Abstract
Przewalski’s gazelle (Procapra przewalskii) is an endangered ungulate in the Qinghai–Tibet Plateau of China. This study aimed to determine the influence of selenium (Se) deprivation in the natural habitat on the immune index and antioxidant capacity of P. przewalskii. Samples of soil and forage were collected from affected and healthy areas, and animal tissues were collected from affected and healthy P. przewalskii. The samples were used for measuring mineral content and for hematological and biochemical analyses. The results showed that Se concentrations were significantly lower in the soil and mixed forage samples from the affected area than in those from the healthy area. The Se concentrations were significantly lower in blood and hair samples from affected P. przewalskii than in those from healthy P. przewalskii. Meanwhile, hemoglobin, packed cell volume, and platelet count of affected P. przewalskii were significantly lower than those of healthy P. przewalskii. The serum level of glutathione peroxidase and total antioxidant capacity were significantly lower and the serum levels of malondialdehyde, total nitric oxide synthase, and lipid peroxide were significantly higher in affected P. przewalskii. The serum levels of interleukin (IL)-1β, IL-2, tumor necrosis factor-alpha, immunoglobulin A (IgA), and IgG significantly decreased and the serum levels of IL-6 and IgM significantly reduced in affected P. przewalskii compared with healthy P. przewalskii. Therefore, the findings indicated that Se deprivation in soil and forage caused oxidative stress damage and posed a serious threat to the immune function of P. przewalskii.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Przewalski’s gazelle (Procapra przewalskii) is endemic and a well-known endangered ungulate in the Qinghai–Tibet Plateau of China. It belongs to Artiodactyla (order), Bovidae (family), Antilopinae (subfamily), and Procapra (genus) [1, 2]. Historically, the species was widely distributed throughout the provinces or autonomous regions of Tibet, Inner Mongolia, Ningxia, Qinghai, Xinjiang, and Gansu [3]. Its population has decreased sharply, and its range has shrunk due to habitat fragmentation, resource competition, pasture fencing, excessive poaching, and disorder in the last century. Such that this gazelle is found only in the deserts around the Qinghai Lake on the Tibetan plateau; fewer than 300 remained from 1986 to 1994 [4,5,6,7]. Of China’s endemic mammals, the P. przewalskii has become the least populous species. Hence, it has been classified as endangered by the International Union for Conservation of Nature Red List of Threatened Species and has been listed as a category I species under the Wild Animal Protection Law in China [6, 7].
Selenium (Se) is an essential trace element in wildlife and livestock. It occurs in selenoproteins in the form of selenocysteine, which is involved in antioxidant activity, immune modulation, endocrine function, bone metabolism, iodine metabolism, and reproductive processes [8]. The Qinghai Lake Basin of Tibetan Plateau is the only existing natural habitat of P. przewalskii, with the total population size estimated at 1860 individuals in the 2017 survey. Previous studies reported Se deprivation–related illnesses of livestock in the Qinghai Lake Basin. Li et al. (2018) found that the Se concentrations in the blood, liver, and muscles of Tibetan sheep were significantly lower than the reference values of healthy animals in the Hudong area of the Qinghai Lake Basin (P < 0.01) [9]. Huo et al. (2019) reported an illness related to the Se-deficient pasture in yaks in the Hudong area of the Qinghai Lake. The main signs of the illness included indigestion, emaciation, diarrhea, loss of appetite, allotriophagia, growth retardation, and fecundity decline. The most serious case was abortion or sudden death. The pathological symptoms included degeneration and necrosis of skeletal muscle, myocardium, and liver tissue. This sickness was controlled by sodium selenite [10]. Shen et al. (2018) reported that the natural habitat of P. przewalskii in the upper reaches of the Buha River area was Se deprived; the Se concentrations in the mixed forage were significantly lower compared with the reference values of ruminants (P < 0.01) [11]. However, to date, no study has investigated the influence of Se-deficient environment on the P. przewalskii.
The objective of this study was to explore the effects of Se-deficient pasture on the immune index and antioxidant capacity of P. przewalskii, thus providing the scientific basis for protecting the remaining P. przewalskii populations.
Material and Methods
Study Site
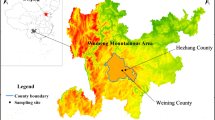
This study was conducted in the Kuaierma area (98° 42′–98° 50′ E, 37° 21′–37° 28′ N) and the Bird Island area (99° 44′–99° 52′ E, 36° 59′–37° 01′ N). Kuaierma is located in the remote upper reaches of the Buha River. The average Se concentrations in the soil and forage are (0.033 ± 0.011) μg/g and (0.029 ± 0.005) μg/g, respectively, based on the preliminary analysis. This site was chosen as an affected area. The elevations of this area range from 3200 to 3800 m [12]. The climate is characterized by dry and cold winters, strong winds, strong ultraviolet radiation, and a short frost-free period, with the annual precipitation of 350–450 mm. The average atmospheric temperature is 0.3–1.1 °C, and the temperature extremes are −40 and 25 °C [13, 14]. The main grassland species include shining speargrass (Achnatherum splendens), wheatgrass (Agropyron cristatum), fringed sagebrush (Artemisia frigida), drilled wormwood (Artemisia anethifolia), moorcraft sedge (Carex mooscroftii), Chinese iris (Iris lactea), Kokono orinus (Orinus kokonorica), wood betony (Pedicularis resupinata), and Chinese stellera (Stellera chamaejasme) [12, 14]. Most of the plants are herbaceous and good food resources for P. przewalskii.
Experimental Animals
Sample Collections
Samples from soils, forages, and animals were collected in June 2018 in the Kuaierma area (affected pasture) and Bird Island area (healthy pasture). Ten soil samples were taken from the surface layer (0–30 cm) of randomly distributed locations in each area using a 30-mm-diameter cylindrical corer. Each soil sample comprised four soil cores collected at one site, each site with an interval of 500 m. Twenty mixed forage samples were collected randomly from the same locations in quadrants of l × l m2. The forage samples were cut 1–2 cm above the ground level to reduce soil contamination. The mixed forage and soil samples were dried at 20–25 °C until a constant weight was achieved, crushed, and passed through a 2-mm sieve; a 0.075-mm sieve was used to remove silver sand. The soil and mixed pasture samples were kept in a vacuum desiccator until chemical analysis.
The use of P. przewalskii in these experiments was approved by the Institutional Animal Care and Use Committee of Southwest University of Science and Technology in China (Project A00865) and guided by acceptable practices outlined in the Guide for the Care and Use of Wildlife Animals in Wildlife Research and Teaching Consortium (2012). P. przewalskii was caught using a Model-l50 anesthesia gun with ketamine hydrochloride (Anesthetic Medicinal Fujian Gutian Pharmaceutical Co., Ltd., Fujian, China) between 17 and 20 o’clock on a sunny day in June. When the animal was at a distance of 20–25 m, the ketamine hydrochloride injection was continuously fired from a tranquilizer gun. The animal was basically anesthetized when five shots (30 mg per shot) were continuously emitted within 3 min, and the anesthesia was maintained for about 20 min. Seven P. przewalskii were selected from the Kuaierma area and Bird Island area, respectively. The hair samples of selected P. przewalskii were taken from the animal necks. Each sample was washed with acetone, rinsed five times with deionized water, and then kept on a silica gel in a desiccator until analyses. The blood samples were obtained from the jugular vein using a vacuum blood collection tube without anticoagulant for biochemical analysis and a vacuum blood collection tube containing 1% sodium heparin as an anticoagulant for hematological and trace element analyses. The serum samples were separated by centrifugation (at 1200×g for 5 min) and stored in plastic vials at − 20 °C for subsequent experiments. The selected P. przewalskii were released after the samples were collected. No P. przewalskii was injured or died during the entire sampling process.
Mineral Content Analysis
The sample solvent was prepared using a microwave digestion system (Touchwin4.0, APL Instrument Co., Ltd., Chengdu, China). The soil samples were heated in a microwave with a mixture of nitric acid (HNO3), hydrofluoric acid (HF), and perchloric acid (HClO4) (5:2:5) to dissolve the sample. The forage and animal tissues (hair and whole blood) were dissolved in HNO3 and HClO4 (4:1) mixture by microwave heating [15].
The concentrations of copper (Cu), iron (Fe), zinc (Zn), and manganese (Mn) in the samples of soil, forage, and animal tissues were measured using an AA-7000 atomic absorption spectrophotometer (Shimadzu Corporation, Japan). The concentrations of Se and molybdenum (Mo) were determined using a flameless atomic absorption spectrophotometer (Perkin-Elmer 3030 graphite furnace with a Zeeman background correction). It was difficult to accurately determine Mo concentrations in samples due to “memory” or carryover effects. Therefore, two blanks (deionized water) were run after each sample was tested to reduce memory effects. The accuracy of the analytical values was checked by referring to the certified values of elements in the National Institute of Standards and Technology Standard Reference Material bovine liver SRM 1577a.
Hematological Examination
An automatic animal hematology analyzer (BC2800Vet, Mindray Biomedical Electronics Co., Ltd., Shenzhen, China) was used to measure routine hematological indexes in the whole blood following the manufacturer’s instructions. These routine blood indexes included hemoglobin (Hb), red blood cell count (RBC), packed cell volume (PCV), mean corpuscular hemoglobin (MCH), mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), white blood cell count (WBC), and platelet count (PLT).
Biochemical Examination
The serum antioxidant capacity and immune index were determined using diagnostic kits from the Jianchen Bioengineering Institute (Nanjing, China) following the manufacturer’s protocols. The activities of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) in the serum were measured by the xanthine oxidase method and 5,5′-dithio-2-dinitrobenzoicacid chromogenic reaction, respectively. The serum catalase (CAT) and total antioxidant capacity (T-AOC) assays were based on ammonium molybdate colorimetry. The serum malondialdehyde (MDA) content was measured with a thiobarbituric acid chromatometer. The serum level of nitric oxide (NO) was determined by the nitric acid reduction method. The serum total nitric oxide synthase (T-NOS) activity was assayed according to the NOS-catalyzed L-Arg method. The serum levels of lipid peroxide (LPO), interleukin (IL)-1β, IL-2, interleukin 6 (IL-6), tumor necrosis factor-alpha (TNF-α), immunoglobulin (Ig)A, IgG, and IgM were quantitatively determined using enzyme-linked immunosorbent assay.
Statistical Analysis
Data were statistically analyzed using the Statistical Package for the Social Sciences (SPSS, version 23.0, Inc., Chicago, Illinois, USA) software. They were expressed as mean ± standard deviation. The differences between the two groups were analyzed using the Student’s t test, with the threshold of P = 0.05 (*P < 0.05, **P < 0.01).
Results
Mineral Element Concentrations in Soil and Forage
The concentrations of mineral elements in soil and forage are shown in Table 1. The Se concentrations in soil and mixed forage from the affected pastoral grassland were significantly lower than those from the healthy pasture (P < 0.01). The concentrations of Cu and Mo in the soil and mixed forage from the affected pastoral grassland were significantly lower than those from the healthy pasture (P < 0.05). No significant difference was found in the concentrations of other elements.
Mineral Element Concentrations in Animal Tissues
The concentrations of mineral elements in animal tissues are shown in Table 2. The Se concentrations in the blood and hair from affected P. przewalskii were significantly lower than those from healthy P. przewalskii (P < 0.01). The Fe concentrations in the blood from affected P. przewalskii were significantly lower than those from healthy P. przewalskii (P < 0.05).
Hematological Results of P. przewalskii
The hematological values of P. przewalskii are shown in Table 3. Hb, PCV, and PLT of affected P. przewalskii were significantly lower than those of healthy P. przewalskii (P < 0.01). RBC and MCV of affected P. przewalskii were significantly lower than those of healthy P. przewalskii (P < 0.05). No significant difference was observed in MCH, MCHC, and WBC.
Antioxidant Capacity of P. przewalskii
The serum antioxidant capacity of P. przewalskii in the affected and healthy areas is presented in Table 4. The serum levels of GSH-PX and T-AOC in affected P. przewalskii were significantly lower than those in the healthy P. przewalskii (P < 0.01). The serum SOD and CAT activities in affected P. przewalskii were significantly lower than those in healthy P. przewalskii (P < 0.05). The serum levels of MDA, T-NOS, and LPO in affected P. przewalskii were significantly higher than those in healthy P. przewalskii (P < 0.01). The serum levels of NO in affected P. przewalskii were significantly higher than those in healthy P. przewalskii (P < 0.05).
Immune Function of P. przewalskii
Table 5 shows significantly lower levels of IL-1β, IL-2, TNF-α, IgA, and IgG in the serum samples from affected P. przewalskii compared with healthy P. przewalskii (P < 0.01). The serum levels of IL-6 and IgM in affected P. przewalskii were significantly lower than those in healthy P. przewalskii (P < 0.05).
Discussion
The alpine meadow ecosystem of the Qinghai–Tibet Plateau has an important gene pool of wildlife and plants. The Buha River is the largest river in the Qinghai Lake; its upper reach is one of the main natural habitats of P. przewalskii. Mineral elements play an important role in maintaining the evolution, development, and reproduction of livestock and wildlife [16,17,18]. In this study, the Se concentrations in the soil and mixed forage from the Kuaierma area were significantly lower than those from the Bird Island area (P < 0.01). The contents of Cu and Mo in the soil and mixed forage from the Kuaierma area were significantly lower than those from the Bird Island area (P < 0.05). In general, the Se contents in the soil and forage lower than 0.1 μg/g DM should be insufficient. Also, the content lower than 0.040 μg/g and 0.050 μg/g DM in the soil and forage separately should be considered as serious Se deficiency for ruminants [11, 19]. The mineral content in the blood and hair is the most direct indicator of the nutritional status of animals [20, 21]. At the same time, the Se content in the blood and hair of P. przewalskii grazing in the Kuerma area is also significantly lower than that in the Bird Island area and that in the healthy Tibetan yaks, camels, and sheep [21,22,23,24]. The ability of a feed to meet animal requirements for Cu depends more on the absorption of Cu than on the concentration of Cu that the feed contains. This is because Mo first forms thiomolybdate with sulfur and then forms a Cu-containing protein complex with Cu in the rumen, which is not conducive to the absorption of Cu [14]. Normally the requirements for ruminants were met by a Cu content of 7–8 μg/g DM in the forage. However, if ruminants are exposed to a containing no Mo, the Cu content of 1 μg/g DM in the mixed forage could meet the requirements of ruminants [11, 12]. The Cu requirement of P. przewalskii should be lower than normal due to the relatively low Mo content of forage in this study area.
Se is an essential nutrient for animals and performs numerous biological functions in organisms [25,25,27]. Some reports showed a significant relationship between Se deficiency and anemia, which was associated with an increased generation of reactive oxygen species and exposure of erythrocytes to a high degree of oxidative stress [28]. Hematological parameters are the diagnostic indicators used for assessing the degree of anemia in animals [29, 30]. In the present study, Hb, PCV, RBC, and MCV in the affected P. przewalskii significantly decreased, indicating that the affected P. przewalskii had subclinical anemia. Se is an essential component of GSH-Px, an enzyme that catalyzes the reduction of hydrogen peroxide and different organic peroxides to protect cells against peroxidation and to control the concentrations of intracellular peroxides [31]. Thus, the subclinical anemia in Se-deficient P. przewalskii was possibly explained by a much lower activity of GSH-Px in erythrocytes, which caused increased lipid peroxidation of membrane lipids, denaturation of hemoglobin, decreased osmotic resistance of erythrocytes, and ultimately chronic anemia [32,32,33,35]. Moreover, recent studies also showed inefficient erythropoiesis with defective erythroid differentiation and maturation in Se-deficient animals [35,35,37].
The main cause of oxidative stress is the excessive accumulation of free radicals in an animal organism, which may cause impairment of cell structure and organization [25, 38]. The free radicals can be scavenged by antioxidant compounds [39]. The antioxidant system is the defense system for scavenging free radicals, comprising non-enzymatic and enzymatic systems. The non-enzymatic system includes mainly vitamin E, vitamin C, cysteine, glutathione (GSH), Cu, Fe, Zn, and Se. The enzymatic system consists of SOD, GSH-Px, CAT, and other antioxidant enzymes [40,40,41,43]. SOD facilitates efficient dismutation of superoxide radical or hydrogen ion into hydrogen peroxide, which is scavenged by GSH-Px and CAT [44, 45]. GSH-Px plays an important role in reducing organic hydroperoxides such as lipid hydroperoxides to their corresponding alcohols or reducing free hydrogen peroxide to water [45]. The T-AOC is the comprehensive indicator for evaluating levels of antioxidant enzymes and the non-enzymatic system in animal organisms. It can reflect the compensatory capacity to external stimuli and the metabolism capacity of free radicals in organisms [44]. Decreased function of the T-AOC defense system cannot keep the antioxidant system active, leading to the abundance of lipid peroxides and free radicals. MDA is the most common product of lipid peroxidation, and its level can directly reflect the degree of lipid oxidative injury [44,44,46]. The expression level of NOS increases and a large amount of NO is released when the animal remains in an Se-deprived environment for a long time; the change in NO metabolism causes oxidative stress [47, 48]. In the present study, serum biochemical assays indicated that Se deficiency caused a decrease in the levels of GSH-Px, T-AOC, CAT, and SOD and an increase in the levels of MDA, LPO, T-NOS, and NO in the Se-deficient P. przewalskii, indicating that Se deprivation induced the dysfunction of the antioxidant system, disrupted the relative balance between the oxidant and antioxidant systems, and caused severe oxidative stress in P. przewalskii.
The immune system of animals is a defense system involved in immune response and immune function, which maintains the relative stability of the internal environment, including the adaptive and innate immune systems. Selenomethionine and selenocysteine are present in tissues and cells of the immune system, regulating immune functions in vivo through complex biological processes [49, 50]. When the animal is in a state of Se deprivation, the proliferation and differentiation of T and B lymphocytes is inhibited. The decrease in the levels of lymphokines leads to a decrease in the killing ability of natural killer cells and T cells in vitro, and the immune function of the body is significantly reduced [51, 52]. IL-1β is a polypeptide adjustment factor produced by mononuclear macrophages, which has an immunoregulatory function [53, 54]. IL-2 is an important growth factor in the development of T cells. It is produced by activated T cells and combines with the IL-2 receptor on the surface of T lymphocytes to stimulate T cell proliferation further [55, 56]. IL-6 is a lymphatic factor produced by activated T cells and fibroblasts. It can stimulate B cell precursor cells to produce antibodies and enhance the function of natural killer cells [55,55,57]. In the present study, the serum levels of IL-1β, IL-2, IL-6, and TNF-α were lower in the Se-deficient P. przewalskii, suggesting that Se deprivation weakened the immune function of P. przewalskii. IgG is produced mainly by plasma cells in the spleen and lymph nodes and has immune activity with antiviral and antibacterial effects [58]. Serotype IgA is the second most abundant immunoglobulin present in the blood; it can effectively engage polymorphonuclear cells via the interaction with the FcαRI receptor. The ligation of FcαRI by IgA-containing immune complexes can trigger antibody-dependent cell-mediated cytotoxicity by neutrophils, degranulation of eosinophils, and phagocytosis by monocytes and macrophages. Insufficient Se intake results in the decreased synthesis of immunoglobulins in animals [58,58,60]. Ashley et al. found that Se deprivation decreased the content of IgM in the colostrum of sows [61]. Quan et al. demonstrated that Se deprivation induced lower levels of IgA, IgG, and IgM in Keshan patients than in normal people [62]. Wang et al. reported that serum levels of IgG, IgA, and IgM significantly decreased in Se-deficient calves [63]. The present study found that the serum levels of IgA, IgG, and IgM significantly decreased in the Se-deficient P. przewalskii, indicating that Se deficiency led to insufficient immunoglobulin in P. przewalskii.
In conclusion, the findings indicated that Se deficiency in the soil and forage weakened antioxidant capacity and induced oxidative stress damage. It also significantly affected the levels of cytokines, thus posing a serious threat to the immune function of P. przewalskii. Therefore, to protect the remaining P. przewalskii populations and maintain the integrity of their ecological environment, it is extremely important to increase the Se nutrition of mixed pastures by applying Se-containing fertilizers and reseeding and increasing the proportion of plants with high Se concentration. Also, removing fences, establishing habitat corridors, expanding habitats, and increasing feeding areas may be beneficial to maintain the Se balance of P. przewalskii.
References
Li Z, Jiang Z (2008) Sexual segregation in Tibetan gazelle: a test of the activity budget hypothesis. Proc Zool Soc Lond 274(4):327–331
Lian XM, Li XX, Zhou DX, Yan PS (2012) Avoidance distance from Qinghai–Tibet highway in sympatric Tibetan antelope and gazelle. Transport Res Part D-Tr E 17(8):585–587
Bhatnagar YV, Wangchuk R, Mishra C (2006) Decline of the Tibetan gazelle, Procapra picticaudata in Ladakh, India. Oryx 40(02):229–232
Shen XY, Li X, Zhang RD (2010) Studies of “unsteady gait disease” of the Tibetan gazelle (Procapra picticaudata). J Wildl Dis 46(2):560–563
Zhang L, Liu JZ, Wang DJ, Wang H, Wu YL, Lü Z (2018) Fencing for conservation?—The impacts of fencing on grasslands and the endangered Przewalski’s gazelle on the Tibetan Plateau. Sci China Life Sci 61(12):145–147
Zhang F, Jiang Z (2006) Mitochondrial phylogeography and genetic diversity of Tibetan gazelle (Procapra picticaudata): implications for conservation. Mol Phylogenet Evolution 41(2):313–321
Li ZQ, Jiang ZG, Li CW (2008) Dietary overlap of Przewalski’s gazelle, Tibetan gazelle, and Tibetan sheep on the Qinghai-Tibet plateau. J Wildlife Manage 72(4):944–948
Huo B, Wu T, Song CJ, Shen XY (2019) Studies of selenium deficiency in the Wumeng semi-fine wool sheep. Biol Trace Elem Res 194(1):152–158. https://doi.org/10.1007/s12011-019-01751-1
Li XP, Li GP, Wang GH, Li PL, Wang GP, Jin YP, Jian YN (2018) Analysis of causes of copper and selenium deficiency in Sanjiaocheng sheep farms. Qinghai J Anim Sci Vet Med 48(06):38–39
Huo B, Wu T, Song CJ, She XY (2019) Effects of selenium deficiency in alpine meadow on antioxidant systems of yaks. China Anim Husb Vet Med 46(04):1053–1062
Shen XY, Huo B, Min XY, Wu T, Liao JJ (2018) Assessment of mineral nutrition of forage in the natural habitat of Przewalski’s gazelle (Procapra przewalskii). Acta Pratacul Sin 27(3):108–115
Chi YK, Huo B, Shen XY (2020) Distribution characteristics of selenium nutrition on the natural habitat of Przewalski’s gazelle. Pol J Environ Stud 29(1):67–77
Li Z, Beauchamp JG (2010) Nonrandom mixing between groups of Przewalski’s gazelle and Tibetan gazelle. J Mammal 91(3):674–680
Chi YK, Huang DH, Song SZ, Huo B, Wu T, Song CJ, Shen XY (2019) Effect of seasonal variation on mineral nutrient of forage in habitat of Przewalski’s gazelle (Procapra przewalskii). Fresenius Environ Bull 28(2A):1446–1453
Song CJ, Shen XY (2019) Effects of environmental zinc deficiency on antioxidant system function in Wumeng semi-fine wool sheep. Biol Trace Elem Res:1–7. https://doi.org/10.1007/s12011-019-01840-1
Chi YK, Zhang ZZ, Song CJ, Xiong KN, Shen XY (2020) Effects of fertilization on physiological and biochemical parameters of Wumeng sheep in China’s Wumeng prairie. Pol J Environ Stud 29(1):79–85
Liu KY, Liu HL, Zhang T, Mu LL, Liu XQ, Li GY (2019) Effects of vitamin E and selenium on growth performance, antioxidant capacity, and metabolic parameters in growing furring blue foxes (Alopex lagopus). Biol Trace Elem Res 192(2):183–195
Helder L, Egon H, Carolina R, Jimenez PS, Correa DB (2019) Effects of maternal dietary cottonseed on the profile of minerals in the testes of the lamb. Biol Trace Elem Res. https://doi.org/10.1007/s12011-019-01971-5
Huo B, Wu T, Song CJ, Shen XY (2020) Effects of selenium deficiency in the environment on antioxidant systems of Wumen semi-fine wool sheep. Pol J Environ Stud 29(2):1–9
Shen XY, Zhang J, Zhang RD (2014) Phosphorus metabolic disorder of Guizhou semi-fine wool sheep. PLoS One 9(2):e89472
Liu Z (2007) Effect of a copper, selenium and cobalt soluble glass bolus given to grazing yaks. Asian Austral J Anim Sci 20(9):1433–1437
Corbera JA, Morales M, Pulido M, Gutierrez C (2003) An outbreak of nutritional muscular dystrophy in dromedary camels. J Appl Anim Res 23(1):117–122
Grace ND, Knowles SO (2002) A reference curve using blood selenium concentration to diagnose selenium deficiency and predict growth responses in lambs. N Z Vet J 50(4):163–165
Zhang L, Jiao T, Zheng ZC, Liu CQ, Zhou XH, Feng RL (2005) Analysis of Se concentrations in study farm of Sanjiaocheng in Qinghai at different seasons. J Tradit Chin Vet Med 05:17–19
Shen XY, Huo B, Wu T, Song CJ, Chi YK (2019) iTRAQ-based proteomic analysis to identify molecular mechanisms of the selenium deficiency response in the Przewalski’s gazelle. J Proteome 203:103389
Zhang QJ, Zheng SF, Wang SC, Jiang ZH (2019) The effects of low selenium on DNA methylation in the tissues of chickens. Biol Trace Elem Res 191(2):474–484
Wu BY, Muhammad JM, Fang J, Peng X (2019) The protective role of selenium against AFB1-induced liver apoptosis by death receptor pathway in broilers. Biol Trace Elem Res 191(2):453–463
Petkova MTV, Ruseva BK, Atanasova BD (2017) Selenium deficiency as a risk factor for development of anemia. J Biomed Clin Res 10(1):9–17
Huo B, Wu T, Xiao H, Shen XY (2019) Effect of copper contaminated pasture on mineral metabolism in the Wumeng semi-fine wool sheep. Asian J Ecotoxicol 14(6):1–9
Emmanuelchide O, Charle O, Uchenna O (2011) Hematological parameters in association with outcomes in sickle cell anemia patients0. Indian J Med Sci 65(9):393–401
Saban C (2019) Effect of dietary vitamin E, selenium and their combination on concentration of selenium, MDA, and antioxidant enzyme activities in some tissues of laying hens. Pakistan J Zool 51:1155–1161
Aram S, Bahram DN, Siamak AR, Ehsan A (2019) Platelet selenium indices as useful diagnostic surrogate for assessment of selenium status in lambs: an experimental comparative study on the efficacy of sodium selenite vs selenium nanoparticles. Biol Trace Elem Res:1–9. https://doi.org/10.1007/s12011-019-01784-6
Han YH, Kim SU, Kwon TH, Lee DS, Ha HL (2012) Peroxiredoxin II is essential for preventing hemolytic anemia from oxidative stress through maintaining hemoglobin stability. Biochem Biophys Res Commun 426(3):427–432
Zhao J, Xing H, Liu C (2016) Effect of selenium deficiency on nitric oxide and heat shock proteins in chicken erythrocytes. Biol Trace Elem Res 171(1):208–213
Liao C, Hardison RC, Kenentt MJ (2018) Selenoproteins regulate stress erythroid progenitors and spleen microenvironment during stress erythropoiesis. Blood 131(23):2568–2580
Kaushal N, Hegde S, Lumadue J, Paulson RF, Prabhu KS (2011) The regulation of erythropoiesis byselenium in mice. Antioxid Redox Signal 14(8):1403–1412
Liao C, Carlson BA, Paulson RF (2018) The intricaterole of selenium and selenoproteins in erythropoiesis. Free Radical Bio Med 127(1):165–171
Huo B, Wu T, Chi YK, Min XY, Shen XY (2019) Effect of molybdenum fertilizer treatment to copper pollution meadow on copper metabolism in Wumeng semi-fine wool sheep. Acta Ecologiae Animalis Domastici 40(07):44–49
Zeng R, Muhammad UF, Zhang G, Tang ZC (2019) Dissecting the potential of selenoproteins extracted from selenium-enriched rice on physiological, biochemical and anti-ageing effects in vivo. Biol Trace Elem Res. https://doi.org/10.1007/s12011-019-01896-z
Wu L, Zhang H, Xu C, Xia C (2016) Critical thresholds of antioxidant and immune function parameters for Se deficiency prediction in dairy cows. Biol Trace Elem Res 172(2):320–325
Roman M, Jitaru P, Barbante C (2014) Selenium biochemistry and its role for human health. Metallomics 6(1):25–54
Chen M, Mahfuz S, Cui Y, Jia LY, Liu ZJ, Song H (2019) The antioxidant status of serum and egg yolk in layer fed with mushroom stembase (Flammulina velutipes). Pak J Zool 52:389–392
Herena YH, Naghum A, Marla JB, Lucia AS (2019) From selenium absorption to selenoprotein degradation. Biol Trace Elem Res 192(1):26–37
Huma N, Sajid A, Khalid A, Wardah H, Moazama B, Shakeela P, Sadia M, Sajida M (2019) Toxic effect of insecticides mixtures on antioxidant enzymes in different organs of fish, Labeo rohita. Pak J Zool 51:1355–1361
Iqra B, Moolchand M, Pershotam K, Saeed AS, Hira S (2019) Effect of dietary selenium yeast supplementation on morphology and antioxidant status in testes of young goat. Pak J Zool 51:979–988
Meng T, Liu YL, Xie CY (2019) Effects of different selenium sources on laying performance, egg selenium concentration, and antioxidant capacity in laying hens. Biol Trace Elem Res 189(2):548–555
Xu J, Gong Y, Sun Y (2019) Impact of selenium deficiency on inflammation, oxidative stress, and phagocytosis in mouse macrophages. Biol Trace Elem Res. https://doi.org/10.1007/s12011-019-01775-7
Cao C, Fan R, Chen M, Li XJ, Xing MY, Zhu FT (2017) Inflammatory response occurs in veins of broiler chickens treated with a selenium deficiency diet. Biol Trace Elem Res 183(2):1–9
Bakhshalinejad R, Reza AMK, Zoidis E (2018) Effects of different dietary sources and levels of selenium supplements on growth performance, antioxidant status and immune parameters in Ross 308 broiler chickens. Brit Poultry Sci 59(1):81–91
Michael TH, Paul RC (2019) New directions for understanding the codon redefinition required for selenocysteine incorporation. Biol Trace Elem Res 192(1):18–25
Pan TR, Liu TQ, Tan SR, Wan N, Zhang YM, Li S (2018) Lower selenoprotein T expression and immune response in the immune organs of broilers with exudative diathesis due to selenium deficiency. Biol Trace Elem Res 182(2):364–372
Amy S, Michelle J, Louise MCW, Amanda H (2010) Putative GTPase GIMAP1 is critical for the development of mature B and T lymphocytes. Blood 115(16):3249–3257
Mosmann T, Bond M, Coffman R, Ohara J, Paul W (1986) T-cell and mast cell lines respond to B-cell stimulatory factor-I. P Natl Acad Sci USA 83:5654–56628
Chang WC, Chen CH, Yu YM (2010) P385 chlorogenic acid attenuates adhesion molecules upregulation in IL-1β treated huvecs. Atherosclerosis Supp 11(2):98–98
Ahmed KP, Zhang YM, Hang Y, Teng XH, Li S (2018) Selenium deficiency affects immune function by influencing selenoprotein and cytokine expression in chicken spleen. Biol Trace Elem Res 187(2):506–516
Liu LN, Chen F, Qin SY, Ma JF, Li L, Jin TM, Zhao RL (2019) Effects of selenium-enriched yeast improved aflatoxin B1-induced changes in growth performance, antioxidation capacity, IL-2 and IFN-γ contents, and gene expression in mice. Biol Trace Elem Res 191(1):183–188
Michal K, Szabo P, Barbora D, Lukas L (2012) Upregulation of IL-6, IL-8 and CXCL-1 production in dermal fibroblasts by normal/malignant epithelial cells in vitro: Immunohistochemical and transcriptomic analyses. Biol Cell 104(12):738–751
Sackesen C, Veen W, Akdis M, Soyer O, Zumkehr J, Ruckert B (2013) Suppression of B-cell activation and IgE, IgA, IgG1 and IgG4 production by mammalian telomeric oligonucleotides. Allergy 68(5):593–603
Lopez AJR, Rueda CU, Patrucco L, Juan IR (2011) Selective IgA deficiency and multiple sclerosis déficit selectivo de IgA y esclerosis múltiple. Neurología 26(6):375–377
Launay P, Patry C, Lehuen A, Benoit P (1999) Alternative endocytic pathway for immunoglobulin A Fc receptors (CD89) depends on the lack of FcRy association and protects against degradation of bound ligand. J Biol Chem 274(11):7216–7225
Ashley G, Jeffrey C (2014) Effects of inorganic or organic selenium on immunoglobulins in swine. J Anim Sci Biotechno 4(2):47–47
Quan P, Tan W, Xu C (2004) The effects of selenium deficiency, oxidative stress, coxsackievirus B infection on the pathogenesis of Keshan disease. J Xi'an Med Univ Chinese Edition 02:22–29
Wang CR, Wang JQ, Zhao GQ, Zhou ZF, Wei HY (2009) Effects of supplementary vitamin E and selenium for cows on growth and immune of neonatal calves. Chinese J Vet Sci 12:3–14
Funding
This study was supported by the National Natural Science Foundation of China (41671041) and the Project of National Key Research and Development Program of China in the 13th five-year plan (2016YFC0502601).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huo, B., He, J. & Shen, X. Effects of Selenium-Deprived Habitat on the Immune Index and Antioxidant Capacity of Przewalski’s Gazelle. Biol Trace Elem Res 198, 149–156 (2020). https://doi.org/10.1007/s12011-020-02070-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02070-6