Abstract
The fencing device on pasture has seriously restricted the foraging range in grazing animals. As a result, the incidence of selenium (Se) deficiency is rising in grazing Choko yaks in the Shouqu prairie in Northwest China. To study the effect of Se deprivation on antioxidant capacity in the Choko yaks, the mineral contents in soil, forage, blood, and liver have been analyzed. The parameters of physiology and biochemistry in animal were also measured. The tested results showed that Se contents in soil and forage from tested pastures were very greatly lower than those in the control ranges (P < 0.01), and there were no extreme differences in other elements. Se contents in blood and the liver in tested animals were very extremely lower than those in the control yaks (P < 0.01). Levels of hemoglobin (Hb), erythrocyte (RBC), and hematocrit (HCT) were very extremely less than those in the control group (P < 0.01). Activities of glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), catalase (CAT), and total antioxidant capacity (T-AOC) in blood from the tested yaks were very much lower than those in the control animals (P < 0.01). Contents of malondialdehyde (MDA) in tested yaks were extremely higher than those in the control animals (P < 0.01). Therefore, it is suggested that Se-deficient forage in natural habitat not only influenced mineral contents in the blood and the liver but also causes serious harm to antioxidant function in the Choko yaks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Qinghai-Tibet Plateau is the most unique alpine meadow ecosystem of the highest altitude and the largest area in all over the world [1]. It is also the largest pasture land in China [1]. The Shouqu prairie is located in the first bend of the Yellow River in the eastern region of the Qinghai-Tibet Plateau, Northwest China [2]. It is the most beautiful natural ranch with the highest primary productivity in Asia [3]. The famous Hequ horse, Oula sheep, and Choko yak are born and bred in there [2,3,4].
The Choko yaks were vital to the production system in alpine grassland ecosystem in the Shouqu prairie in the eastern region of the Qinghai-Tibetan Plateau. The animals not only provide meat, wool, milk, and hides for local people but are also an indispensable means of transport in the Qinghai-Tibet Plateau [5]. In recent years, to improve the nomadic lifestyle and productivity in local herdsmen, the projects of settlement for herdsmen were implemented [6]. Pasture and livestock were distributed and contracted to individual families. More and more ranches have been fenced. The fencing devices on pasture have strictly restricted the foraging range and seriously interfere with nomadic behavior in grazing Choko yaks [7, 8]. All animals need graze on the same pasture throughout the year. As a result, selenium (Se) deficiency is rising in the Choko yak in the Shouqu prairie in the eastern region of the Qinghai-Tibet Plateau of China [5, 9]. Se element is an essential mineral nutrition in humans and animals. It occurs in selenoproteins, which is involved in antioxidant capacity, endocrine function, and immune modulation [10, 11]. It is a key element in the enzyme, glutathione peroxidase (GSH-Px), which can reduce lipoperoxides and hydrogen peroxide by catalyzing reduced glutathione (GSH) to oxidized glutathione (GSSG), thereby protecting the organism against oxidative damage [10,11,12]. Many researchers have reported Se deficiency in animals. Li et al. (2018) found that the Se contents in the blood, liver, and hair from Se-deprived Tibetan sheep were extremely lower than the reference levels in healthy sheep (P < 0.01) [13]. Huo et al. (2020) reported a pathema related to the Se-deficient pasture in yaks in the Hudong area in the Qinghai-Tibet Plateau, and more and more animals died in abortion or sudden death in Shouqu prairie, all of which were related to Se deprivation in pasture [14]. However, to date, no research has investigated the effects of Se-deprived pastures on antioxidant capacity in the Choko yaks.
The purpose of this research is to explore the relationships between Se-deficient pastures and blood parameter (mineral content, physiological index, biochemical indicator) and study the effects of Se-deprived pastures on antioxidant capacity in the Choko yaks.
Materials and Methods
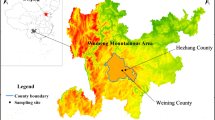
The tested pasture is located in the Shouqu prairie in the Qinghai-Tibet Plateau. The natural pasture is 145,000 km2. Geographical coordinates are 100°45′45″– 104°45′30″ E longitude and 33°06′30″–35°34′00″ N latitude. At an average elevation of 3100 m above sea level, the alpine meadows are the main vegetation types. The average annual total rainfall is 786 mm. Average temperature is 3.2 °C, and the lowest temperature recorded is − 40 °C. The tested and control pasture are located in this area with a wide range of temperatures between day and night, strong ultraviolet radiation, the frost-free period is short and the winter is long and cold [9].
Tested Design
Tested range is the Hequ farm in Maqu county, with a high incidence of Se-deficient signs in grazing Choko yaks, and the control range is the Azi animal husbandry experimental station in Maqu county, without Se-deficient signs in grazing Choko yaks.
Tested animals: Ten Choko yaks (1-year-old male yaks) with obvious Se-deficient signs were selected from the Hequ farm. Ten healthy Choko yaks (1-year-old male yaks) were selected from the Azi animal husbandry tested station as control group.
Sample Collection
On July 22, 2019, sixty soil samples were collected from each tested pasture and control pasture. Soil sample was collected in a quadrangle (1 m × 1 m), with an interval of 200 m. Each sample was 500 g.
On July 25, 2019, sixty mixed forage samples were collected from the tested ranges and the control ranges, and each sample was 500 g. Distributions of the forage samples were the same as the soil samples.
On July 26, 2019, twenty blood samples (20 mL) were collected from jugular vein in the animals of tested and control group. Blood samples were kept cool at 4–8 °C and reached the Lanzhou University animal nutrition lab within 4 h for analysis.
On July 29, 2019, twenty liver samples (about 100 mg) were collected. Before sample collection, animal platelets and clotting time were measured, and the puncture points were located by ultrasound. On the day of surgery, 10 mg of vitamin K was injected [15].
Sample Preparation
Samples of soil and forage were dried at 25–30 °C in air, crushed and sifted through 0.155 mm screen. The sample (0.5 g) was put into a digestive tube, and 6 mL of nitric acid (HNO3) and 1 mL of hydrogen peroxide (H2O2) were added. The sample was shaken well and sat quietly for 10 min. The samples were dissolved according to a microwave digestion program and transferred into a 100 mL of volumetric flask. The solution was diluted to scale and then marked. Samples of blood and the liver were dried at 25–30 °C in air to a constant weight; sample was added to a mixture (5:2:5) of nitric acid (HNO3), perchloric acid (HClO4), hydrofluoric acid (HF), and dissolved in a microwave [15, 16].
Sample Analysis
An atomic emission spectroscopy (HK9600, Huaketiancheng Co., Ltd, China) was used to measure the mineral contents of manganese (Mn), zinc (Zn), copper (Cu), molybdenum (Mo), Se, calcium (Ca), potassium(K), sodium (Na), and phosphorus(P) in soil, forage, blood, and the liver using inductively coupled plasma atomic emission spectroscopy (ICP-AES).
We have been used to analyzing the physiological parameters, including hemoglobin (Hb), white blood cell count (WBC), red blood cell count (RBC), and hematocrit value (HCT) by an automatic blood analyzer (Sysmex Poch-100i, Japan).
We have been used to analyzing the biochemical parameters, including alanine aminotransferase (ALT), albumin (ALB), alkaline phosphatase (AKP), lactate dehydrogenase (LDH), aminotransferase (AST), inorganic phosphorus (IP), glutathione peroxidase (GSH-Px), malondialdehyde (MDA), and total antioxidant capacity (T-AOC) using an automatic biochemical analyzer (Mindraybs-420, China). All assay kits were used according to their respective instructions and purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China).
Data Analysis
Tested data were analyzed by SPSS 23.0 for Windows and were expressed as mean ± standard deviation (S). P < 0.01 was considered a very extreme difference.
Results
Mineral Element Content in Soil and Forage
The main grassland type was swamp meadow, with the content of organic matter up to 43.87% and pH 5.6–6.3. Contents of Se in soil and forage from the tested pastures are extremely lower than those from the control ranges (P < 0.01), and there are no extreme difference in other mineral elements, as given in Table 1 for details.
Contents of Se in the blood and the liver from the Se-deficient Choko yaks are extremely lower than those in the healthy Choko yaks in control (P < 0.01), and there are no extreme differences in other mineral elements, as given in Table 2 for details.
Physiological Parameters
Levels of Hb, HCT, MCV, and MCH were extremely lower than those in the control yaks (P < 0.01), and there were no extreme differences in other physiological parameters. The above parameters indicate that the affected Choko yaks present with microcytic anemia, as given in Table 3 for details.
Biochemical Parameters
Levels of LDH, AST, and AKP are extremely higher than those in the control yaks (P < 0.01), and there are no extreme differences in other biochemical parameters, as given in Table 4 for details.
Antioxidant Capacities
The activities of GSH-Px, CAT, T-AOC, and SOD in the tested Choko yaks were very much lower than those in the control Choko yaks (P < 0.01), and MDA contents were very extremely higher than those in the control group (P < 0.01) (Table 5).
Discussion
Se contents in soil from the Hequ farm in the Shouqu prairie were very obviously lower than those in the healthy pasture. The swamp meadow was the main pasture type in the Shouqu prairie. The contents of organic matter in soil were 43.87% and pH = 5.6–6.3. Absorption and utilization of Se nutrient by plant will rapidly decrease in acidic soil which is rich in organic matter, and further very much aggravate the impact of Se deprivation on animals, which will inevitably cause Se deprivation in the grazing Choko yaks [17, 18]. During the long withering period, grazing Choko yaks obtain very little vitamin C, vitamin E, and carotene from the forage, and other nutrients are also very insufficient, extremely aggravating the symptoms of Se deprivation in the grazing Choko yaks [19,20,21].
The Se contents in blood and the liver were very much lower than those in the control yaks. The low contents of Se in blood and liver will extremely decrease the activity of GSH-Px. Se nutrients are a key component of GSH-Px; there are 4 g of Se/a gram of crystalline molecule [22, 23]. Peroxides are produced in the metabolism of organisms, and destroy the lipid membrane of cell and subcellular structures of the organism (mitochondria, centrosomes, and lysosomes), and cause rapidly cell degeneration and necrosis [24,25,26]. GSH-Px can catalyze the decomposition of peroxides and protects the cells and subcellular structures in animal organisms from peroxides [12]. When Se nutrient is deficient in organisms, the activity of GSH-Px will be very much reduced, and the peroxides generated in the life activities of the organisms will not be rapidly decomposed in time, resulting in very serious damage to the cell and subcellular structure of organisms, cell degeneration, and a series of severe pathological processes [12, 27, 28].
The antioxidant system of animal organisms is the defense system of the organisms for rapidly scavenging free radicals in mainly comprising non-enzymatic and enzymatic systems. The non-enzymatic systems include mainly vitamin E, vitamin C, cysteine, glutathione (GSH), Cu, Mn, Mo, Zn, Fe, and Se. The enzymatic system consists of SOD, GSH-Px, CAT, and other antioxidant enzymes [29,30,31,32,33,34]. The SOD is a kind of key metal enzyme which widely exists in aerobic organism (needs oxygen to metabolize) and plays a very key role in the oxidation and oxidation resistance of animal organism, and it is a key antioxidant too and can catalyze rapidly the superoxide anion (O2-) to produce disproportionation reaction, remove superoxide anion, and protect animal cells from damage from free radicals [30, 35, 36]. The GSH-Px is also a key antioxidant enzyme that catalyzes the decomposition of hydrogen peroxide (H2O2), and it is the key scavenger of H2O2 and ROOH [37, 38]. The CAT is a key antioxidant enzyme too, which can make H2O2 decompose into molecular oxygen and water, very clear H2O2 in animal organism, so as to protect animal cells from H2O2 [39, 40]. The T-AOC is the comprehensive parameter for evaluating capacities of antioxidant system in animal organisms [41]. It can rapidly reflect the compensatory capacity to external stimuli and the metabolism capacity of free radicals in organisms [41, 42]. Extremely decreased level of the T-AOC will very much increase the abundance of lipid peroxides and free radicals of animal organism [11, 24, 43]. The MDA is a lipid peroxide of organism, which can reflect rapidly the degree of lipid peroxidation in organism [41,42,43,44,45]. In this research, Se deprivation in animals will cause a very rapid decrease in the capacities of GSH-Px, T-AOC, CAT, and SOD and a very rapid increase in the content of MDA in the tested yaks. It indicates that Se deprivation in animal will induce obviously the dysfunction in the antioxidant system, rapidly disrupt the relative balance between the oxidant and antioxidant systems, and cause severe oxidative stress in the control yaks.
Conclusion
Based on the analysis of the mineral content in soil, forage and animal tissues, combined with the studied results of the blood parameters and antioxidant capacity, we can draw the following conclusions: Se deprivation of forage in natural ranges will not only influence the Se content in the blood and other tissues but also severely disrupt the blood parameters and antioxidant function in organism.
References
Fan JW, Shao QQ, Liu JY, Wang JB, Harris W (2010) Assessment of effects of climate change and grazing activity on grassland yield in the three rivers headwaters region of Qinghai–tibet plateau, china. Environ Monit Assess 170(1-4):571–584
Li Y (2009) Investigation on breed resources of hequ horse. Anim Husb Vet Sci Tech Info 6(06):132–134
Yan X, Cui TB (2002) Discussion on the relationship between some characters and ecological environment in Hequ horse. Pratacul Sci 19(06):71–77
Shen XY, Qin RF, Wan MCD (2007) Survey of variety resources in Hequ horse. China Herbivores 27(6):62–66
Shen XY, Jiang ZG (2008) Studies of dynamics of content of trace elements in herbage on Shouqu grassland in Yellow River. Chinese J Grassl 4:118–120
Cao JJ, Xiong YC, Sun J, Du XGZ (2011) Differential benefits of multi- and single-household grassland management patterns in the Qinghai-Tibetan Plateau of China. Human Ecology 39(2):217–227
Xiao YS (2009) Sulfur-induced copper deficiency in the yaks. Agricul Ences China 9(08):1000–1003
Shen XY, Du GZ, Chen YM (2006) Copper deficiency in yaks on pasture in western China. Can Vet J 47(9):902–906
Zhao K, Chi YK, Shen XY (2020) Studies on edema pathema in Hequ horse in the Qinghai-Tibet Plateau. Biol Trace Elem Res 198(1):142–148
Streeter RM, Divers TJ, Mittel L, Korn AE, Wakshlag JJ (2012) Selenium deficiency associations with gender, breed, serum vitamin E and creatine kinase, clinical signs and diagnoses in horses of different age groups: a retrospective examination 1996-2011. Equine Vet J 44(S43):31–35
Huo B, Wu T, Song CJ, Shen XY (2020) Effects of selenium deficiency in the environment on antioxidant systems of Wumen semi-fine wool sheep. Pol J Environ Stud 29(2):1649–1657
Shen XY, Huo B, Gan SQ (2020) Effects of nano-selenium on antioxidant capacity in Se-deprived Tibetan gazelle (Procapra picticaudata) in the Qinghai–Tibet Plateau. Biol Trace Elem Res. https://doi.org/10.1007/s12011-020-02206-8
Li XP, Li GP, Wang GH, Li PL, Wang GP, Jin YP, Jian YN (2018) Analysis of causes of copper and selenium deficiency in Sanjiaocheng sheep farms. Qinghai J Anim Sci Vet Med 48(06):38–39
Huo B, Wu T, Song CJ, She XY (2019) Effects of selenium deficiency in alpine meadow on antioxidant systems of yaks. China Anim Husb Vet Med 46(04):1053–1062
Shen XY, Song CJ, Wu T (2020) Effects of nano-copper on antioxidant function in copper-deprived Guizhou black goats. Biol Trace Elem Res. https://doi.org/10.1007/s12011-020-02342-1
Huo B, Shen XY (2020) Study of rickets and osteomalacia in Tibetan gazelle. Pak J zool 52(5):1751–1759
Chi YK, Huang DH, Song SZ (2019) Effect of seasonal variation in mineral nutrient of forage in habitat of Przewalski’s gazelle (procapra przewalskii). Fresen Environ Bull 28(2A):1446–1453
Luo JC, Mei Z, Wang ZL, Zhang YJ (2017) Fluxion and regulation of selenium in soil, plants, and animals in grassland grazing system. Pratacul Sci 34(4):869–880
Wu T, Song M, Shen XY (2020) Seasonal dynamics of copper deficiency in Wumeng semi-fine wool sheep. Bioll Trace Elem Res 197(1):487–494
Shen XY, Chi YK, Huo B (2018) Effect of fertilization on ryegrass quality and mineral metabolism in grazing the Wumeng semi-fine wool sheep. Fresen Environ Bull 27(10):6824–6830
Shen XY, Chi YK, Huo B, Xiong K (2019) Studies on phosphorus deficiency in the Qianbei-Pockmarked goat. Asian Austral J Anim 32(6):896–903
Fatmagül Y, Dede S, Deger Y, Kilicalp D (2008) Effects of vitamin E and selenium on serum trace and major elements in horses. Biol Trace Elem Res 125(3):223–228
Youssef MA, El-Khodery SA, Ibrahim HMM (2013) Effect of selenium and vitamin C on clinical outcomes, trace element status, and antioxidant enzyme activity in horses with acute and chronic lower airway disease. a randomized clinical trial. Biol Trace Elem Res 152(3):333–342
Huo B, Wu T, Song CJ, Shen X (2019) Studies of selenium deficiency in the Wumeng semi-fine wool sheep [J]. Biol Trace Elem Res 194(1):152–158
Adebayo OL, Adenuga GA, Sandhir R (2016) Selenium and zinc protect brain mitochondrial antioxidants and electron transport chain enzymes following postnatal protein malnutrition. Life Sci 152(1):145–155
Jin X, Xu Z, Zhao X (2017) The antagonistic effect of selenium on lead-induced apoptosis via mitochondrial dynamics pathway in the chicken kidney. Chemosphere 180(1):259–264
Chi YK, Huo B, Shen XY (2019) Distribution characteristics of selenium nutrition on the natural habitat of Przewalski’s gazelle. Polish J Environ Stud 29(1):67–77
Shen XY, Huo B, Wu T, Song C, Chi Y (2019) iTRAQ-based proteomic analysis to identify molecular mechanisms of the selenium deficiency response in the Przewalski’s gazelle. J Proteomics 203:103389
Huo B, He J, Shen XY (2020) Effects of selenium-deprived habitat on the immune index and antioxidant capacity of Przewalski’s Gazelle. Biol Trace Elem Res 198(1):149–156
Song CJ, Shen XY (2020) Effects of environmental zinc deficiency on antioxidant system function in Wumeng semi-fine wool sheep. Biol Trace Elem Res 195(1):110–116
Huo B, Wu T, Chi YK, Min XY, Shen XY (2019) Effect of molybdenum fertilizer treatment to copper pollution meadow on copper metabolism in Wumeng semi-fine wool sheep. Acta Ecologiae Animalis Domastici 40(07):44–49
Song CJ, Jiang Q, Shen XY (2020) Responses of Przewalski’s gazelle (Procapra przewalskii) to zinc nutrition in physical habitat. Biol Trace Elem Res. https://doi.org/10.1007/s12011-020-02137-4
Chen M, Mahfuz S, Cui Y, Jia LY, Liu ZJ, Song H (2019) The antioxidant status of serum and egg yolk in layer fed with mushroom stembase (Flammulina velutipes). Pak J Zool 52:389–392
Herena YH, Naghum A, Marla JB, Lucia AS (2019) From selenium absorption to selenoprotein degradation. Biol Trace Elem Res 192(1):26–37
Shen XY, Chi YK, Xiong KN (2019) The effect of heavy metal contamination on humans and animals in the vicinity of a zinc smelting facility. Plos One 14(10):e0207423
Huma N, Sajid A, Khalid A, Wardah H, Moazama B, Shakeela P, Sadia M, Sajida M (2019) Toxic effect of insecticides mixtures on antioxidant enzymes in different organs of fish, Labeo rohita. Pak J Zool 51:1355–1361
Chi YK, Xiong KN, Chen H, Min X, Xiao H, Liao J, Shen X (2019) Effect of grazing to copper pollution meadow on copper metabolism in Wumeng semi-fine wool sheep. Pol J Environ Stud 28(3):1083–1091
Iqra B, Moolchand M, Pershotam K, Saeed AS, Hira S (2019) Effect of dietary selenium yeast supplementation on morphology and antioxidant status in testes of young goat. Pak J Zool 51:979–988
Chi YK, Zhang ZZ, Song CJ, Xiong K, Shen X (2019) Effects of fertilization on physiological and biochemical parameters of Wumeng sheep in China’s Wumeng prairie [J]. Pol J Environ Stud 29(1):79–85
Song CJ, Gan SQ, Shen XY (2020) Effects of nano-copper poisoning on immune and antioxidant function in the Wumeng semi-fine wool sheep. Biol Trace Elem Res 198:515–520
Shen XY, Min XY, Zhang SH, Song C, Xiong K (2020) Effect of heavy metal contamination in the environment on antioxidant function in Wumeng semi-fine wool sheep in Southwest China. Biol Trace Elem Res 198:505–514
Shen XY, Song CJ (2020) Responses of Chinese merino sheep (junken type) on copper-deprived natural pasture. Biol Trace Elem Res. https://doi.org/10.1007/s12011-020-02214-8
Xu J, Gong Y, Sun Y (2019) Impact of selenium deficiency on inflammation, oxidative stress, and phagocytosis in mouse macrophages. Biol Trace Elem Res 194:237–243
Meng T, Liu YL, Xie CY (2019) Effects of different selenium sources on laying performance, egg selenium concentration, and antioxidant capacity in laying hens. Biol Trace Elem Res 189(2):548–555
Song CJ, Gan SQ, He J et al (2020) Effects of nano-zinc on immune function in Qianbei-Pockmarked Goats. Biol Trace Elem Res. https://doi.org/10.1007/s12011-020-02182-z
Funding
This work was supported by the Project of the National Natural Science Foundation of China (41671041) and the National Key Research and Development Program of China in 13th Five-Year Plan (2016YFC0502601).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, K., Huo, B. & Shen, X. Studies on Antioxidant Capacity in Selenium-Deprived the Choko Yak in the Shouqu Prairie. Biol Trace Elem Res 199, 3297–3302 (2021). https://doi.org/10.1007/s12011-020-02461-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02461-9