Abstract
Supplementation of selenium (Se) is a common practice in the poultry industry via sodium selenite (SS) and selenium yeast (SY), while the effects of nano-selenium (NS) on laying hens are poorly known. This study aimed to compare the effects of NS, SS, and SY on productivity; selenium (Se) deposition in eggs; and antioxidant capacity in laying hens. A total of 288 30-week-old Brown Hy-line laying hens were randomly assigned into four dietary treatments, which included corn-soybean meal basal diet (Con) without Se sources and basal diets supplemented with 0.3 mg Se/kg as SS, SY, or NS, respectively. The results exhibited that Se-supplemented treatments achieved greater egg production, egg weight, and daily egg mass, also better feed conversion ratio than Con group (p < 0.05). Se supplementation significant increased egg Se concentration and decreased the egg Se deposition efficiency (p < 0.05), while SY or NS supplementation had higher Se deposition efficiency than SS group at 35 days (p < 0.05). Moreover, serum glutathione peroxidase (GSH-Px) activity increased in SS or NS group compared to Con group (p < 0.05). The glutathione peroxidase 4 (GPX-4) mRNA levels in liver were significantly higher (p < 0.05) in SS or SY group than in NS group, and mRNA levels of the methionine (Met) metabolism gene glycine N-methyltranserfase (GNMT) were markedly upregulated (p < 0.05) in SY group compared to SS or NS group. Taken together, the results revealed Se from SY is deposited into eggs more efficiently than Se from NS or SS, probably via enhancing the route of Met metabolism. Meanwhile, it might be concluded that SS or SY supplementation directly regulated GSH-Px activity via enhancing GPx4 level, whereas NS via GPx1, thus affecting body oxidation and development.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As an essential trace element, selenium (Se) is vital to several biological processes in animals, such as antioxidant defense, immune function, reproduction, and thyroid hormone metabolism. Se carries out its biological effects within mammalian systems mainly through selenocysteine, which is incorporated into selenoproteins [1]. Se is involved in the formation of glutathione peroxidases (GSH-Px), and at least another seven selenoproteins may play a role in protection against oxidative stress and elimination of toxins associated with reactive oxygen species [2]. Supplementation of Se is a common practice in the poultry industry and has traditionally been added to poultry diets via inorganic sources (such as sodium selenite (SS)) and organic sources (such as selenium yeast (SY)). Recently, research has shown that SY results in less Se being transferred to the environment through feces, and more Se deposited into body tissues and eggs [3,4,5]. Nano-selenium (NS) appears to be more effective than other forms of Se at increasing selenoprotein expression, scavenging free radicals, and preventing oxidative damage to DNA in addition to providing benefits such as low toxicity and high bioavailability [6, 7]. In the small intestines, organic Se is actively absorbed through the amino-acid transport mechanisms, and NS has been reported to exhibit high specific surface area, small particle size, and good intestinal absorption due to the formation of nanoemulsion droplets [8].
Most studies showed that supplementation 0.3 mg of Se/kg of diet in laying hens will receive better egg Se deposition efficiency [9, 10]. Therefore, the main objective of this study was to compare the effects of Se supplementation in the form of NS, SY, and SS on productivity, Se deposition in eggs, and antioxidant capacity in laying hens over a 5-week period.
Materials and Methods
Ethics Statement
The methods used in this study were approved by the Animal Care Committee of the Institute of Subtropical Agriculture at the Chinese Academy of Science.
Birds and Management
A total of 288 Brown Hy-line laying hens of similar body size were selected from a commercial flock (Changsha County, Changsha City, China) at 30 weeks of age, and divided into four equal groups, for 35 days, each containing six replicates with 12 hens each. Prior to the start of the experiment, average egg production did not differ among treatment groups (p = 0.989) and was approximately 94%. Throughout the experimental period (March to April in 2017), hens were housed in pairs in 39 × 35 × 38 cm wire cages equipped with three ladders. Experimental units comprised of six wire cages were randomly distributed throughout the housing shed and were kept on a 16-h light and 8-h dark lighting regimen, with lights beginning at 06:00 local time [11]. Hens were fed twice daily (07:30 and 15:30) and allowed ad libitum access to water and treatment diets during the experimental period. Eggs were collected from egg trays and the total egg weight of each replicate was calculated once daily.
Diets
The four treatment diets used in the study were formulated by Se unsupplemented, corn-soybean meal basal diet (Con) and basal diets plus 0.3 mg/kg of Se from SS (analytical grade, 1% Se content, Xingjia Bio-Engineering Co., Ltd., Changsha, China), SY (2000 mg/kg Se content, Angel Yeast Co., Ltd., Hubei, China) or NS (180 mg/kg Se content, Xingjia Bio-Engineering Co., Ltd., Changsha, China). The particle size of NS ranged from 40 to 75 nm. The total analyzed Se concentrations of the Con, SS, SY, and NS diets were 0.157, 0.413, 0.422, and 0.408 mg Se/kg diet, respectively. The basal diet was formulated to meet nutritional requirements for brown laying hens suggested in the National Research Council (1994) and feeding standard of chickens (NY/T 33–2004). The ingredients and nutrient content of the basal diet are shown in Table 1.
The Concentration of Egg Se Analyses
Two eggs in each replicate were randomly collected on days 9, 18, 27, and 35 and stored until Se analysis. It was measured using a fluorescence spectrophotometer (AFS 830, Titan, Beijing, China). In short, 1 g of homogenized egg sample in 10-ml of HNO3-HClO4 (4:1) was added in to a 50-ml Erlenmeyer flask and heated at 180 °C until white fumes appeared. After which, 15 ml of 5 M hydrochloric acid solution was added to the flask and the mixture was again heated until white fumes appeared. Once cooled, the digested sample was transferred to a cuvette and ultrapure water was added to make a final volume of 25 ml. The supernatant was then measured directly on the spectrophotometer with the measured parameters follow: 270 V of negative high voltage, 30 mA of the current of hollow cathode lamp, 7 mm of electrothermal atomizer height, high pure Ar of carrier, 800 mL/min of carrier flow, 1.0 mL of injecting sample.
Measurement of Antioxidant Enzymes Activity and Product of Oxidative Injury
Total superoxide dismutase (T-SOD), glutathione peroxidase (GSH-Px), total antioxidant capability (T-AOC), catalase (CAT), and malondialdehyde (MDA) in serum and the liver were determined with the use of assay kits (Nanjing Jiancheng Bioengineering Institute, China) according to the manufacturer instructions.
cDNA Synthesis and mRNA Quantification
To quantify mRNA, approximately 100 mg of liver tissue was pulverized in liquid nitrogen and total RNA was isolated from the liver homogenate with the use of TRIzol and DNase I according to manufacturer instructions. First-strand cDNA was then synthesized using Oligo (dT) 20 and Superscript II reverse transcriptase. TRIzol, DNase I, and reverse transcriptase were obtained from Takara Bio Inc. (Kusatsu, Shiga, Japan). Primers were designed in NCBI using the chick gene sequence (http://www.ncbi.nlm.nih.gov/pubmed/) to produce an amplification product (Table 2). Real-time PCR was then performed as previously described. The relative level of mRNA expression was calculated using the 2-ΔΔCt method after normalization with β-actin as a housekeeping gene [12].
Statistical Analyses
This study was performed using a completely randomized design. All statistical analyses were performed using SPSS 17.0 software. All data are shown as mean ± standard error of the mean (SEM). Significant differences among treatment means were determined using one-way analysis of variance (ANOVA) followed by Duncan’s multiple-ranges test. Results were considered significant when p < 0.05. Laying performance and laying ratios were average values over 5 weeks.
Results
Laying Performance
As shown in Table 3, Se supplementation significantly increased the average egg production, average daily egg mass, and decreased feed conversion ratios (p < 0.05). Also, hens in SY group increased markedly the average daily egg mass than those in NS group (p < 0.05). Meanwhile, supplementation with SS and NS significantly increased the average egg weight when compared with Con or SY group (p < 0.05).
Egg Se Concentration and Se Deposition Efficiency
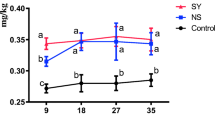
The egg Se concentration was presented in Fig. 1. Egg in SY group had higher Se concentration than those in Con or SS group at 9 days, 18 days, 27 days, and 35 days (p < 0.05). Meanwhile, egg in NS group had higher Se concentration than those in Con group at 18 days, 27 days, and 35 days (p < 0.05), while egg in SS group had higher Se concentration than those in Con group at 18 days only (p < 0.05).
The egg Se deposition efficiency in Con group was significantly higher (p < 0.001) than SS, SY, or NS group at 9, 18, 27, and 35 days (Table 4). The hens supplemented with SY or NS had higher (p < 0.05) Se deposition efficiency than SS group at 35 days. Meanwhile, the SY diet tended to increase Se deposition efficiency compared to SS or NS diet at 27 and 35 days, respectively (increase 11.83%, p = 0.067; increase 26.06%, p = 0.065).
Antioxidant Capacity in Serum and Liver
The effects of dietary supplementation with different Se sources on GSH-Px activity, T-SOD activity, CAT activity, T-AOC, and MDA content in serum and liver are shown in Table 5. Serum T-AOC and CAT activities in SY group were significantly higher than those in other groups (p < 0.05). In addition, supplementation with SS or NS led to significant increase in serum GSH-Px activity compared to that in Con group (p < 0.05). And the MDA content in NS or SY group was significantly lower than that in SS group (p < 0.05). However, SY supplementation led to significant increase in hepatic T-SOD activity compared to SS supplementation (p < 0.05).
Antioxidant Enzymes Genes mRNA Levels in Liver
On the basis of above results, the Cu/Zn-SOD, CAT, GPX-1, and GPX-4 mRNA levels in liver were investigated. The mRNA level of GPX-4 in liver was significantly upregulated in SY or SS group when compared to NS group (p < 0.05) (Table 6).
Methionine Metabolism Genes mRNA Levels in Liver
Several methionine (Met) metabolism genes (Mat1α, GNMT, Ahcy, BHMT, Mtr or CBS) in liver were tested via RT-PCR. As shown in Table 7, the mRNA level of GNMT in liver was significantly upregulated in SY group compared to SS or NS group (p < 0.05), while was markedly downregulated in NS group compared to Con or SY group (p < 0.05). No significant differences were observed among treatment groups in terms of Mat1α, Ahcy, BHMT, Mtr or CBS mRNA levels in liver.
Discussion
In the present study, the results showed that supplementing with SS, SY, or NS can improve laying performance, egg weight, and daily egg mass in hens compared to those observed in the low-Se group. Similarly, Han showed that the combined supplementation of SS or SY improved egg production [13]. Pavlović reported that supplementation of SY resulted in a higher egg production of hens than SS from the ninth week on to the end of the trial [14]. As shown in the results of this study, supplementation with 0.3 mg Se/kg of SS, SY, or NS was beneficial for the performance of laying hens. It is contrast to the results that dietary supplementation with different levels and sources of Se (0.18 to 0.51 mg/kg of SS, SY, Se-enriched kale sprouts, or other organic Se) had no effect on egg production or feed intake [3, 4, 15,16,17,18,19]. A potential reason for such discrepancies may be due to differences in the duration and process control of the experiments.
Selenium supplementation with SS, SY, or NS sources increased Se concentration in eggs, which is consistent with the findings from previous studies [5, 15, 16]. Moreover, SY was found to be more effective in increasing Se concentration than SS, which is also consistent with the results reported by Lu, et al., who showed that the egg Se concentration was significantly higher in the SY-supplemented group than the SS-supplemented group after 3 days [20]. Utterback also noted that the SY-supplemented diet yielded an approximate 4.8-fold increase in egg Se concentration over a Con diet, compared with a 2.8-fold increase with the SS diet after 56 days [16]. Notably, our results indicated that the egg Se efficiency in SY or NS group was higher than in SS. It is likely that organic sources of Se, such as SY, can be absorbed by active transport and nonspecifically incorporated into proteins in place of Met, and is preferentially absorbed and utilized by the body over inorganic Se [21].
Selenoenzymes appears to be GSH-Px which works as an antioxidant by removing hydrogen peroxides and organic hydroperoxides. Adequate intake of bioavailable forms of Se is therefore critical for maintaining appropriate Se and antioxidant levels in animals [22]. It has previously been reported that organic, inorganic, or Nano-Se affects GSH-Px activity [23,24,25,26]. The present study showed that dietary SS or NS supplementation led to significant increases in serum GSH-Px activity compared to that in Con or SY group, and GSH-Px activity in hens supplemented with NS was higher than that in other groups. Those results are consistent with previous studies. Previous work has suggested that SS may be more biologically available for GSH-Px activity than SY [10, 27], whereas GSH-Px activity in NS-supplemented group of broilers was significantly higher than those in basal diet group [26, 28]. Several studies reported that no difference was observed in GSH-Px activity of liver and kidney in broiler breeders and their offspring when feeding SY- or SS-supplemented diet [29], which is consistent with the present study. Meanwhile, the results from the present study found that the Se sources significantly affected T-AOC and CAT activities and MDA content in serum. Jing, et al. showed that hens fed diets supplemented with SS or SY showed lower MDA content in plasma than basal diet group [25]. Therefore, Se supplementation can improve the antioxidant capacity of laying hens, thereby increasing anti-stress ability and ensuring the maintenance of egg laying performance.
To further explain the differences of antioxidant capacity in serum and the liver in four groups, the mRNA levels of Cu/Zn-SOD, CAT, GPX-1, and GPX-4 were investigated. The results revealed that GPx1 mRNA level in liver was the greatest in NS group, whereas GPx4 mRNA level decreased, which increased in SS or SY group compared with Con diet. Similarly, Chen, et al. reported that organic Se or SS supplementation led to significant increase in GPX-4 mRNA levels compared to those of the control group in broilers [30]. Therefore, it might be concluded that SS or SY supplementation directly regulated GSH-Px activity via GPx4, whereas NS supplementation via GPx1, thus affecting body oxidation and development. Meanwhile, the expressions of Cu/Zn-SOD and CAT were not markedly changed in the liver of the Se sources treated hens. A previous study showed that a Se-deficient diet caused a significant decrease in mRNA expression for SOD, but Se supplementation increased the expression of SOD in tissues [31]. Thus, it may be explained that Se supplementation just decreased oxidative stress via the transcription level of GPx1 or GPx4 in the liver of hens.
Selenomethionine, a Se analog of Met, is the predominant form of Se in SY, and is metabolized along with Met by the same enzymes and at similar rates until selenocysteine is formed [22]. In our study, the mRNA level of the Met metabolism gene GNMT in liver significantly was upregulated in SY group compared to SS- or NS-supplemented group, which indicated that SY may affect Met metabolism, and ultimately influence the egg Se concentration. In contrast, inorganic Se sources are passively absorbed into the body and typically have lower rates of absorption [32]. Nanoparticles, due to smaller particle size, have larger surface areas, can penetrate the tissue gap, and also move through the smallest capillaries, and thus resulting in beneficial absorption [8]. One of the possible mechanisms of nano-Se action could be mediated by the gut microbiota which could convert nano-Se into selenite, H2Se or Se-phosphate with the synthesis of selenoproteins [33]. Therefore, it may explain that why supplementing with SY is more effective for egg Se deposition than supplementing with SS and NS. Further studies need to be performed to reveal the underlying mechanisms of NS in bird nutrition.
Conclusions
The present results showed that dietary supplementation with SS, SY, or NS improved laying performance, antioxidant capacity, and Se concentration in eggs of laying hens. Further, SY was most effective in increasing egg Se concentration, probably via influencing Met metabolism. Meanwhile, it might be concluded that SS or SY supplementation directly regulating GSH-Px activity via upregulating GPx4 level, whereas NS supplementation maybe via increasing GPx1 level, thus affecting body oxidation and development.
References
Holben DH, Smith AM (1999) The diverse role of selenium within selenoproteins. J Am Diet Assoc 99:836–843. https://doi.org/10.1016/s0002-8223(99)00198-4
Yang Z, Liu C, Liu C, Teng X, Li S (2016) Selenium deficiency mainly influences antioxidant selenoproteins expression in broiler immune organs. Biol Trace Elem Res 172:209–221. https://doi.org/10.1007/s12011-015-0578-y
Jiakui L, Xiaolong W (2004) Effect of dietary organic versus inorganic selenium in laying hens on the productivity, selenium distribution in egg and selenium content in blood, liver and kidney. J Trace Elem Med Biol 18:65–68. https://doi.org/10.1016/j.jtemb.2004.04.002
Payne RL, Lavergne TK, Southern LL (2005) Effect of inorganic versus organic selenium on hen production and egg selenium concentration. Poult Sci 84:232–237. https://doi.org/10.1093/ps/84.2.232
Pan CL, Huang KH, Zhao YX, Qin SY, Chen F, Hu QH (2007) Effect of selenium source and level in hen’s diet on tissue selenium deposition and egg selenium concentrations. J Agric Food Chem 55:1027–1032. https://doi.org/10.1021/jf062010a
Peng D, Zhang J, Liu Q, Taylor EW (2007) Size effect of elemental selenium nanoparticles (Nano-Se) at supranutritional levels on selenium accumulation and glutathione S-transferase activity. J Inorg Biochem 101:1457–1463. https://doi.org/10.1016/j.jinorgbio.2007.06.021
Zhang J, Wang X, Xu T (2008) Elemental selenium at nano size (Nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: comparison with se-methylselenocysteine in mice. Toxicol Sci 101:22–31. https://doi.org/10.1093/toxsci/kfm221
Hu CH, Li YL, Xiong L, Zhang HM, Song J, Xia MS (2012) Comparative effects of nano elemental selenium and sodium selenite on selenium retention in broiler chickens. Anim Feed Sci Technol 177:204–210. https://doi.org/10.1016/j.anifeedsci.2012.08.010
Chantiratikul A, Chinrasri O, Chantiratikul P (2018) Effect of selenium from selenium-enriched kale sprout versus other selenium sources on productivity and selenium concentrations in egg and tissue of laying hens. Biol Trace Elem Res 182:105–110
Leeson S, Namkung H, Caston L, Durosoy S, Schlegel P (2008) Comparison of selenium levels and sources and dietary fat quality in diets for broiler breeders and layer hens. Poult Sci 87:2605–2612. https://doi.org/10.3382/ps.2008-00174
Liu Y, Lin X, Zhou X, Wan D, Wang Z, Wu X, Yin Y (2017) Effects of dynamic feeding low and high methionine diets on egg quality traits in laying hens. Poult Sci 96:1459–1465. https://doi.org/10.3382/ps/pew398
Xie C, Wu X, Long C, Wang Q, Fan Z, Li S, Yin Y (2016) Chitosan oligosaccharide affects antioxidant defense capacity and placental amino acids transport of sows. BMC Vet Res 12:243. https://doi.org/10.1186/s12917-016-0872-8
Han XJ, Qin P, Li WX, Ma QG, Ji C, Zhang JY, Zhao LH (2017) Effect of sodium selenite and selenium yeast on performance, egg quality, antioxidant capacity, and selenium deposition of laying hens. Poult Sci 96:3973–3980
Pavlović Z, Miletić I, Jokić Ž, Šobajić S (2009) The effect of dietary selenium source and level on hen production and egg selenium concentration. Biol Trace Elem Res 131:263–270. https://doi.org/10.1007/s12011-009-8369-y
Paton ND, Cantor AH, Pescatore AJ, Ford MJ, Smith CA (2002) The effect of dietary selenium source and level on the uptake of selenium by developing chick embryos. Poult Sci 81:1548–1554. https://doi.org/10.1093/ps/81.10.1548
Utterback PL, Parsons CM, Yoon I, Butler J (2005) Effect of supplementing selenium yeast in diets of laying hens on egg selenium content. Poult Sci 84:1900–1901. https://doi.org/10.1093/ps/84.12.1900
Chantiratikul A, Chinrasri O, Chantiratikul P (2008) Effect of sodium selenite and zinc-L-selenomethionine on performance and selenium concentrations in eggs of laying hens. Asian Australas J Anim Sci 21:1048–1052. https://doi.org/10.5713/ajas.2008.70576
Chantiratikul A, Chinrasri O, Chantiratikul P (2017) Effect of selenium from selenium-enriched kale sprout versus other selenium sources on productivity and selenium concentrations in egg and tissue of laying hens. Biol Trace Elem Res 182:105–110. https://doi.org/10.1007/s12011-017-1069-0
Bennett DC, Cheng KM (2010) Selenium enrichment of table eggs. Poult Sci 89:2166–2172. https://doi.org/10.3382/ps.2009-00571
Lu J, Qu L, Shen MM, Hu YP, Guo J, Dou TC, Wang KH (2018) Comparison of dynamic change of egg selenium deposition after feeding sodium selenite or selenium-enriched yeast. Poult Sci 97:3102–3108
Schrauzer GN (2003) The nutritional significance, metabolism and toxicology of selenomethionine. Adv Food Nutr Res 47:73–112. https://doi.org/10.1016/s1043-4526(03)47002-2
Petrovič V, Boldižárová K, Faix Š, Mellen M, Arpášová H, Leng L (2006) Antioxidant and selenium status of laying hens fed with diets supplemented with selenite or Se-yeast. J Anim Feed Sci 15:435–444. https://doi.org/10.22358/jafs/66914/2006
Qin SY, Chen F, Guo YG, Huang BX, Zhang JB, Ma JF (2014) Effects of nano-selenium on kindey selenium contents, glutathione peroxidase activities and GPx-1 mRNA expression in mice. Adv Mater Res 1051:383–387 https://doi.org/10.4028/www.scientific.net/AMR.1051.383
Cantor AH, Moorhead PD, Musser MA (1982) Comparative effects of sodium selenite and selenomethionine upon nutritional muscular-dystrophy, selenium-dependent glutathione-peroxidase, and tissue selenium concentrations of Turkey poults. Poult Sci 61:478–484. https://doi.org/10.3382/ps.0610478
Jing CL, Dong XF, Wang ZM, Liu S, Tong JM (2015) Comparative study of DL-selenomethionine vs sodium selenite and seleno-yeast on antioxidant activity and selenium status in laying hens. Poult Sci 94:965–975. https://doi.org/10.3382/ps/pev045
Boostani A, Sadeghi AA, Mousavi SN, Chamani M, Kashan N (2015) Effects of organic, inorganic, and nano-Se on growth performance, antioxidant capacity, cellular and humoral immune responses in broiler chickens exposed to oxidative stress. Livest Sci 178:330–336. https://doi.org/10.1016/j.livsci.2015.05.004
Mahan DC, Parrett NA (1996) Evaluating the efficacy of selenium-enriched yeast and sodium selenite on tissue selenium retention and serum glutathione peroxidase activity in grower and finisher swine. J Anim Sci 74:2967–2974. https://doi.org/10.2527/1996.74122967x
Zhou X, Wang Y (2011) Influence of dietary nano elemental selenium on growth performance, tissue selenium distribution, meat quality, and glutathione peroxidase activity in Guangxi yellow chicken. Poult Sci 90:680–686. https://doi.org/10.3382/ps.2011-01921
Yuan D, Zhan XA, Wang YX (2012) Effect of selenium sources on the expression of cellular glutathione peroxidase and cytoplasmic thioredoxin reductase in the liver and kidney of broiler breeders and their offspring. Poult Sci 91:936–942. https://doi.org/10.3382/ps.2010-00977
Chen F, Zhu L, Qiu H, Qin S (2016) Selenium-enriched Saccharomyces cerevisiae improves growth, antioxidant status and selenoprotein gene expression in Arbor Acres broilers. J Anim Physiol Anim Nutr (Berl) 101:259–266. https://doi.org/10.1111/jpn.12571
Kurz B, Jost B, Schünke M (2002) Dietary vitamins and selenium diminish the development of mechanically induced osteoarthritis and increase the expression of antioxidative enzymes in the knee joint of STR/1N mice. Osteoarthr Cartil 10:119–126. https://doi.org/10.1053/joca.2001.0489
Reasbeck PG, Barbezat GO, Weber FL Jr, Robinson MF, Thomson CD (1985) Selenium absorption by canine jejunum. Dig Dis Sci 30:489–494
Surai PF, Kochish II, Velichko OA (2017) Nano-Se assimilation and action in poultry and other monogastric animals: is gut microbiota an answer? Nanoscale Res Lett 12:612. https://doi.org/10.1186/s11671-017-2383-3
Acknowledgements
This research received financial support from national key research and development program of China (2016YFD0501200, 2016YFD0200900, 2016YFD0500500), Agricultural innovation project of Hunan Province (2017YC03) and Science and Technology Service Network Initiative program of Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The methods used in this study were approved by the Animal Care Committee of the Institute of Subtropical Agriculture at the Chinese Academy of Science.
Rights and permissions
About this article
Cite this article
Meng, T., Liu, Yl., Xie, Cy. et al. Effects of Different Selenium Sources on Laying Performance, Egg Selenium Concentration, and Antioxidant Capacity in Laying Hens. Biol Trace Elem Res 189, 548–555 (2019). https://doi.org/10.1007/s12011-018-1490-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1490-z