS-Na treatment for 3 weeks restored Mn increased the ratio of Glu and GABA, but no effects on the GABAA protein expression. PAS-Na treatment for 6 weeks significantly reversed Mn-induced alteration of GAT-1 mRNA expression levels, but no effects on GABAA mRNA expression levels. These results confirmed our previous study which showed that PAS-Na (200 mg/kg) treatment for 6 weeks or PAS-Na (100 or 200 mg/kg) restored Glu, Gln, and Gly levels of the Mn-exposed rats to the normal levels [30].
In conclusion, our data show that Mn exposure caused the long-lasting spatial learning and memory abilities impairment. Moreover, Mn exposure produced ultrastructural alterations in the basal ganglia. The results also indicate that Mn exposure increased the Glu/GABA values by feedback loops controlling GAT-1 and GABAA mRNA, GABAA protein expression. PAS-Na treatment effectively restored the above-mentioned adverse effects induced by Mn. These findings suggest the involvement of GABA metabolism and ultrastructural alterations of basal ganglia in PAS-Na’s protective effects on the spatial learning and memory abilities.
Reference
Anderson JG, Cooney PT, Erikson KM (2007) Brain manganese accumulation is inversely related to gamma-amino butyric acid uptake in male and female rats. Toxicol Sci 95(1):188–195
Anderson JG, Fordahl SC, Cooney PT, Weaver TL, Colyer CL, Erikson KM (2008) Manganese exposure alters extracellular GABA, GABA receptor and transporter protein and mRNA levels in the developing rat brain. Neurotoxicology 29(6):1044–1053
Bhang SY, Cho SC, Kim JW, Hong YC, Shin MS, Yoo HJ, Cho IH, Kim Y, Kim BN (2013) Relationship between blood manganese levels and children’s attention, cognition, behavior, and academic performance—a nationwide cross-sectional study. Environ Res 126:9–16
Bikashvili TZ, Shukakidze AA, Kiknadze GI (2001) Changes in the ultrastructure of the rat cerebral cortex after oral doses of manganese chloride. Neurosci Behav Physiol 31(4):385–389
Burton NC, Schneider JS, Syversen T, Guilarte TR (2009) Effects of chronic manganese exposure on glutamatergic and GABAergic neurotransmitter markers in the nonhuman primate brain. Toxicol Sci 111(1):131–139
Crawford S, Davis K, Saddler C, Joseph J, Catapane EJ, Carroll MA (2011) The ability of PAS, acetylsalicylic acid and calcium disodium EDTA to protect against the toxic effects of manganese on mitochondrial respiration in gill of Crassostrea virginica. In Vivo (Brooklyn) 33(1):7–14
Defazio G, Soleo L, Zefferino R, Livrea P (1996) Manganese toxicity in serumless dissociated mesencephalic and striatal primary culture. Brain Res Bull 40(4):257–262
Dong, X., D. Zhang and X. Meng 2006. The effects of Glu/GABA horizontal correlation on learning and memory ability. J Chin Gerontol (02):283–285
Erikson KM, Aschner M (2003) Manganese neurotoxicity and glutamate-GABA interaction. Neurochem Int 43(4–5):475–480
Fitsanakis VA, Au C, Erikson KM, Aschner M (2006) The effects of manganese on glutamate, dopamine and gamma-aminobutyric acid regulation. Neurochem Int 48(6–7):426–433
Guilarte TR (2013) Manganese neurotoxicity: new perspectives from behavioral, neuroimaging, and neuropathological studies in humans and non-human primates. Front Aging Neurosci 5:23
Gwiazda RH, Lee D, Sheridan J, Smith DR (2002) Low cumulative manganese exposure affects striatal GABA but not dopamine. Neurotoxicology 23(1):69–76
Horning KJ, Caito SW, Tipps KG, Bowman AB, Aschner M (2015) Manganese is essential for neuronal health. Annu Rev Nutr 35:71–108
Jiang Y, Zheng W, Long L, Zhao W, Li X, Mo X, Lu J, Fu X, Li W, Liu S, Long Q, Huang J, Pira E (2007) Brain magnetic resonance imaging and manganese concentrations in red blood cells of smelting workers: search for biomarkers of manganese exposure. Neurotoxicology 28(1):126–135
Jiang YM, Mo XA, Du FQ, Fu X, Zhu XY, Gao HY, Xie JL, Liao FL, Pira E, Zheng W (2006) Effective treatment of manganese-induced occupational Parkinsonism with p-aminosalicylic acid: a case of 17-year follow-up study. J Occup Environ Med 48(6):644–649
Kim Y, Jeong KS, Song HJ, Lee JJ, Seo JH, Kim GC, Lee HJ, Kim HJ, Ahn JH, Park SJ, Kim SH, Kwon YJ, Chang Y (2011) Altered white matter microstructural integrity revealed by voxel-wise analysis of diffusion tensor imaging in welders with manganese exposure. Neurotoxicology 32(1):100–109
King C, Myrthil M, Carroll MA, Catapane EJ (2008) Effects of p-aminosalicylic acid on the neurotoxicity of manganese and levels of dopamine and serotonin in the nervous system and innervated organs of Crassostrea virginica. In Vivo (Brooklyn) 29(3):26–34
Ky SQ, Deng HS, Xie PY, Hu W (1992) A report of two cases of chronic serious manganese poisoning treated with sodium para-aminosalicylic acid. Br J Ind Med 49(1):66–69
Li, S. J., Y. Li, J. W. Chen, Z. X. Yuan, Y. H. Mo, G. D. Lu, Y. M. Jiang, C. Y. Ou, F. Wang, X. W. Huang, Y. N. Luo, S. Y. Ou and Y. N. Huang 2015a. Sodium para-aminosalicylic acid protected primary cultured basal ganglia neurons of rat from manganese-induced oxidative impairment and changes of amino acid neurotransmitters. Biol Trace Elem Res
Li SJ, Meng HY, Deng XF, Fu X, Chen JW, Huang S, Huang YS, Luo HL, Ou SY, Jiang YM (2015b) Protective effects of sodium p-aminosalicylic acid on learning and memory via increasing the number of basal forebrain choline acetyltransferase neurons in manganese-exposed rats. Hum Exp Toxicol 34(3):240–248
Lipe GW, Duhart H, Newport GD, Slikker W Jr, Ali SF (1999) Effect of manganese on the concentration of amino acids in different regions of the rat brain. J Environ Sci Health B 34(1):119–132
Long Z, Jiang YM, Li XR, Fadel W, Xu J, Yeh CL, Long LL, Luo HL, Harezlak J, Murdoch JB, Zheng W, Dydak U (2014) Vulnerability of welders to manganese exposure--a neuroimaging study. Neurotoxicology 45:285–292
Lu CS, Huang CC, Chu NS, Calne DB (1994) Levodopa failure in chronic manganism. Neurology 44(9):1600–1602
Lucchini RG, Albini E, Benedetti L, Borghesi S, Coccaglio R, Malara EC, Parrinello G, Garattini S, Resola S, Alessio L (2007) High prevalence of Parkinsonian disorders associated to manganese exposure in the vicinities of ferroalloy industries. Am J Ind Med 50(11):788–800
Lucchini RG, Guazzetti S, Zoni S, Benedetti C, Fedrighi C, Peli M, Donna F, Bontempi E, Borgese L, Micheletti S, Ferri R, Marchetti S, Smith DR (2014) Neurofunctional dopaminergic impairment in elderly after lifetime exposure to manganese. Neurotoxicology 45:309–317
McKinney AM, Filice RW, Teksam M, Casey S, Truwit C, Clark HB, Woon C, Liu HY (2004) Diffusion abnormalities of the globi pallidi in manganese neurotoxicity. Neuroradiology 46(4):291–295
Meyer-Baron M, Schaper M, Knapp G, Lucchini R, Zoni S, Bast-Pettersen R, Ellingsen DG, Thomassen Y, He S, Yuan H, Niu Q, Wang XL, Yang YJ, Iregren A, Sjogren B, Blond M, Laursen P, Netterstrom B, Mergler D, Bowler R, van Thriel C (2013) The neurobehavioral impact of manganese: results and challenges obtained by a meta-analysis of individual participant data. Neurotoxicology 36:1–9
Nachtman JP, Delor S, Brennan CE (1987) Manganese neurotoxicity: effects of varying oxygen tension and EDTA on dopamine auto-oxidation. Neurotoxicology 8(2):249–253
Noristani HN, Meadows RS, Olabarria M, Verkhratsky A, Rodriguez JJ (2011) Increased hippocampal CA1 density of serotonergic terminals in a triple transgenic mouse model of Alzheimer’s disease: an ultrastructural study. Cell Death Dis 2:e210
Ou CY, Huang ML, Jiang YM, Luo HL, Deng XF, Wang C, Wang F, Huang XW (2011) [Effect of sodium para-aminosalicylic on concentrations of amino acid neurotransmitters in basal ganglia of manganese-exposed rats]. Zhonghua Yu Fang Yi Xue Za Zhi 45(5):422–425
Racette BA, Aschner M, Guilarte TR, Dydak U, Criswell SR, Zheng W (2012) Pathophysiology of manganese-associated neurotoxicity. Neurotoxicology 33(4):881–886
Rodriguez-Barranco M, Lacasana M, Aguilar-Garduno C, Alguacil J, Gil F, Gonzalez-Alzaga B, Rojas-Garcia A (2013) Association of arsenic, cadmium and manganese exposure with neurodevelopment and behavioural disorders in children: a systematic review and meta-analysis. Sci Total Environ 454-455:562–577
Roels HA, Bowler RM, Kim Y, Claus Henn B, Mergler D, Hoet P, Gocheva VV, Bellinger DC, Wright RO, Harris MG, Chang Y, Bouchard MF, Riojas-Rodriguez H, Menezes-Filho JA, Tellez-Rojo MM (2012) Manganese exposure and cognitive deficits: a growing concern for manganese neurotoxicity. Neurotoxicology 33(4):872–880
Rosenstock HA, Simons DG, Meyer JS (1971) Chronic manganism. Neurologic and laboratory studies during treatment with levodopa. JAMA 217(10):1354–1358
Sidoryk-Wegrzynowicz M, Aschner M (2013) Role of astrocytes in manganese mediated neurotoxicity. BMC Pharmacol Toxicol 14:23
Stanwood GD, Leitch DB, Savchenko V, Wu J, Fitsanakis VA, Anderson DJ, Stankowski JN, Aschner M, McLaughlin B (2009) Manganese exposure is cytotoxic and alters dopaminergic and GABAergic neurons within the basal ganglia. J Neurochem 110(1):378–389
Struve MF, McManus BE, Wong BA, Dorman DC (2007) Basal ganglia neurotransmitter concentrations in rhesus monkeys following subchronic manganese sulfate inhalation. Am J Ind Med 50(10):772–778
Tandon SK (1978) Chelation in metal intoxication. VI. Influence of PAS and CDTA on the excretion of manganese in rabbits given MnO2. Toxicology 9(4):379–385
Wade A, Jacobs P, Morton AJ (2008) Atrophy and degeneration in sciatic nerve of presymptomatic mice carrying the Huntington’s disease mutation. Brain Res 1188:61–68
Wang F, Wang C, Jiang Y, Deng X, Lu J, Ou S (2014) Protective role of sodium para-amino salicylic acid against manganese-induced hippocampal neurons damage. Environ Toxicol Pharmacol 37(3):1071–1078
Yamada M, Ohno S, Okayasu I, Okeda R, Hatakeyama S, Watanabe H, Ushio K, Tsukagoshi H (1986) Chronic manganese poisoning: a neuropathological study with determination of manganese distribution in the brain. Acta Neuropathol 70(3–4):273–278
Yoon H, Kim DS, Lee GH, Kim JY, Kim DH, Kim KW, Chae SW, You WH, Lee YC, Park SJ, Kim HR, Chae HJ (2009) Protective effects of sodium para-amino salicylate on manganese-induced neuronal death: the involvement of reactive oxygen species. J Pharm Pharmacol 61(11):1563–1569
Zheng W, Jiang YM, Zhang Y, Jiang W, Wang X, Cowan DM (2009) Chelation therapy of manganese intoxication with para-aminosalicylic acid (PAS) in Sprague-Dawley rats. Neurotoxicology 30(2):240–248
Zwingmann C, Leibfritz D, Hazell AS (2007) Nmr spectroscopic analysis of regional brain energy metabolism in manganese neurotoxicity. Glia 55(15):1610–1617
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Grant Support
ᅟ
ᅟ
This study was supported by grants from the National Natural Science Foundation of China (NSFC 81072320, 81460505, 30760210), Guangxi Natural Science Foundation (GXNSFAA 118232, 2015GXNSFAA139181) and the Innovation Project of Guangxi Graduate Education.
Additional information
Drs. Chao-Yan Ou, Yi-Ni Luo, Sheng-Nan He and Xiang-Fa Deng contributed equally to this article.
Rights and permissions
About this article
Cite this article
Ou, CY., Luo, YN., He, SN. et al. Sodium P-Aminosalicylic Acid Improved Manganese-Induced Learning and Memory Dysfunction via Restoring the Ultrastructural Alterations and γ-Aminobutyric Acid Metabolism Imbalance in the Basal Ganglia. Biol Trace Elem Res 176, 143–153 (2017). https://doi.org/10.1007/s12011-016-0802-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-016-0802-4