Abstract
In the present study, we investigated the effects of ascorbic acid on lead-exposed developing cerebellum. Female rats were divided into the following three groups: control (distilled water), lead (0.2% lead acetate), and lead plus ascorbic acid (100 mg/kg/day, 10% solution). To evaluate the effect of lead exposure and ascorbic acid treatment accurately on the cerebellar development for the gestational period, we halted further treatment with lead and ascorbic acid in the dams after delivery of the pups. Although the ascorbic acid slightly decreased the lead level in pups, lead level was still high in the group treated with lead plus ascorbic acid group compared with the control group. The blood lead levels indicated that the ascorbic acid could facilitate both the excretion and transfer of lead from a dam to its pups via milk. At postnatal day 21, lead exposure significantly reduced the number of Purkinje cells in the cerebellar cortex of pups. Additionally, lead treatment induced degenerative changes such as reduction of glutamic acid decarboxylase (GAD67) and c-kit expressions are observed in the developing cerebellar cortex. In the cerebellum of the pups from the lead plus ascorbic acid group, reduction of the number of Purkinje cells, GAD67 expression, and c-kit immunopositivity were remarkably restored compared with the lead group. Our present results suggested that ascorbic acid treatment to lead-exposed dam exerted protective effects on the developing cerebellum against lead-induced neurotoxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The frequent exposure to heavy metals including lead, mercury, arsenic, and cadmium has become a health-threatening issue [1]. Among these, lead is easily found in the environment and is considered dangerous at low doses [2]. In particular, the wide use of lead in water infrastructures increases the possibility of contamination of drinking water and chances of lead exposure [3]. Additionally, lead can be absorbed into the body via ingestion of lead-contaminated food and inhalation of lead particles in the air [4]. In pregnant woman, lead crosses the placenta and subsequently causes adverse effects such as abortion, preterm delivery, premature rupture of membranes, and developmental delays in children [5]. While the efforts to decrease the lead content in paints, gasoline, batteries, and insecticides have been successful in decreasing the prevalence of lead poisoning, low level of lead exposure remains a health-threatening concern [2].
Lead-induced toxicities are caused by its accumulation in multiple body organs including hematopoietic, digestive, renal, and reproductive systems [6,7,8,9]. Lead is also a potent neurotoxicant and chronic lead exposure deteriorates the structure and function of the central nervous system (CNS) [10]. Compared to the immature brain of children, the adult brain is resistant to lead-induced neurotoxicity and the lead-related alterations may be reversed by preventing its accumulation via chelation [11]. However, the developing fetal and postnatal brains are much more vulnerable to lead-induced neurotoxicity and the effects are permanent or fatal [10]. In severe cases, lead intoxication in children resulted in encephalopathy, convulsions, coma, and death [12, 13]. The harmful effects of lead on the brain are induced by various mechanisms including apoptosis, excitotoxicity, and neurotransmitter alterations [10]. In addition to neuronal mitochondria, lead poisoning structurally impairs cellular proliferation, neuronal differentiation, neurogenesis and synaptogenesis in the developing brain [10, 14, 15]. Functionally, exposure to low lead levels has been associated with behavioral abnormalities, learning impairment, decreased hearing, and impaired cognitive functions in humans and in experimental animals [14, 15]. Moreover, long-term lead exposure has been associated with the development of neurodegenerative diseases such as Parkinson’s disease and Alzheimer’s disease [16, 17].
Metal chelating materials and phytochemicals have been used to prevent heavy metal precipitation and subsequent toxicity. Among these, ascorbic acid, which is a phytochemical, has received increasing attention due to its protective effects against heavy metal intoxication [18,19,20]. Ascorbic acid, also known as vitamin C, is an essential nutrient for its role as a cofactor of numerous enzymes and a building block in the biosynthesis of collagen, carnitine, and catecholamines [21]. Ascorbic acid possesses additional health benefits such as the prevention of scurvy, common cold, cancer, myocardial infarction, and stroke [22]. Interestingly, we previously demonstrated that ascorbic acid could effectively ameliorate the detrimental effects of lead-induced histopathology and neuronal apoptosis in the hippocampus [18]. Our previous data also demonstrated that anti-oxidative effect of ascorbic acid on lead-exposed hippocampus [19]. Lead induces toxicity by competing with biologically essential elements such as calcium and zinc [23]. Additionally, Flora and Tandon reported that the chemical binding of ascorbic acid with lead is one of the important mechanisms of protection against lead poisoning [24].
However, to date, most studies regarding the effects of lead poisoning have focused on the hippocampus, and only a few studies investigated on the cerebellum. Based on the beneficial effects of ascorbic acid on the hippocampal development [18, 19], we hypothesized that ascorbic acid treatment may ameliorate the harmful effect of lead on the cerebellar development. Normal cerebellar development is important for its role in motor control, integration of proprioceptive information, and cognitive functions [25]. In the developing cerebellum of rats, the two main neurons move from their primordium to their destination; the Purkinje cells, which migrate from the neuroepithelium to the surface of the cerebellum and the granule cells, which originate from the rhombic lip, form the external granular layer, and migrate to the internal granular layer [26]. The neurotransmitter γ-amino butyric acid (GABA) acts as a trophic factor for the cerebellar development by activating GABAA receptors, and acts as an inhibitory synaptic transmitter to regulate the neuronal excitatory activity after the developmental period [27]. Lead exposure is known to exert adverse influences on the GABA neurotransmission [28].
Because there are only few studies on the lead-induced changes in the GABAergic signal in the developing cerebellum, the present study aimed to investigate the effect of lead exposure during the gestational period with focusing on the GABAergic synapse in the cerebellum of pups. In addition, ascorbic acid was co-administrated to evaluate the protective effect against lead-induced neurodegeneration.
Materials and Methods
Animals and Experimental Treatment
The experiment described here was performed in accordance with the guidelines provided by the Experimental Animal Laboratory and approved by the Animal Care and Use Committee of the Konkuk University School of Veterinary Medicine (Korea). Sprague-Dawley rats were housed under conditions of constant temperature (22 ± 2 °C) and humidity (60%) with a 12-h light/12-h dark cycle. The animals were mated at 70–90 days of age. On pregnancy day 0, which was determined by the presence of sperm in vaginal smears (or vaginal plugs), 11 pregnant rats were chosen and randomly divided into three groups: control (n = 3), lead (n = 4), and lead + ascorbic acid (n = 4). Control dams were administered tap water and did not receive lead and/or ascorbic acid treatment. The lead group and lead + ascorbic acid group were given 0.2% lead acetate (Sigma-Aldrich, USA) in the drinking water throughout pregnancy. Among various lead compounds, we selected lead acetate for its water solubility and high bioavailability [29]. Ascorbic acid (100 mg/kg, Sigma-Aldrich, USA) dissolved in saline was orally administered once a day. The present doses of lead acetate and ascorbic acid were adopted based on previous studies demonstrating the toxicity and protection in the rat brain [18, 19]. Rats in the control and lead groups were orally provided with same volume of physiological saline. Twelve pups per group were selected and used for the analysis. Lead and ascorbic acid exposure was discontinued after birth until postnatal day (PND) 21. The body weight was measured once a week, and an average value was calculated. All of the experiments were conducted to minimize the number of animals used and the suffering caused by the procedures of the present study.
Blood Lead Level Analysis
At PND 21, animals (n = 12) were anesthetized with ketamine (50 mg/kg) and xylazine (1 mg/kg) and blood was collected from the abdominal aorta. The measurement of the blood lead level was carried out by an atomic absorption spectrophotometer (Perkin Elmer Zeeman 5100, Norwalk, USA) using an HGA-600 graphite furnace with Zeeman background correction. Blood samples were diluted using deionized water. The absorption wavelength was 283.3 nm. The r 2 of the calibration curve was at least above 0.995.
Cresyl Violet Staining and Immunohistochemical Analysis
At PND 21, the pups (n = 12) were anesthetized with ketamine (50 mg/kg) and xylazine (1 mg/kg). Subsequently, pups were perfused transcardially with heparinized phosphate-buffered saline (PBS, 0.1 M, pH 7.4) followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The cerebellum was removed and post-fixed in the same fixative overnight at 4 °C. Brains were cut into 5 μm thick sagittal sections using a microtome (Leica, Wetzlar, Germany). The first three sections in each series of 10 were mounted on gelatin-coated slides. For the cresyl violet staining, the deparaffinized paraffin sections were stained in filtered cresyl violet solution and processed further according to routine procedures. Immunohistochemistry was conducted under the same conditions. For antigen retrieval, sections were placed in 400-mL jars filled with citrate buffer (pH 6.0) and heated in the microwave oven (three heating cycles of 5 min each). After cooling to room temperature, the slides were washed in PBS and sequentially treated with 0.3% hydrogen peroxide (H2O2) in PBS for 30 min and subsequently in 5% normal horse serum in PBS for 30 min. The primary antibodies used here are glutamic acid decarboxylase 67 (GAD67) (1:500, Stressgen, USA), and c-kit (1:100, Santa Cruz, USA). Subsequently, they were exposed to biotinylated anti-IgG (1:500, Vector, Burlingame, CA) and streptavidin peroxidase complex (1:500, Vector). They were then visualized by a reaction to 3,3′-diaminobenzidine tetrachloride (Sigma) in 0.1 M Tris-HCl buffer (pH 7.2); several sections were counterstained with hematoxylin. For the double staining, sections stained with GAD67 and c-kit antibodies were incubated again with calbindin-28kd (1:1000, Sigma-Aldrich, USA) antibody using a different chromogenic substrate. The SG substrate kit (Vector) was used and the reaction was stopped after a blue-gray staining developed. Following dehydration, the sections were mounted in toluene-based-mounting medium (Richard-Allan Scientific, Thermo Scientific).
Quantitative Analysis
All histopathological analyses described below were performed by an investigator blinded to the treatment allocation. The numbers of Purkinje, GAD67/calbindin, and c-kit/calbindin-immunopositive cells were counted in micrographs obtained at ×100 magnification. The observations were carried out in the three lobules (2nd, 5th, and 8th lobules) from a sagittal section of the cerebellar vermis. Results were recorded in Microsoft office Excel spreadsheets.
Statistical Analysis
Values were expressed as the means ± standard error of the mean (SEM) for rats in each group, and significance levels of the differences between the mean values were determined by one-way analysis of variance (ANOVA) followed by Turkey’s multiple test for multiple comparisons using GraphPad Prism 5.01 software (GraphPad Software, Inc., La Jolla CA, USA). Values of P < 0.05 were considered to be statistically significant.
Results
Body Weights and Blood Lead Levels
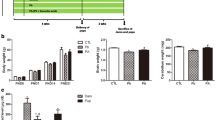
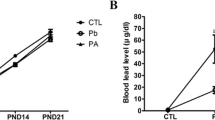
There was no significant difference in body weight gain between the control and experimental groups throughout all the time points, i.e., PND 0, 7, 14, and 21. The blood lead level measurements using atomic absorption spectrometry indicated a significant increase in both lead-exposed groups, regardless of ascorbic acid administration, compared with controls. Additionally, co-administration of ascorbic acid with lead in dams resulted in a significant decrease in blood lead level in dams and a marginal decrease in the pups, respectively. These results suggested that treatment with ascorbic acid may have resulted in the excretion of lead from the dams, which may have been subsequently transferred to the suckling offspring through the milk. However, compared to our previous studies [14, 15], the termination of lead exposure from delivery did not result in significant blood lead level changes among pups in all experimental groups (Fig. 1).
Body weight of pups at postnatal day (PND) 21 from in the control, lead, and lead + ascorbic acid groups. Blood lead level of dams and pups (n = 12 pups per group) at the time point of PND 21 (*P < 0.05, control group versus lead group, # P < 0.05, lead group versus lead + ascorbic acid group). The bars indicate the means ± SE
Degenerative Effect of Lead on Purkinje Cells in the Developing Cerebellum
As evidenced by the microscopic cell count results, the number of Purkinje cells in the group that was treated with lead and ascorbic acid was significantly higher than that in the group that was treated with lead and their shape was more circular. In both the lead and lead + ascorbic acid groups, the relative number of Purkinje cells in the cerebellum was 78.32 and 94.48% of that in the control group, respectively (Fig. 2).
Cresyl violet staining of the cerebellum of pups at PND 21 from in the control, lead, and lead + ascorbic acid groups. Bar = 20 μm. Mean number of Purkinje cells was decreased in the lead group, while ascorbic acid treatment ameliorated this reduction in the cerebellar cortex (n = 12 per group; *P < 0.05, control group versus lead group, # P < 0.05, lead group versus lead + ascorbic acid group). The bars indicate the means ± SEM
Immunolocalization of GABA Synthesizing Enzyme (GAD67)
GABAergic Purkinje cells and glutamatergic granule cells are primary neurons in the cerebellar cortex. GABAergic interneurons such as the basket cells and stellate cells reside in the molecular layer and they synapse with the Purkinje cells. Other GABAergic interneurons include the Golgi and Lugaro cells, which are located in the granular layer of the cerebellar cortex. We investigated the expression of GAD67 which synthesizes GABA from glutamate in the cerebellum. The expression of GAD67 was observed in the axonal projections of the Purkinje cells in the granule cell layer of the cerebellum. The GAD67 immunoreactivity was strongly detected in the somata regions of the Purkinje cells. GAD67-positive signals were also detected in the molecular layer of the cerebellum, where the dendrites and spines of Purkinje cells extend. Furthermore, GAD67 was colocalized with calbindin in the somata of the Purkinje cells. In addition, GAD67 expression was the lowest in the Purkinje cells of rats in the lead group. In contrast, treatment with ascorbic acid ameliorated the lead-induced reduction in GAD67 immunoreactivity in the cerebellar cortex, as observed in the lead + ascorbic acid group (Fig. 3).
Immunolocalization of GAD67 in the cerebellum of pups at PND 21 from in the control, lead, and lead + ascorbic acid groups. Double staining results for GAD67 and calbindin (C, F, and I). GAD67 expressions are colocalized in calbindin immunoreactive somata of Purkinje cells. C; Cell marked by arrow is magnified and the magnified image shows calbindin staining (asterisks) and colocalization of GAD67 and calbindin are stained dark blue. Bar = 20 μm. Mean number of GAD67 and calbindin immunoreacitve synapse in the cerebellar cortex (n = 12 per group; *P < 0.05, control group versus lead group, # P < 0.05, lead group versus lead + ascorbic acid group). The bars indicate the means ± SEM
Immunolocalizaton of c-Kit
c-Kit is a receptor tyrosine kinase, which is expressed in various stem cells and is involved in their maintenance [30]. c-Kit is expressed both in the peripheral nervous system and in the CNS [31, 32] and is functionally important for neuronal migration and axonal extension in the cerebral cortex [31]. In the present study, the c-kit expression was mainly restricted to the cytoplasmic membrane of the Purkinje cells. To a lesser extent, c-kit was also detected in the molecular and granule cell layers of the cerebellum. As evidenced by the c-kit and calbindin colabeling analysis in the cerebellum of PND 21 pups, c-kit was located in the periphery of the soma and dendritic tree of the Purkinje cells. Similar to our present results, Manova et al. reported the c-kit expression in the pinceau structures, which are generated by basket cells by entwining the soma and the initial segment of the Purkinje cells axons. c-Kit has been reported to be also expressed in the other interneurons including stellate cells and Golgi cells in the cerebellar cortex [33]. Based on these results, we suggest that these c-kit immunoreactive structures might be the parts of basket cells and stellate cells that in turn synapse with soma and dendrites of Purkinje cells, in a respective way. In addition, lead-induced reduction of c-kit expression was restored by ascorbic treatment in the lead + ascorbic acid group (Fig. 4).
Immunolocalization of c-kit in the cerebellum of pups at PND 21 from in the control, lead, and lead + ascorbic acid groups. Double staining results for c-kit and calbindin (C, F, and I). Calbindin immunoreactive somata of Purkinje cells (asterisks) are stained blue whereas c-kit immunoreactive pinceaus are stained brown (arrows). Bar = 20 μm. Mean number of c-kit and calbindin immunoreactive synapse in the cerebellar cortex (n = 12 per group; *P < 0.05, control group versus lead group, # P < 0.05, lead group versus lead + ascorbic acid group). The bars indicate the means ± SEM
Discussion
Maternal lead exposure deteriorates the embryonic and fetal development. Particularly, the brain is susceptible to low-dose lead-induced toxicity with ensuing mental retardation, memory and learning deficits, and abnormal behaviors [34]. Lead exposure during in childhood indicated persistent effects such as neurobehavioral deficits [35]. Developmentally, the immature postnatal cerebellum is more susceptible to the deleterious effects of environmental factors [10, 12, 13]. The relatively simple cytoarchitecture of the cerebellum had merits in a developmental study of cell specification, patterning, migration, and synapse formation [36]. Moreover, the cerebellum exerts vital functions such as motor coordination, motor learning, and cognitive functions [25]. Given the importance of the cerebellum, the present study aimed to investigate the effect of maternal lead exposure during gestation to the cerebellar development of pups.
We evaluated the effect of lead exposure on the body weight gain and the blood lead level in dams and pups. There were no significant differences among all experimental groups in the body weight of pups. Following lead exposure to the dam, the blood lead level was increased in pups in the lead group and lead + ascorbic acid groups; blood lead level was highest in the lead group. Consistent with previous studies, treatment of pregnant dams with ascorbic acid increased the blood lead level in the pups [18, 19, 37]. However, the relatively high blood lead level in the pups of the lead + ascorbic acid group indicated the possible transfer of the accumulated lead from mother to offspring via milk. Compared to the pups, the blood lead level of dams was significantly lowered by the ascorbic acid treatment. According to a previous study in dairy cows, ascorbic acid treatment decreased the blood lead level by enhancing the lead secretion in milk [38]. Chemically, ascorbic acid can bind to lead [24] and hence the coadministration of lead and ascorbic acid decreased lead absorption [39]. Additionally, even though we did not evaluate the level of lead in the developing embryos and fetuses, lead was reported to be transferred via placenta, while its serum level reached equilibrium between dam and fetus 24 h after lead poisoning [40]. Therefore, we can suggest that gestational exposure to lead and ascorbic acid differentially affected the lead exposure level in the embryo and fetus in utero. Compared to the low lead dose (0.2%) used in the present study, gestational exposure to high lead dose (0.54%, 1%) caused abnormal fetal development, teratogenicity, and lethality [41].
Subsequently, we investigated the structural changes in the cerebellum by focusing on the Purkinje cells for the reason that they are the only output from the cerebellar cortex and are involved the regulation of the cerebellar function [42]. Low-dose lead in the drinking water impaired the normal development of the cerebellum owing to a reduction in the number of Purkinje cells and their dendritic branches. In particular, in the cerebellum, all Purkinje cells are formed at prenatal stages (around embryonic days E13–E16) and the outgrowth and dendritic trees of Purkinje cells occur during the first three postnatal weeks [43]. The reduced number of Purkinje cells suggested that lead exposure in a pregnant dam is transferred across the placenta [43] and hence it affected the formation of Purkinje cells in the cerebellum of pups. Based on the impaired dendritic arbor of the Purkinje cells and the high concentration level of lead in the pups, we suggest that the effect of lead persisted even though lead exposure was terminated at birth. Additionally, the detained migration of Purkinje cells was observed in the granular cell layer of the cerebellum in the lead-exposed pups. Remarkably, ascorbic acid treatment ameliorated on the development of Purkinje cells by enhancing their numbers, the complexity of their dendritic branches, and their migration to the Purkinje cell layer in the cerebellum. Although we did not evaluate the functional consequence of the impaired development of Purkinje cells in the cerebellum, previous studies reported that dysfunctional Purkinje cells were highly correlated with impairments in motor coordination in cerebellar ataxia [42]. Cerebellar ataxia from lead-induced encephalopathy is more frequent in children than in adults [44]. In children, cerebellar encephalopathy develops due to their susceptibility to lead-induced disruption of the blood-brain barrier [45]. Additionally, the immature brain is much more susceptible to lead-induced neurotoxicity due to the lack of lead sequestering capacity of astrocytes [11].
In this study, we focused on the GABergic signaling in the cerebellum because of its importance in normal development. In the cerebellar cortex, Purkinje, stellate, basket, and Golgi cells release GABA. We investigated the effect of lead poisoning and the protective effect of ascorbic acid on the morphology of the inhibitory synapses between Purkinje cells and interneurons. The GABA synthesizing GAD67 enzyme was detected in the somata and dendrites of Purkinje cells and in the molecular layer of the cerebellum. The expression of GAD67 in the lead group was the lowest, while ascorbic acid treatment restored the immunoreactivity of GAD67 in the cerebellum. Similarly, Bernocchi et al. demonstrated that cerebellar insults by cisplatin in the developing periods resulted in a reduction of GAD67 expression and structural deterioration of Purkinje cells [46]. Based on these results, we suggest that the reduction of the GABA synthesizing enzyme level is highly correlated with changes in the Purkinje cells. In addition, c-kit, which is tyrosine kinase receptor and it has been reported to be related to the development of synaptic connection [47], was abundantly expressed in the molecular layer, periphery of somata of Purkinje cells and pinceau structures. Pinceau is formed via synapses between the axonal terminals of basket cells and the initial segments of the axons of Purkinje cells. Functionally, this synapse negatively regulates firing of an action potential in inhibitory Purkinje cells with consequent activation [33]. In the present study, c-kit showed the same pattern of change with GABAergic signals in the cerebellar cortex. Along with the reduction of pinceau structures, lead exposure reduced the expression of c-kit in the molecular layer of the cerebellum. We assumed that the reduction of c-kit immunoreactive inhibitory synapse was correlated with the reduction in the number of Purkinje cells. Indeed, upregulation of c-kit in the cerebellar inhibitory synapse of a kainic acid-induced seizure model was highly correlated with the expression of stem cell factor in the axonal process of Purkinje cells [48]. In particular, ascorbic acid-induced restoration of c-kit immunoreactivity in the periphery of the Purkinje cells suggested that the GABAergic synapse was protected against lead-induced developmental defects.
In the present study, we did not further investigate the underlying molecular mechanisms and functional consequences of the effects of lead and ascorbic acid treatment on the GABAergic synapses in the cerebellum. It is known that the GABAergic system and glutamatergic systems interact with each other and that the regulatory glutamate-glutamine (GABA) cycle develops at postnatal age of 3–4 weeks [49]. Therefore, future studies addressing whether the present reduction of the GABAergic synapse is related to the glutamatergic synapse in the cerebellum are warranted. Yeganeh-Doost et al. reported that alterations in the glutamatergic N-methyl-D-aspartate receptor complex lead to dysfunction of GABAergic signaling and subsequent decrease in the inhibitory action in the cerebellum [50]. Gestational exposure to lead acetate impaired K+-stimulated hippocampal glutamate and GABA release [51]. The neuromodulatory role of ascorbic acid has been linked with its protective effect against glutamate-induced neuronal damage by removing extracellular glutamate [52].
Conclusion
Our present results indicated that co-administration of ascorbic acid with lead was effective in ameliorating the detrimental effects of lead intoxication in the developing cerebellum. In particular, the ascorbic acid protected the Purkinje cells from degeneration and spared more abundant GABAergic signals in the cerebellar cortex as a result of more synapses in the periphery of Purkinje cells.
References
Duruibe JO, Ogwuegbu MOC, Egwurugwu JN (2007) Heavy metal pollution and human biotoxic effects. International Journal of Physical Sciences 2:112–118
Silergeld EK (1990) Implications of new data on lead toxicity for managing and preventing exposure. Eviron Health Perspect 89:49–54
Hanna-Attisha M, LaChance J, Sadler RC, Champney Schnepp A (2016) Elevated blood lead levels in children associated with the Flint drinking water crisis: a spatial analysis of risk and public health response. Am J Public Health 106:283–290
D'souza HS, Dsouza SA, Menezes G, Venkatesh T (2011) Diagnosis, evaluation, and treatment of lead poisoning in general population. Indian J Clin Biochem 26:197–201
Vigeh M, Yokoyama K, Shinohara A, Afshinrokh M, Yunesian M (2010) Early pregnancy blood lead levels and the risk of premature rupture of the membranes. Reprod Toxicol 30:477–480
Vij AG, Satija NK, Flora SJ (1998) Lead induced disorders in hematopoietic and drug metabolizing enzyme system and their protection by ascorbic acid supplementation. Biomed Environ Sci 11:7–14
Liu B, Zhang H, Tan X, Yang D, Lv Z, Jiang H, Lu J, Baiyun R, Zhang Z (2017) GSPE reduces lead-induced oxidative stress by activating the Nrf2 pathway and suppressing miR153 and GSK-3β in rat kidney. Oncotarget
Mobarak YMS, Sharaf MM (2010) Lead acetate-induced histopathological changes in the gills and digestive system of silver sailfin molly (Poecilia latipinna). Int J Zool Res 7:1–18
Fahim MA, Tariq S, Adeghate E (2013) Vitamin E modifies the ultrastructure of testis and epididymis in mice exposed to lead intoxication. Ann Anat 195:272–277
Lidsky TI, Schneider JS (2003) Lead neurotoxicity in children: basic mechanisms and clinical correlates. Brain 126:5–19
Holtzman D, DeVries C, Nguyen H, Olson J, Bensch K (1984) Maturation of resistance to lead encephalopathy: cellular and subcellular mechanisms. Neurotoxicology 5:97–124
Goyer R (1990) Lead toxicity: from overt to subclinical to subtle health effects. Environ Health Perspect 86:177–181
World Health Organization (2010) Childhood lead poisoning. World Health Organization http://www.who.int/ceh/publications/childhoodpoisoning/en/
Bellinger D, Sloman J, Leviton A, Rabinowitz M, Needleman HL, Waternaux C (1991) Low-level lead exposure and children’s cognitive function in the preschool years. Pediatrics 87:219–227
Al-Shimali HM, Al-Musaileem AF, Rao MS, Khan KM (2016) Low-dose exposure to lead during pregnancy affects spatial learning, memory and neurogenesis in hippocampus of young rats. J Neurol Neurosci 7:3
Coon S, Stark A, Peterson E, Gloi A, Kortsha G, Pounds J, Chettle D, Gorell J (2006) Whole-body lifetime occupational lead exposure and risk of Parkinson’s disease. Environ Health Perspect 114:1872–1876
Wu J, Basha MR, Brock B, Cox DP, Cardozo-Pelaez F, McPherson CA, Harry J, Rice DC, Maloney B, Chen D, Lahiri DK, Zawia NH (2008) Alzheimer’s disease (AD)-like pathology in aged monkeys after infantile exposure to environmental metal lead (Pb): evidence for a developmental origin and environmental link for AD. J Neurosci 28:3–9
Han JM, Chang BJ, Li TZ, Choe NH, Quan FS, Jang BJ, Cho IH, Hong HN, Lee JH (2007) Protective effects of ascorbic acid against lead-induced apoptotic neurodegeneration in the developing rat hippocampus in vivo. Brain Res 1185:68–74
Chang BJ, Jang BJ, Son TG, Cho IH, Quan FS, Choe NH, Nahm SS, Lee JH (2012) Ascorbic acid ameliorates oxidative damage induced by maternal low-level lead exposure in the hippocampus of rat pups during gestation and lactation. Food Chem Toxicol 50:104–108
Hounkpatin ASY, Johnson RC, Guédénon P, Domingo E, Alimba CG, Boko M, Edorh PA (2012) Protective effects of vitamin C on haematological parameters in intoxicated wistar rats with cadmium, mercury and combined cadmium and mercury. Int Res J Biol Sci 1:76–81
Wilson JX (2002) The physiological role of dehydroascorbic acid. FEBS Lett 527:5–9
Chambial S, Dwivedi S, Shukla KK, John PJ, Sharma P (2013) Vitamin C in disease prevention and cure: an overview. Indian J Clin Biochem 28:314–328
Prasanthi RPJ, Reddy GH, Reddy GR (2006) Calcium or zinc supplementation reduces lead toxicity: assessment of behavioral dysfunction in young and adult mice. Nutr Res 26:537–545
Flora SJ, Tandon SK (1986) Preventive and therapeutic effects of thiamine, ascorbic acid and their combination in lead intoxication. Acta Pharmacol Toxicol (Copenh) 58:374–378
Buckner RL (2013) The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron 80:807–815
Altman J, Bayer SA (1985) Embryonic development of the rat cerebellum. III. Regional differences in the time of origin, migration, and settling of Purkinje cells. J Comp Neurol 231:42–65
Takayama C, Inoue Y (2004) Extrasynaptic localization of GABA in the developing mouse cerebellum. Neurosci Res 50:447–458
Silbergeld EK, Hruska RE, Miller LP, Eng N (1980) Effects of lead in vivo and in vitro on GABAergic neurochemistry. J Neurochem 34:1712–1718
Dieter MP, Matthews HB, Jeffcoat RA, Moseman RF (1993) Comparison of lead bioavailability in F344 rats fed lead acetate, lead oxide, lead sulfide, or lead ore concentrate from Skagway, Alaska. J Toxicol Environ Health 39:79–93
Goldstein BJ, Goss GM, Hatzistergos KE, Rangel EB, Seidler B, Saur D, Hare JM (2015) Adult c-Kit(+) progenitor cells are necessary for maintenance and regeneration of olfactory neurons. J Comp Neurol 523:15–31
Guijarro P, Wang Y, Ying Y, Yao Y, Jieyi X, Yuan X (2013) In vivo knockdown of cKit impairs neuronal migration and axonal extension in the cerebral cortex. Dev Neurobiol 73:871–887
Tamada H, Kiyama H (2016) Suppression of c-Kit signaling induces adult neurogenesis in the mouse intestine after myenteric plexus ablation with benzalkonium chloride. Sci Rep 6:32100
Manova K, Bachvarova RF, Huang EJ, Sanchez S, Pronovost SM, Velazquez E, McGuire B, Besmer P (1992) C-kit receptor and ligand expression in postnatal development of the mouse cerebellum suggests a function for c-kit in inhibitory interneurons. J Neurosci 12:4663–4676
Hossain S, Bhowmick S, Jahan S, Rozario L, Sarkar M, Islam S, Basunia MA, Rahman A, Choudhury BK, Shahjalal H (2016) Maternal lead exposure decreases the levels of brain development and cognition-related proteins with concomitant upsurges of oxidative stress, inflammatory response and apoptosis in the offspring rats. Neurotoxicology 56:150–158
Needleman HL, Schell A, Bellinger D, Leviton A, Allred EN (1990) The long-term effects of exposure to low doses of lead in childhood. An 11-year follow-up report. N Engl J Med 322:83–88
Millen KJ, Wurst W, Herrup K, Joyner AL (1994) Abnormal embryonic cerebellar development and patterning of postnatal foliation in two mouse Engrailed-2 mutants. Development 120:695–706
Sadeghi A, Ebrahimzadeh Bideskan A, Alipour F, Fazel A, Haghir H (2013) The effect of ascorbic acid and garlic administration on lead-induced neural damage in rat offspring’s hippocampus. Iran J Basic Med Sci 16:157–164
Naresh R, Dwivedi SK, Swarup D, Patra RC (2003) Effect of ascorbic acid on milk lead and cadmium level on subclinical cases of mastitis. Environ Contam Toxicol 71:899–904
Simon JA, Hudes ES (1999) Relationship of ascorbic acid to blood lead levels. JAMA 281:2289–2293
McClain RM, Becker BA (1975) Teratogenicity, fetal toxicity, and placental transfer of lead nitrate in rats. Toxicol Appl Pharmacol 31:72–82
Mousa AM, Al-Fadhli AS, Rao MS, Kilarkaje N (2015) Gestational lead exposure induces developmental abnormalities and up-regulates apoptosis of fetal cerebellar cells in rats. Drug Chem Toxicol 38:73–83
Sausbier M, Hu H, Arntz C, Feil S, Kamm S, Adelsberger H, Sausbier U, Sailer CA, Feil R, Hofmann F, Korth M, Shipston MJ, Knaus HG, Wolfer DP, Pedroarena CM, Storm JF, Ruth P (2004) Cerebellar ataxia and Purkinje cell dysfunction caused by Ca2+-activated K+ channel deficiency. Proc Natl Acad Sci U S A 101:9474–9478
Hosseini-Sharifabad M, Sabahi A (2014) Stereological estimation of granule cell number and Purkinje cell volume in the cerebellum of noise-exposed young rat. Iran J Med Sci 39:387–390
Mani J, Chaudhary N, Kanjalkar M, Shah PU (1998) Cerebellar ataxia due to lead encephalopathy in an adult. J Neurol Neurosurg Psychiatry 65:797
Brochin R, Leone S, Phillips D, Shepard N, Zisa D, Angerio A (2008) The cellular effect of lead poisoning and its clinical picture. GU Journal of Healh Science 5:2
Bernocchi G, Bottone MG, Piccolini VM, Dal Bo V, Santin G, De Pascali SA, Migoni D, Fanizzi FP (2011) Developing central nervous system and vulnerability to platinum compounds. Chemother Res Pract 2011:315418
Takeda H, Yoshiki A, Nishikawa SI, Nishikawa S, Kunisada T, Sakakura T, Amanuma H, Kusakabe M (1992) Expression of c-kit, a proto-oncogene of the murine W locus, in cerebella of normal and neurological mutant mice: immunohistochemical and in situ hybridization analysis. Differentiation 51:121–127
Kim D, Im JO, Won YJ, Yoon SY, Lee EJ, Lee JH, Hong HN (2003) Upregulation of c-Kit receptor and stem cell factor in cerebellar inhibitory synapses in response to kainic acid. J Neurosci Res 71:72–78
Hertz L (2013) The glutamate-glutamine (GABA) cycle: importance of late postnatal development and potential reciprocal interactions between biosynthesis and degradation. Front Endocrinol (Lausanne) 4:59
Yeganeh-Doost P, Gruber O, Falkai P, Schmitt A (2011) The role of the cerebellum in schizophrenia: from cognition to molecular pathways. Clinics (Sao Paulo) 66:71–77
Lasley SM, Gilbert ME (2002) Rat hippocampal glutamate and GABA release exhibit biphasic effects as a function of chronic lead exposure level. Toxicol Sci 66:139–147
Yusa T (2001) Increased extracellular ascorbate release reflects glutamate re-uptake during the early stage of reperfusion after forebrain ischemia in rats. Brain Res 897:104–113
Acknowledgements
This research was supported by the faculty research fund of Konkuk University in 2009.
Author information
Authors and Affiliations
Contributions
SMN, SCA, THG, JSS, and JHL conceived and conducted the experiments, and collected the data. SMN and JHL analyzed the data and wrote the manuscript. SSN and BJC participated in designing and discussing the study. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Nam, S.M., Ahn, S.C., Go, TH. et al. Ascorbic Acid Ameliorates Gestational Lead Exposure-Induced Developmental Alteration in GAD67 and c-Kit Expression in the Rat Cerebellar Cortex. Biol Trace Elem Res 182, 278–286 (2018). https://doi.org/10.1007/s12011-017-1086-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-1086-z