Abstract
The manufacture, purification, and applications of hyaluronic acid (HA) are discussed in this article. Concerning the growing need for affordable, high-quality HA, it is essential to consider diverse production techniques using renewable resources that pose little risk of cross-contamination. Many microorganisms can now be used to produce HA without limiting the availability of raw materials and in an environmentally friendly manner. The production of HA has been associated with Streptococci A and C, explicitly S. zooepidemicus and S. equi. Different fermentation techniques, including the continuous, batch, fed-batch, and repeated batch culture, have been explored to increase the formation of HA, particularly from S. zooepidemicus. The topic of current interest also involves a complex broth rich in metabolites and residual substrates, intensifying downstream processes to achieve high recovery rates and purity. Although there are already established methods for commercial HA production, the anticipated growth in trade and the diversification of application opportunities necessitate the development of new procedures to produce HA with escalated productivity, specified molecular weights, and purity. In this report, we have enacted the advancement of HA technical research by analyzing bacterial biomanufacturing elements, upstream and downstream methodologies, and commercial-scale HA scenarios.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In terms of biopolymers, hyaluronic acid belongs to the family of polysaccharides, which are non-sulfated glycosaminoglycans. Hyaluronic acid contains recurrent units (2000–2500) of β d-glucuronic acid and β N-acetylglucosamine [122]. Many bacterial extracellular matrices, including Streptococcus, contain HA as a crucial component and facilitate adherence and protection. Additionally, it can fool the host’s immune system while infected by acting as a molecular spoof [147]. HA’s molecular weight (MW) contributes to its predominant role in its biological activities and utility [68]; HA with an MW higher than 10kDa is preferable for applications in orthopedics, engineering of tissues, cosmetics, and ophthalmology [3, 49, 79]. Meanwhile, MW under 5kDa helps form the products essential in angiogenesis and obstruct tumor furtherance [68, 134]. Due to its biological functions, which include biocompatibility, angiogenic, and immunostimulatory provinces, HA has a wide variety of utilization in medicines encircling plastic surgery, osteoarthritis (OA) treatment, targeted drug conveyance, skin moisturizers, ophthalmic surgery, and wound alleviation; it also has some practical applications in drug formulations and targeted drug delivery [36, 55, 79].
Karl Meyer and John Palmer discovered an unknown polysaccharide in the vitreous humor of cattle in 1934. They also reported that the isolated polysaccharide contains uronic acid and amino sugar. As a result, they gave the recently registered polysaccharide the name “HA” [10], even though the term “HA” was first used to agree on the nomenclature of the polysaccharide in 1986. Traditionally, HA has been distilled from tissues of animals like an umbilical cord, synovial fluid, rooster combs, and the vitreous humor of bovid animals [106]. Even though the structure of HA is straightforward, it has several properties like semi-flexibility. Also, it acquires a stretched worm-like arbitrary coil-like arrangement in solution [40], showing a very different rheological nature. In the 1950s and 1960s, the administration of the physicochemical properties of HA was concluded. HA chains were entangled even at the lower concentration of 0.1%, resulting in an exceptionally elevated and shears-reliant viscosity [82]. With the help of these properties, HA governed flow resistance and water balance, stabilized structures, and acted as a lubricant [36].
Endre Balazs blew the first thoroughly purified, anti-inflammatory high MW HA across rooster combs and umbilical cords, marking HA’s veritable evolution as a therapeutic product [12]. Plastic intraocular lenses were made using HA in the early 1980s, becoming a vital component in ophthalmic surgery. Numerous other applications of HA have been introduced based on its characteristics and molecular weight. HA helps safeguard the exposed tissues during eye surgery and empowers the reformation of the functional form of the operated site [97]. Eyes lens treatment utilizing lower molecular weight HA derivate–based solution directs to the elevated in vitro lens hydrophilicity [156]. Many clinical investigations have manifested HA’s adequacy in treating osteoarthritis. HA can also be used as an emphatic bone cement supplement component to obtain preferential biological and physicochemical characteristics compared to traditional calcium phosphate cement [41]. It also reduces swelling and helps in the continual production of HA, ultimately enhancing mobility and strength. It also has a critical role in the wound alleviating procedure. After an injury, the healing procedure depends upon a synchronized chain of events: inflammation, granular tissue formation, reepithelization, and remodeling. For the arbitration of these cellular and matrix events, a versatile role is played by HA [151]. Therefore, HA is utilized in abrasions, burns, pressure sores, and metabolic ulcers. HA-based nanofiber mats are also made for clinical wound treatment [158]. It has also been used for skin treatments for ages. With the aging of humans, skin is also affected adversely, and HA content is declined, especially after 50 years. HA with a lower molecular weight can penetrate effortlessly across the skin and restore the HA content. A visco-elastic film is formed after applying HA to the skin’s surface, preventing the penetration of any foreign materials and retaining the skin’s moisture [151]. It also helps in curing skin lesions [87]. These are the reasons that the market value of HA is comparatively higher than other microbial extracellular polysaccharides.
Extraction of HA from rooster combs and other sources is a challenging, costly, and contamination-prone process [22] with many technical impediments. To avoid this, contamination-free microbial production of HA is being practiced nowadays using the bacteria Streptococcus zooepidemicus [94]. It gives enhanced productivity and more adequate recovery processes with the lowest chances of viral contamination [155]. S. zooepidemicus is gram-positive, catalase, and oxidase-negative, facultative anaerobic cocci but also an aerotolerant species [37]. Because of the remarkable productivity of HA using bacterial strains, fermentation is more suitable for large-scale production. Although microbial production has many advantages, it must be cost-effective compared to HA extraction from animal sources. However, this production process is associated with the simultaneous production of other secondary metabolites including lactic acid, which further complicates the downstream processing. To solve this problem, researchers sought genes accountable for HA biomanufacturing, and bacteria such as Agrobacterium, Lactococcus, E. coli, and Bacillus were used as a tool for genetic modification. Genetic modifications were done in these bacteria to articulate HA genes, so they synthesize a good amount of HA [44, 45, 100, 147].
There are numerous techniques for isolating and purifying HA. Maintaining the intrinsic qualities of polysaccharides throughout the process is crucial when choosing an isolation method. Numerous techniques have been researched recently, including scorching water extraction, which is frequently utilized on the theory that most polysaccharides are more soluble in water and more durable in warm water [126]. Using enzymes for digestion is another approach. When cost, purification level, and environmental impact are considered, HA extraction methods have benefits and drawbacks. Enzymatic extraction methods are more expensive and time-consuming than the first two methods discussed previously. It also requires a substantial amount of chemicals to hydrolyze the tissue and further heating to terminate the procedure. On the contrary, employing organic solvents eliminates the need for enzymes and heat treatment, and it is also a rapid, more straightforward, and less expensive process. Present extraction methods need optimization to guarantee an effective separation for the highest purity today; this calls for a low-cost, quick, and environmentally safe procedure [1].
This review presents an in-depth investigation of HA, highlighting potential production and purification techniques, its industrial applications, and current technological advancements to fulfill market requirements. Accordingly, the present review summarizes HA development into its marketed product and discusses future anticipated segments for further improving HA production for industrial uses. These targets included methods for producing and purifying HA and industrial prospects for accountable commercial goods.
Production Strategies
Different HA production processes have been well-researched and employed for mass production (Fig. 1, Table 1). In the beginning, the procedure of removing HA from animal tissues was employed for laboratory tests to characterize and identify the polymer and learn more about its biological potential and applications. HA was extracted from all the vertebrate tissues and described, such as the cartilage of sharks, the pericardial fluid of the rabbit, pigskin, the umbilical cord, synovial fluid, and vitreous humor of the eye [22]. As an alternative source of animal husbandry, HA extraction from fish’s eyes was reported [6]. Pharmaceutical-grade HA was achieved in 1979, despite the several extraction techniques that had been used in the past. Balazs found an effective way to extract and purify HA from the human umbilical cords and rooster combs which became the base for the commercial fabrication of HA [13]. HA is water-soluble. Water-soluble components are mostly stored in compartmentalization or binding to membranes in the cells, so for their purification polar organic solvents are used in the extraction process, like methanol or ethanol [128]. Mostly HA when extracted is found in a complex form with different biopolymers; for instance, members of the lectican family, such as aggrecan and versican, as well as other proteoglycans, can combine to form a complex with HA outside the cell [73, 116] which is non-desirable; therefore, extracting pure and high molecular weight HA from animal tissues is a challenging procedure [22]. The first challenge is the difficult extraction processes brought on by grinding, acid treatment, and repeated extraction with organic solvents. Thus, the extraction processes have always had technological limits. This unregulated degrading process has a significant negative impact on both the yield and the polydispersity of HA [22]. Secondly, removing all the undesirable contaminants which form complexes with HA outside the cell during its isolation process [52]. Isolating HA from these complexes is a complex process that involves various purification steps including use of detergents, nonsolvent precipitation, precipitation with organic solvents, HA ion-pair precipitation, a proteolytic enzyme, and so forth [22]. Other contaminants and degradation products were removed by using ultrafiltration and chromatography techniques.
Schematic representation of commonly used methods utilized for hyaluronic acid production including extraction from animal tissues, and microbial and in vitro production [22]
Despite the thorough purification, the chances of contamination in HA extracted from animal tissues with nucleic acid and proteins are high. The nature and quantity of the contaminants can vary with the source of the extraction, as the content of proteins and nucleic acid was high in the HA extracted from bovine vitreous humor and human umbilical cord as compared to the one isolated from the bacterial capsule [127]. These impurities can lead to different diseases also, so the purification process was constantly improved over the years to accomplish the desired high standards of the product for various medical applications. An essential source for the industrial synthesis of HA is animal waste which offers up to multiple tones of pharmaceutical-grade HA every year. Diosynth of the Netherlands, Genzyme, Pfizer of the USA, and Pharmacia of Sweden are the primitive corporations that manufactured HA from animal tissue waste at the commercial level. High MW HA varying from a few thousand to 2.5 MDa is obtained from the extraction process and is available in the markets [79]. Extraction of HA from animals was the first technique implied. Biotechnological production of HA was an alternate process as it was cost-effective, less contamination-prone, and environmentally friendly [32, 33, 37, 96].
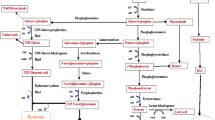
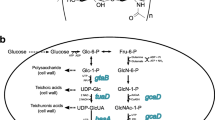
Over the past two decades, bacterial fermentation evolved as an upstanding procedure for producing HA, as shown in Table 2. Understanding their biosynthetic pathways is essential to the fermentative production of secondary metabolites (Fig. 2). HA has a sugar backbone that is made up of fructose-6-phosphate and glucose-6-phosphate. Two different sets of reactions lead to the formation of HA. In the initial set, glucose-6-phosphate is transformed into UDP-glucuronic acid in a series of reactions governed by various enzymes at each step; this is the first precursor of HA, while in the second assortment, to form the second precursor of HA, fructose-6-phosphate is transformed into UDP-N-acetylglucosamine. Therefore, the higher growth rate of cells is not suitable for HA synthesis (Fig. 3) [7].
Biosynthetic pathway for HA in S. zooepidemicus. With the help of hexokinase, glucose is first converted into glucose-6-phosphate, which indistinctly follows two different routes to form UDP-glucuronic acid and UDP-N-acetylglucosamine. With the help of HA synthase both bound together to form hyaluronic acid
Biosynthetic pathway of hyaluronic acid in S. zooepidemicus. This figure shows the conversion of glucose into hyaluronic acid as well as the microbial biomass production running parallelly [94]
Microorganism-based HA synthesis has been the subject of extensive research. Nevertheless, there are lots of limitations of organisms that may prevent its mass manufacturing, which can be avoided by using enzymes from the class I HAS and class II HAS family members in in vitro synthesis. Furthermore, due to their extremely low yields and inability to serve as a substitute for industrial production, these cell-free systems are still not optimal [36, 133]. The production of HA has been increased using specific nanoparticles and genetic engineering, which involves introducing crucial genes (such as hasA, hasB, hasS, and glmU) [133] associated with HA synthesis. The HA synthase genes from the Streptococci are incorporated into various microbial hosts which include E. coli [161], L. lactis [32], Bacillus sp. [150], and Agrobacterium sp. [100] through different plasmid vectors such as pSJR3 (co-expressing hasA, hasB, and hasC genes). HA production could sometimes be boosted sevenfold or its molecular weight increased by partially blocking the glycolytic route and deflecting carbon flux toward HA formation [124]. The main obstacle of generating HA with a comparatively uniform length has not yet been overcome.
Consequently, the medium’s viscosity at HA concentration greater than 4g/L restricts oxygen transfer, resulting in an anaerobic surrounding that regulates carbon flow more toward biomass formation than HA, resulting in polluting byproducts such as lactate [160]. Streptococci sp., peculiarly Streptococcus equi sub sp. zooepidemicus, are typically the leading producers of HA. Streptococci, a gram-positive bacterium with 49 species and eight subspecies, is exceptionally diverse and heterogeneous. It is based on serological responses to various polysaccharide compositions of cell walls to categorize the Lancefield Streptococci group. The S. zooepidemicus has been significantly explored by many research groups for HA production.
Various fermentation modes have been studied for S. zooepidemicus, including continuous, fed-batch, and repeated batch fermentation [92, 93, 95]. Regulating the bacterial growth rate utilizing continuous or fed-batch mechanisms to achieve increased metabolite yield can counteract the specific growth rate’s intrusive effect on metabolic products [92]. The traditional method of producing HA in batches [29, 102] was changed to a fed-batch module; a decrease in the fermentation time was observed with increased output [92]. Since the fermenter responds quickly, fermentation in a continuous mode for HA generation helps extend the growth cycle, minimize waste, and reduce MW polydispersity [64, 145]. To synthesize HA, a two-stage fermentation method with a fragmented control approach was used since HA chain extension happens in the primary stage of fermentation, and HA accumulation proceeds in the latter. The initial fermentation stage (31°C, pH 8.0) and the accumulation stage (37°C, pH 7.0) were designed to increase the MW of HA. The suggested two-stage fermentation produced an ideal outcome with high HA titers [91].
The HA (>1MDa) produced on an industrial scale, either by the fermentation of genetically engineered bacteria or through the extraction of animal tissue, is suitable for use in aesthetic and biological applications [94]. The potential of contamination with other viruses, which have compatibility difficulties and require time-consuming, expensive DSP removal techniques, affects the extraction of HA from animal tissue. The Streptococci C and A groups started the bacterial fermentation process that produced HA, but the toxic byproducts hampered them. The primary by-product of HA fermentation is lactic acid, and as it accumulates, cell growth and HA synthesis are severely inhibited. Acetic acid production also hampers HA production [94]. The HA biosynthesis pathway genes were inserted into the genetically engineered bacterium (Gram +ve bacteria), which began producing HA. The microbial biotechnological HA is manufactured using a B. subtilis-dependent synthesizing system that combines three overexpressed native B. subtilis precursor genes with expression constructs of the hasA gene from S. equisimilis. The HA manufactured by genetically engineered B. subtilis is recognized as GRAS because there are no exo-endo-toxins in the output streams [150]. Different plasmid vectors have been designed for the expression of HA synthase genes and to produce HA from various host bacteria. Artificial operons were created and then introduced into the B. subtilis genome using the plasmids pNNBT20 and pNNBT21, respectively, to express HA synthase proteins [150]. L. lactis has introduced the plasmids pEIrkA, pEIrkB, and pEIrkAB, each of which contains the hasA, hasB, and hasA together with hasB genes from S. equi subsp. zooepidemicus [32]. Small-scale fermenters successfully synthesize HA with genetically engineered bacterial cultures up to 6–7g/L yields. Intense media viscosity of the large-scale fermenters causes impoverished mass-transfer rates and inadequate mixing for better yields [94]. Additionally, because it relies on the growth circumstances, obtaining a monodisperse-HA in the microbial biotechnological production process is troublesome.
Due to the disadvantages of mass manufacturing, through either bacterial or animal origins, utilizing hyaluronan synthase (has), a cell-free synthesis technique has been created. Class I-has of P. multocida is integral membrane proteins that necessitate time-consuming obstructions and have impeded functions without being intimately associated with the phospholipid layer, making them the most practical options for commercial cell-free HA propagation. After the membrane motifs (residues 704–972) were deleted, class II created a soluble enzyme that catalyzes “pmhas1–703” that could manufacture the HA [71]. Class II-has-based in vitro (cell-free) production methods can combine HA oligomers to produce 1–2 MDa HA with tuned processability and low polydispersity [72]. By forbearing the accelerated accumulation of sugars at the oligomeric ends, the inclusion of HA oligomers skips the first glycosidic linkage development, which is frequently a rate-limiting step and results in the acquisition of a high MW-HA but falls short of creating larger quantities of HA. Since then, numerous researchers have experimented with cloning the genes of host bacteria (non-pathogenic), such as E. coli, that code for Class-I or -II enzymes [101]. Enzymes from classes I or II work together in bacterial expression systems to lengthen the HA polymer [161].
One of the more environmentally friendly methods for manufacturing high-value HA is the creation of HA biosynthesis pathways employing fundamental molecular building blocks in recombinant microorganisms [43]. In order to build a cell factory that produces HA using a synthetic biology technique, it is essential to understand how HA’s biosynthesis quality is primarily based on the “has” enzyme activity. A nucleoside inosine-based synthesis approach was suggested using a genome-scale model (GEM), which highlighted the need for carefully designed GEMs for further development of HA biogenesis and led to a treble enhancement in HA titer ratio [10]. Using a two-stage induction technique and two synthetic operons harboring the P. multocida hasA gene and B. subtilis hasB and hasC precursor genes, the rational design-based biogenesis, a neoteric development in synthetic biology, offers the foundation for static HA yield with 6.8 g/L. A new synthetic biology technique produces twice more substantial HA outputs by keeping up with the glucose-6-phosphate isomerase in S. zooepidemicus [30]. Carbon flow rerouting is one technique for increasing HA yields; as part of the technique, numerous HA syntheses are accomplished by reducing the expression of glycolytic pathway enzymes and enabling the basic physiological requirements of bacteria to be satisfied [162]. The expression of the driven pathway enzymes, knock-out routes, antisense RNA–mediated attenuation, and additional promoter inclusions have all been shown to work together genetically to shift the carbon flux to HA generation, culminating in a 28.7 g/L HA concentration [31]. The recommended efficient synthetic biology techniques were lab-scale results that urgently needed scaling by applying synthetic biology machinery to create dominant HA-producing microbial strains [111]. Streptomyces sp. might be utilized in a biorefinery context to establish a microbial conversion strategy for the synthesis of HA as an enhancement to the lignocellulosic biorefinery. The fermentation technical advancements toward larger HA productivity can be further expedited by creating a flawless downstream approach for expanded commercial uses of HA.
Purification Strategies
Downstream and purification operations are crucial to overcoming obstacles and creating HA with a high purity index and MW [160]. Most papers on HA downstream procedures depend on laboratory-scale procedures, whereas only a few depend on industrial-scale procedures [118]. When under physiological pH, HA’s molecular structure causes it to hold onto pollutants in its highly hydrated and negatively charged form. Since the publication of the research, HA from different origins has been used to study the purification and separation procedures involving numerous downstream processes (Fig. 4). The filtration, adsorption, precipitation, and ion exchange procedure are the HA downstream process operations that have received the most attention.
Flow sheet diagram of purification strategies for extraction of HA from the fermented broth. The purification process is divided into three main steps pre-purification, separation, and final purification process [146]
Rangaswami et al. [114] improvised a single solvent precipitation system for the purification procedure. In order to reduce the amount of solvent needed in other unit operations, single-solvent precipitation was utilized. The ability to produce pure and uniform material is the principal benefit of the precipitation process. However, the main drawback is the requirement for the separation of salts following the precipitation procedure. If the precipitation occurs intermittently, it is especially challenging to maintain stable product quality throughout the entire precipitation process. Utilizing a single solvent precipitation system decreases the proportion of salts in the solution and reduces the usage of solvent. Specifically, the solvent was used to dilute the HA in diafiltration, typically done at low concentrations to enhance the HA’s quality. It resulted in 65% of the HA recovery (Mw ̴ 4 × 106 Da) with less than 0.1% protein contamination [114]. Cleland and Sherblom [39] isolated HA from the bovine nasal septum through precipitation utilizing cetylpyridinium chloride, and 96% glucosamines were obtained [39].
Similarly, Amagai et al. utilized the cetylpyridinium chloride precipitation technique for HA extraction from fish eyeballs, yielding 10.5 mg of hyaluronan from a single tuna eye [6]. The method of precipitating human umbilical cord remains using ammonium quaternary salt solution after treating it with sodium chloride solution was developed by Lago et al. The hyaluronan ammonium quaternary salt complex is then dissociated from the solid using a calcium chloride solution, and ethanol is used to precipitate it [81]. Yang and Lee used the co-precipitation method to aid HA recovery by conjugating chitosan with magnetic nanoparticles. Here, a pH lower than a point of zero charges favored capturing of HA; a total of about 39 mg per gram of particle was captured at pH 6 [157].
Organic solvent-based purification techniques were once considered expensive for large-scale downstream operations. As precipitation results are very effective, organic solvents are frequently used in laboratory-level HA production studies. However, the cost is unsuitable for abundant quantities; many research reports suggest membrane technologies like membrane filtration (MF), ultrafiltration (UF), or diafiltration (DF) are the best for large-scale HA purification instead of using organic solvents. It has been found that tangential flow MF and UF effectively separate HA from S. zooepidemicus broth medium. At this stage, a serial procedure using microfiltration and ultrafiltration membranes was used that produced not only a high yield (89%) but also used less water [164]. In a different method, DF was used to extract HA that was produced by microbes. The highest purity grade (about 90%), with a yield greater than 90%, was reached after seven diavolumes. For example, HA purity stayed unchanged with six diavolumes, whereas HA production declined by 20% after ten diavolumes. The number of diavolumes explicitly characterized HA’s evolution in yield and purity. Controlling the concentration of salts in the solution was essential for the DF because salts could change the structure of hyaluronan due to the electrostatic shielding of the carboxyl groups [107].
In a study, purification of HA was carried out by passing the HA solution through a filter (0.22mm) and then UF and DF were carried out utilizing a 300-kDa membrane for the further sterilization of HA after dilution with pyrogen free water [74]. The relevant impact of electrostatic interactions between membrane materials and the solutes must be considered when working with the purification and recovery of molecules via membrane processes [27, 59]. A polyethersulfone UF membrane cassette with a nominal molecular weight cutoff (NMWCO) of 300 kDa was used for HA separation under a pressure range of 1-0-1.5 bar. After that, a 0.22-m cellulose acetate filter was used to filter the diafiltered broth after it had been subjected to an adsorbent treatment of 1% activated charcoal for 2–3 h while being continuously stirred. The end outcomes revealed a high-purity HA with a MW ranging from 0.6 to 1.8 MDa and an overall HA yield of roughly 0.8–1.0 g/L [113].
A low-cost procedure utilizing fish eyeballs is used to produce HA with a clinical grade purity (more than 99.5%) by utilizing DF methodology [105]. In order to improve the accumulation of the final yield and lessen environmental problems associated with conventional methods, electrofiltration has been investigated as a post-synthesis step for HA. Comparing electrofiltration-based HA extraction to filtration tests without an electrical field while maintaining the same molecular weight and structure has increased concentration factors [56].
The capacity of chemicals to be selectively retained on the surface of porous substances serves as the foundation for the adsorption process. Adsorption is typically utilized in batch mode to purify HA, followed by precipitation and filtration. Activated charcoal, silica gel, alumina, and resins are the most common adsorbents utilized for HA purification [35, 60, 114]. With a yield of 2.3 g/L, the glucuronic acid imprinted particles can be reused repeatedly without significantly decreasing adsorption capabilities [2]. In a different method, a stiff and durable substance was made for the separation and isolation of HA by fusing the mechanical properties of cryogel with the discriminating of glucuronic acid imprinted polymer particles, and HA was isolated from the fish eye and microbially fermented broth [139]. Wibowo and Lee carried out the HA adsorption with maximum adsorption capacities of 184 mg/g and 351 mg/g for Si-Quaternary ammonium-containing compounds and choline surface-functionalized cotton fibers, respectively [148]. In the situation of high levels of contamination, HA could be efficiently retrieved from a B. subtilis culture with a 15 mg/g capacity utilizing Si-QAC-modified antimicrobial cotton fiber.
The effectiveness of electrophoresis, a technique frequently used for protein separation and identification, depends on the gel employed as well as the density, size, and purity of the target molecules. Because electrophoresis has a poor protein removal capability compared to other processes, it is uncommon to employ it for HA purification. Hong et al. achieved HA quantification through capillary electrophoresis; hyaluronate oligomers (80 kDa) were accomplished by using columns packed with a highly viscous polyacrylamide matrix [61]. Grundmann et al. demonstrated that strong electrical field strengths, short-length, and small-ID capillaries, as well as an adjusted buffer composition, may be used to achieve high separation efficiency in conjunction with quick migration times. Additional tests revealed no worsening effects when injecting samples containing protein, and the approach was effectively used on hyaluronan digest samples [57]. In order to purify and recover HA, at the commencement of the processing stage, Murado et al. documented employing protein electrodeposition in conjunction with diafiltration [105].
Pure HA is a commodity employed in the pharmaceutical, medical, biomaterial, and cosmetic industries, which are discussed in the subsequent sections.
Applications
Its elemental composition and physical attributes determine the end-use of HA and characteristics like viscoelasticity, lubricity, biocompatibility, immunostimulation, and many more properties (Fig. 5, Table 3). OA treatment, joint injections, eye, and plastic surgery, components for skin burns, and anti-aging treatments all contain HA [1, 42, 43, 47, 117]. Due to its role in the extracellular matrix and the variety of derivatization scenarios it can undergo, HA is frequently employed in drug delivery through various channels, including cutaneous, topical, and ocular (intravitreal, periocular, subretinal), oral, and nasal. HA can be integrated into various molecular architectures or coupled with therapeutic molecules (as prodrugs) (microparticles, nanoparticles, gels, microspheres, polymersomes, polyplexes, micelles, liposomes, implants, and many more). Higher therapeutic efficacy and improved physicochemical characteristics are found in the HA constructs. Targeting for skin disorders, the regulated release of proteins, cancer therapy, antibiotics, and antiseptics are just a few examples of how HA is used in drug delivery [19, 48, 50, 62, 63, 138, 142].
Applications of hyaluronic acid in different biomedical aspects [22]
The realm of oncology is another area where HA may see more application. The stroma of many tumors and the surrounding tissue matrix exhibit an elevated HA concentration, which poses a challenge. Tumors of epithelial origin have a particularly notable rise in HA levels. A dismal prognosis typically accompanies this process. Apoptosis, drug resistance, and invasiveness are all caused by an increase in HA production. Increased interstitial pressure is linked to an increase in HA in the tumor environment; the blood vessels can narrow due to this condition. This condition causes drug resistance and hypoxia. In addition to the aforementioned physicochemical characteristics, HA plays a significant part in the physiology of tumors, particularly concerning its impact on the receptors of tumor cells [67, 70, 80]. This information can result in the development of several HA-based cancer therapeutic strategies. First, it is essential to mention the conjugation of paclitaxel (PXT) and docetaxel (DOX). Paclitaxel (PXT) and docetaxel (DXT) are anti-cancer chemotherapy drugs. Due to its hydrophobicity and undesirable side effects, PXT alone is not suited for intravenous administration [9, 83, 154]. The PXT-HA combination appears to overcome restrictions and is sufficiently hydrophilic. Hydrophobic drug molecules can be added to HA micelles for targeted drug delivery to cancer cells.
Drugs that are hydrophilic and lipophilic can both be put into polymersomes. The primary benefits of the previously described structural modulations are the improvement in solubility and the ability to target CD44 receptors on tumor cells. When HA is used to alter mesoporous silica nanoparticles, cells overexpressing CD44 are more likely to take them up. Dendrimers and liposomes are different nanomaterials with the potential to be effective in cancer treatment. Moreover, HA-coated nanoparticles are highly desirable for cancer treatment. NIR-loaded nanoparticles, oxide nanoparticles, gold nanoparticles, Prussian Blue nanoparticles, functionalized graphene, and other particles are employed in hyperthermia, which increases the temperature of tumor cells to around 42–46°C (related to magnetic hyperthermia treatment). Immunotherapy, photodynamic, and sonodynamic therapy also used HA-based nanoparticles [34, 76,77,78, 85, 89, 149].
As HA occurs naturally in the joint capsule, synovial fluid, and articular cartilage, orthopedics frequently uses it. This substance mainly treats joint conditions like OA or rheumatoid arthritis. The most prevalent joint disease, OA, causes significant impairment and reduces the caliber of living. In this disorganization, there is an imbalance in the middle of the formation and degradation of articular cartilage, with the latter occurring more frequently [58]. In this state, intra-articular modifications such as a reduction in GAG, a growth in proteoglycans and collagen-degrading enzymes, and maybe a rise in deposited water may be observed. Endogenous HA changes depend on variations in its molecular weight and amount [15, 104]. Reactive oxygen species are produced in more significant quantities as a result of inflammation, and they are what cause collagen, laminin, and HA to break down [17]. HA is a high molecular mass molecule naturally occurring in synovial fluid and can neutralize free radicals [120]. By reducing chemotaxis and migration of inflammatory cells, high molecular mass HA serves as an excellent barrier to the inflammatory process.
The crucial issue about the etiopathogenesis of OA relates to the inhibitory and stimulatory effects of HA on chondrocyte death and proteoglycan production, respectively. HA directly contributes to the analgesic action [14, 15, 54, 103, 104]. The therapeutic effects (pain reduction) of intra-articular HA preparations are well tolerated and supported by randomized trials [4, 104]. The absence of systemic side effects from intra-articular injections is a crucial benefit [132]. Patients are increasingly interested in oral HA administration in addition to HA injections. On the other hand, oral formulations do not have any proven therapeutic benefits for OA. According to the research that is now available on oral HA formulations, oral supplementation may reduce pain and improve quality of life.
Numerous uses for HA exist in ophthalmology, both from a conservative and from a practical standpoint. Due to its viscoelastic characteristics, it is widely employed as the “lubricant” component and frequently makes up most artificial tear formulations used to treat dry eyes. It soothes discomfort, hydrates the eye, and makes up for any sodium hyaluronate deficiency in the tear film. The substance is frequently offered in an unpreserved form. People who wear contact lenses utilize eye drops. The symptoms of dry eye are significantly lessened by its noteworthy qualities, which include securing the tear film, reducing friction while blinking, and preventing dangerous particles from adhering to the eye. More than 50% of respondents say they no longer want to wear contact lenses due to dry eye. Because most ophthalmic solutions contain artificial ingredients and preservatives, they leave residues on the eye’s surface, making it easy to distinguish treatments containing HA from other eye drops. Additionally, the fluid frequently does not disperse evenly on the eye’s surface, resulting in visible blurring and decreased vision. Because HA is hydrophilic and viscoelastic, it reduces friction and slows the evaporation of tears. HA replaces water in these medications because they do not dilate conjunctival blood vessels, making them safe to use during the winter [79, 98].
Orthokeratology is a method of treating refractive errors in patients by having them wear a specific lens nightlong. Viscous artificial tears (established upon HA) were superior to the saline solution when used to fit orthokeratology lenses. Amido bonds were used to bind nisin to HA. This modified polysaccharide’s biocidal capacity (added in gels or solutions) was testified on Gram-positive microorganisms with encouraging outcomes. In order to prevent infections, eye surgery was carried out using HA conjugated with ciprofloxacin and vancomycin. HA has an important role in the rapid restoration of a healthy ocular epithelium and it also improves the amount of moisture retention and hence relieve dry eye syndrome after surgery; it can also be added to artificial tears [20, 110, 121]. Since HA is found in the vitreous humor of the eye, it can be utilized in the artificial vitreous humor [112, 123]. Because of its biocompatibility and biodegradability, HA can substitute silicone oil in vitrectomy and prevent adverse effects, cytotoxicity, silicone oil emulsification, and second surgery [16]. Because of their many advantages, it is common for cataract surgeries to use ophthalmic viscoelastic devices (OVDs).
On the other hand, prolonged OVD retention durations may increase intraocular pressure (IOP). IOP did not significantly increase using two OVDs (Healon 5, 2.3% sodium hyaluronate Healon GV, and 1.8% sodium hyaluronate). The most popular treatment for dry eye conditions is artificial tears. Comparing HA and carmellose (carboxymethylcellulose)-based tears to regular saline solution, researchers found that the latter was superior in terms of tear film stability and visual clarity [19, 26, 88, 99, 141, 163].
HA is currently among the active components in cosmetic drafting that are most frequently used. Both industry professionals and consumers are constantly interested in the general perception of skin regeneration. It is obvious that HA is one of the critical components of good skin and serves as a health indicator for individuals [18]. Today, research is being done to produce biopolymers with the proper molecular weight. Studies in the literature suggest that this particular element depends precisely on biological processes. Even though HA was created long ago, its physical, chemical, and biological characteristics still need to be studied [152]. The nasolabial folds and wrinkles can be removed, the horizontal forehead lines can be reduced, the eyebrows can be raised, the nose can be positioned, the lips’ shape and volume can be changed, the cheeks and chin can be modeled, and the body contouring (enlarging and modeling the thighs, breasts, buttocks, and calves) can all result in beautiful results. More recently, boosting the shape of the labia has also been shown to be effective (labiaplasty). The fill effect lasts for around 6 months after intradermal or subcutaneous injections of small amounts of HA. Products for the eyes, face, neck, and body, as well as in anticellulite and anti-stretch mark cosmetics, use a high molecular mass HA composition to build a protective layer that makes skin texture smoother and feel softer to the touch [75, 136].
Numerous in vitro and in vivo studies have demonstrated the efficacy of HA therapy, including its skin regeneration, chondroprotective, anti-aging, anti-inflammatory, and immunosuppressive properties. Hyaluronan has many uses, but further study and technical advancement are still required to understand some current problems fully. In order to understand the numerous biological functions and predict the consequences that can fluctuate with the molecular mass of HA, additional thought must first be given to features of HA metabolism and receptor clustering analysis. Diverse molecular weights of HA can be included in some medications and cosmetic products. Therefore, research must determine molecular weight’s relevance to HA’s effects. The primary goal is to develop next-generation products with high biocompatibility, a prolonged half-life, and permanent in situ performance using HA-conjugated polymers. In order to properly designate the efficacy profile and safety of these drugs, a clinical investigation is essential. So far, the safety and effectiveness of these exciting and innovative substances have been the subject of encouraging in vitro studies [50, 142].
Industrial and Market Aspects
The first HA product extracted from rooster combs patented was Healon [13]. The product was a huge success when it was utilized as a viscoelastic material to replace and replenish the deficiency of vitreous body fluid following an eye operation. This non-inflammatory HA product’s introduction into the market led the way for many other HA-derived products that are beneficial in pharmaceutical, cosmetic, and biomedical applications. US Food and Drug Administration approved the pioneer single-injection HA visco-supplementation product Synvisc-One, which Genzyme manufactured in 2009. It gained massive popularity because of its appurtenance and efficacy in alleviating pain in knee joints due to OA. Following the significant quantum leap, the demand for easily usable HA-derived products has increased in several parts of the world, specifically Asia-Pacific and Europe. The first HA dermal filler Restylane was created to treat minor to severe wrinkles and folds. This product grew incredibly popular and is now widely used in over 65 nations. The use of a production technique known as NASHA (non-animal stabilized HA), which forgoes the utilization of animal tissues when HA is removed, is its distinguishing feature [140]. Many other HA products are on the market, such as wound dressing equipment to assist wound medication after surgery, skin moisturizers [79], and scaffolds for administered drug release and tissue engineering [3].
A collaborative industry-academic research team predicted that the worldwide market for HA would be over $1 billion in 2005 after discovering that genetically engineered B. subtilis can yield HA with a molecular weight of over 1000 kDa [150]. Hyaluronan’s efficacy, lack of toxicity, and soaring future demand are driving the market, which grew to $9.1 billion in 2020 and was anticipated to grow to $17 billion by 2027, expanding at a compound annual growth rate of 8.1% [129]. Pharmaceutical-grade hyaluronan’s export price in China, where most bioproduct is now produced, ranged between $2700 and $50,000 per kilogram in 2020, based on pureness and molecular mass [38]. Around 500 million people worldwide suffer from knee OA, approximately 7% of the global population, so the demand for viscosupplements has increased rapidly. The viscosupplementation market has seen a growth of approx one point three billion in the past decade and is estimated to rise at a CAGR of 6.36% through 2025 [115]. In the USA, Synvisc-One, the first single-injection HA viscosupplementation merchandise, was authorized in February 2009, and since then, its acceptance and demand for the product by medical and patients have been rapid due to its convenience [94]. The convenience of HA single injection was studied and was validated for its good efficacy, tolerance, and safety of one bigger dosage of knee intra-articular injection by comparing a single 5-ml dose regime with typical three doses of 2.5 ml of intra-articular HA administered at weekly intervals [131]. A simple cost analysis study also revealed that single injections are cheaper than the 2-injection method [21]. In 2015, GenVisc 850 of Orthogen Rx got FDA approval for OA-suffering patients. By the name of brand ADANT, outside the USA, it has been endorsed in 60 other countries with the help of Meiji Seika Pharma, and its partners are distributed well [129]. Bestowing to the American Society of Plastic Surgeons (ASAPS), dermal fillers are the utmost prominent and nonsurgical injectable method, significantly applied to rectify the soft tissue defects of the face. HA injections are simple to use, productive, and also cost-effective (in comparison to surgeries). The aesthetic sentience is expected to boost the HA market in the coming years.
Future perspectives-
Due to its numerous cosmetic, pharmacological, and medicinal applications, HA is experiencing a considerable increase in market demand. It is thought to contribute to virulence and is the primary element of group A Streptococcal extracellular capsule. Additionally, fermentation reactions using renewable carbon crude materials as a source of carbon and nitrogen could lower production costs while having a more negligible carbon impact on fulfilling the expanding demand for HA. Cetylpyridinium chloride and cold ethanol (4 °C) are widely employed in the downstream process to extract the higher purity HA on a laboratory scale. A low molecular weight of HA is produced using isopropanol as the solvent before long ultrafiltration (100 kDa) and activated carbon adsorption, which lowers the cost of producing HA on an industrial scale. To produce HA, the techno-economic study recommends employing fed-batch manufacturing, wild strains, and solvent recycling after recovery.
HA has some remarkable properties, and with the help of those, it has proven to be exceptional for numerous and distinct applications in medical, cosmetics, and pharmaceutics. Tremendous advancements have been made in the last few years in all HA-related domains, ranging from diverse HA oligomer and polymer synthesis techniques to the evolution of the top statistics for use in medicine. Most recent achievements in the yield of HA and, more specifically, the illustration of the biosynthetic pathways in microorganisms producing HA brings up undiscovered routes for the advanced optimization of the biotechnological processes used for the synthesis of HA with secured hosts. Furthermore, knowledge of the process of regulation of molecular weight (in vivo) of HA has profited the biotechnological yield of the well-defined HA merchandise with parochial polydispersity. Inflamed by the evolution of the technology of producing HA and increased knowledge of the HA biological functions, research to gain ground on current medical supplies and to accrediting the new ideas in medical treatments will be set off.
Conclusion
The significant improvements in manufacturing and extracting HA from various sources with its application and industrial scenario are explained in this paper. HA is utilized substantially in the pharmaceutical, medical, biomaterial, and cosmetic industries due to its hydrophilic biopolymer with exceptional biocompatibility, viscoelasticity qualities, and non-immunogenic and water retention capabilities. If natural microbe selection, fermentation, and downstream qualities are considered, fermentation is the practical method for producing HA and can satisfy industrial expectations. Microbial fermentation appears to be the reassuring route for yielding high-MW HA as of right now. Even though there are many sources of HA, the variety of bacteria that can make this chemical encourages more extraordinary efforts in this area. The primary pre-downstream procedures involve standard unit operations like centrifugation and numerous precipitations with polar solvents when discussing the principal strategies for extraction and purification.
In contrast, size exclusion chromatography is the most popular final purification technique. As demonstrated, hyaluronic acid’s effectiveness mostly depends on its molecular weight, with various effects, including moisturizing, renewing, and anti-aging. It is necessary to give more consideration to HA metabolism, receptor clustering studies, and explanations of the multiple biological alterations and potential outcomes connected to HA’s molecular weight. It is necessary to conduct more research on the mechanism by which HA exerts its biological effects, probable applicant for drug-delivery formulations, and biomaterial entities with potential HA utilization domain extensions.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- HA:
-
Hyaluronic acid
- MW:
-
Molecular weight
- OA:
-
Osteoarthritis
- MWCO:
-
Molecular weight cutoff
- UDP:
-
Uridine diphosphate
- TMP:
-
Transmembrane pressure
- OVD:
-
Ophthalmic viscoelastic devices
- GAG:
-
Glycosaminoglycan
References
Abdallah, M. M., Fernández, N., Matias, A. A., & do Rosário Bronze, M. (2020). Hyaluronic acid and Chondroitin sulfate from marine and terrestrial sources: Extraction and purification methods. Carbohydrate Polymers, 243, 116441.
Akdamar, H. A., Sarıözlü, N. Y., Özcan, A. A., Ersöz, A., Denizli, A., & Say, R. (2009). Separation and purification of hyaluronic acid by glucuronic acid imprinted microbeads. Materials Science and Engineering: C, 29, 1404–1408.
Allison, D. D., & Grande-Allen, K. J. (2006). Hyaluronan: A powerful tissue engineering tool. Tissue engineering, 12, 2131–2140.
Altman, R. (1998). Intra-articular sodium hyaluronate (Hyalgan®) in. The Journal of Rheumatology, 25, 2203–2212.
Amado, I. R., Vázquez, J. A., Pastrana, L., & Teixeira, J. A. (2016). Cheese whey: A cost-effective alternative for hyaluronic acid production by Streptococcus zooepidemicus. Food Chemistry, 198, 54–61.
Amagai, I., Tashiro, Y., & Ogawa, H. (2009). Improvement of the extraction procedure for hyaluronan from fish eyeball and the molecular characterization. Fisheries Science, 75, 805–810.
Armstrong, D., Cooney, M., & Johns, M. (1997). Growth and amino acid requirements of hyaluronic-acid-producing Streptococcus zooepidemicus. Applied Microbiology and Biotechnology, 47, 309–312.
Arslan, N. P., & Aydogan, M. N. (2021). Evaluation of sheep wool protein hydrolysate and molasses as low-cost fermentation substrates for hyaluronic acid production by Streptococcus zooepidemicus ATCC 35246. Waste and Biomass Valorization, 12, 925–935.
Auzenne, E., Ghosh, S. C., Khodadadian, M., Rivera, B., Farquhar, D., Price, R. E., Ravoori, M., Kundra, V., Freedman, R. S., & Klostergaard, J. (2007). Hyaluronic acid-paclitaxel: antitumor efficacy against CD44 (+) human ovarian carcinoma xenografts. Neoplasia, 9, 479–486.
Badri, A., Raman, K., & Jayaraman, G. (2019). Uncovering novel pathways for enhancing hyaluronan synthesis in recombinant Lactococcus lactis: Genome-scale metabolic modeling and experimental validation. Processes, 7, 343.
Bajaj, G., Kim, M. R., Mohammed, S. I., & Yeo, Y. (2012). Hyaluronic acid-based hydrogel for regional delivery of paclitaxel to intraperitoneal tumors. Journal of Controlled Release, 158, 386–392.
Balazs, E., & Sweeney, D. (1968). New and controversial aspects of retinal detachment. Edited by: McPherson A. Harper and Row.
Balazs, E. A. (1979). Ultrapure hyaluronic acid and the use thereof. Google Patents.
Balazs, E. A. and Laurent, T. (1998) Chemistry, biology and medical applications of hyaluronan and its derivatives .
Barbucci, R., Lamponi, S., Borzacchiello, A., Ambrosio, L., Fini, M., Torricelli, P., & Giardino, R. (2002). Hyaluronic acid hydrogel in the treatment of osteoarthritis. Biomaterials, 23, 4503–4513.
Barth, H., Crafoord, S., Andréasson, S., & Ghosh, F. (2016). A cross-linked hyaluronic acid hydrogel (Healaflow®) as a novel vitreous substitute. Graefe's Archive for Clinical and Experimental Ophthalmology, 254, 697–703.
Bates, E., Harper, G., Lowther, D., & Preston, B. (1984). Effect of oxygen-derived reactive species on cartilage proteoglycan-hyaluronate aggregates. Biochemistry international, 8, 629–637.
Baumann, L. S., & Baumann, L. (2009). Cosmetic dermatology. McGraw-Hill Professional Publishing.
Bayer, I. S. (2020). Hyaluronic acid and controlled release: A review. Molecules, 25, 2649.
Beck, R., Stachs, O., Koschmieder, A., Mueller-Lierheim, W. G., Peschel, S., & van Setten, G.-B. (2019). Hyaluronic acid as an alternative to autologous human serum eye drops: Initial clinical results with high-molecular-weight hyaluronic acid eye drops. Case Reports in Ophthalmology, 10, 244–255.
Belzile, E. L., Deakon, R. T., Vannabouathong, C., Bhandari, M., Lamontagne, M., & McCormack, R. (2017). Cost-utility of a single-injection combined corticosteroid-hyaluronic acid formulation vs a 2-injection regimen of sequential corticosteroid and hyaluronic acid injections. Clinical Medicine Insights: Arthritis and Musculoskeletal Disorders, 10, 1179544117712993.
Boeriu, C. G., Springer, J., Kooy, F. K., van den Broek, L. A., & Eggink, G. (2013). Production methods for hyaluronan. International Journal of Carbohydrate Chemistry, 2013.
Bronstone, A., Neary, J. T., Lambert, T. H., & Dasa, V. (2019). Supartz (sodium hyaluronate) for the treatment of knee osteoarthritis: A review of efficacy and safety. Clinical Medicine Insights: Arthritis and Musculoskeletal Disorders, 12, 1179544119835221.
Bui, H. T., Friederich, A. R., Li, E., Prawel, D. A., & James, S. P. (2018). Hyaluronan enhancement of expanded polytetrafluoroethylene cardiovascular grafts. Journal of Biomaterials Applications, 33, 52–63.
Cai, S., Thati, S., Bagby, T. R., Diab, H.-M., Davies, N. M., Cohen, M. S., & Forrest, M. L. (2010). Localized doxorubicin chemotherapy with a biopolymeric nanocarrier improves survival and reduces toxicity in xenografts of human breast cancer. Journal of Controlled Release, 146, 212–218.
Carracedo, G., Villa-Collar, C., Martin-Gil, A., Serramito, M., & Santamaría, L. (2018). Comparison between viscous teardrops and saline solution to fill orthokeratology contact lenses before overnight wear. Eye & contact lens, 44, S307–S311.
Castro-Muñoz, R., García-Depraect, O., León-Becerril, E., Cassano, A., Conidi, C., & Fíla, V. (2021). Recovery of protein-based compounds from meat by-products by membrane-assisted separations: A review. Journal of Chemical Technology & Biotechnology, 96, 3025–3042.
Chahuki, F. F., Aminzadeh, S., Jafarian, V., Tabandeh, F., & Khodabandeh, M. (2019). Hyaluronic acid production enhancement via genetically modification and culture medium optimization in Lactobacillus acidophilus. International Journal of Biological Macromolecules, 121, 870–881.
Chen, S.-J., Chen, J.-L., Huang, W.-C., & Chen, H.-L. (2009). Fermentation process development for hyaluronic acid production by Streptococcus zooepidemicus ATCC 39920. Korean Journal of Chemical Engineering, 26, 428–432.
Chen, W. Y., Marcellin, E., Hung, J., & Nielsen, L. K. (2009). Hyaluronan molecular weight is controlled by UDP-N-acetylglucosamine concentration in Streptococcus zooepidemicus. Journal of Biological Chemistry, 284, 18007–18014.
Cheng, F., Yu, H., & Stephanopoulos, G. (2019). Engineering Corynebacterium glutamicum for high-titer biosynthesis of hyaluronic acid. Metabolic Engineering, 55, 276–289.
Chien, L.-J., & Lee, C.-K. (2007). Hyaluronic acid production by recombinant Lactococcus lactis. Applied Microbiology and Biotechnology, 77, 339–346.
Chien, L. J., & Lee, C. K. (2007). Enhanced hyaluronic acid production in Bacillussubtilis by coexpressing bacterial hemoglobin. Biotechnology Progress, 23, 1017–1022.
Chis, A. A., Dobrea, C., Morgovan, C., Arseniu, A. M., Rus, L. L., Butuca, A., Juncan, A. M., Totan, M., Vonica-Tincu, A. L., & Cormos, G. (2020). Applications and limitations of dendrimers in biomedicine. Molecules, 25, 3982.
Choi, S., Choi, W., Kim, S., Lee, S.-Y., Noh, I., & Kim, C.-W. (2014). Purification and biocompatibility of fermented hyaluronic acid for its applications to biomaterials. Biomaterials Research, 18, 1–10.
Chong, B. F., Blank, L. M., Mclaughlin, R., & Nielsen, L. K. (2005). Microbial hyaluronic acid production. Applied Microbiology and Biotechnology, 66, 341–351.
Chong, B. F., & Nielsen, L. K. (2003). Aerobic cultivation of Streptococcus zooepidemicus and the role of NADH oxidase. Biochemical Engineering Journal, 16, 153–162.
Ciriminna, R., Scurria, A., & Pagliaro, M. (2021). Microbial production of hyaluronic acid: the case of an emergent technology in the bioeconomy. Biofuels, Bioproducts and Biorefining, 15, 1604–1610.
Cleland, R. L., & Sherblom, A. P. (1977). Isolation and physical characterization of hyaluronic acid prepared from bovine nasal septum by cetylpyridinium chloride precipitation. Journal of Biological Chemistry, 252, 420–426.
Cowman, M. K., Lee, H.-G., Schwertfeger, K. L., McCarthy, J. B., & Turley, E. A. (2015). The content and size of hyaluronan in biological fluids and tissues. Frontiers in Immunology, 6, 261.
Cui, X., Huang, C., Chen, Z., Zhang, M., Liu, C., Su, K., Wang, J., Li, L., Wang, R., & Li, B. (2021). Hyaluronic acid facilitates bone repair effects of calcium phosphate cement by accelerating osteogenic expression. Bioactive Materials, 6, 3801–3811.
De Bartolo, L., Leindlein, A., Hofmann, D., Bader, A., de Grey, A., Curcio, E., & Drioli, E. (2012). Bio-hybrid organs and tissues for patient therapy: A future vision for 2030. Chemical Engineering and Processing: Process Intensification, 51, 79–87.
de Oliveira, J. D., Carvalho, L. S., Gomes, A. M. V., Queiroz, L. R., Magalhães, B. S., & Parachin, N. S. (2016). Genetic basis for hyper production of hyaluronic acid in natural and engineered microorganisms. Microbial Cell Factories, 15, 1–19.
DeAngelis, P. L., Jing, W., Drake, R. R., & Achyuthan, A. M. (1998). Identification and molecular cloning of a unique hyaluronan synthase from Pasteurella multocida. Journal of Biological Chemistry, 273, 8454–8458.
DeAngelis, P. L., Papaconstantinou, J., & Weigel, P. H. (1993). Isolation of a Streptococcus pyogenes gene locus that directs hyaluronan biosynthesis in acapsular mutants and in heterologous bacteria. Journal of Biological Chemistry, 268, 14568–14571.
Don, M. M., & Shoparwe, N. F. (2010). Kinetics of hyaluronic acid production by Streptococcus zooepidemicus considering the effect of glucose. Biochemical Engineering Journal, 49, 95–103.
Dovedytis, M., Liu, Z. J., & Bartlett, S. (2020). Hyaluronic acid and its biomedical applications: A review. Engineered Regeneration, 1, 102–113.
Dubashynskaya, N., Poshina, D., Raik, S., Urtti, A., & Skorik, Y. A. (2019). Polysaccharides in ocular drug delivery. Pharmaceutics, 12, 22.
Fagien, S., & Cassuto, D. (2012). Reconstituted injectable hyaluronic acid: expanded applications in facial aesthetics and additional thoughts on the mechanism of action in cosmetic medicine. Plastic and Reconstructive Surgery, 130, 208–217.
Fallacara, A., Baldini, E., Manfredini, S., & Vertuani, S. (2018). Hyaluronic acid in the third millennium. Polymers, 10, 701.
Flores-Méndez, D. A., Ramos-Ibarra, J. R., Toriz, G., Arriola-Guevara, E., Guatemala-Morales, G., & Corona-González, R. I. (2021). Bored coffee beans for production of hyaluronic acid by Streptococcus zooepidemicus. Fermentation, 7, 121.
Fraser, J. R. E., Laurent, T. C., & Laurent, U. (1997). Hyaluronan: its nature, distribution, functions and turnover. Journal of internal medicine, 242, 27–33.
Gedikli, S., Güngör, G., Toptaş, Y., Sezgin, D. E., Demirbilek, M., Yazıhan, N., Aytar Çelik, P., Denkbaş, E. B., Bütün, V., & Çabuk, A. (2018). Optimization of hyaluronic acid production and its cytotoxicity and degradability characteristics. Preparative Biochemistry and Biotechnology, 48, 610–618.
Ghosh, P., Holbert, C., Read, R., & Armstrong, S. (1995). Hyaluronic acid (hyaluronan) in experimental osteoarthritis. The Journal of rheumatology, 43, 155–157.
Goa, K. L., & Benfield, P. (1994). Hyaluronic acid. Drugs, 47, 536–566.
Gözke, G., Kirschhöfer, F., Prechtl, C., Brenner-Weiss, G., Krumov, N. V., Obst, U., & Posten, C. (2017). Electrofiltration improves dead-end filtration of hyaluronic acid and presents an alternative downstream processing step that overcomes technological challenges of conventional methods. Engineering in Life Sciences, 17, 970–975.
Grundmann, M., Rothenhöfer, M., Bernhardt, G., Buschauer, A., & Matysik, F.-M. (2012). Fast counter-electroosmotic capillary electrophoresis–time-of-flight mass spectrometry of hyaluronan oligosaccharides. Analytical and Bioanalytical Chemistry, 402, 2617–2623.
Gupta, S., Hawker, G., Laporte, A., Croxford, R., & Coyte, P. C. (2005). The economic burden of disabling hip and knee osteoarthritis (OA) from the perspective of individuals living with this condition. Rheumatology, 44, 1531–1537.
Hadidi, M., Buckley, J. J., & Zydney, A. L. (2016). Effect of electrostatic interactions on the ultrafiltration behavior of charged bacterial capsular polysaccharides. Biotechnology Progress, 32, 1531–1538.
Han, H.-Y., Jang, S.-H., Kim, E.-C., Park, J.-K., Han, Y.-J., Lee, C., Park, H.-S., Kim, Y.-C., & Park, H.-J. (2009). Microorganism producing hyaluronic acid and purification method of hyaluronic acid. Google Patents.
Hong, M., Sudor, J., Stefansson, M., & Novotny, M. V. (1998). High-resolution studies of hyaluronic acid mixtures through capillary gel electrophoresis. Analytical Chemistry, 70, 568–573.
How, K. N., Yap, W. H., Lim, C. L. H., Goh, B. H., & Lai, Z. W. (2020). Hyaluronic acid-mediated drug delivery system targeting for inflammatory skin diseases: A mini review. Frontiers in Pharmacology, 11, 1105.
Huang, G., & Huang, H. (2018). Application of hyaluronic acid as carriers in drug delivery. Drug Delivery, 25, 766–772.
Huang, W.-C., Chen, S.-J., & Chen, T.-L. (2008). Production of hyaluronic acid by repeated batch fermentation. Biochemical Engineering Journal, 40, 460–464.
Izawa, N., Hanamizu, T., Sone, T., & Chiba, K. (2010). Effects of fermentation conditions and soybean peptide supplementation on hyaluronic acid production by Streptococcus thermophilus strain YIT 2084 in milk. Journal of Bioscience and Bioengineering, 109, 356–360.
Izawa, N., Serata, M., Sone, T., Omasa, T., & Ohtake, H. (2011). Hyaluronic acid production by recombinant Streptococcus thermophilus. Journal of Bioscience and Bioengineering, 111, 665–670.
Jacobetz, M. A., Chan, D. S., Neesse, A., Bapiro, T. E., Cook, N., Frese, K. K., Feig, C., Nakagawa, T., Caldwell, M. E., & Zecchini, H. I. (2013). Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut, 62, 112–120.
Jagannath, S., & Ramachandran, K. (2010). Influence of competing metabolic processes on the molecular weight of hyaluronic acid synthesized by Streptococcus zooepidemicus. Biochemical Engineering Journal, 48, 148–158.
Jeong, E., Shim, W. Y., & Kim, J. H. (2014). Metabolic engineering of Pichia pastoris for production of hyaluronic acid with high molecular weight. Journal of Biotechnology, 185, 28–36.
Jiang, P., Li, X., Thompson, C. B., Huang, Z., Araiza, F., Osgood, R., Wei, G., Feldmann, M., Frost, G. I., & Shepard, H. M. (2012). Effective targeting of the tumor microenvironment for cancer therapy. Anticancer Research, 32, 1203–1212.
Jing, W., & DeAngelis, P. L. (2000). Dissection of the two transferase activities of the Pasteurella multocida hyaluronan synthase: two active sites exist in one polypeptide. Glycobiology, 10, 883–889.
Jing, W., & DeAngelis, P. L. (2004). Synchronized chemoenzymatic synthesis of monodisperse hyaluronan polymers. Journal of Biological Chemistry, 279, 42345–42349.
Kakehi, K., Kinoshita, M., & Yasueda, S.-I. (2003). Hyaluronic acid: separation and biological implications. Journal of Chromatography B, 797, 347–355.
Kanala, J. R., Panati, K., & Narala, V. R. (2011). Efficient recovery of hyaluronic acid from highly viscous culture broth. IUP Journal of Life Sciences, 5, 15–20.
Kanchwala, S. K., Holloway, L., & Bucky, L. P. (2005). Reliable soft tissue augmentation: A clinical comparison of injectable soft-tissue fillers for facial-volume augmentation. Annals of Plastic Surgery, 55, 30–35.
Kim, J. H., Moon, M. J., Kim, D. Y., Heo, S. H., & Jeong, Y. Y. (2018). Hyaluronic acid-based nanomaterials for cancer therapy. Polymers, 10, 1133.
Kim, K., Choi, H., Choi, E. S., Park, M.-H., & Ryu, J.-H. (2019). Hyaluronic acid-coated nanomedicine for targeted cancer therapy. Pharmaceutics, 11, 301.
Kim, S., Moon, M. J., Poilil Surendran, S., & Jeong, Y. Y. (2019). Biomedical applications of hyaluronic acid-based nanomaterials in hyperthermic cancer therapy. Pharmaceutics, 11, 306.
Kogan, G., Šoltés, L., Stern, R., & Gemeiner, P. (2007). Hyaluronic acid: A natural biopolymer with a broad range of biomedical and industrial applications. Biotechnology Letters, 29, 17–25.
Kultti, A., Li, X., Jiang, P., Thompson, C. B., Frost, G. I., & Shepard, H. M. (2012). Therapeutic targeting of hyaluronan in the tumor stroma. Cancers, 4, 873–903.
Lago, G., Oruna, L., Cremata, J. A., Pérez, C., Coto, G., Lauzan, E., & Kennedy, J. F. (2005). Isolation, purification and characterization of hyaluronan from human umbilical cord residues. Carbohydrate Polymers, 62, 321–326.
Laurent, T. C., Laurent, U. B., & Fraser, J. R. E. (1996). The structure and function of hyaluronan: An overview. Immunology and Cell Biology, 74, a1–a7.
Lee, H., Lee, K., & Park, T. G. (2008). Hyaluronic acid− paclitaxel conjugate micelles: Synthesis, characterization, and antitumor activity. Bioconjugate Chemistry, 19, 1319–1325.
Lee, H., Mok, H., Lee, S., Oh, Y.-K., & Park, T. G. (2007). Target-specific intracellular delivery of siRNA using degradable hyaluronic acid nanogels. Journal of Controlled Release, 119, 245–252.
Lee, S. Y., Kang, M. S., Jeong, W. Y., Han, D.-W., & Kim, K. S. (2020). Hyaluronic acid-based theranostic nanomedicines for targeted cancer therapy. Cancers, 12, 940.
Leighton, R., Fitzpatrick, J., Smith, H., Crandall, D., Flannery, C. R., & Conrozier, T. (2018). Systematic clinical evidence review of NASHA (Durolane hyaluronic acid) for the treatment of knee osteoarthritis. Open Access Rheumatology: Research and Reviews, 10, 43.
Leite, M. N., & Frade, M. A. C. (2021). Efficacy of 0.2% hyaluronic acid in the healing of skin abrasions in rats. Heliyon, 7, e07572.
Lequeux, I., Ducasse, E., Jouenne, T., & Thebault, P. (2014). Addition of antimicrobial properties to hyaluronic acid by grafting of antimicrobial peptide. European Polymer Journal, 51, 182–190.
Li, M., Sun, J., Zhang, W., Zhao, Y., Zhang, S., & Zhang, S. (2021). Drug delivery systems based on CD44-targeted glycosaminoglycans for cancer therapy. Carbohydrate Polymers, 251, 117103.
Li, R., Liu, H., Huang, H., Bi, W., Yan, R., Tan, X., Wen, W., Wang, C., Song, W., & Zhang, Y. (2018). Chitosan conduit combined with hyaluronic acid prevent sciatic nerve scar in a rat model of peripheral nerve crush injury. Molecular Medicine Reports, 17, 4360–4368.
Liu, J., Wang, Y., Li, Z., Ren, Y., Zhao, Y., & Zhao, G. (2018). Efficient production of high-molecular-weight hyaluronic acid with a two-stage fermentation. RSC Advances, 8, 36167–36171.
Liu, L., Du, G., Chen, J., Wang, M., & Sun, J. (2008). Enhanced hyaluronic acid production by a two-stage culture strategy based on the modeling of batch and fed-batch cultivation of Streptococcus zooepidemicus. Bioresource Technology, 99, 8532–8536.
Liu, L., Du, G., Chen, J., Wang, M., & Sun, J. (2009). Comparative study on the influence of dissolved oxygen control approaches on the microbial hyaluronic acid production of Streptococcus zooepidemicus. Bioprocess and Biosystems Engineering, 32, 755–763.
Liu, L., Liu, Y., Li, J., Du, G., & Chen, J. (2011). Microbial production of hyaluronic acid: current state, challenges, and perspectives. Microbial Cell Factories, 10, 99.
Liu, L., Sun, J., Xu, W., Du, G., & Chen, J. (2009). Modeling and optimization of microbial hyaluronic acid production by Streptococcus zooepidemicus using radial basis function neural network coupling quantum-behaved particle swarm optimization algorithm. Biotechnology Progress, 25, 1819–1825.
Liu, L., Wang, M., Du, G., & Chen, J. (2008). Enhanced hyaluronic acid production of Streptococcus zooepidemicus by an intermittent alkaline-stress strategy. Letters in Applied Microbiology, 46, 383–388.
Ludwig, A., & Van Ooteghem, M. (1989). Evaluation of sodium hyaluronate as viscous vehicle for eye drops. Journal de Pharmacie de Belgique, 44, 391–397.
Maltese, A., Borzacchiello, A., Mayol, L., Bucolo, C., Maugeri, F., Nicolais, L., & Ambrosio, L. (2006). Novel polysaccharides-based viscoelastic formulations for ophthalmic surgery: Rheological characterization. Biomaterials, 27, 5134–5142.
Malvankar-Mehta, M. S., Fu, A., Subramanian, Y., & Hutnik, C. (2020). Impact of ophthalmic viscosurgical devices in cataract surgery. Journal of Ophthalmology, 2020.
Mao, Z., & Chen, R. R. (2007). Recombinant synthesis of hyaluronan by Agrobacterium sp. Biotechnology Progress, 23, 1038–1042.
Mao, Z., Shin, H.-D., & Chen, R. (2009). A recombinant E. coli bioprocess for hyaluronan synthesis. Applied Microbiology and Biotechnology, 84, 63–69.
Marcellin, E., Chen, W. and Nielsen, L. K. (2009) Microbial hyaluronic acid biosynthesis.
Moore, A., & Willoughby, D. (1995). Hyaluronan as a drug delivery system for diclofenac: A hypothesis for mode of action. International Journal of Tissue Reactions, 17, 153–156.
Moreland, L. W. (2003). Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: Mechanisms of action. Arthritis Research and Therapy, 5, 1–14.
Murado, M., Montemayor, M., Cabo, M., Vázquez, J., & González, M. (2012). Optimization of extraction and purification process of hyaluronic acid from fish eyeball. Food and Bioproducts Processing, 90, 491–498.
O'Regan, M., Martini, I., Crescenzi, F., De Luca, C., & Lansing, M. (1994). Molecular mechanisms and genetics of hyaluronan biosynthesis. International Journal of Biological Macromolecules, 16, 283–286.
Oueslati, N., Leblanc, P., Bodin, A., Harscoat-Schiavo, C., Rondags, E., Meunier, S., Marc, I., & Kapel, R. (2015). A simple methodology for predicting the performances of hyaluronic acid purification by diafiltration. Journal of Membrane Science, 490, 152–159.
Pan, N. C., Pereira, H. C. B., da Silva, M. D. L. C., Vasconcelos, A. F. D., & Celligoi, M. A. P. C. (2017). Improvement production of hyaluronic acid by Streptococcus zooepidemicus in sugarcane molasses. Applied Biochemistry and Biotechnology, 182, 276–293.
Pape, L. G., & Balazs, E. A. (1980). The use of sodium hyaluronate (Healon®) in human anterior segment surgey. Ophthalmology, 87, 699–705.
Pinto-Fraga, J., López-de la Rosa, A., Blázquez Arauzo, F., Urbano Rodríguez, R., & González-García, M. J. (2017). Efficacy and safety of 0.2% hyaluronic acid in the management of dry eye disease. Eye & Contact Lens: Science & Clinical Practice, 43, 57–63.
Pourzardosht, N., & Rasaee, M. J. (2017). Improved yield of high molecular weight hyaluronic acid production in a stable strain of Streptococcus zooepidemicus via the elimination of the hyaluronidase-encoding gene. Molecular biotechnology, 59, 192–199.
Raia, N. R., Jia, D., Ghezzi, C. E., Muthukumar, M., & Kaplan, D. L. (2020). Characterization of silk-hyaluronic acid composite hydrogels towards vitreous humor substitutes. Biomaterials, 233, 119729.
Rajendran, V., Puvendran, K., Guru, B. R., & Jayaraman, G. (2016). Design of aqueous two-phase systems for purification of hyaluronic acid produced by metabolically engineered Lactococcus lactis. Journal of Separation Science, 39, 655–662.
Rangaswamy, V., & Jain, D. (2008). An efficient process for production and purification of hyaluronic acid from Streptococcus equi subsp. zooepidemicus. Biotechnology letters, 30, 493–496.
Raulkar, K. P. (2020). Viscosupplementation. Regenerative Medicine for Spine and Joint Pain, 29–41.
Reddy, K. J., & Karunakaran, K. (2013). Purification and characterization of hyaluronic acid produced by Streptococcus zooepidemicus strain 3523-7. Journal of BioScience & Biotechnology, 2.
Robert, L. (2015). Hyaluronan, a truly “youthful” polysaccharide. Its medical applications. Pathologie Biologie, 63, 32–34.
Rodriguez-Marquez, C. D., Arteaga-Marin, S., Rivas-Sánchez, A., Autrique-Hernández, R., & Castro-Muñoz, R. (2022). A review on current strategies for extraction and purification of hyaluronic acid. International Journal of Molecular Sciences, 23, 6038.
Rohit, S. G., Jyoti, P. K., Subbi, R. R. T., Naresh, M., & Senthilkumar, S. (2018). Kinetic modeling of hyaluronic acid production in palmyra palm (Borassus flabellifer) based medium by Streptococcus zooepidemicus MTCC 3523. Biochemical Engineering Journal, 137, 284–293.
Saari, H., & Konttinen, Y. (1989). Determination of synovial fluid hyaluronate concentration and polymerisation by high performance liquid chromatography. Annals of the rheumatic diseases, 48, 565–570.
Salzillo, R., Schiraldi, C., Corsuto, L., D’Agostino, A., Filosa, R., De Rosa, M., & La Gatta, A. (2016). Optimization of hyaluronan-based eye drop formulations. Carbohydrate polymers, 153, 275–283.
Schiraldi, C., Annalisa, L., & Mario, D. (2010). In M. Elnashar (Ed.), ISBN: 978-953-307-109-1 Biotechnological production and application of hyaluronan, biopolymers. InTech.
Schramm, C., Spitzer, M. S., Henke-Fahle, S., Steinmetz, G., Januschowski, K., Heiduschka, P., Geis-Gerstorfer, J., Biedermann, T., Bartz-Schmidt, K. U., & Szurman, P. (2012). The cross-linked biopolymer hyaluronic acid as an artificial vitreous substitute. Investigative Ophthalmology & Visual Science, 53, 613–621.
Shah, M. V., Badle, S. S., & Ramachandran, K. (2013). Hyaluronic acid production and molecular weight improvement by redirection of carbon flux towards its biosynthesis pathway. Biochemical Engineering Journal, 80, 53–60.
Shelma, R. (2022). A holistic and integrated approach to lifestyle diseases (pp. 319–332). Apple Academic Press.
Shi, L. (2016). Bioactivities, isolation and purification methods of polysaccharides from natural products: A review. International Journal of Biological Macromolecules, 92, 37–48.
Shiedlin, A., Bigelow, R., Christopher, W., Arbabi, S., Yang, L., Maier, R. V., Wainwright, N., Childs, A., & Miller, R. J. (2004). Evaluation of hyaluronan from different sources: Streptococcus z ooepidemicus, rooster comb, bovine vitreous, and human umbilical cord. Biomacromolecules, 5, 2122–2127.
Shimizu, Y., & Li, B. (2006). Natural products isolation (pp. 415–438). Springer.
Size, H. A. M. (2020). Share & trends analysis report by application (dermal fillers, osteoarthritis (single injection, three injection, five injection), ophthalmic, vesicoureteral reflux), by region, and segment forecasts, 2020–2027 (p. 150). Grand View Research.
Sunguroğlu, C., Sezgin, D. E., Aytar Çelik, P., & Çabuk, A. (2018). Higher titer hyaluronic acid production in recombinant Lactococcus lactis. Preparative Biochemistry and Biotechnology, 48, 734–742.
Suppan, V. K. L., Wei, C. Y., Siong, T. C., Mei, T. M., Chern, W. B., Nanta Kumar, V. K., Sheng, K. R., & Sadashiva Rao, A. (2017). Randomized controlled trial comparing efficacy of conventional and new single larger dose of intra-articular viscosupplementation in management of knee osteoarthritis. Journal of Orthopaedic Surgery, 25, 2309499017731627.
Szabó, A., Zelkó, R., & Antal, I. (2011). Treatment of rheumatic diseases with intraarticular drug delivery systems. Acta Pharmaceutica Hungarica, 81, 77–86.
Sze, J. H., Brownlie, J. C., & Love, C. A. (2016). Biotechnological production of hyaluronic acid: A mini review. 3 Biotech, 6, 1–9.
Tammi, R. H., Kultti, A., Kosma, V.-M., Pirinen, R., Auvinen, P., & Tammi, M. I. (2008). Hyaluronan in human tumors: Pathobiological and prognostic messages from cell-associated and stromal hyaluronan. In Seminars in cancer biology (pp. 288–295). Elsevier.
Tarricone, E., Elia, R., Mattiuzzo, E., Faggian, A., Pozzuoli, A., Ruggieri, P., & Brun, P. (2021). The viability and anti-inflammatory effects of hyaluronic acid-chitlac-tracimolone acetonide-β-cyclodextrin complex on human chondrocytes. Cartilage, 13, 920S–924S.
Tezel, A., & Fredrickson, G. H. (2008). The science of hyaluronic acid dermal fillers. Journal of Cosmetic and Laser Therapy, 10, 35–42.
Thomas, R. G., Moon, M., Lee, S., & Jeong, Y. Y. (2015). Paclitaxel loaded hyaluronic acid nanoparticles for targeted cancer therapy: In vitro and in vivo analysis. International Journal of Biological Macromolecules, 72, 510–518.
Trombino, S., Servidio, C., Curcio, F., & Cassano, R. (2019). Strategies for hyaluronic acid-based hydrogel design in drug delivery. Pharmaceutics, 11, 407.
Ünlüer, Ö. B., Ersöz, A., Denizli, A., Demirel, R., & Say, R. (2013). Separation and purification of hyaluronic acid by embedded glucuronic acid imprinted polymers into cryogel. Journal of Chromatography B, 934, 46–52.
van Eijk, T., & Braun, M. (2007). A novel method to inject hyaluronic acid: The Fern Pattern Technique. Journal of Drugs in Dermatology: JDD, 6, 805–808.
Vandermeer, G., Chamy, Y., & Pisella, P.-J. (2018). Comparison of objective optical quality measured by double-pass aberrometry in patients with moderate dry eye: Normal saline vs. artificial tears: A pilot study. Journal Francais d'ophtalmologie, 41, e51–e57.
Vasvani, S., Kulkarni, P., & Rawtani, D. (2020). Hyaluronic acid: A review on its biology, aspects of drug delivery, route of administrations and a special emphasis on its approved marketed products and recent clinical studies. International Journal of Biological Macromolecules, 151, 1012–1029.
Vázquez, J. A., Montemayor, M. I., Fraguas, J., & Murado, M. A. (2010). Hyaluronic acid production by Streptococcus zooepidemicus in marine by-products media from mussel processing wastewaters and tuna peptone viscera. Microbial Cell Factories, 9, 1–10.
Wang, J., He, W., Wang, T., Li, M., & Li, X. (2021). Sucrose-modified iron nanoparticles for highly efficient microbial production of hyaluronic acid by Streptococcus zooepidemicus. Colloids and Surfaces B: Biointerfaces, 205, 111854.
Wang, S., Zhang, J., Wang, Y., & Chen, M. (2016). Hyaluronic acid-coated PEI-PLGA nanoparticles mediated co-delivery of doxorubicin and miR-542-3p for triple negative breast cancer therapy. Nanomedicine: Nanotechnology. Biology and Medicine, 12, 411–420.
Wang, Y., Zhang, J., & Liu, H. (2014). Separation and purification of hyaluronic acid from fermentation broth. In Proceedings of the 2012 International Conference on Applied Biotechnology (ICAB 2012) (pp. 1523–1530). Springer.
Wessels, M. R., Moses, A. E., Goldberg, J. B., & DiCesare, T. J. (1991). Hyaluronic acid capsule is a virulence factor for mucoid group A streptococci. Proceedings of the National Academy of Sciences, 88, 8317–8321.
Wibowo, D., & Lee, C.-K. (2010). Nonleaching antimicrobial cotton fibers for hyaluronic acid adsorption. Biochemical Engineering Journal, 53, 44–51.
Wickens, J. M., Alsaab, H. O., Kesharwani, P., Bhise, K., Amin, M. C. I. M., Tekade, R. K., Gupta, U., & Iyer, A. K. (2017). Recent advances in hyaluronic acid-decorated nanocarriers for targeted cancer therapy. Drug discovery today, 22, 665–680.
Widner, B., Behr, R., Von Dollen, S., Tang, M., Heu, T., Sloma, A., Sternberg, D., DeAngelis, P. L., Weigel, P. H., & Brown, S. (2005). Hyaluronic acid production in Bacillus subtilis. Applied and environmental microbiology, 71, 3747–3752.
Willoughby, D. A. (1994) First International Workshop on Hyaluronan in Drug Delivery: Proceedings of an Extended Panel Discussion Held in Windsor, UK on 29 September, 1992. ed. Royal Society of Medicine Services with financial support from Hyal.
Witting, M., Boreham, A., Brodwolf, R., Vavrova, K., Alexiev, U., Friess, W., & Hedtrich, S. (2015). Interactions of hyaluronic acid with the skin and implications for the dermal delivery of biomacromolecules. Molecular pharmaceutics, 12, 1391–1401.
Woo, J. E., Seong, H. J., Lee, S. Y., & Jang, Y.-S. (2019). Metabolic engineering of Escherichia coli for the production of hyaluronic acid from glucose and galactose. Frontiers in Bioengineering and Biotechnology, 7, 351.
Wu, J., Deng, C., Meng, F., Zhang, J., Sun, H., & Zhong, Z. (2017). Hyaluronic acid coated PLGA nanoparticulate docetaxel effectively targets and suppresses orthotopic human lung cancer. Journal of Controlled Release, 259, 76–82.
Yamada, T., & Kawasaki, T. (2005). Microbial synthesis of hyaluronan and chitin: New approaches. Journal of Bioscience and Bioengineering, 99, 521–528.
Yamasaki, K., Drolle, E., Nakagawa, H., Hisamura, R., Ngo, W., & Jones, L. (2021). Impact of a low molecular weight hyaluronic acid derivative on contact lens wettability. Contact Lens and Anterior Eye, 44, 101334.
Yang, P.-F., & Lee, C.-K. (2007). Hyaluronic acid interaction with chitosan-conjugated magnetite particles and its purification. Biochemical Engineering Journal, 33, 284–289.
Yang, Q., Xie, Z., Hu, J., & Liu, Y. (2021). Hyaluronic acid nanofiber mats loaded with antimicrobial peptide towards wound dressing applications. Materials Science and Engineering: C, 128, 112319.
Yao, J., Fan, Y., Du, R., Zhou, J., Lu, Y., Wang, W., Ren, J., & Sun, X. (2010). Amphoteric hyaluronic acid derivative for targeting gene delivery. Biomaterials, 31, 9357–9365.
Yao, Z.-Y., Qin, J., Gong, J.-S., Ye, Y.-H., Qian, J.-Y., Li, H., Xu, Z.-H., & Shi, J.-S. (2021). Versatile strategies for bioproduction of hyaluronic acid driven by synthetic biology. Carbohydrate Polymers, 264, 118015.
Yu, H., & Stephanopoulos, G. (2008). Metabolic engineering of Escherichia coli for biosynthesis of hyaluronic acid. Metabolic Engineering, 10, 24–32.
Zhang, W., Sun, Q., Hao, S., Geng, J., & Lv, C. (2016). Experimental study on the variation of physical and mechanical properties of rock after high temperature treatment. Applied Thermal Engineering, 98, 1297–1304.
Zhang, Z., Suner, S. S., Blake, D. A., Ayyala, R. S., & Sahiner, N. (2020). Antimicrobial activity and biocompatibility of slow-release hyaluronic acid-antibiotic conjugated particles. International Journal of Pharmaceutics, 576, 119024.
Zhou, H., Ni, J., Huang, W., & Zhang, J. (2006). Separation of hyaluronic acid from fermentation broth by tangential flow microfiltration and ultrafiltration. Separation and Purification Technology, 52, 29–38.
Acknowledgements
It is acknowledged that the Central Instrument Facility Center (CIFC) of the Indian Institute of Technology (BHU) Varanasi has offered technical assistance.
Author information
Authors and Affiliations
Contributions
PS designed and completed the manuscript. All authors supervised, read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable
Consent for Publication
Not applicable
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
•In the medical, pharmaceutical, and polymer sectors, HA, a glycosaminoglycan, holds a prominent position.
•The ideal method for producing sustainable HA is microbial production.
•Due to their advantages in downstream processing, precipitation and ultrafiltration are frequent processes.
•HA production potential and the industrial-scale scenario are discussed.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shukla, P., Sinha, R., Anand, S. et al. Tapping on the Potential of Hyaluronic Acid: from Production to Application. Appl Biochem Biotechnol 195, 7132–7157 (2023). https://doi.org/10.1007/s12010-023-04461-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04461-6