Abstract
We evaluated the effect of lead (Pb) and ascorbic acid treatment of pregnant female rats on cerebellar development in pups. Pb was administered in drinking water (0.2% Pb acetate), and ascorbic acid (100 mg/kg) was administered through oral intubation. Fifteen female rats were randomly classified into control, Pb, and Pb plus ascorbic acid (PA) groups. The treatment of Pb and ascorbic acid treatments were terminated after birth to evaluate the effects on the gestational development of the cerebellum. At postnatal day 21 (PND21), pups were sacrificed, and blood Pb level was analyzed. Blood Pb levels of pups and dams were highest in the Pb group and reduced in the PA group. Immunohistochemistry and immunoblot assays were conducted to study the cerebellar expression levels of synaptic proteins. Along with a significant reduction in Purkinje cells, the reduction in presynaptic (synaptophysin) and postsynaptic (postsynaptic density protein 95, N-methyl-d-aspartate receptor subtype 1) marker proteins was observed in Pb-exposed pups. Ascorbic acid treatment significantly prevented Pb-induced impairment in the cerebellar synaptic proteins. Hypothesizing that brain-derived neurotrophic factor (BDNF) might be affected by Pb exposure given its importance in the regulation of synaptogenesis, we observed a Pb-induced decrease and ascorbic acid-mediated increase of BDNF in the cerebellum. Luxol fast blue staining and myelin basic protein analysis suggest that ascorbic acid treatment ameliorated the Pb exposure-induced reduction in the axonal fibers in the developing cerebellum. Overall, we conclude that ascorbic acid treatment during pregnancy can prevent Pb-induced impairments in the cerebellar development in rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since ancient times, humans have used lead (Pb) for its malleable, ductile characteristics, low melting point, and sweet taste. The use of Pb in petrol in industrial times has significantly increased the risk of Pb exposure [1]. Although regulations have successfully reduced the amount of Pb in gasoline, Pb is still a threat to human health [2]. Environmentally, Pb is widely dispersed in water, soil, and the atmosphere because of the incineration of Pb-containing waste and the continued widespread use of Pb-containing products, such as batteries, solder in food cans, ceramic glazes, cosmetics, toys, pigments, and water pipelines [1,2,3,4]. Though Pb is noxious to people of all ages, the highest risk of complications in children, for whom no safe levels of Pb exist [3]. In particular, the immature brain is vulnerable to Pb-induced neurotoxicity because of the still developing blood-brain barrier and astrocytes. Prenatal Pb exposure increases the risk of developing mental illnesses such as schizophrenia and attention deficit hyperactivity disorder [5, 6]. In a study in monkeys, 23 years of Pb exposure from infancy increased the chances of developing Alzheimer’s disease pathology [7].
Synaptophysin is expressed in the presynaptic vesicular membrane as integral protein [8]. Synaptophysin is an ideal synaptic marker and is involved in synaptogenesis, synaptic neurotransmitter release, and synaptic plasticity [9, 10]. Synaptic impairment in neurodegenerative diseases is characterized by a reduction in synaptophysin [11]. Postsynaptically, the N-methyl-d-aspartate receptor (NMDAR) is linked with postsynaptic density protein 95 (PSD95), which is involved in neurotransmission and signal conduction. Many studies have suggested that expression levels of pre- and postsynaptic proteins are correlated, and these proteins are involved in the processes of learning and memory formation in the hippocampus [12, 13]. Pb exposure diminishes the synaptic synaptophysin, PSD-95, and NMDAR in the hippocampus, and this reduction is associated with the learning and memory impairments [14, 15]. Therefore, we hypothesized that Pb exposure may also interfere with the normal development of cerebellar synapses by affecting pre- and postsynaptic proteins. As newly generated neurons mature, their dendrites, perikarya, and axons participate in synapse formation. In the developing cerebellum, proper synaptogenesis along the inhibitory Purkinje cells and excitatory granule cells is important for the elaborate regulation of balance, motor coordination, and motor learning.
Pb-induced encephalopathy has been reported in adults and children [16]. Low and high doses of Pb exposure alter the development of two major neuron types in the rat cerebellum: Purkinje cells and granule cells [17, 18]. Additionally, ascorbic acid has been reported to act as an effective remedy for Pb-induced toxicity in several body systems. Ascorbic acid directly enhances the excretion of Pb and prevents Pb-induced oxidative stress by acting as antioxidant [19, 20]. In previous studies, ascorbic acid was effective in detoxifying the Pb-induced impairments in hippocampus and cerebellum [18, 19]. However, the effects of ascorbic acid on Pb-induced changes in synaptic protein expression and axonal development in the developing cerebellum have not been clearly determined. Therefore, to investigate the beneficial effects of ascorbic acid, we examined the synaptic proteins (synaptophysin, PSD95, and NMDAR1) and axonal myelin basic protein (MBP) in the developing cerebella of the pups from Pb-exposed pregnant rats.
Materials and Methods
Experimental Animals
All procedures involving animal models and treatments were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals issued by the Institute of Laboratory Animal Resources, USA, 1996, and a protocol for it was approved by the Experimental Animal Laboratory and the Animal Care and Use Committee of the Konkuk University School of Veterinary Medicine. Female (n = 15) and male (n = 5) Sprague-Dawley (SD) rats (8 weeks old) were purchased from Koatech (Pyeongtaek, Gyeonggi-do, Korea) and they were housed under conditions of adequate temperature (23 °C) and humidity (60%) with a 12-h light/12-h dark cycle. After 1-week acclimation to the animal facility, rats were mated. Pregnancy was determined by confirming the presence of sperm in vaginal smears (or vaginal plugs), designating onset of pregnancy as day 0. Drug treatment to dams was started from day 0 until the end of the gestational period.
Drug Treatment
Pregnant SD female rats were randomly divided into three groups; the control group (n = 5), Pb group (n = 5), and Pb plus ascorbic acid (PA) group (n = 5). The pregnant rats were housed individually in the cage. The doses of Pb acetate and ascorbic acid were the same as in previous studies [18, 19]. Because people from ancient Rome to modern times have been exposed to Pb in water [2, 21], we administered Pb to rats via their drinking water. Accordingly, Pb acetate (0.2%, Sigma-Aldrich, USA) was dissolved in distilled water with glacial acetic acid (0.05%, Junsei Chemical Co., Tokyo, Japan) to prevent Pb precipitation. Ascorbic acid (100 mg/kg, Sigma-Aldrich) was freshly prepared in physiological saline for oral administration. To account for the effect of stress due to the process of oral intubation, rats in the control and Pb groups were orally administered the same volume of saline. Pb and ascorbic acid treatment was stopped from the time of the delivery of the pups until the end of the experiment on postnatal day (PND) 21. To exclude the effect of litter size on body weight gain, we selected 6 rat pups per dam. Body weight of pups (n = 24 per group) was measured on the last day of the experiment.
Luxol Fast Blue Stain and Immunohistochemistry
For histological analysis, the pups (n = 12 per group) were randomly selected and anesthetized on PND21 with ketamine (50 mg/kg) and xylazine (1 mg/kg). Then, the animals were perfused transcardially with heparinized phosphate-buffered saline (PBS, 0.1 M, pH 7.4), followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The rat cerebella were removed and postfixed in the same fixative overnight at 4 °C. Tissues were embedded in paraffin blocks and cut into 5-μm sections at multiple levels. Three slides per pup were selected, and a total of 36 sections per group were used in histological analysis. Luxol fast blue and hematoxylin and eosin (HE) staining was conducted using routine procedures. Briefly, to detect myelinated axonal fibers in the cerebellar cortex, deparaffinized sections were incubated in 0.1% Luxol fast blue solution in a 56 °C oven overnight. Then, sections were washed in graded ethyl alcohol and differentiated in a 0.05% lithium carbonate solution.
To detect synaptic proteins in the cerebellar cortex, immunohistochemistry for presynaptic synaptophysin and postsynaptic PSD95 and NMDAR1 was conducted. Additionally, to compare the effects of Pb and ascorbic acid on the development of myelinated axonal fibers, immunohistochemistry for myelin basic protein (MBP) was conducted. Briefly, deparaffinized sections were processed for antigen retrieval using citrate buffer (pH 6.0). These sections were sequentially treated with 0.3% hydrogen peroxide (H2O2) for quenching of endogenous peroxidase activity and 5% normal horse serum for blocking. Then, sections were incubated with primary antibodies against synaptophysin (rabbit, 1:1000, Abcam, Cambridge, UK), PSD95 (rabbit, 1:1000, Abcam), NMDAR1 (rabbit, 1:500, Millipore, Billerica, MA, USA), brain-derived neurotrophic factor (BDNF, rabbit, 1:500, Proteintech, IL, USA), or MBP (rabbit, 1:3000, Millipore) overnight at 4 °C. Subsequently, sections were exposed to biotinylated horse anti-rabbit IgG (1:400, Vector, Burlingame, CA, USA) and streptavidin peroxidase complex (1:400, Vector). They were then visualized by reaction to 3,3′-diaminobenzidine tetrachloride (DAB, Sigma) in 0.1 M Tris-HCl buffer (pH 7.2), counterstained with hematoxylin. Following dehydration and clearing, the sections were mounted in toluene-based mounting medium (Richard-Allan Scientific, Thermo Scientific).
All histopathological analyses described henceforth were performed by an investigator blind to rat treatment. The numbers of Purkinje cells were counted in micrographs obtained at × 100 magnification. The observations were carried out in two lobules (5th and 8th lobules) from the sagittal section of the cerebellar vermis [22].
Atomic Absorption Spectrometry for Blood Pb Level Analysis
At PND21, remaining pups (n = 12 per group) and dams were anesthetized with ketamine (50 mg/kg) and xylazine (1 mg/kg). Blood samples for Pb level analysis were collected through cardiac puncture. The measurement was conducted by an atomic absorption spectrophotometer (Perkin Elmer Zeeman 5100, Norwalk, USA) using an HGA-600 graphite furnace with Zeeman background correction. The absorption wavelength was 283.3 nm, and the r2 of a calibration curve was at least 0.995.
Western Blot Analysis
To detect synaptophysin, PSD95, NMDAR1, BDNF, and MBP expression in the cerebellum, brains of PND21 pups (n = 12 per group) were immediately dissected. The samples were frozen until use. The cerebellar tissues were homogenized in lysis buffer and centrifuged at 15,000g and the supernatant was separated. Proteins were quantified using Thermo Pierce® BCA protein assay kit (Thermo Fisher Scientific, Rockford, IL, USA). Proteins were separated on a 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel and transferred to polyvinylidene fluoride (PVDF) membranes. The membranes were blocked by incubating with 5% skim milk in Tris-buffered saline (TBS, pH 7.4) for 1 h, followed by incubation with primary antibody against synaptophysin (rabbit, 1:5000, Abcam), PSD95 (rabbit, 1:2000, Abcam), NMDAR1 (rabbit, 1:1000, Millipore), MBP (rabbit, 1:3000, Millipore), or BDNF (rabbit, 1:2000, Proteintech) overnight at 4 °C. The blots were washed three times in TBS containing 0.1% Tween-20 and then incubated with a horseradish peroxidase-conjugated secondary antibody (1:2000). Bands were visualized using SuperSignal® West Pico Chemiluminescent Substrate (Thermo Fisher Scientific, Rockford, IL, USA). After several rounds of blotting, the relative optical density (ROD) of each band was measured using NIH ImageJ software.
Statistical Analysis
Values are expressed as the means ± standard error of the mean (SEM) for rats in each group, and significance levels of the differences between mean values were determined by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test for body weights and Bonferroni’s post hoc test for other data. Data were analyzed in GraphPad Prism 5.01 software (GraphPad Software, Inc., La Jolla CA, USA). Values of P < 0.05 were considered to be significant.
Results
Body Weight and Blood Pb Levels
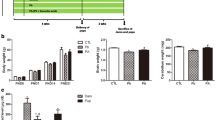
Pb exposure during the gestational period did not affect the body weight gain of pups at PND21. Additionally, no signs of Pb-induced reproductive toxicity, such as abortion, stillbirth, reduction in the number of pups, and malformation of pups, were observed. Similar to our previous studies [18, 23], mean blood Pb level of pups was significantly increased by Pb exposure during the fetal period and was slightly but not significantly decreased by ascorbic acid treatment. However, the Pb-induced increase in blood Pb level in dams was significantly reduced by ascorbic acid treatment (Fig. 1).
a Body weight of pups at postnatal day (PND) 0, 7, 14, and 21 from control, lead (Pb), and Pb+ ascorbic acid (PA) groups. b Blood Pb level of pups (n = 12 pups per group) and dams at PND21 (aP < 0.05, control group versus Pb group; bP < 0.05, Pb group versus PA group). The bars indicate the means ± SE
Effect of Pb and Ascorbic Acid on Presynaptic Synaptophysin
Luxol fast blue and HE staining showed that a significant Pb-induced reduction in Purkinje cells in the cerebellar cortex was reversed by ascorbic acid treatment. Synaptophysin is expressed in the presynaptic vesicular membrane, and its pattern of change corresponds with synaptic changes [9]. In the present study, synaptophysin immunohistochemistry and immunoblot results suggest that Pb exposure deteriorated the development of synapses in the cerebellum, and ascorbic acid treatment ameliorated the reduction in synaptophysin immunoreactivity and protein expression (Figs. 2 and 5).
Luxol fast blue and HE stain (a–f) and immunohistochemistry for synaptophysin (d–f) in the cerebella of pups from control, Pb, and PA groups. GCL granule cell layer, ML molecular layer, PL Purkinje cell layer. Bar = 25 μm. Myelinated fibers were stained blue in the white matter of the cerebellum. Synaptophysin was stained brown and detected mainly in the molecular layer in the cerebellar cortex. g Mean number of Purkinje cells in the cerebellar cortex was decreased in the Pb group, and ascorbic acid treatment ameliorated this Pb-induced reduction (n = 12 per group; aP < 0.05, control group versus Pb group; bP < 0.05, Pb group versus PA group). The bars indicate the means ± SEM
Effect of Lead/Ascorbic Acid on PSD95 and NMDAR1
In the cerebellum, postsynaptic PSD95 and NMDAR1 showed patterns of changes similar to those of presynaptic synaptophysin. Consistent with results from Li and colleagues [24], immunostaining primarily detected PSD95 and NMDAR1 in the somata of Purkinje cells. PSD95 is also located in the molecular layer where dendrites of Purkinje cells synapse with climbing and parallel fibers (Fig. 3). Respectively, Pb exposure and ascorbic acid co-treatment reduced and restored PSD95 and NMDAR1 immunoreactivity in the cerebellar cortex (Fig. 3). Accordingly, from immunoblotting, we observed that both PSD95 and NMDAR1 were reduced in the cerebellum by Pb exposure via placental transfer during the fetal period. However, ascorbic acid co-administration to gestational female rats prevented the Pb-induced reduction in the PSD95 and NMDAR1 protein levels in the cerebella of pups (Fig. 5).
Immunohistochemistry for postsynaptic density 95 (PSD95) (a–c) and N-methyl-d-aspartate receptor subtype 1 (NMDAR1) (d–f) in the cerebella of pups from control, Pb, and PA groups. GCL granule cell layer, ML molecular layer, PL Purkinje cell layer. Bar = 25 μm. g Mean number of PSD95+ and NMDAR1+ Purkinje cells in the cerebellar cortex was reduced in the Pb group. However, Pb-induced decrease of PSD95+ and NMDAR1+ Purkinje cells was ameliorated after ascorbic acid treatment (n = 12 per group; aP < 0.05, control group versus Pb group; bP < 0.05, Pb group versus PA group). The bars indicate the means ± SEM
Effect of Pb and Ascorbic Acid on Axonal Fibers and MBP
Figure 2 shows representative photographs of Luxol fast blue staining in the cerebellum. In the control group, myelinated fibers were mainly detected in the central core of white matter and surrounding granule cell layer in the cerebellar cortex. In Pb-exposed pups, the density of myelinated fibers was slightly reduced in the cerebellum. However, ascorbic acid treatment attenuated Pb-induced impairment of fiber development in the cerebellum. MBP immunoblot results showed a similar pattern of changes. MBP level was significantly reduced by gestational Pb exposure in the developing cerebellum, while ascorbic acid treatment restored MBP protein expression level in the cerebellum (Figs. 4 and 5).
Immunohistochemistry for myelin basic protein (MBP) (a–c) and brain-derived neurotrophic factor (BDNF) (d–i) in the cerebella of pups from control, Pb, and PA groups. GCL granule cell layer, ML molecular layer, PL Purkinje cell layer. Bar = 25 μm. MBP+ myelinated fibers were detected in the granule cell layer in the gray matter (GM-GCL) and white matter (WM). BDNF immunoreactivity was detected in the Purkinje cell and fibers in the white mater. j Mean number of BDNF+ Purkinje cells in the cerebellar cortex was reduced in the Pb group. Reduction of the BDNF+ Purkinje cells was ameliorated in the PA group (n = 12 per group; aP < 0.05, control group versus Pb group; bP < 0.05, Pb group versus PA group). The bars indicate the means ± SEM
Immunoblot for synaptophysin and NMDAR1, PSD95, MBP, and BDNF proteins (a) in the cerebella of pups from control, Pb, and PA groups. Relative optical density (ROD) of immunoblot bands (b–f) is demonstrated as a percentage of the value of the control group. Synaptic proteins (synaptophysin, NMDAR1, PSD95), axonal MBP, and BDNF were reduced by Pb exposure. Pb-induced reduction of these proteins was ameliorated by ascorbic acid treatment (n = 12 per group; aP < 0.05, control group versus Pb group; bP < 0.05, Pb group versus PA group). The bars indicate the means ± SEM
Effect of Pb and Ascorbic Acid on BDNF
During brain development, BDNF is essential for the synaptic formation, synaptic differentiation, and branching of dendritic arbor [25]. Therefore, we focused on the Pb and ascorbic acid co-treatment-induced changes in cerebellar BDNF expression in relation to synaptic development. Previous study reported that BDNF is expressed in the Purkinje cells, inhibitory interneurons, and Bergman glia in adult cerebellum [26]. After immunostaining, BDNF immunoreactivity was primarily observed in the somata of Purkinje cells and fibers in the white matter of cerebellum at PND21. Pb exposure during gestational period negatively affected cerebellar development by reducing the BDNF-immunoreactive Purkinje cells and the expression of BDNF protein in the cerebellum. Compared to that in the Pb group, the reduction of BDNF-immunoreactive Purkinje cells and BDNF protein level was significantly attenuated by ascorbic acid administration in the cerebellum (Figs. 4 and 5).
Discussion
The brain is the primary location of Pb exposure-induced toxicity, and the immature developing brain is more susceptible than the mature adult brain. Neurons in the early postnatal cerebellum are immature, and glia and the blood-brain barrier are not completely developed. Normal synapse formation in the developing cerebellum is especially important for the cerebellar regulation of movement and balance. Therefore, we investigated the effects of gestational Pb exposure on cerebellar synapse development.
To investigate blood Pb level in pups and dams, we used atomic absorptive spectrometry. Low level of Pb exposure during the gestational period significantly increased the blood level of Pb both in pups and dams. However, ascorbic acid treatment differentially affected Pb level by nonsignificant and significant reductions in pups and dams, respectively. The difference between pups and dams may result from Pb excretion via milk, which reduces the Pb level in the dam and increases Pb level in the pups after cessation of Pb treatment. In a previous study, Hossain et al. reported that low Pb exposure in pregnant females increased the level of Pb in milk and pups’ brain [27].
Synaptogenesis in the rat cerebellum spans from the embryonic stage to the early postnatal period [28, 29]. In maturing neurons of the postnatal cerebellum, the growth of dendritic branches and axons is followed by synaptic formation. Synaptic maturation is associated with increased vesicles and density of pre- and postsynaptic membranes [28]. Synpatophysin is a molecular marker of the synapse, and it regulates neurotransmitter exocytosis from synaptic vesicles and recycling of synaptic vesicles [9, 10]. Normally, the cerebellar expression of presynaptic synaptophysin increases during the postnatal period and then stabilizes during adulthood [30]. At PND21, we investigated the expression pattern of synaptophysin in the cerebellum, detecting it in the molecular layer and granule cell layer of the cerebellar cortex. In the molecular layer, the dendrites of Purkinje cells receive parallel fibers, which are the axonal fibers of granule cells. In previous studies, the expression of synaptophysin in the postnatal rat cerebellum showed a similar pattern of distribution consistent with present results [31, 32]. Synaptic vesicles are present in neurons prior to and during synaptogenesis [33]. In the present study, Pb exposure during the fetal period impaired synaptophysin-immunoreactive synapses and synaptophysin expression level in the cerebellum. The detrimental effects of Pb exposure on synapses and synaptophysin were ameliorated by ascorbic acid treatment. In a previous study, synaptophysin expression increased with the maturation of cerebellar cells and synapse formation between granule cells and Purkinje cells or between mossy fibers and granule cells [32]. From these results, we suggest that gestational Pb exposure induces impairment in postnatal synaptophysin and synapse formation, and ascorbic acid treatment prevents these alterations.
Similar to presynaptic synaptophysin, low-level Pb exposure during prenatal and postnatal periods impaired the increase in postsynaptic PSD95 in the developing cerebellum. Gąssowska et al. also reported a similar pattern of change by showing a reduction in both synaptophysin and PSD95 levels in the Pb-exposed cerebellum [34]. Comparable to the cerebellum, long-term 0.2% Pb acetate treatment significantly reduced hippocampal expression of synaptophysin and PSD95 [15]. Compared to Pb-induced changes in postsynaptic PSD95 in the developing brain, the effect of ascorbic acid treatment on PSD95 expression has not yet been determined. In the present study, ascorbic acid administration prevented Pb-induced reduction in the cerebellar expression of synaptophysin and PSD95. PSD95 is an abundant PSD protein that can bind to other postsynaptic membrane proteins and cytoplasmic proteins. PSD95 functions as a scaffolding protein to assemble a specific set of signaling proteins, including NMDAR [35]. PSD95 is particularly abundant in excitatory synapses and is involved in synaptic plasticity in association with glutamatergic receptors, such as NMDAR and AMPAR [36].
Along with PSD95 expression levels, levels of the essential NMDAR1 were also decreased by gestational Pb exposure in the developing cerebellum. NMDAR is located in the postsynaptic membrane, and its substrate glutamate is released from presynaptic vesicles. PSD95 anchors and gathers NMDARs with downstream signals stimulating the transcription of BDNF and subsequent increase of synaptic proteins [37]. In the developing rat cerebellum, Pb exposure reduced the number of NMDAR1-immunoractive Purkinje cells and protein expression level of NMDAR1, and ascorbic acid treatment ameliorated these reductions. Consistent with these results, Alkondon et al. previously reported that Pb is an inhibitor of NMDAR-activated channel currents [38]. Along with decreasing NMDAR1 expression, Pb exposure also delays the switch from NMDAR1/NMDAR2B to NMDAR1/NMDAR2A during synaptogenesis [39]. Recent study additionally reported that ascorbic acid treatment was effective in activating NMDAR in the retinal cell cultures [40]. Moreover, we previously observed a reduction in inhibitory synaptic proteins in the developing cerebellum following gestational Pb exposure [18].
In a previous in vitro study, Pb reduced BDNF levels and induced cell death signaling pathway [37]. In the present in vivo study, a similar pattern was observed in the cerebellum; Pb reduced the protein expression level of BDNF, while ascorbic acid ameliorated the Pb-induced reduction. In addition, BDNF was expressed in the Purkinje cell and fibers in the white mater of the cerebellum. Pb-induced reduction in the BDNF-expressing Purkinje cells was ameliorated by ascorbic acid co-administration. From these results, we suggest that the change in BDNF expression explains the Pb-induced impairment and ascorbic acid-induced protection of cerebellar synaptic proteins. The ascorbic acid-mediated increase in BDNF ameliorates peroxide-induced oxidative stress in neurons [41]. In addition, ascorbic acid treatment at the same or twice the dose in stressed rats was effective in enhancing BDNF levels in brain homogenate [42]. In agreement with these results, the importance of BDNF in normal development of the cerebellum was shown through alterations in the arborization of Purkinje cells, pre- and postsynaptic specialization, layer formation, and foliation in the cerebella of BDNF-knockout mice [43].
The results of Luxol fast blue staining of lipoproteins in the myelin sheath and MBP immunoblot analysis showed prominent decreases in the cerebellum. MBP is an abundant protein in myelin and is used as a marker protein for oligodendrocytes, which form myelin around axonal fibers. The present findings suggest that Pb exposure during the gestational period deteriorates the development of myelinated axon fibers in the cerebellum. Ascorbic acid treatment protected myelinated fibers from Pb-induced impairment. In previous studies, ascorbic acid treatment initiated myelination by regulating basal lamina and myelin formation [44, 45]. The reduction of axonal fibers in the present study may be related to the Pb-induced reduction of the Purkinje cells which project axonal output from cortex to deeper area of the cerebellum [18]. Previous in vitro study also supported present positive effect of ascorbic acid by showing induction of myelination [46].
Summarizing, low-level Pb exposure during the gestational period negatively affects the normal development of the cerebellum by impairing synaptic protein expression and axonal fibers. Additionally, ascorbic acid treatment in Pb-exposed pregnant females protected the developing cerebellum of offsprings from Pb-induced alterations. The pattern of BDNF expression suggests that this neurotrophic factor is highly correlated with the respective Pb- and ascorbic acid-induced deterioration and restoration of cerebellar development. Therefore, we suggest that these observations will help us understand the mechanisms of Pb toxicity in the immature brain and support the therapeutic use of ascorbic acid in preventing Pb intoxication.
References
World Health Organization (2010) Childhood lead poisoning http://www.who.int/ceh/publications/childhoodpoisoning/en
Boucher O, Muckle G, Jacobson JL, Carter RC, Kaplan-Estrin M, Ayotte P, Dewailly É, Jacobson SW (2014) Domain-specific effects of prenatal exposure to PCBs, mercury, and lead on infant cognition: results from the Environmental Contaminants and Child Development Study in Nunavik. Environ Health Perspect 122:310–316
Bellinger DC (2016) Lead contamination in flint—an abject failure to protect public health. N Engl J Med 374:1101–1103
Mielke HW, Gonzales CR, Powell ET (2017) Soil lead and children’s blood lead disparities in pre- and post-hurricane Katrina New Orleans (USA). Int J Environ Res Public Health 14:e407
Froehlich TE, Lanphear BP, Auinger P, Hornung R, Epstein JN, Braun J, Kahn RS (2009) Association of tobacco and lead exposures with attention-deficit/hyperactivity disorder. Pediatrics 124:e1054–e1063
Opler MG, Buka SL, Groeger J, McKeague I, Wei C, Factor-Litvak P, Bresnahan M, Graziano J, Goldstein JM, Seidman LJ, Brown AS, Susser ES (2008) Prenatal exposure to lead, delta-aminolevulinic acid, and schizophrenia: further evidence. Environ Health Perspect 116:1586–1590
Wu J, Basha MR, Brock B, Cox DP, Cardozo-Pelaez F, McPherson CA, Harry J, Rice DC, Maloney B, Chen D, Lahiri DK, Zawia NH (2008) Alzheimer’s disease (AD)-like pathology in aged monkeys after infantile exposure to environmental metal lead (Pb): evidence for a developmental origin and environmental link for AD. J Neurosci 28:3–9
Wiedenmann B, Franke WW (1985) Identification and localization of synaptophysin, an integral memebrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell 41:1017–1028
Saito S, Kobayashi S, Ohashi Y, Igarashi M, Komiya Y, Ando S (1994) Decreased synaptic density in aged brains and its prevention by rearing under enriched environment as revealed by synaptophysin contents. J Neurosci Res 39:57–62
Valtorta F, Pennuto M, Bonanomi D, Benfenati F (2004) Synaptophysin: leading actor or walk-on role in synaptic vesicle exocytosis? BioEssays 26:445–453
Schwalm MT, Pasquali M, Miguel SP, Dos Santos JP, Vuolo F, Comim CM, Petronilho F, Quevedo J, Gelain DP, Moreira JC, Ritter C, Dal-Pizzol F (2014) Acute brain inflammation and oxidative damage are related to long-term cognitive deficits and markers of neurodegeneration in sepsis-survivor rats. Mol Neurobiol 49:380–385
Matosin N, Fernandez-Enright F, Lum JS, Engel M, Andrews JL, Gassen NC, Wagner KV, Schmidt MV, Newell KA (2016) Molecular evidence of synaptic pathology in the CA1 region in schizophrenia. NPJ Schizophr 2:16022
Wu DM, Lu J, Zheng YL, Zhou Z, Shan Q, Ma DF (2008) Purple sweet potato color repairs d-galactose-induced spatial learning and memory impairment by regulating the expression of synaptic proteins. Neurobiol Learn Mem 90:19–27
Yu H, Li T, Cui Y, Liao Y, Wang G, Gao L, Zhao F, Jin Y (2014) Effects of lead exposure on D-serine metabolism in the hippocampus of mice at the early developmental stages. Toxicology 325:189–199
Yu H, Liao Y, Li T, Cui Y, Wang G, Zhao F, Jin Y (2016) Alterations of synaptic proteins in the hippocampus of mouse offspring induced by developmental lead exposure. Mol Neurobiol 53:6786–6798
Holtzman D, DeVries C, Nguyen H, Olson J, Bensch K (1984) Maturation of resistance to lead encephalopathy: cellular and subcellular mechanisms. Neurotoxicology 5:97–124
Mousa AM, Al-Fadhli AS, Rao MS, Kilarkaje N (2015) Gestational lead exposure induces developmental abnormalities and up-regulates apoptosis of fetal cerebellar cells in rats. Drug Chem Toxicol 38:73–83
Nam SM, Ahn SC, Go TH, Seo JS, Nahm SS, Chang BJ, Lee JH (2018) Ascorbic acid ameliorates gestational lead exposure-induced developmental alteration in GAD67 and c-Kit expression in the rat cerebellar cortex. Biol Trace Elem Res 182:278–286
Chang BJ, Jang BJ, Son TG, Cho IH, Quan FS, Choe NH, Nahm SS, Lee JH (2012) Ascorbic acid ameliorates oxidative damage induced by maternal low-level lead exposure in the hippocampus of rat pups during gestation and lactation. Food Chem Toxicol 50:104–108
Goyer RA, Cherian MG (1979) Ascorbic acid and EDTA treatment of lead toxicity in rats. Life Sci 24:433–438
Needleman HL (1999) History of lead poisoning in the world. In: George AM (ed) Lead poisoning prevention and treatment in developing countries. The George Foundation, Bangalore, pp 17–25
Volk B (1984) Cerebellar histogenesis and synaptic maturation following pre- and postnatal alcohol administration. An electron-microscopic investigation of the rat cerebellar cortex. Acta Neuropathol 63:57–65
Nam SM, Chang BJ, Kim JH, Nahm SS, Lee JH (2018) Ascorbic acid ameliorates lead-induced apoptosis in the cerebellar cortex of developing rats. Brain Res 1686:10–18
Li J, Ma Y, Teng YD, Zheng K, Vartanian TK, Snyder EY, Sidman RL (2006) Purkinje neuron degeneration in nervous (nr) mutant mice is mediated by a metabolic pathway involving excess tissue plasminogen activator. Proc Natl Acad Sci U S A 103:7847–7852
Cohen-Cory S, Kidane AH, Shirkey NJ, Marshak S (2010) Brain-derived neurotrophic factor and the development of structural neuronal connectivity. Dev Neurobiol 70:271–288
Vazquez-Sanroman D, Leto K, Cerezo-Garcia M, Carbo-Gas M, Sanchis-Segura C, Carulli D, Rossi F, Miquel M (2015) The cerebellum on cocaine: plasticity and metaplasticity. Addict Biol 20:941–955
Hossain S, Bhowmick S, Jahan S, Rozario L, Sarkar M, Islam S, Basunia MA, Rahman A, Choudhury BK, Shahjalal H (2016) Maternal lead exposure decreases the levels of brain development and cognition-related proteins with concomitant upsurges of oxidative stress, inflammatory response and apoptosis in the offspring rats. Neurotoxicology 56:150–158
Aghajanian GK, Bloom FE (1967) The formation of synaptic junctions in developing rat brain: a quantitative electron microscopic study. Brain Res 6(4):716–727
Zimatkin SM, Karnyushko OA (2017) Synaptogenesis in the developing rat cerebellum. Neurosci Behav Physiol 47:631–636
McClatchy DB, Liao L, Park SK, Venable JD, Yates JR (2007) Quantification of the synaptosomal proteome of the rat cerebellum during post-natal development. Genome Res 17:1378–1388
Fujita M, Kadota T, Sato T (1996) Developmental profiles of synaptophysin in granule cells of rat cerebellum: an immunohistocytochemical study. J Electron Microsc 45:185–194
Leclerc N, Beesley PW, Brown I, Colonnier M, Gurd JW, Paladino T, Hawkes R (1989) Synaptophysin expression during synaptogenesis in the rat cerebellar cortex. J Comp Neurol 280:197–212
Fletcher TL, Cameron P, De Camilli P, Banker G (1991) The distribution of synapsin I and synaptophysin in hippocampal neurons developing in culture. J Neurosci 11:1617–1626
Gąssowska M, Baranowska-Bosiacka I, Moczydłowska J, Frontczak-Baniewicz M, Gewartowska M, Strużyńska L, Gutowska I, Chlubek D, Adamczyk A (2016) Perinatal exposure to lead (Pb) induces ultrastructural and molecular alterations in synapses of rat offspring. Toxicology 373:13–29
Sheng M (2001) The postsynaptic NMDA-receptor--PSD-95 signaling complex in excitatory synapses of the brain. J Cell Sci 114:1251
Steiner P, Higley MJ, Xu W, Czervionke BL, Malenka RC, Sabatini BL (2008) Destabilization of the postsynaptic density by PSD-95 serine 73 phosphorylation inhibits spine growth and synaptic plasticity. Neuron 60:788–802
Stansfield KH, Pilsner JR, Lu Q, Wright RO, Guilarte TR (2012) Dysregulation of BDNF-TrkB signaling in developing hippocampal neurons by Pb2+: implications for an environmental basis of neurodevelopmental disorders. Toxicol Sci 127:277–295
Alkondon M, Costa AC, Radhakrishnan V, Aronstam RS, Albuquerque EX (1990) Selective blockade of NMDA-activated channel currents may be implicated in learning deficits caused by lead. FEBS Lett 261:124–130
Neal AP, Worley PF, Guilarte TR (2011) Lead exposure during synaptogenesis alters NMDA receptor targeting via NMDA receptor inhibition. Neurotoxicology 32:281–289
Domith I, Socodato R, Portugal CC, Munis AF, Duarte-Silva AT, Paes-de-Carvalho R (2018) Vitamin C modulates glutamate transport and NMDA receptor function in the retina. J Neurochem 144:408–420
Grant MM, Barber VS, Griffiths HR (2005) The presence of ascorbate induces expression of brain derived neurotrophic factor in SH-SY5Y neuroblastoma cells after peroxide insult, which is associated with increased survival. Proteomics 5:534–540
Ashwin R, Sampath M, Gayathri R, Rajalakshmi R, Sudhanshu S (2013) A comparison of resveratrol and vitamin C therapy on expression of BDNF in stressed rat brain homogenate. IOSR J Pharm 10:22–27
Schwartz PM, Borghesani PR, Levy RL, Pomeroy SL, Segal RA (1997) Abnormal cerebellar development and foliation in BDNF−/− mice reveals a role for neurotrophins in CNS patterning. Neuron 19:269–281
Eldridge CF, Bunge MB, Bunge RP, Wood PM (1987) Differentiation of axon-related Schwann cells in vitro. I. Ascorbic acid regulates basal lamina assembly and myelin formation. J Cell Biol 105:1023–1034
Zhang L, Ma Z, Smith GM, Wen X, Pressman Y, Wood PM, Xu XM (2009) GDNF-enhanced axonal regeneration and myelination following spinal cord injury is mediated by primary effects on neurons. Glia 57:1178–1191
Kim JY, Choi CS, Hong SK (2014) Coculture of Schwann cells and neuronal cells for myelination in rat. Rapid Commun Photoscience 3:48–49
Contributions
All authors conceived and designed the study. SMN, JSS, and JHL conducted animal modeling and histological analysis. ISC, THG, and BJC conducted animal caring and immunoblot analysis. SMN, ISC, BJC, and JHL collected and analyzed the data and wrote the manuscript. JHK and SSN participated in designing of the study, discussing of results, and revising the manuscript. All authors approved the final version of the manuscript.
Funding
This research was supported by the faculty research fund of Konkuk University and the Veterinary Science Research Institute of Konkuk University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Nam, S.M., Cho, IS., Seo, J.S. et al. Ascorbic Acid Attenuates Lead-Induced Alterations in the Synapses in the Developing Rat Cerebellum. Biol Trace Elem Res 187, 142–150 (2019). https://doi.org/10.1007/s12011-018-1354-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1354-6