Abstract
Lead exposure can cause cognitive dysfunction in children, thus it still raises important public health concerns in China and other countries. However, the underlying molecular mechanisms are still not well defined. In this study, we aimed to elucidate the mechanisms underlying lead neurotoxicity by focusing on alterations of synaptic proteins in the mouse hippocampus at the early life. Mother mice and their offspring were exposed to 0, 0.5, 1.0, and 2.0 g/L lead via drinking water from the first day of gestation until postnatal day (PND) 40. Synaptic ultrastructure and expressions of postsynaptic density protein-95 (PSD-95), neuronal nitric oxide synthase (nNOS) and synaptophysin (SYP) at both protein and gene levels in the hippocampus were analyzed. The results revealed that developmental lead exposure caused a diminished postsynaptic density in the hippocampus. Moreover, the protein levels of PSD-95, nNOS, and SYP decreased significantly due to developmental lead exposure. On the other hand, the messenger RNA (mRNA) levels of PSD-95 and SYP decreased significantly in PND 40 mice exposed to lead. Collectively, developmental lead exposure might result in decreased protein and gene expressions of both presynaptic and postsynaptic proteins. Our findings raised a possibility that alterations of synaptic proteins in the hippocampus induced by lead exposure at the early life might serve an important role for the subsequent intellectual impairments, e.g., deficits in spatial learning and memory ability at later ages shown in our recently published paper.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The developing brain is highly susceptible to lead exposure, and deficits in cognitive function are the principal effects of lead exposure in children, thus it still raises important public health concerns in both China and other countries [1]. However, the underlying molecular mechanisms are still not well defined. Learning and memory are important aspects of cognitive function, and the synaptic plasticity plays an essential role in this process [2, 3]. A variety of studies have demonstrated that lead is a potent non-competitive antagonist of the N-methyl-d-aspartate receptor (NMDAR) due to the chemical similarity of lead with calcium, and developmental lead exposure could result in decreased gene and protein expression of NMDAR subunits in the hippocampus that exhibit deficits in long-term potentiation (LTP) and spatial learning ability [4–6].
The NMDAR is one of the three main types of glutamatergic receptors in the brain and is composed of an obligatory NR1 subunit and accessory subunits. It is targeted to excitatory synapses where it functions in neural plasticity [7]. It is well documented that NMDAR is linked by the postsynaptic density protein 95 (PSD-95) with the downstream signaling proteins such as neuronal nitric oxide synthase (nNOS) in the postsynaptic membranes [8–10]. It was suggested that the physical approximation of nNOS to NMDAR subunits permitted via PSD-95 determines the specificity for nNOS to synthesize NO induced by Ca2+ influx through NMDAR [9, 11, 12]. The cumulated data have demonstrated that the association of nNOS with NMDAR, via PSD-95, plays an important role in a range of normal neuronal functions including formation of learning and memory [10, 13]. Thus, the previous results raised a possibility that NMDAR-mediated nNOS-NO signaling might be influenced by developmental lead exposure.
It is well established that NO is produced from l-arginine in three distinct isoforms of NOS, namely, nNOS, inducible NOS (iNOS), and endothelial NOS (eNOS). However, nNOS is the principal form expressed in the brain, and the predominant source of NO in the neurons [14, 15]. Studies have shown that the enzymatic activity of nNOS is primarily regulated by interaction with Ca2+ and calmodulin complex when intracellular Ca2+ increases [16, 17]. Although there are several distinct Ca2+ pools at the synapse, only Ca2+ influx through the NMDAR efficiently activates nNOS. It has been reported that reduced PSD-95 expression might block Ca2+-dependent nNOS activation in the brain [18].
As an important gaseous signaling molecule, NO has the potential to act as an anterograde or retrograde modulator in the brain and is involved in various cellular functions in the brain [15, 19, 20]. It exhibits properties ideally suited for a transcellular messenger since NO is highly soluble and mobile, widely synthesized, and of limited lifetime. It has been extensively studied that NO functions as a retrograde messenger in the hippocampal LTP. Of which, NO is synthesized in the postsynaptic membranes in response to Ca2+-influx induced by postsynaptic NMDAR activation, travels through the extracellular space, and acts directly in the presynaptic neuron to enhance transmitter release during LTP [14, 21]. The major physiological target of NO is the soluble guanylyl cyclase-coupled receptor. The ensuing accumulation of cyclic guanosine monophosphate (cGMP) may then engage protein kinases to initiate phosphorylation cascades, leading to synaptic vesicle exocytosis and transmitter release at presynaptic terminals [22].
Synaptophysin (SYP) is a synaptic vesicle protein proposed to regulate the endocytosis and exocytosis of neurotransmitters and participate in recycling of synaptic vesicles [23, 24]. Expression of SYP is regarded as a reliable marker of presynaptic elements and has been used to evaluate synaptogenesis or synapse loss induced by the exogenous chemical treatment [24]. In addition, studies have suggested that decreased expression of SYP is correlated positively with impaired learning and memory capacity in animal models [25–27].
Taken in aggregate, all the available evidence seems to suggest that alterations of NMDAR subunits expressions found in the developing hippocampus of lead-exposed offspring might be related to changes in some important synaptic proteins, such as PSD-95, nNOS, and SYP. However, to date, there are no relevant studies specifically addressing alterations of these synaptic proteins in the developing brain induced by lead exposure. We thus undertook this study to investigate the effects of developmental lead exposure on these synaptic proteins at protein and gene levels in the hippocampus of mice during developmental stages.
Materials and Methods
Animal Care and Use Statement
All animal studies were approved by the Scientific Research Committee of China Medical University and have been conducted in accordance with the Chinese National Guidelines for the Care and Use of Laboratory Animal in animal experiments.
Animals
Albino mice with body weights ranging from 23 to 27 g were obtained from the Experimental Animal Centre of China Medical University. Animal room was kept at a temperature of 20 ± 2 °C with a 12-h light/dark cycle and a relative humidity of 50–60 %. Mice were housed in the sterilized plastic cages with wood shaving bedding and given food and water ad libitum.
Experimental Procedures
After 1-week adaptation, female mice were mated with healthy male mice. Gestation was determined by checking vaginal plug and vaginal smears twice daily. Conception was estimated by vaginal plug or sperm positive. Twenty-four pregnant mice were randomly divided into four groups, six pregnant mice in each group. They were fed separately (one per cage) and given drinking water containing 0, 0.5, 1.0, or 2.0 g/L lead in lead acetate from the first day of gestation until the end of lactation. The day of birth was designated as postnatal day (PND) 1. The pups were weaned and housed in a colony room on PND 21, and continually drank water with lead acetate until PND 40. Pregnant mice and their offspring in the control group drank distilled water.
During the course of study, the pups were carefully observed for the signs of abnormality, and weighed on PND 10, 20, and 40. Lead addition did not apparently affect the consumption of drinking water compared to the control group.
On PND 10, 20, and 40, the pups taken one per litter were decapitated under deep ether anesthesia. The hippocampal formation was dissected rapidly, and stored at −80 °C for western blot and real-time reverse transcription polymerase chain reaction (RT-PCR) analysis. At the same time of sacrifice, another six pups from different litters in each group were randomly selected, three pups for transmission electron microscopy, and the others were for immunofluorescence analysis.
Reagents and Laboratory Wares
All reagents used in the present study were analytical grade, and acids were of specific grade for lead analysis. All glasses and plastic wares were washed with detergent and acid, and rinsed with redistilled water to be free of metal leaching. Water used in this study was doubly distilled.
Analysis Procedures
Brain Lead Levels
About 50 mg of cerebral samples obtained from PND 10, 20, and 40 mice were wet digested by heating with 1 mL of nitric acid, and finally diluted to 10 mL with ultrapure water. Brain lead levels were detected by atomic absorption spectrophotometry-Graphite Furnace (Hitachi Z-2000, Japan). Instrumental parameters used for sample analysis were as follows: drying for 65 s between 85 and 120 °C, charring for 30 s between 300 and 480 °C, atomization for 3 s at 1850 °C, and cleaning for 4 s at 2700 °C. Photometry was performed at a wavelength of 283.3 nm, using a lead hollow cathode lamp with a current supply of 7.5 mA, taking advantage of Zeeman background correction. Duplicate determinations were carried out for each sample and the average was taken as a measure. Quality control was performed by determination of the reference samples from the Ministry of Environmental Protection Key Lab of Environment, PR China. The test results were in good agreement with the reference values.
Synaptic Ultrastructure Observation
Mice were anesthetized by ether and then transcardially perfused with glutaraldehyde. After perfusion, the mice were decapitated and the entire brain was taken and rapidly fixed in glutaraldehyde. After fixation for 24 h, the hippocampal tissues of PND 10, 20, and 40 mice were quickly dissected and separated into thin slices. They were fixed immediately with 2.5 % glutaraldehyde at 4 °C overnight. Washed three times in phosphate-buffered saline (PBS), these slices were fixed in 1 % osmium tetroxide, stained with 2 % aqueous solution of uranyl acetate, and then dehydrated with different concentrations of ethanol and acetone gradient, finally embedded in epoxy resin. Ultra-thin sections (70 nm) were cut with ultramicrotome, collected on copper grids, and then stained with 4 % uranyl acetate and lead citrate. Synaptic ultrastructure was viewed under a transmission electron microscope (Hitachi 7650, Japan). The thickness of the postsynaptic density and the width of synaptic cleft were measured using an image analyzing software (NIS-Elements BR 3.2).
Immunofluorescence
Mice were deeply anesthetized with ether, and perfused through the heart with PBS (pH = 7.4) containing 0.02 % heparin, followed by 4 % paraformaldehyde in PBS. Then, the brains were quickly removed in a cold plate, washed in PBS, and fixed overnight in the 4 % paraformaldehyde. The fixed samples were embedded in paraffin and sectioned into 5 μm thickness when intact structure of hippocampus was observed in the slices. After deparaffinizing in xylene, the slices were incubated for 30 min with normal donkey serum (Jackson ImmunoResearch, PA, USA) to block nonspecific binding of antiserum. Then, the sections were incubated with rabbit antibody against PSD-95 (1:100, Abcam, MA, USA); goat antibody against nNOS (1:100, Abcam, MA, USA); and rabbit antibody against SYP (1:50, Abcam, MA, USA) at 4 °C overnight. On the following day, donkey anti-rabbit Alexa Fluor 488 (green) and donkey anti-goat Alexa Fluor 594 (red) conjugated secondary antibodies (1:100, Abcam, MA, USA), were added in dark room and incubated for 30 min at 37 °C. Hoechst 33258 (Sigma) was used to stain the nucleus for 5 min at room temperature. Finally, they were observed under a fluorescence microscope (Olympus BX50). Digital images were captured using digital camera system (Olympus SC35). For negative controls, the primary antibodies were omitted.
Western Blot Analysis
At each experimental age, the protein expressions of PSD-95, nNOS, and SYP were analyzed using western blot techniques. Briefly, the hippocampal tissues of mice were homogenized, and then the lysates were centrifuged at 4 °C, 12,000×g for 20 min. Protein concentrations were measured with a BCA protein assay kit (Pierce, IL, USA). An equal amount of protein (30 μg/lane) were loaded on the 7 % SDS-polyacrylamide gels and resolved by standard electrophoresis. Subsequently, the gels were transferred on the polyvinylidene difluoride (PVDF) filters (Millipore, MA, USA). The filters were incubated with a specific primary antibody against PSD-95 (1:1000, Abcam, MA, USA); nNOS (1:500, Abcam, MA, USA); SYP (1:500, Abcam, MA, USA); and β-actin (1:2000, Santa Cruz, CA, USA), and then visualized by chemiluminescence. For quantization of immunoblot signals, the band intensity was measured by densitometry using an image analyzing software (Gel-Pro analyzer v4.0), and normalized to β-actin level (served as the internal standard) from the same blot.
Quantitative Real-Time RT-PCR
Total RNA was extracted from the hippocampal tissues of mice using TRIzol Reagent (Invitrogen, CA, USA). First strand of complementary DNA (cDNA) was synthesized from the total RNA using the PrimeScript RT reagent Kit (Takara, Tokyo, Japan) and the random primers of PSD-95, nNOS, SYP, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (as the housekeeping gene). Thereafter, the cDNA was served as templates for real-time PCR amplification using the SYBR Premix Ex Taq II (Takara, Tokyo, Japan) and ABI 7500 real-time PCR System (Applied Biosystems, CA, USA). To amplify a fragment of PSD-95, nNOS, SYP, and GAPDH, the following primer pairs detailed in Table 1 were used. Amplification was conducted for 40 cycles of 5 s at 95 °C and 34 s at 60 °C. Results were analyzed using the comparative Ct method. RNA abundance were expressed as 2−ΔΔCt for the target messenger RNA (mRNA) relative to those of the GAPDH gene (as the internal control), and presented as fold change versus contralateral control samples.
Statistical Analysis
All values were expressed as means ± standard deviation (SD), and analyzed using the SPSS for Windows, version 16.0 (SPSS Inc, IL, USA). Significant difference was evaluated by analysis of variance test (one-way ANOVA). Post hoc tests were analyzed by Student-Newman-Keuls test (SNK). The level of statistical significance was defined as p < 0.05.
Results
Our previously published paper has reported that the body weight of mice in both control and lead-exposed groups increased along with development. There was no significant difference among groups except for PND 40 mice in 2.0 g/L lead-exposed group (decrease to 87.4 % of the control group, p < 0.05). Furthermore, none of the lead-exposed mice showed any neurological, gastroenterology, or other symptoms of lead poisoning. On the other hand, brain lead levels increased in a dose-dependent manner, of which, brain lead levels in 1.0 and 2.0 g/L lead-exposed group and in PND 40 mice of 0.5 g/L lead-exposed group increased significantly. In addition, brain lead levels in 2.0 g/L lead-exposed group were also significantly higher than those in 0.5 and 1.0 g/L lead-exposed groups at the same age.
The spatial learning ability tested by place trail in Morris water maze could be significantly impaired by 0.5 g/L lead exposure (the escape latency increased to 138.1 % of the control group from the second training day, p < 0.05).
Changes in Synaptic Ultrastructure of the Hippocampus Induced by Developmental Lead Exposure
The representative photomicrographs shown in Figs. 1, 2, and 3 illustrated the synaptic ultrastructure in the hippocampus of PND 10, 20, and 40 mice, respectively. As shown in these photomicrographs, the presynaptic and postsynaptic membranes and synaptic clefts were clearly visible, and the postsynaptic densities were abundant in the postsynaptic membrane of hippocampus in the control group at different developmental stages. However, the hippocampal postsynaptic densities in the lead-exposed groups decreased obviously as compared to the control group at the same age. Furthermore, as shown in Table 2, the thickness of postsynaptic densities in 1.0 and 2.0 g/L lead-exposed groups and those of PND 40 mice in 0.5 g/L lead-exposed group were significantly thinner than in the control group at the same age. In addition, those in 1.0 and 2.0 g/L lead-exposed groups were also significantly thinner than in 0.5 g/L lead-exposed group at the same age. But as illustrated in Figs. 1, 2, and 3 and in Table 3, the width of synaptic cleft in hippocampus of lead-exposed groups did not differ significantly with the control group at the same age.
Changes in the hippocampal synaptic ultrastructure of PND 10 mice induced by developmental lead exposure. PND postnatal day, PSD postsynaptic density, SC synaptic cleft, and SV synaptic vesicle. The representative photomicrographs were from three independent experiments and captured with a Hitachi 7650 transmission electron microscope (×30,000). Scale bar = 0.5 μm. a Control group; b 0.5, c 1.0, and d 2.0 g/L lead-exposed group
Changes in the hippocampal synaptic ultrastructure of PND 20 mice induced by developmental lead exposure. PND postnatal day, PSD postsynaptic density, SC synaptic cleft, and SV synaptic vesicle. The representative photomicrographs were from three independent experiments and captured with a Hitachi 7650 transmission electron microscope (×30,000). Scale bar = 0.5 μm. a Control group; b 0.5, c 1.0, and d 2.0 g/L lead-exposed group
Changes in the hippocampal synaptic ultrastructure of PND 40 mice induced by developmental lead exposure. PND postnatal day, PSD postsynaptic density, SC synaptic cleft, and SV synaptic vesicle. The representative photomicrographs were from three independent experiments and captured with a Hitachi 7650 transmission electron microscope (×30,000). Scale bar = 0.5 μm. a Control group; b 0.5, c 1.0, and d 2.0 g/L lead-exposed group
Changes in Protein and mRNA Expression of PSD-95 Induced by Developmental Lead Exposure
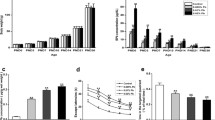
Effects of developmental lead exposure on PSD-95 expression at protein and gene levels in the hippocampus at different developmental stages were summarized in Figs. 4, 5, 6, and 7. The photomicrographs shown in Figs. 4, 5, and 6, illustrated the double immunofluorescence staining on coexpression of PSD-95 with nNOS in the hippocampal CA1 region of mice at the early developmental stages. It was disclosed that both PSD-95 and nNOS were positively stained in the cytoplasm of pyramidal layer neurons and overlapped with each other well. Of which the immunoreactivities to PSD-95 decreased obviously in 2.0 g/L lead-exposed group as compared to each control group. Consistent with the results of immunofluorescence staining, the protein levels of PSD-95 in 2.0 g/L lead-exposed group decreased significantly compared to the control group and the other lead-exposed groups at the same age (Fig. 7b). Likewise, the mRNA levels of PSD-95 in PND 10 and 20 mice of 2.0 g/L lead-exposed group decreased significantly as compared to the control and the other lead-exposed groups at the same age (Fig. 7c). In addition, levels of PSD-95 mRNA in PND 40 mice of lead-exposed groups decreased significantly compared to the control group, but the differences among the lead-exposed groups were not significant (Fig. 7c).
Changes of immunofluorescence staining for PSD-95 (green) and nNOS (red) in the hippocampal CA1 region of PND 10 mice induced by developmental lead exposure. PND postnatal day, PSD-95 postsynaptic density protein-95, and nNOS neuronal nitric oxide synthase. The representative photomicrographs were from three independent experiments and captured with Olympus BX50 microscope (×400). Scale bar = 50 μm. a Control group; b 0.5, c 1.0, and d 2.0 g/L lead-exposed group
Changes of immunofluorescence staining for PSD-95 (green) and nNOS (red) in the hippocampal CA1 region of PND 20 mice induced by developmental lead exposure. PND postnatal day, PSD-95 postsynaptic density protein-95, and nNOS neuronal nitric oxide synthase. The representative photomicrographs listed above were from three independent experiments and captured with Olympus BX50 microscope (×400). Scale bar = 50 μm. a Control group; b 0.5, c 1.0, and d 2.0 g/L lead-exposed group
Changes of immunofluorescence staining for PSD-95 (green) and nNOS (red) in the hippocampal CA1 region of PND 40 mice induced by developmental lead exposure. PND postnatal day, PSD-95 postsynaptic density protein-95, and nNOS neuronal nitric oxide synthase. The representative photomicrographs were from three independent experiments and captured with Olympus BX50 microscope (×400). Scale bar = 50 μm. a Control group; b 0.5, c 1.0, and d 2.0 g/L lead-exposed group
Changes in the expression of PSD-95 induced by developmental lead exposure. PND postnatal day and PSD-95 postsynaptic density protein-95. Mice were sacrificed on PND 10, 20, and 40, and their hippocampal tissues were immediately dissected out. Total proteins (30 μg/lane) in the hippocampus were collected and separated by SDS-PAGE; lastly they were transferred to PVDF membranes and immunoblotted for PSD-95. a Western blot analysis. Images were the representative results of six separate experiments for each group. b Densitometric analysis of western blots. The relative intensity in arbitrary units compared to β-actin. c Quantitation of mRNA by real-time RT-PCR. Gene expression were normalized to GAPDH and presented as fold change versus the control group. Data were given as mean ± SD, n = 6. Significant difference was defined as p < 0.05 *vs control group, #vs 0.5 g/L lead-exposed group, and +vs 1.0 g/L lead-exposed group
Changes in Protein and mRNA Expression of nNOS Induced by Developmental Lead Exposure
Effects of developmental lead exposure on nNOS expression at protein and gene levels in the hippocampus at different developmental stages were summarized in Figs. 4, 5, 6, and 8. As shown in Figs. 4, 5, and 6, the immunoreactivities to nNOS decreased obviously in 1.0 and 2.0 g/L lead-exposed groups compared to each control group. Consistent with the results of immunofluorescence staining, the protein levels of nNOS in 1.0 and 2.0 g/L lead-exposed groups, and those of PND 40 mice in 0.5 g/L lead-exposed group decreased significantly as compared to each control group (Fig. 8b). Moreover, those of PND 20 and 40 mice in 2.0 g/L lead-exposed group were also significantly lower than in the 0.5 and 1.0 g/L lead-exposed groups at the same age (Fig. 8b). However, mRNA levels of nNOS in the lead-exposed groups did not differ significantly with each control group during the early developmental stages (Fig. 8c).
Changes in the expression of nNOS induced by developmental lead exposure. PND postnatal day and nNOS neuronal nitric oxide synthase. Mice were sacrificed on PND 10, 20, and 40, and their hippocampal tissues were immediately dissected out. Total proteins (30 μg/lane) in the hippocampus were collected and separated by SDS-PAGE; lastly they were transferred to PVDF membranes and immunoblotted for nNOS. a Western blot analysis. Images were the representative results of six separate experiments for each group. b Densitometric analysis of western blots. The relative intensity in arbitrary units compared to β-actin. c Quantitation of mRNA by real-time RT-PCR. Gene expression were normalized to GAPDH and presented as fold change vs the control group. Data were given as mean ± SD, n = 6. Significant difference was defined as p < 0.05 *vs control group, #vs 0.5 g/L lead-exposed group, and +vs 1.0 g/L lead-exposed group
Changes in Protein and mRNA Expression of SYP Induced by Developmental Lead Exposure
Effects of developmental lead exposure on SYP expression at protein and gene levels in the hippocampus at different developmental stages were summarized in Figs. 9 and 10. The photomicrographs shown in Fig. 9, illustrated the immunofluorescence staining of SYP in the pyramidal layer neurons of hippocampal CA1 region at different developmental stages. Of which, the immunoreactivities to SYP in the lead-exposed groups decreased gradually in a dose-dependent manner at the early developmental stages. Consistent with the results of immunofluorescence staining, except for PND 10 mice in 0.5 g/L lead-exposed group, the protein levels of SYP in the lead-exposed groups were significantly lower than those in the control group at the same age (Fig. 10b). In addition, those in 2.0 g/L lead-exposed group decreased significantly compared to both 0.5 and 1.0 g/L lead-exposed groups at the same age (Fig. 10b). On the other hand, mRNA levels of SYP in hippocampus of PND 40 mice in the lead-exposed groups decreased significantly as compared to the control group (Fig. 10c). Furthermore, those of PND 10 and 20 mice subjected with lead exposure exhibited no statistical differences compared to each control group (Fig. 10c).
Changes of immunofluorescence staining for SYP (green) in the hippocampal CA1 region of mice induced by developmental lead exposure. PND postnatal day and SYP synaptophysin. The representative photomicrographs were from three independent experiments and captured with Olympus BX50 microscope (×400). Scale bar = 50 μm. a Control group; b 0.5, c 1.0, and d 2.0 g/L lead-exposed group
Changes in the expression of SYP induced by developmental lead exposure. PND postnatal day and SYP synaptophysin. Mice were sacrificed on PND 10, 20, and 40, and their hippocampal tissues were immediately dissected out. Total proteins (30 μg/lane) in the hippocampus were collected and separated by SDS-PAGE; lastly, they were transferred to PVDF membranes and immunoblotted for SYP. a Western blot analysis. Images were the representative results of six separate experiments for each group. b Densitometric analysis of western blots. The relative intensity in arbitrary units compared to β-actin. c Quantitation of mRNA by real-time RT-PCR. Gene expression were normalized to GAPDH and presented as fold change vs the control group. Data were given as mean ± SD, n = 6. Significant difference was defined as p < 0.05 *vs control group, #vs 0.5 g/L lead-exposed group, +vs 1.0 g/L lead-exposed group
Discussion
Although considerable researches have been devoted to the understanding of how developmental lead exposure influences the expressions of NMDAR subunits, few studies have addressed how the changes of NMDAR subunits expressions correlate with the other synaptic proteins in both presynaptic and postsynaptic membranes. In the recently published paper, we have demonstrated that developmental lead exposure could inhibit the expressions of NMDAR subunits in the hippocampus, and furthermore contribute to deficits in learning and memory ability in mice [6]. In this study, we characterized the alterations of some important synaptic proteins, such as PSD-95, nNOS, and SYP in the hippocampus of mice subjected to developmental lead exposure.
The observation of synaptic ultrastructure in this study strongly indicated that the synaptic morphology in the hippocampus of lead-exposed mice were abnormal. Alterations in the synaptic morphology can cause synaptic dysfunction and leads to abnormality in synaptic transmission efficiency. The changes of synapses in structure and function in response to the stimuli are referred to as synaptic plasticity [28], which may occur at several levels, including alterations in postsynaptic proteins and receptors, postsynaptic dendrites, and presynaptic function [29, 30]. It has been reported that the thickness of postsynaptic density is an important morphological indicator of synaptic plasticity and closely correlated with the synaptic transmission efficiency [31]. Thus, it was speculated that the morphological changes of postsynaptic density observed in this study might result from the decreased expression of postsynaptic proteins and receptors, such as PSD-95 and NMDAR subunits, and contribute to deficit in learning and memory ability of lead-exposed mice.
Furthermore, results of this study also disclosed that the protein expressions of PSD-95, nNOS, and SYP could be suppressed by developmental lead exposure. Of which, the changes of SYP were the most sensitive to lead exposure, and nNOS were more sensitive than PSD-95. On the other hand, especially in the PND 40 mice, the mRNA expression of PSD-95 and SYP in the hippocampus could be downregulated by lead exposure, which suggested that lead effects on PSD-95 expression were at the transcriptional level. Whereas, lead effects on nNOS and SYP expression were mainly at the translational level.
An increasing body of evidence suggested that PSD-95 is the major component of the postsynaptic density and appears to be important for molecular organization of postsynaptic complex in neurons [32–34]. Studies on synaptic development in cultured neurons indicated that PSD-95 clusters at synapses before other postsynaptic proteins [35, 36], suggesting that PSD-95 may play a primary role in organizing and localizing proteins to the postsynaptic density. It is well established that PSD-95 consists of five protein interaction domains, via these domains, PSD-95 links the NMDAR subunits with the signaling proteins and assembles them into a macromolecular complex [10, 12]. In this study, although developmental lead exposure could downregulate PSD-95 expression at the transcriptional level, the changes of PSD-95 at both protein and gene levels are not sensitive to lead effects, which were induced only by the high levels of lead exposure. Thus, it was speculated that the lead effects on nNOS expression might not be related to the changes of PSD-95. One possible mechanism underlying lead-induced downregulation of PSD-95 might be due to the inhibitory effects of lead on NMDAR function, which may reduce Ca2+ influx via NMDAR channels, subsequently influence Ca2+/calmodulin-dependent signaling pathways, and finally hamper the activation of transcription factors that regulate PSD-95 gene expression. Consistent with our findings, studies performed by Guilarte and McGlothan [4] have shown that decreased mRNA levels of NR1 and NR2A subunits of NMDAR in the hippocampus were associated with deficits in spatial learning ability in the rats exposed to environmentally relevant levels of lead; however, absence of changes in PSD-95 gene expression was presented in the same animals. Moreover, results of an in vitro study reported by Neal et al. [37] have shown that PSD-95 puncta density did not change during lead exposure, although their data suggested that lead exposure could reduce the levels of NR2A-NMDAR due to arrest or delay, the critical developmental switch from NR2B- to NR2A-NMDAR during synaptogenesis [5, 37, 38].
Further, since NMDAR-mediated Ca2+ signaling is one of the most potent activators of nNOS, alteration in expression of NMDAR subunits might result in abnormal expression and activity of nNOS in the hippocampus of lead-exposed animals. Consistent with this hypothesis, our findings suggested that nNOS protein levels in the hippocampus were lowered obviously by developmental lead exposure, which was further supported by the studies reported by other researchers [39–41]. Taken together, our present and previous studies demonstrated that by decreasing the expressions of NMDAR subunits, developmental lead exposure might modify NMDAR-mediated Ca2+ signaling at the postsynaptic membranes, which is likely to alter the localization and activation of nNOS; furthermore affecting NO production and NO-cGMP signaling transmission in the presynaptic membranes. The candidate molecule mechanism of these changes might result from the competition of lead with Ca2+ at the calmodulin binding sites.
It has been reported that NO-cGMP signaling in the presynaptic membranes is associated with enhanced neurotransmitter release, increased release volume, and increased SYP expression at glutamatergic synapses in both animal and cell culture models [22, 42–44]. Study reported by Neal et al. [38, 45] showed that protein levels of SYP and vesicular neurotransmitter release in lead-exposed neurons were decreased compared to the control; however, this loss of SYP protein could be recovered by the exogenous NO, suggesting that SYP expression is linked to NO-cGMP signaling, and lead exposure might inhibit NO production. Therefore, alteration in SYP protein expression might be a result of inhibition of NMDAR-dependent NO signaling, and may be responsible for the impaired vesicular release in lead-exposed neurons [38]. However, NMDARs mediate multiple trans-synaptic pathways besides NO-mediated signaling and these alternated pathways may also be implicated in the effects of lead on SYP protein expression. Studies reported by Neal et al. [38] disclosed that effects of lead exposure on both synaptic protein expressions and vesicular release could be recovered after treatment with exogenous brain-derived neurotrophic factor (BDNF) in vitro. Their results suggested that dysfunction of the BDNF-mediated pathway may also play an important role in the effects of lead exposure on synaptic development and function. Thus, changes of NO-cGMP signaling might only contribute to the part reason for decreased protein levels of SYP, which may explain why SYP protein expression was more sensitive to lead effects than nNOS.
In summary, our results provided an experimental evidence for the characteristics of PSD-95, nNOS, and SYP expressions associated with developmental lead exposure, which raised a possibility that lead exposure during synaptogenesis may result in changes in both presynaptic and postsynaptic protein levels. However, the detailed mechanism through which lead exposure affects the synaptic protein levels remains to be explored. Since alterations in the specific localization and expression of these proteins in the hippocampus can influence the properties of synaptic transmission and even cause the deficit in the learning and memory ability, a better understanding of the changes of these synaptic proteins may promote to elucidate the mechanism underlying lead-induced cognitive deficits in children.
References
Zhang S, Dai Y, Xie X, Fan Z, Tan Z, Zhang Y (2009) Surveillance of childhood blood lead levels in 14 cities of China in 2004–2006. Biomed Environ Sci 22(4):288–296
Liu H, Zhang J, Zheng P, Zhang Y (2005) Altered expression of MAP-2, GAP-43, and synaptophysin in the hippocampus of rats with chronic cerebral hypoperfusion correlates with cognitive impairment. Brain Res Mol Brain Res 139(1):169–177
Silva AJ (2003) Molecular and cellular cognitive studies of the role of synaptic plasticity in memory. J Neurobiol 54(1):224–237
Guilarte TR, McGlothan JL (2003) Selective decrease in NR1 subunit splice variant mRNA in the hippocampus of Pb2+-exposed rats: implications for synaptic targeting and cell surface expression of NMDAR complexes. Brain Res Mol Brain Res 113(1–2):37–43
Toscano CD, Guilarte TR (2005) Lead neurotoxicity: from exposure to molecular effects. Brain Res Brain Res Rev 49(3):529–554
Yu H, Li T, Cui Y, Liao Y, Wang G, Gao L, Zhao F, Jin Y (2014) Effects of lead exposure on D-serine metabolism in the hippocampus of mice at the early developmental stages. Toxicology 325:189–199
Carroll RC, Zukin RS (2002) NMDA-receptor trafficking and targeting: implications for synaptic transmission and plasticity. Trends Neurosci 25(11):571–577
Kornau H-C, Schenker LT, Kennedy MB, Seeburg PH (1995) Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science 269(5231):1737–1740
Niethammer M, Kim E, Sheng M (1996) Interaction between the C terminus of NMDA receptor subunits and multiple members of the PSD-95 family of membrane-associated guanylate kinases. J Neurosci 16(7):2157–2163
Christopherson KS, Hillier BJ, Lim WA, Bredt DS (1999) PSD-95 assembles a ternary complex with the N-Methyl-D-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain. J Biol Chem 274(39):27467–27473
Jaffrey SR, Snowman AM, Eliasson MJ, Cohen NA, Snyder SH (1998) CAPON: a protein associated with neuronal nitric oxide synthase that regulates its interactions with PSD95. Neuron 20(1):115–124
Sattler R, Xiong Z, Lu WY, Hafner M, MacDonald JF, Tymianski M (1999) Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science 284(5421):1845–1848
Migaud M, Charlesworth P, Dempster M, Webster LC, Watabe AM, Makhinson M, He Y, Ramsay MF, Morris RG, Morrison JH, O’Dell TJ, Grant SG (1998) Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature 396(6710):433–439
Prast H, Philippu A (2001) Nitric oxide as modulator of neuronal function. Prog Neurobiol 64(1):51–68
Boehning D, Snyder SH (2003) Novel neural modulators. Annu Rev Neurosci 26:105–131
Bredt DS, Snyder SH (1990) Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A 87(2):682–685
Abu-Soud HM, Yoho LL, Stuehr DJ (1994) Calmodulin controls neuronal nitric-oxide synthase by a dual mechanism. Activation of intra- and interdomain electron transfer. J Biol Chem 269(51):32047–32050
Yan X, Song B, Zhang G (2004) Postsynaptic density protein 95 mediates Ca2+/calmodulin-dependent protein kinase II-activated serine phosphorylation of neuronal nitric oxide synthase during brain ischemia in rat hippocampus. Neurosci Lett 355(3):197–200
Garthwaite J (2008) Concepts of neural nitric oxide-mediated transmission. Eur J Neurosci 27(11):2783–2802
Taqatqeh F, Mergia E, Neitz A, Eysel UT, Koesling D, Mittmann T (2009) More than a retrograde messenger: nitric oxide needs two cGMP pathways to induce hippocampal long-term potentiation. J Neurosci 29(29):9344–9350
Hopper RA, Garthwaite J (2006) Tonic and phasic nitric oxide signals in hippocampal long-term potentiation. J Neurosci 26(45):11513–11521
Arancio O, Kiebler M, Lee CJ, Lev-Ram V, Tsien RY, Kandel ER, Hawkins RD (1996) Nitric oxide acts directly in the presynaptic neuron to produce long-term potentiation in cultured hippocampal neurons. Cell 87(6):1025–1035
Greengard P, Valtorta F, Czernik AJ, Benfenati F (1993) Synaptic vesicle phosphoproteins and regulation of synaptic function. Science 259(5096):780–785
Valtorta F, Pennuto M, Bonanomi D, Benfenati F (2004) Synaptophysin: leading actor or walk-on role in synaptic vesicle exocytosis? Bioessays 26(4):445–453
Smith TD, Adams MM, Gallagher M, Morrison JH, Rapp PR (2000) Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. J Neurosci 20(17):6587–6593
Schmitt U, Tanimoto N, Seeliger M, Schaeffel F, Leube RE (2009) Detection of behavioral alterations and learning deficits in mice lacking synaptophysin. Neuroscience 162(2):234–243
Rao JS, Kellom M, Kim HW, Rapoport SI, Reese EA (2012) Neuroinflammation and synaptic loss. Neurochem Res 37(5):903–910
Marrone DF, Petit TL (2002) The role of synaptic morphology in neural plasticity: structural interactions underlying synaptic power. Brain Res Brain Res Rev 38(3):291–308
Lynch MA (2004) Long-term potentiation and memory. Physiol Rev 84(1):87–136
Rabenstein RL, Addy NA, Caldarone BJ, Asaka Y, Gruenbaum LM, Peters LL, Gilligan DM, Fitzsimonds RM, Picciotto MR (2005) Impaired synaptic plasticity and learning in mice lacking beta-adducin, an actin-regulating protein. J Neurosci 25(8):2138–2145
Di G, Zheng Y (2013) Effects of high-speed railway noise on the synaptic ultrastructure and phosphorylated-CaMKII expression in the central nervous system of SD rats. Environ Toxicol Pharmacol 35(1):93–99
El-Husseini AE-D, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS (2000) PSD-95 involvement in maturation of excitatory synapses. Science 290(5495):1364–1368
Valtschanoff JG, Weinberg RJ (2001) Laminar organization of the NMDA receptor complex within the postsynaptic density. J Neurosci 21(4):1211–1217
Kellom M, Basselin M, Keleshian VL, Chen M, Rapoport SI, Rao JS (2012) Dose-dependent changes in neuroinflammatory and arachidonic acid cascade markers with synaptic marker loss in rat lipopolysaccharide infusion model of neuroinflammation. BMC Neurosci 13:50
Che Y, Tamatani M, Tohyama M (2000) Changes in mRNA for post-synaptic density-95 (PSD-95) and carboxy-terminal PDZ ligand of neuronal nitric oxide synthase following facial nerve transection. Brain Res Mol Brain Res 76(2):325–335
Rao A, Kim E, Sheng M, Craig AM (1998) Heterogeneity in the molecular composition of excitatory postsynaptic sites during development of hippocampal neurons in culture. J Neurosci 18(4):1217–1229
Neal AP, Worley PF, Guilarte TR (2011) Lead exposure during synaptogenesis alters NMDA receptor targeting via NMDA receptor inhibition. Neurotoxicology 32(2):281–289
Neal AP, Stansfield KH, Worley PF, Thompson RE, Guilarte TR (2010) Lead exposure during synaptogenesis alters vesicular proteins and impairs vesicular release: potential role of NMDA receptor-dependent BDNF signaling. Toxicol Sci 116(1):249–263
Chetty CS, Reddy GR, Murthy KS, Johnson J, Sajwan K, Desaiah D (2001) Perinatal lead exposure alters the expression of neuronal nitric oxide synthase in rat brain. Int J Toxicol 20(3):113–120
Nava-Ruíz C, Alcaraz-Zubeldia M, Méndez-Armenta M, Vergara P, Díaz-Ruìz A, Ríos C (2010) Nitric oxide synthase immunolocalization and expression in the rat hippocampus after sub-acute lead acetate exposure in rats. Exp Toxicol Pathol 62(3):311–316
Nava-Ruíz C, Méndez-Armenta M, Ríos C (2012) Lead neurotoxicity: effects on brain nitric oxide synthase. J Mol Histol 43(5):553–563
Wang H, Lu FM, Jin I, Udo H, Kandel ER, de Vente J, Walter U, Lohmann SM, Hawkins RD, Antonova I (2005) Presynaptic and postsynaptic roles of NO, cGK, and RhoA in long-lasting potentiation and aggregation of synaptic proteins. Neuron 45(3):389–403
Steinert JR, Kopp-Scheinpflug C, Baker C, Challiss RAJ, Mistry R, Haustein MD, Griffin SJ, Tong H, Graham BP, Forsythe ID (2008) Nitric oxide is a volume transmitter regulating postsynaptic excitability at a glutamatergic synapse. Neuron 60(4):642–656
Ota KT, Monsey MS, Wu MS, Schafe GE (2010) Synaptic plasticity and NO-cGMP-PKG signaling regulate pre- and postsynaptic alterations at rat lateral amygdale synapses following fear conditioning. PLoS One 5(6):e11236
Neal AP, Stansfield KH, Guilarte TR (2012) Enhanced nitric oxide production during lead (Pb2+) exposure recovers protein expression but not presynaptic localization of synaptic proteins in developing hippocampal neurons. Brain Res 1439:88–95
Acknowledgments
This work was supported by The National Natural Science Foundation of China (no. 31070992), Program for Liaoning Innovative Research Team in University (LT2015028), Liaoning Provincial Natural Science Foundation (no. 20102263), and Science and Technology Plan Project of Educational Department of Liaoning Province (no. L2010559).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declared no competing interests.
Rights and permissions
About this article
Cite this article
Yu, H., Liao, Y., Li, T. et al. Alterations of Synaptic Proteins in the Hippocampus of Mouse Offspring Induced by Developmental Lead Exposure. Mol Neurobiol 53, 6786–6798 (2016). https://doi.org/10.1007/s12035-015-9597-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9597-0